Introduction
Carcinoma of the tongue is the most common malignant
tumor in the oral and maxillofacial region, of which tongue
squamous cell carcinoma (TSCC) is the most prevalent, seriously
affecting the patients' quality of life (1). The underlying mechanism of occurrence
and development of TSCC has been a focus of oral and maxillofacial
surgery; however, additional studies are required to understand the
etiology to improve treatment and prognosis of TSCC (2).
The tumor microenvironment (TME) includes tumor
cells and their surrounding cellular and non-cellular components. T
cells, granulocytes and myeloid-derived suppressor cells (MDSC) all
serve a role in the formation of the TME (2). In addition, non-immune cells, protein
molecules, inflammatory cytokines and chemokines are also present
in the TME (2–4). The TME makeup is dynamic with the
nature of the matrix and cells present constantly changing. This
results in a large number of immunosuppressive cells such as Treg,
MDSC, tumor infiltrating lymphocyte and inflammatory molecules and
mediators such as interleukin (IL)-6, IL-10 and transforming growth
factor (TGF)-β aggregating in the TME (5). The body mobilizes a wide array of
immunosuppressive strategies to control and limit tumor
development. In addition, cancer cells can activate various
signaling factors through a series of pathways to avoid destruction
resulting in immune escape (6–8).
Regulatory T cells (Treg) are a subset of immune
cells with immunoregulatory effects. These cells primarily secrete
IL-10 and TGF-β, which exhibit a strong immunosuppressive function
and serve an important role in tumor immune escape (9). T helper cell 17 (Th17) is another
recently discovered subgroup of CD4+ T cells named due to their
characteristic secretion of IL-17. Th17 cells exhibit a strong
pro-inflammatory effect and participate in the development of many
immune-associated diseases and tumors (10). Zhou et al (11), found that, B cells convert CD4+ CD25-
T cells into Tregs in co-culture with TCSS cells and influence the
prognosis of patients with TSCC, which may be associated with the
activation of immune cells and immune escape of tumor cells.
Therefore, in the present study, the presence of Treg and Th17
cells in the peripheral blood of patients with TSCC and their
related cytokines was measured. The distribution characteristics of
Treg and Th17 cells in the TME were also examined to provide novel
insight into the development of treatments for clinical
immune-targeting TSCC therapies.
Materials and methods
Patients
A total of 40 patients with TSCC who were admitted
to The Department of Oral and Maxillofacial Surgery, Second
Affiliated Hospital of Jinzhou Medical University (Liaoning, China)
between January 2017 and June 2018 were used in the present study.
The cohort consisted of 24 males and 16 females, aged 40–76 years
old, with a median age of 54 years. The inclusion criteria were
that all patients were diagnosed with TSCC by pathology and no
radiation therapy or chemotherapy was performed prior to biopsy.
Patients with one or more of the following conditions were
excluded: i) Infectious disease; ii) acute cardiovascular and
cerebrovascular diseases; iii) rheumatic disease; iv) diabetes; and
v) other tumors. The present study was approved by The Ethical
Committee of Jinzhou Medical University (Liaoning, China). Informed
consent was obtained from all patients included in the study. In
addition, 16 healthy individuals with no statistical difference in
age and sex were used as the control group. All the participants
were informed and blood samples of patients were collected prior to
clinical treatment.
Sample collection
A total of 3 ml venous blood of each participant was
collected and heparin sodium (10 mg/ml, Sigma-Aldrich; Merck KGaA)
was used as an anticoagulant. Half of each blood sample was
centrifuged (200 × g; 10 min; room temperature) to prepare serum
samples with the remainder used to prepare peripheral blood
mononuclear cells (PBMCs). All blood samples were processed within
6 h of collection.
Hematoxylin and eosin (H&E)
staining
TSCC tissues were fixed (>24 h at room
temperature) in 4% paraformaldehyde and embedded in paraffin.
Paraffin-embedded samples were cut into 4 µm sections and resected
specimens were dewaxed in xylene, rehydrated, washed in distilled
water and then stained with hematoxylin and eosin at room
temperature for 5 min. Pathological alterations of myocardial
tissue were observed under a light microscope (magnification,
×200).
