Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic,
progressive and severe lung disease characterized by aggressive
progression and poor prognosis (1).
Although its exact etiology is poorly understood, IPF is
characterized by abnormal extracellular matrix (ECM) deposition,
including excessive collagen deposition (2,3). Lung
transplantation used to be the only effective treatment known for
IPF prior to 2014, when a novel antifibrotic agent, pirfenidone,
was approved by the Food and Drug Administration in the United
States and by the European Medicines Agency for the treatment of
IPF (4). A previous study
demonstrated that pirfenidone treatment was effective in extending
survival time and stabilizing pulmonary function in patients with
IPF (5). Pirfenidone was able to
suppress the infiltration of inflammatory cells into the
bronchoalveolar lavage fluid (BALF) and reduce the transcription of
transforming growth factor-β (TGF-β) in a hamster model of IPF
(6,7). In addition, pirfenidone could protect
mice from endotoxic shock by reducing the levels of tumor necrosis
factor-α (TNF-α) and increasing the levels of interleukin (IL)-10
(8). However, the potential
mechanism responsible for the antifibrotic properties of
pirfenidone is not fully understood.
Cannabinoid receptor 2 (CB2R) is a G protein-coupled
receptor that is predominantly expressed in the spleen and cells of
the immune system (9), although CB2R
is also expressed in non-immune cells such as pulmonary endothelial
cells (10). Unlike CB1R, CB2R
agonists do not exhibit psychoactive effects, which suggests that
targeting CB2R may be a potential treatment of a wide range of
inflammation-related diseases (11).
A previous study demonstrated that CB2R was activated in cirrhotic
livers, and CB2R−/− mice developed increased fibrosis,
indicating that CB2R may have an antifibrogenic role (12). Activation of CB2R by JWH133
alleviates bleomycin (BLM)-induced IPF in mice (13). In addition, a recent study has
demonstrated that treatment with pirfenidone for 2 years could
decrease fibrosis and cytokine levels, as well as enhance CB2R gene
expression, in patients with chronic hepatitis C (14). However, whether CB2R is involved in
the antifibrotic effect of pirfenidone remains to be fully
elucidated.
The present study aimed to investigate whether CB2R
was involved in the antifibrotic effect of pirfenidone in
BLM-induced IPF in mice. The results indicated that pirfenidone
could attenuate IPF and activate CB2R in BLM-treated mice, as well
as inhibit fibroblast cell proliferation in vitro, which
could be reversed by the CB2R antagonist SR144528. These data
indicated that activation of CB2R may be considered a mechanism of
the antifibrotic effects of pirfenidone.
Materials and methods
Animals
Male C57BL/6 mice (weight, 18–20 g; age, 8 weeks;
n=10 in each group) were provided by the Model Animal Research
Center of Nanjing University. Animals were kept under a 12-h
light/dark cycle at room temperature (22±2°C) and 55±5% humidity,
with food and water provided ad libitum. All the procedures
used in the present study were performed according to the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (15). The animal protocols
were approved by the Baodi Clinical College of Tianjin Medical
University Animal Care Committee.
Animal model of IPF
A well-established animal model of IPF was used in
the present study, as previously described (3). Animals were randomly assigned into four
groups: i) Control group, which was intravenously injected with 50
µl 0.9% saline; ii) BLM group, which was intravenously injected
with 5 mg/kg/day BLM (Nippon Kayaku Co., Ltd.) in 50 µl saline for
28 consecutive days; iii) BLM + pirfenidone group, which was orally
administered 300 mg/kg/day pirfenidone (Shionogi & Co., Ltd.)
in 0.5% carboxymethylcellulose (CMC; 40 mg/ml; 7.5 ml/kg/day,
Cayman Chemical Co.); and iv) BLM + CMC group, which was orally
administered with equal amounts of CMC for 14 days after the 28-day
treatment with BLM. The dosage was determined according to a
previous study (16). On days 0, 5,
10 and 15 since the first pirfenidone administration, mice were
sacrificed by exsanguination under deep anesthesia (sodium
pentobarbital intraperitoneal injection, 50 mg/kg). Lung tissues
were then removed from each mouse via a midline incision and the
left lung lobes were fixed in 4% paraformaldehyde (Wako Pure
Chemical Industries, Ltd.) for 48 h at room temperature, embedded
in paraffin and processed to obtain 5-µm sections for Masson's
trichrome staining, and the right lung lobes were frozen in liquid
nitrogen at −80°C for reverse transcription-quantitative PCR
(RT-qPCR) and western blotting. The time points used in the current
study were selected according to previous studies that investigated
fibrocyte accumulation ~14 days after BLM treatment (17–19).