Preparation of PBMCs
PBMCs were isolated from the blood samples of
patients with TSCC and healthy controls using lysing buffer (BD
Biosciences) according to the manufacturer's protocol. Briefly, 3
ml 1× lysing solution was added to 3 ml venous blood, samples were
incubated on ice for 5 min, centrifuged at 200 × g for 5 min at
room temperature and then, the supernatant was carefully aspirated.
The PBMCs were resuspended in RPMI-1640 medium (HyClone; GE
Healthcare Life Sciences) and counted with the density of cells
adjusted to 1×106 cells/ml for cell culture or flow
cytometry analysis.
Flow cytometric analysis of cluster of
differentiation (CD)4+/CD25+/forkhead box protein 3 (Foxp3)+ Treg
cells
The PBMCs were resuspended in PBS. For surface
staining, fluorescein isothiocyanate-conjugated anti CD4 antibody
(cat. no. 555346; BD Biosciences) and allophycocyanin-conjugated
anti CD25 antibodies (cat. no. 555434; BD Biosciences) were used.
The cells were incubated at room temperature for 20 min protected
from light, washed with 1X PBS twice then fixed and permeabilized
for 40 min at room temperature using a fixation/permeabilization
kit (cat. no. 555028, BD Biosciences) according to the
manufacturer's protocol. After fixation and permeabilization, a
phycoerythrin (PE)-conjugated anti-Foxp3 antibody (cat. no.
72-5774-40; eBioscience; Thermo Fisher Scientific, Inc.) was added.
The cells were incubated at room temperature for 45 min protected
from light then washed twice. After discarding the supernatant,
cells were resuspended in 300 µl PBS for analysis using a flow
cytometer. The results were analyzed using FlowJo version 10.1
(Tree Star, Inc.).
Cell culture
PBMCs were resuspended to a density of
2×106 cells/ml, supplemented with 10% heat-inactivated
fetal bovine serum (HyClone; GE Healthcare Life Sciences) and
treated with phorbol-12-myristate-13-acetate (PMA; 10 ng/ml;
Sigma-Aldrich; Merck KGaA), ionomycin (0.5 µg/ml; Sigma-Aldrich;
Merck KGaA), and Brefedin A (1 µl/ml; BD Biosciences) in a 37°C, 5%
CO2 incubator for 5 h. The cultured cells were harvested
and stained for surface markers and intracellular IL-17a.
Flow cytometric analysis of
CD4+IL-17a+ Th17 cells
Surface staining was described as above. The cells
were washed once with PBS then fixed and permeabilized for 40 min
at room temperature using a fixation/permeabilization kit (cat. no.
555028; BD Biosciences) following the manufacturer's protocols.
Following fixation and permeabilization, anti-IL-17a PE (cat. no.
560436; BD Biosciences) was added. The cells were incubated at room
temperature for 45 min protected from light then washed twice.
After discarding the supernatant, cells were resuspended in 300 µl
PBS for flow cytometry. Data analysis was performed using FlowJo
software (version 10.6.0; FlowJo LLC).
Reverse transcription-quantitative PCR
(RT-qPCR)
PBMCs were collected and total RNA was extracted
with TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was reverse transcribed from total RNA
using a RT kit (cat. no. K1622; Thermo Fisher Scientific, Inc.),
according to the manufacturers' protocol. qPCR was performed using
SYBR Select Master mix (Thermo Fisher Scientific, Inc.), according
to the manufacturer's protocol. The primer sequences were as
follows: Foxp3 forward, 5′-GCAGCTCTCAACGGTGGAT-3′ and reverse,
5′-GGGATTTGGGAAGGTGCAGA-3′; RAR-related orphan receptor-γ (RORγt)
forward, 5′-GCCAAGGCTCAGTCATGAGA-3′ and reverse,
5′-CCTCACAGGTGATAACCCCG-3′; and GAPDH forward,
5′-TGTTGCCATCAATGACCCCTT-3′ and reverse, 5′-CTCCACGACGTACTCAGCG-3′.
The PCR thermocycling conditions were 50°C for 2 min, 95°C for 2
min, 95°C for 15 sec, and 60°C for 1 min, for a total of 40 cycles.
The 2−ΔΔCq method was used to calculate the relative
gene expression (12).
Cytometric bead array (CBA)
Serum samples were isolated from the blood of
patients with TSCC and healthy controls via centrifugation at 450 ×
g for 10 min at room temperature. IL-10 and IL-17a levels were
detected using the BD™ CBA Human Th1/Th2/Th17 Cytokine
kit (cat. no. 560484; BD Biosciences) according to the
manufacturer's protocol. IL-10 and IL-17a levels were analyzed
using FCAP Array v3.0 software (BD Biosciences).