Cell culture
The human embryonic lung fibroblast cell line WI38
was purchased from Shanghai Institutes for Biological Sciences.
WI38 cells were maintained in Dulbecco's modified Eagle medium
(DMEM) containing 10% fetal bovine serum (both Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 U/ml streptomycin at
37°C in a humidified atmosphere with 5% CO2. At 50%
confluency, the culture medium was replaced with serum-free DMEM
for 24 h prior to treatment with BALF extracted from BLM-treated
mice. BALF was prepared as previously described (20). Briefly, after 7 days of treatment
with BLM, mice were sacrificed, and a plastic cannula was inserted
into the trachea. A cold sterile saline solution was gently
injected to perform bronchoalveolar lavage (BAL). The BALF was
centrifuged at 700 × g at 4°C for 5 min, and the supernatant was
stored at −80°C until further use.
WI38 cells were incubated with an equal volume of
DMEM and BALF containing 200 µg/ml pirfenidone, 20 µM JWH-015 (a
CB2R-selective agonist) or 1 µM SR144528 (a CB2R antagonist) for 48
h at 37°C. JWH-015 and SR144528 were obtained from Cayman Chemical
Co. The concentration of drugs was determined according to a
previous study (21).
Histological analysis
Lung tissues were fixed in 4% paraformaldehyde and
then embedded in paraffin as described above. Paraffin sections
(5-µm thick) were prepared and stained with Masson's trichrome
stain at room temperature for 10 min. Stained sections were
observed using a light microscope equipped with a DFC490 digital
camera (magnification, ×400; Leica Microsystems GmbH).
RT-qPCR
Total RNA was isolated from mouse lung tissue and
WI38 cells with TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc) according to the manufacturer's
instructions. Complementary DNA synthesis was performed using the
PrimeScript™ RT Master Mix reagent kit (Takara Biotechnology Co.,
Ltd.) at the following conditions: Initial incubation at 37°C for
15 min, followed by incubation at 85°C for 5 sec, and then analyzed
using SYBR® Premix Ex Taq™ kit (Takara Biotechnology
Co., Ltd.) in a 7500 Fast Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). qPCR was performed using the
following protocol: Initial denaturation at 94°C for 1 min; 45
cycles of denaturation at 94°C for 30 sec, annealing at 58°C for 30
sec and extension at 72°C for 30 sec. The primers used were as
follows: Mouse CB2R forward, 5′-ATGGCCGTGCTCTATATTATCCT-3′ and
reverse, 5′-ATGGTCACACTGCCGATCTTC-3′; human embryonic lung
fibroblast cell line WI38 CB2R forward,
5′-GGGTGACAGAGATAGCCAATGG-3′ and reverse,
5′-TGAACAGGTATGAGGGCTTCC-3′; collagen I forward,
5′-AACTTTGCTTCCCAGATGTCCT-3′ and reverse,
5′-TCGGTGTCCCTTCATTCCAG-3′; and GAPDH forward,
5′-GGATTTGGTCGTATTGGG-3′ and reverse, 5′-GGAAGATGGTGATGGGATT-3′.
GAPDH was used as the endogenous control. The relative expression
level of the target gene was calculated using the 2−ΔΔCq
method (22).
Western blotting
Lung tissues and cells were lysed in SDS sample
buffer (62.5 mM Tris-HCl pH 6.8, 2.5% SDS, 0.002% Bromophenol Blue,
5% β-mercaptoethanol and 10% glycerol) and subjected to western
blot analysis as previously described (16). Protein concentrations were determined
using the BCA method. Protein samples were separated by SDS-PAGE
(10% gel) and then transferred to polyvinylidene fluoride membranes
(EMD Millipore), which were then blocked with 5% dry milk for 1 h
at room temperature. Membranes were subsequently incubated with
primary antibodies against CB2R (40 kDa; cat. no. ab3561; 1:1,000;
Abcam) and GAPDH (37 kDa; cat. no. ab8245; 1:1,000; Abcam)
overnight at 4°C. Horseradish peroxidase-conjugated secondary goat
anti-mouse immunoglobulin G antibody (1:2,000; cat. no. 1015-05,
Southern Biotech) was added for 1 h at room temperature. Each
sample was measured in triplicate. Enhanced chemiluminescence was
used to detect the proteins. Images were captured using ChemiDoc™
XRS (Bio-Rad Laboratories, Inc.). The density of bands was
determined using the Image J software (version 1.46; National
Institutes of Health).
ELISA
The levels of inflammatory cytokines IL-6 (cat. no.
550950; BD Biosciences), IL-1β (IL-1β, cat. no. MLB00C; R&D
systems, Inc.) and TNF-α (TNF-α; cat. no. 560478; BD Biosciences)
in the supernatant of cultured lung fibroblasts and in mouse serum
were analyzed with corresponding ELISA kits, according to the
manufacturer's protocols.
Cell proliferation assay
MTT assay was used to assess the proliferation of
fibroblasts. WI38 cells were plated onto 96-well plates at a
density of 8×103 cells per well and cultured for 24 h at
37°C. Vybrant® MTT Cell Proliferation Assay kit (Thermo
Fisher Scientific, Inc.) was used 24, 48 and 72 h after drug
administration (BALF with pirfenidone (0, 100, 200, 400, 600, and
800 µg/ml) according to the manufacturer's protocol. A total of 20
µl MTT (5 mg/ml) was added to each well for a further 4-h
incubation at 37°C, and 150 µl dimethyl sulfoxide (Sigma Aldrich;
Merck KGaA) was added to each well. Subsequently, the absorbance
(A) was measured at 570 nm on a microplate reader (Thermo Fisher
Scientific, Inc.). The cell viability was calculated using the
following equation: Cell viability=A treatment/A
control ×100%. Additionally, prior to the MTT assay,