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis was performed using GraphPad Prism
6.0 (GraphPad Software, Inc.). The data was compared using unpaired
2-tailed Student's t-test. One-way analysis of variance followed by
Kruskal-Wallis test was used to compare differences amongst
multiple groups. P<0.05 was considered to indicate statistical
significance. Experiments were performed in triplicate.
Results
Clinical characteristics of patients
with TSCC
Clinical characteristics of patients with TSCC are
presented in Table I. According to
the Tumor-Node-Metastasis (TNM) classification for TSCC [Union for
International Cancer Control, UICC 2010, 7th Edition (13)], the patients were divided into four
stages: Stage I (n=4); stage II (n=12); stage III (n=17); and stage
IV (n=7). H&E staining demonstrated that all patients had
squamous cell carcinoma (Fig.
1).
 | Table I.Characteristic features of patients
included in the present study. |
Table I.
Characteristic features of patients
included in the present study.
| Characteristics | Patients with tongue
squamous cell carcinoma | Controls |
|---|
| Age, year |
|
|
|
Range | 38–76 | 35–71 |
| Mean ±
SD | 57±9.25 | 59±11.38 |
| Smoking | 21 | 20 |
| Non-smoking | 19 | 20 |
| Drinking | 15 | 20 |
| Non-drinking | 25 | 20 |
| Local
stimulation | 5 | – |
| Residual
roots and crowns of teeth | 3 |
|
| Bad
prosthesis | 2 |
|
| Tumor location |
| – |
| Lingual
margin | 26 |
|
| Lingual
root | 8 |
|
| Ventral
of tongue | 6 |
|
| Tumor size |
| – |