cell morphology in each group was observed using a light microscope
(magnification, ×200; Leica Microsystems GmbH).
Statistical analysis
SPSS version 17.0 software (SPSS, Inc.) was used to
conduct the statistical analysis. All data are generated from
experiments that was repeated at least 3 times, and expressed as
the mean ± standard error of the mean. Statistical significance
among groups was evaluated using one-way analysis of variance
followed by the Tukey-Kramer post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
CB2R expression is increased in a
mouse model of BLM-induced IPF
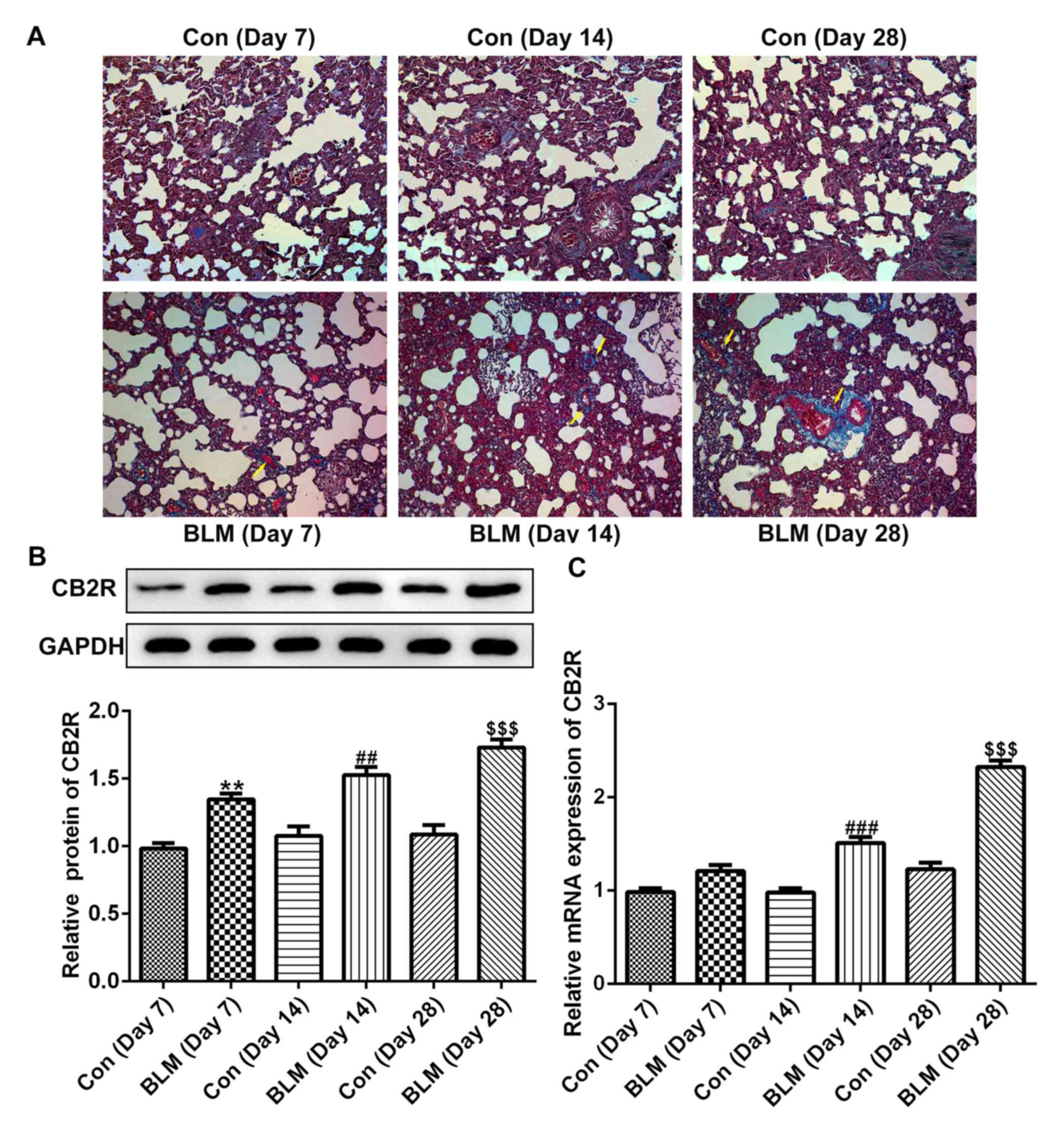
As shown in Fig. 1A,
compared with the control group, the pulmonary fibrotic area in the
experimental mice was markedly increased on days 7, 14 and 28 after
BLM injection. In addition, the protein and mRNA levels of CB2R
were increased at days 14 and 28 after BLM injection compared with
the respective control groups (Fig. 1B
and C).
Pirfenidone activates CB2R in mice
with BLM-induced IPF
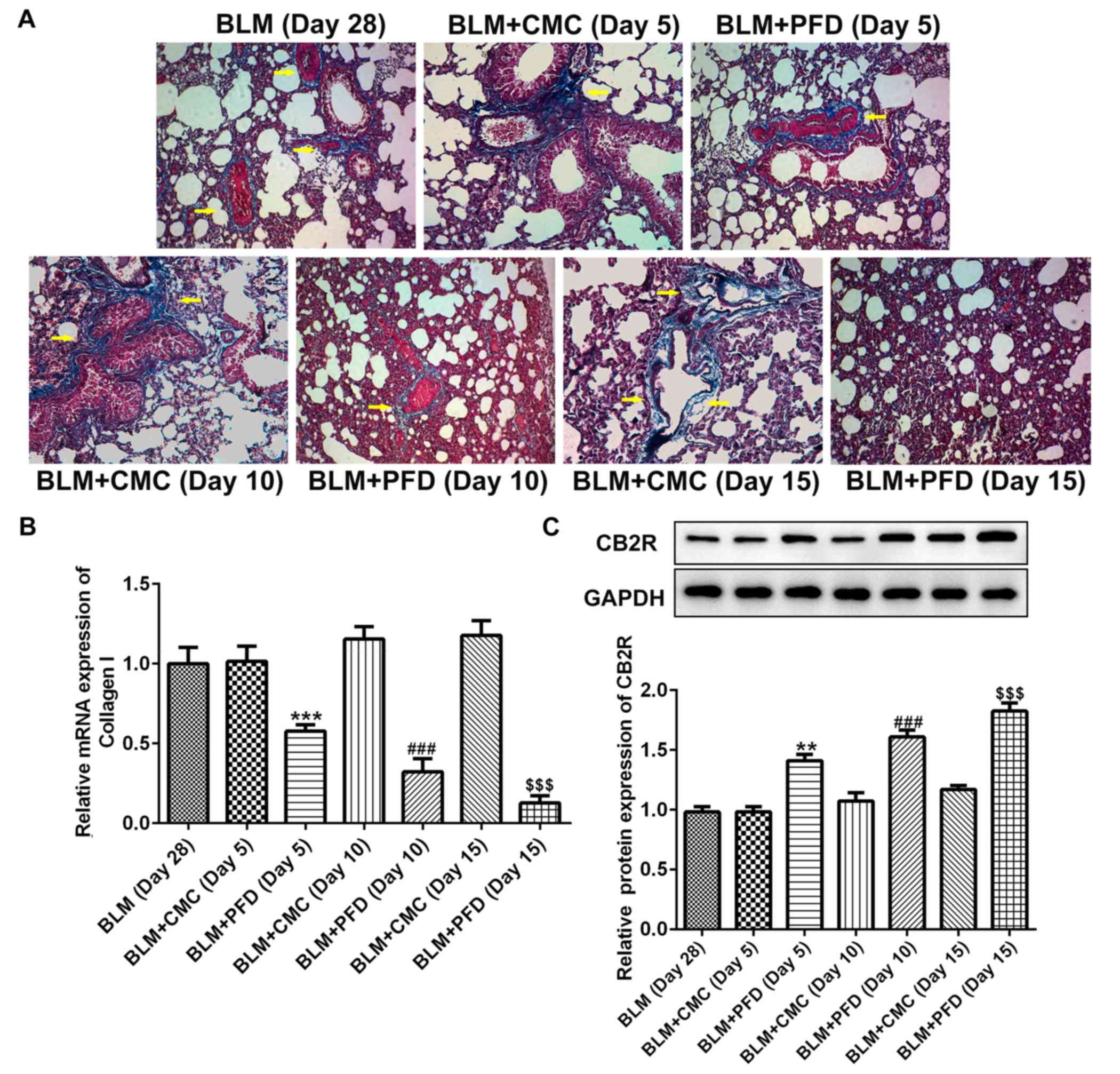
As shown in Fig. 2A,
histological examination revealed that the fibrotic areas in the
BLM + PFD mice were markedly reduced compared with the BLM + CMC
group 10 or 15 days after the administration of pirfenidone.
Furthermore, type I collagen content was quantified in the lungs to
evaluate the antifibrotic effects of pirfenidone. As shown in
Fig. 2B, the mRNA level of type I
collagen in the lungs of pirfenidone-treated mice was significantly
reduced compared with that observed in BLM + CMC-treated mice at
days 5, 10 and 15. In addition, administration of pirfenidone
significantly increased the protein level of CB2R compared with
that exhibited by the BLM + CMC group at days 5, 10 and 15
(Fig. 2C). These results indicated
that CB2R expression was upregulated by pirfenidone treatment.
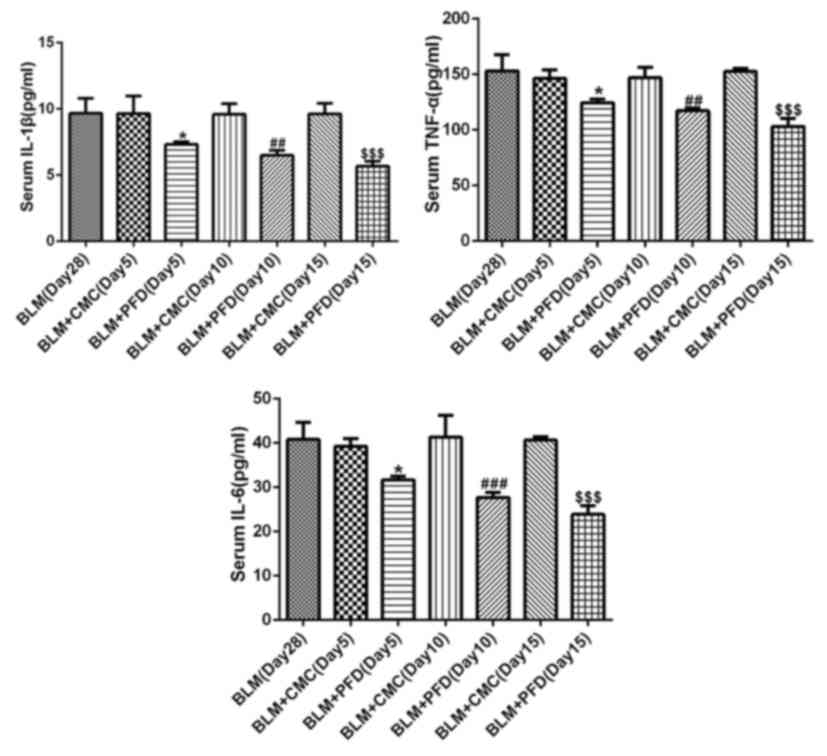
Furthermore, the levels of the inflammatory factors IL-1β, IL-6 and
TNF-α in mouse serum were detected by ELISA. The results revealed
that administration of pirfenidone significantly reduced the serum
concentration of the inflammatory cytokines at days 5, 10 and 15
compared with the respective BLM + CMC groups (Fig. 3). These results suggested that the
activation of CB2R may be considered a mechanism of the
antifibrotic effects of pirfenidone.