| T1 | 4 |
|
| T2 | 21 |
|
| T3 | 10 |
|
| T4 | 5 |
|
| Lymph node
involvement |
| – |
| N0 | 23 |
|
| N+ | 17 |
|
| Pathological
classification |
| – |
| Squamous cell
carcinoma | 40 |
|
| Histological
classification |
| – |
| Well
differentiated | 11 |
|
|
Moderately differentiated | 24 |
|
| Poorly
differentiated | 5 |
|
| Clinical stage |
| – |
| І | 4 |
|
| II | 12 |
|
|
III | 17 |
|
| IV | 7 |
|
CD4+/CD25+/Foxp3+ Treg cells and
CD4+/IL-17a+ Th17 cells in patients with TSCC
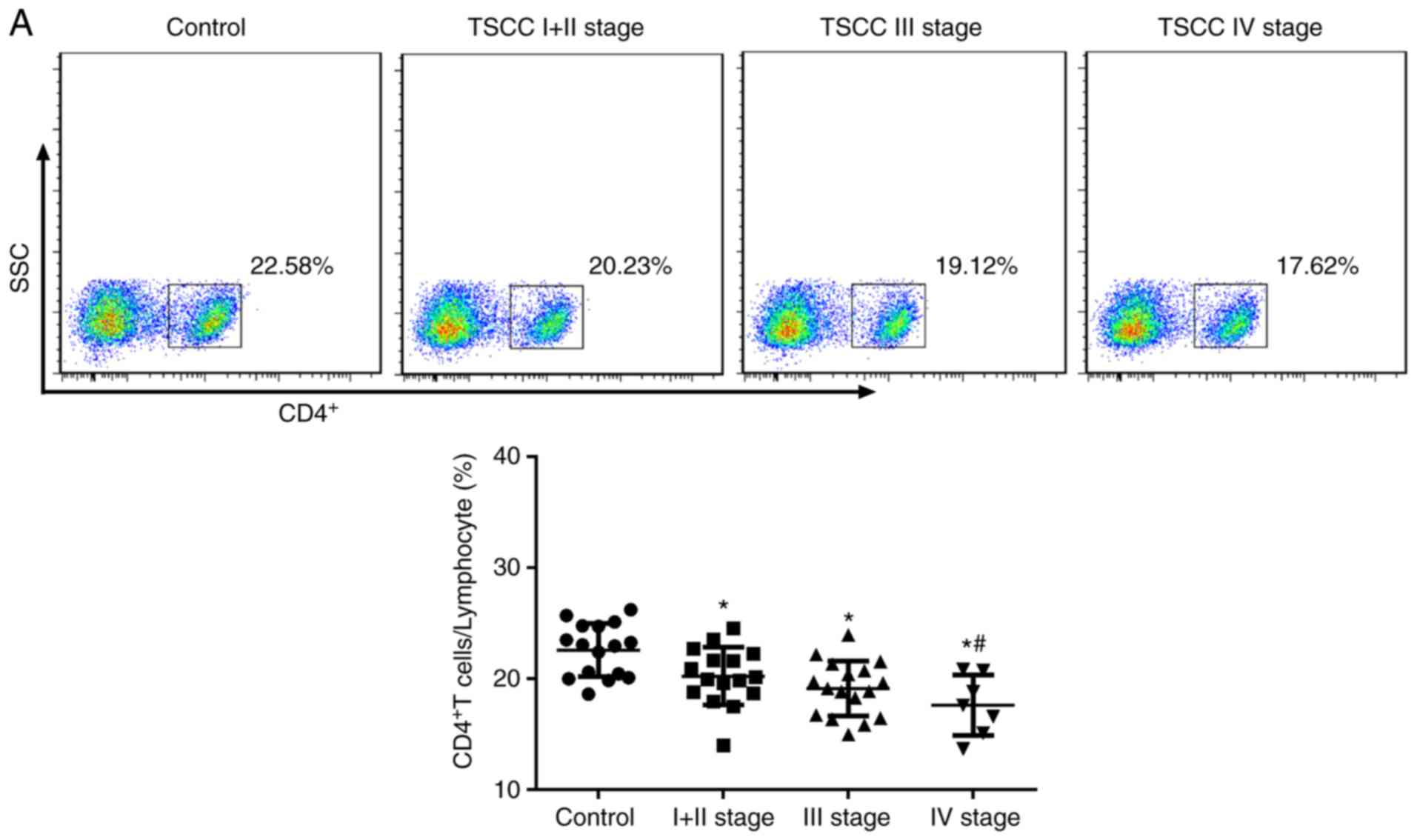
To investigate the function of the immune system in
patients with TSCC, CD4+ T cells were detected using flow
cytometry. The results showed that the expression of CD4+T cells
decreased in all TSCC patients. The capacity of patients to express
CD4+T cells was relatively low (Fig.
2A). The percentage of Treg cells in the peripheral blood of
patients with TSCC was significantly increased compared with the
control group (control, 1.70±0.44%; stage I+II, 2.37±0.58%; stage
III, 3.64±0.61%; and stage IV, 3.92±0.77%; Fig. 2B). The patients with advanced staged
cancer (III or IV) exhibited significantly increased expression
compared with patients with early stage cancer (I + II). The
percentage of Th17 cells in the peripheral blood of patients with
TSCC was significantly increased compared with the control. The
percentage of Th17 cells increased significantly in with stage of
cancer (Fig. 2C). These results
indicated that T cells and Th17 cells affected the development of
TSCC.