Activation of CB2R mediates the
protective effect of pirfenidone on BALF-treated WI38 cells
To further confirm whether the activation of CB2R
mediated the protective effect of pirfenidone on BLM-induced IPF,
human embryonic lung fibroblast WI38 cells were incubated with BALF
from BLM-treated mice. Increasing concentrations of pirfenidone (0,
100, 200, 400, 600, and 800 µg/ml) were added to the culture medium
for 24, 48 or 72 h. The MTT assay was used to assess the effects of
pirfenidone on the growth kinetics of BALF-treated WI38 cells.
Pirfenidone reduced BALF-treated WI38 cell viability in a time- and
concentration-dependent manner (Fig.
4A). In addition, to establish the role of pirfenidone in CB2R
expression in BLM-induced mice pulmonary fibrosis, pirfenidone (200
µg/ml) was added to BALF-treated WI38 cells. As shown in Fig. 4B and C, administration of 200 µg/ml
pirfenidone to BALF-treated WI38 cells for 48 h significantly
increased the protein and mRNA levels of CB2R compared with the
untreated control group. This effect was reversed by the CB2R
antagonist SR144528. In addition, compared with the untreated
control group, pirfenidone significantly decreased the viability of
WI38 cells, which was reversed by the CB2R antagonist SR144528
(Fig. 4D and E). Furthermore, WI38
cells treated with pirfenidone secreted less TNF-α, IL-1β and IL-6
than untreated control cells. The anti-inflammatory effect of
pirfenidone could also be reversed by the CB2R antagonist SR144528
(Fig. 4F-H). The effects of
pirfenidone on BALF-treated WI38 cells were similar to those caused
by the CB2R-selective agonist JWH-015 (Fig. 4B-H).
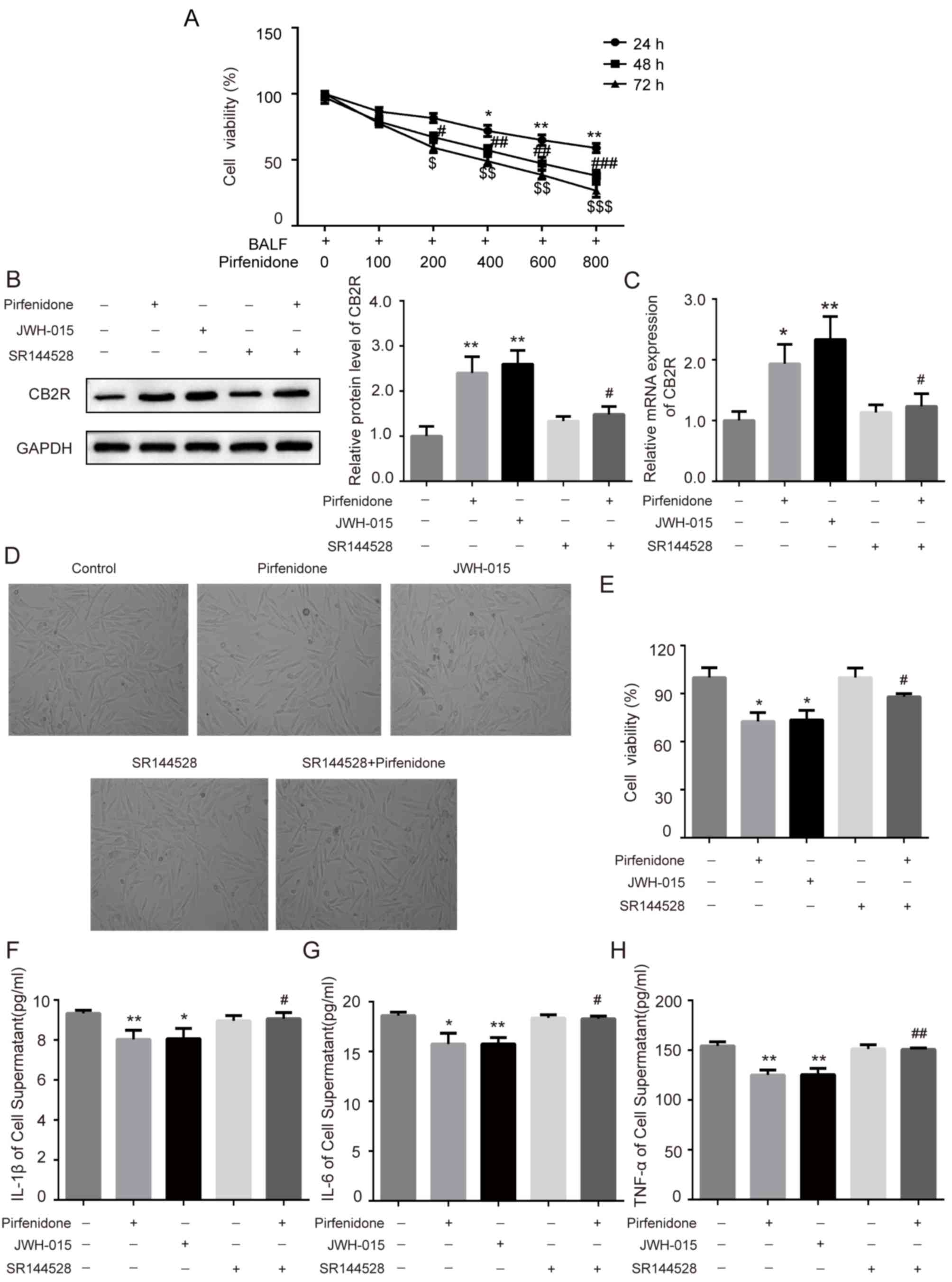 | Figure 4.Activation of CB2R mediates the
protective effect of PFD on BALF-treated WI38 cells. WI38 cells
were incubated with BALF from bleomycin-treated mice and treated
with 200 µg/ml pirfenidone, 20 µM JWH-015, 1 µM SR144528, and 200
µg/ml pirfenidone + 1 µM SR144528. (A) The MTT assay was performed
to determine the viability of WI38 cells treated with various