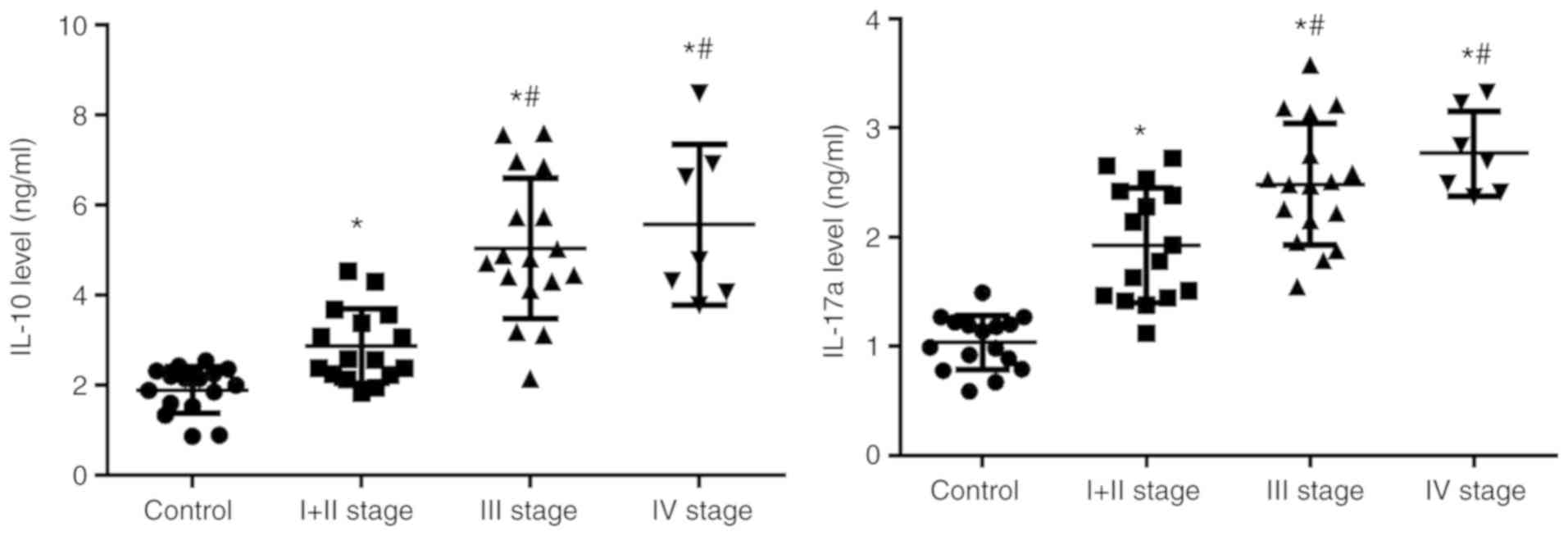
IL-10 and IL-17a expression levels
increase in patients with TSCC
Tregs primarily exert immunoregulatory effects by
producing IL-10 and Th17 cells primarily serve a role by secreting
IL-17. IL-10 and IL-17a were detected in the peripheral blood of
patients with TSCC using CBA. The results demonstrated that IL-10
(control 1.90±0.52%; stage I + II, 2.87±0.82%; stage III,
5.04±1.53%; and stage IV, 5.57±1.78%) and IL-17a (control
1.04±0.25%; stage I + II, 1.93±0.52%; stage III, 2.48±0.56%; and
stage IV, 2.77±0.29%) in peripheral blood of patients with TSCC was
significantly higher compared with the control (Fig. 3), Patients with advanced stage cancer
(III or IV) exhibited significantly increased expression compared
with patients with early stage cancer (I + II). These results
suggested that, in line with the alterations in Treg and Th17
cells, the serum levels of IL-10 and IL-17 were increased gradually
in patients with TSCC.
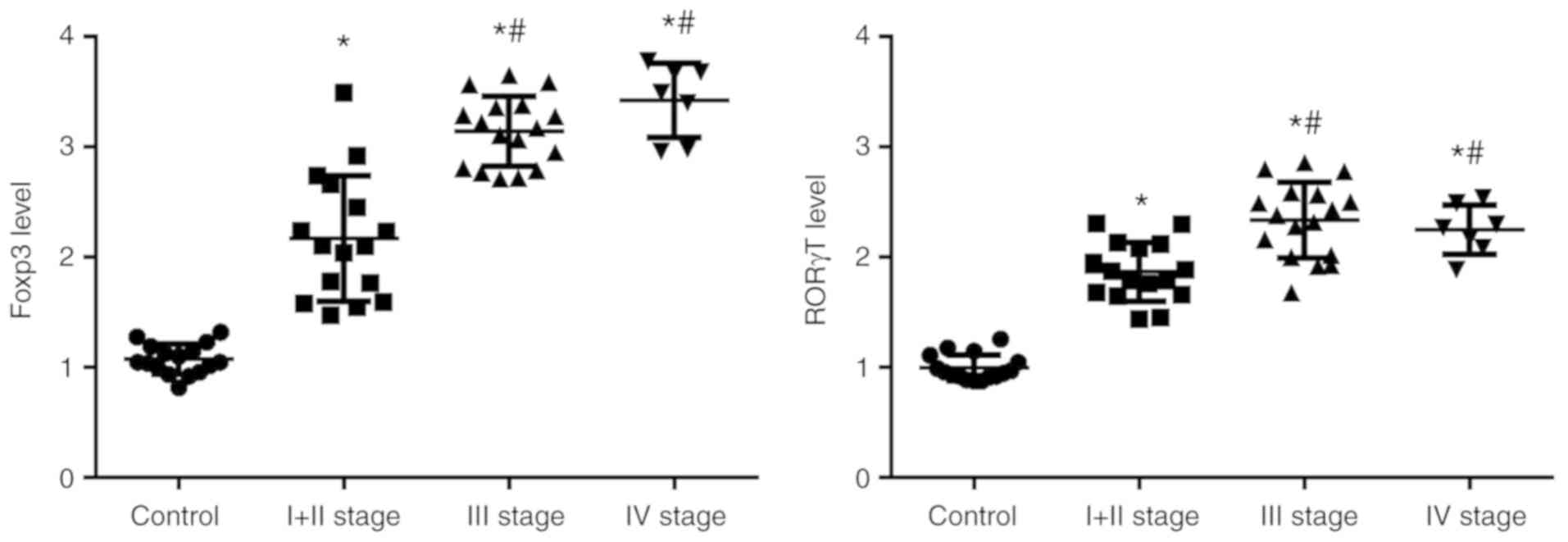
Foxp3 and RORγt levels increase in
patients with TSCC
Foxp3 and RORγt are transcription factors in Treg
and Th17 cells, respectively. The levels of Foxp3 and RORγt in
PBMCs from patients with TSCC were detected using RT-qPCR. The
results demonstrated that the levels of Foxp3 (control 1.07±0.13%;
stage I + II, 2.17±0.57%; stage III, 3.14±0.31%; and stage IV,
3.42±0.33%) and RORγt (control 0.99±0.12%; stage I + II,
1.87±0.27%; stage III, 2.34±0.34%; and stage IV, 2.25±0.23%) in
peripheral blood of patients with TSCC were significantly higher
compared with the control (Fig. 4).
Expression in patients with advanced stages of TSCC (III or IV) was
significantly higher compared with patients with early stage TSCC
(I + II). These results were consistent with the changes in Treg
cells and Th17 cells. These results further demonstrated that Treg
cells and Th17 cells affected the development of TSCC.
Discussion
The immune status of cancer patients is closely
associated with the occurrence of cancer (14). Traditionally, it is considered that
the immune response and immune surveillance can inhibit the
occurrence of tumors but increasing evidence has demonstrated that
chronic inflammation caused by the immune response may promote the
occurrence and development of tumors (14). As an important immune cell in the
tumor microenvironment, Th17 cells have strong pro-inflammatory
functions (9). Conversely, Tregs
exhibit strong immunosuppressive functions (10). Tumor cells can directly induce Treg
cells to produce chemokines, creating a favorable environment for
tumor growth and inhibiting the immune response. Therefore, Treg
cells can be used as an indicator of disease progression and
prognosis and also as a therapeutic index for TSCC (15). The Treg/Th17 cell imbalance is
associated with the development of many diseases and tumors
(16). Increasing Treg cell count
and IL-10 levels may inhibit other effector T cells, interfering
with the Treg/Th17 balance and leading to disease progression
(15). In the present study, the
Treg and Th17 cell counts were measured in the peripheral blood of
patients with TSCC, with the findings potentially providing insight
into understanding the immune response in patients with TSCC.
Tregs are a subset of cells that control the body's
autoimmune response. Numerous studies have determined that Treg
serve an important role in tumor immunity. Tregs can inhibit the
anti-tumor immune response and promote the development of an
immunosuppressive TME, thus promoting immune escape and cancer
progression (17–19). Rasku et al (20) identified that transient Treg
depletion induces regression of metastatic lesions in advanced
stage melanoma patients. Ladoire et al (21) found that Treg depletion prior to
treatment is associated with an anti-tumor immune response and
improved clinical outcomes in breast cancer patients undergoing
tumor resection and radiotherapy. The most prominent transcription
factor in Tregs is Foxp3. Recent studies have identified that Foxp3
is expressed in TSCC cell lines (22,23). In
the present study, the results demonstrated that the percentage of
Treg cells in the peripheral blood of patients with TSCC increased
significantly. Taken together, these findings may explain immune
escape and proliferation of cancer cells. However, further
experiments are required to verify this proposed mechanism.
Th17 cells are a group of cells different from Th1
and Th2 cell subsets. Th17 cells produce IL-17 to promote and
stabilize the transcription of other inflammatory factors such as
tumor necrosis factor-α to promote tissue inflammation (24,25).
Wang et al (26) determined
that the growth rate of melanoma and bladder cancer decreases in
IL-17 deficient mice. The results suggest that Th17 cells can
promote the growth of tumors. De Simone et al (27) found that Th17-type cytokines activate
signal transducer and activator of transcription 3 and NF-κB to
promote colorectal cancer cell growth. In the present study
compared with the control, the percentage of Th17 cells in the
peripheral blood of patients with TSCC was significantly increased
with the level of IL-17 produced by Th17 and the expression of
RORγt, a specific transcription factor of Th17 cells also
significantly increased. These results indicated that Th17 cells
contributed to the proliferation of tumor cells. Treg cell
transcription factor Foxp3 can bind to RORγt, thus inhibiting the
activity of RORγt. RORγt is a specific transcription factor of Th17
cells and the two transcription factors are mutually inhibited
(28,29). Under normal circumstances, Treg/Th17
can maintain balance (30). However,
when the balance of Treg/Th17 cells is lost, it may result in the
promotion and development of tumors. The results of the present
study demonstrated that Treg/Th17 cell counts and IL-10/IL-17
levels increased, providing an additional mode of analysis to
improve prediction of the prognosis of patients with TSCC.
In conclusion, the present study determined that the
percentage of Treg/Th17 cells and IL-10/IL-17 levels increased
significantly in patients with TSCC. The immune balance of Treg and
Th17 cells was lost, possibly resulting in rapid proliferation of
tumor cells. The detection of Treg and Th17 cells may be useful in
diagnosing TSCC and predicting its pathogenesis. The results of the
present study suggested that adoptive immunotherapy may be
developed through modulation of Treg and Th17 populations in the
future.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Nature Science
Foundation of Liaoning Province (grant no. 2015020326).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
CFL and ZXX conceived and designed the study. CFL
drafted the manuscript. Flow cytometry was completed by ZT. Cell
culture was completed by JYT and ZT. Reverese
transcription-quantitative PCR was performed by JYT. Data analysis
was performed by ZXX. All authors read and approved the final
version.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Jinzhou Medical University (Liaoning, China; approval
no. JZH2016052). Informed consent was obtained from all patients
included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TSCC
|
tongue squamous cell carcinoma
|
|
Treg
|
regulatory T cells
|
|
Th17
|
T helper cell 17
|
|
TME
|
tumor microenvironment
|
|
TAM
|
tumor infiltrating lymphocyte
|
|
MDSC
|
myeloid-derived suppressor cells
|
|
PBMCs
|
peripheral blood mononuclear cells
|
References
|
1
|
Yu X and Li Z: MicroRNA expression and its
implications for diagnosis and therapy of tongue squamous cell
carcinoma. J Cell Mol Med. 20:10–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Finotello F and Eduati F: Multi-omics
profiling of the tumor microenvironment: paving the way to
precision immuno-oncology. Front Oncol. 8:4302018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim J and Bae JS: Tumor-associated
macrophages and neutrophils in tumor microenvironment. Mediators
Inflamm. 2016:60581472016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang W, Chan CK, Weissman IL, Kim BYS and
Hahn SM: Immune priming of the tumor microenvironment by radiation.
Trends Cancer. 2:638–645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sahoo SS, Zhang XD, Hondermarck H and
Tanwar PS: The emerging role of the microenvironment in endometrial
cancer. Cancers (Basel). 10(pii): E4082018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steven A and Seliger B: The role of immune
escape and immune cell infiltration in breast cancer. Breast Care
(Basel). 13:16–21. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Terry S, Savagner P, Ortiz-Cuaran S,
Mahjoubi L, Saintigny P, Thiery JP and Chouaib S: New insights into
the role of EMT in tumor immune escape. Mol Oncol. 11:824–846.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spranger S: Mechanisms of tumor escape in
the context of the T-cell-inflamed and the non-T-cell-inflamed
tumor microenvironment. Int Immunol. 28:383–391. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barbi J, Pardoll D and Pan F: Treg
functional stability and its responsiveness to the
microenvironment. Immunol Rev. 259:115–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guéry L and Hugues S: Th17 Cell plasticity
and functions in cancer immunity. Biomed Res Int. 2015:3146202015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou X, Su YX, Lao XM, Liang YJ and Liao
GQ: CD19(+)IL-10(+) regulatory B cells affect survival of tongue
squamous cell carcinoma patients and induce resting CD4(+) T cells
to CD4(+)Foxp3(+) regulatory T cells. Oral Oncol. 53:27–35. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: UICC International Union Against Cancer TNM Classification of
Malignant Tumors. 7th. West Sussex, United Kingdom:
Wiley-Blackwell; 2009
|
|
14
|
Zamarron BF and Chen W: Dual roles of
immune cells and their factors in cancer development and
progression. Int J Biol Sci. 7:651–658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jadidi-Niaragh F, Ghalamfarsa G, Memarian
A, Asgarian-Omran H, Razavi SM, Sarrafnejad A and Shokri F:
Downregulation of IL-17-producing T cells is associated with
regulatory T cell expansion and disease progression in chronic
lymphocytic leukemia. Tumour Biol. 34:929–940. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duan MC, Han W, Jin PW, Wei YP, Wei Q,
Zhang LM and Li JC: Disturbed Th17/treg balance in patients with
non-small cell lung cancer. Inflammation. 38:2156–2165. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chaudhary B and Elkord E: Regulatory T
cells in the tumor microenvironment and cancer progression: Role
and therapeutic targeting. Vaccines (Basel). 4(pii): E282016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanaka A and Sakaguchi S: Regulatory T
cells in cancer immunotherapy. Cell Res. 27:109–118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elkord E, Alcantar-Orozco EM, Dovedi SJ,
Tran DQ, Hawkins RE and Gilham DE: T regulatory cells in cancer:
Recent advances and therapeutic potential. Expert Opin Biol Ther.
10:1573–1586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rasku MA, Clem AL, Telang S, Taft B,
Gettings K, Gragg H, Cramer D, Lear SC, McMasters KM, Miller DM and
Chesney J: Transient T cell depletion causes regression of melanoma
metastases. J Transl Med. 6:122008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ladoire S, Arnould L, Apetoh L, Coudert B,
Martin F, Chauffert B, Fumoleau P and Ghiringhelli F: Pathologic
complete response to neoadjuvant chemotherapy of breast carcinoma
is associated with the disappearance of tumor-infiltrating foxp3+
regulatory T cells. Clin Cancer Res. 14:2413–2420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang YJ, Lao XM, Liang LZ and Liao GQ:
Genome-wide analysis of cancer cell-derived Foxp3 target genes in
human tongue squamous cell carcinoma cells. Int J Oncol.
46:1935–1943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li K, Huang SH, Lao XM, Yang L, Liao GQ
and Liang YJ: Interaction of cancer cell-derived Foxp3 and tumor
microenvironment in human tongue squamous cell carcinoma. Exp Cell
Res. 370:643–652. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu S and Qian Y: IL-17/IL-17 receptor
system in autoimmune disease: Mechanisms and therapeutic potential.
Clin Sci (Lond). 122:487–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X and Oppenheim JJ: Th17 cells and
Tregs: Unlikely allies. J Leukoc Biol. 95:723–731. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Yi T, Kortylewski M, Pardoll DM,
Zeng D and Yu H: IL-17 can promote tumor growth through an
IL-6-Stat3 signaling pathway. J Exp Med. 206:1457–1464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Simone V, Franzè E, Ronchetti G,
Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald
TT, Pallone F, et al: Th17-type cytokines, IL-6 and TNF-α
synergistically activate STAT3 and NF-kB to promote colorectal
cancer cell growth. Oncogene. 34:3493–3503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang F, Wang D, Li Y, Sang L, Zhu J, Wang
J, Wei B, Lu C and Sun X: Th1/Th2 balance and Th17/treg-mediated
immunity in relation to murine resistance to dextran
sulfate-induced colitis. J Immunol Res. 2017:70472012017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun J, Li L, Li L, Ding L, Liu X, Chen X,
Zhang J, Qi X, Du J and Huang Z: Metallothionein-1 suppresses
rheumatoid arthritis pathogenesis by shifting the Th17/Treg
balance. Eur J Immunol. 48:1550–1562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao L, Yang J, Wang HP and Liu RY:
Imbalance in the Th17/Treg and cytokine environment in peripheral
blood of patients with adenocarcinoma and squamous cell carcinoma.
Med Oncol. 30:4612013. View Article : Google Scholar : PubMed/NCBI
|