concentrations of pirfenidone (0, 100, 200, 400, 600, and 800
µg/ml) for 24, 48 or 72 h. Data are presented as the mean ± SEM.
*P<0.05, **P<0.01 vs. cell group only treated with BALF for
24 h; #P<0.05, ##P<0.01,
###P<0.001 vs. cell group only treated with BALF for
48 h; $P<0.05, $$P<0.01,
$$$P<0.001 vs. cell group only treated with BALF for
72 h. The protein and mRNA levels of CB2R were detected by (B)
western blotting and (C) RT-qPCR. (D) WI38 cell proliferation was
observed under a light microscope (magnification, ×40) and detected
by MTT assay. (E) Quantitative analysis of cell proliferation rate.
The levels of (F) IL-1β, (G) IL-6 and (H) TNF-α in cell culture
supernatant were detected by ELISA. Data are presented as the mean
± SEM. *P<0.05, **P<0.01 vs. untreated control group;
#P<0.05, ##P<0.01 vs. PFD only group.
PFD, pirfenidone; CB2R, CB2 receptors; BALF, bronchoalveolar lavage
fluid. |
Discussion
The clinical efficacy and safety of pirfenidone in
patients with IPF have been demonstrated in several multinational,
randomized, double-blind, placebo-controlled, phase 3 clinical
trials, and the cumulative data were summarized in a recent study
by Lancaster et al (23). The
present results demonstrated that pirfenidone attenuated
BLM-induced IPF in mice, which is consistent with the results of
previous studies (24,25). In addition, pirfenidone could
activate CB2R in mice with BLM-induced IPF.
IPF is defined as a specific form of chronic
progressive lung disease attributed to multiple factors associated
with inflammation, oxidative stress and accumulation of
fibroblasts/myofibroblasts, leading to abnormal deposition of
extracellular collagen (26), where
a single pharmacological intervention often fails to incur a
multifaceted protective role (27).
The results of the current study revealed that pirfenidone could
attenuate, but not completely reverse, BLM-induced pulmonary
fibrosis, which was consistent with a previous study (24).
Organ fibrosis is the result of excessive and
irreversible deposition of ECM, particularly collagen. Cytokines
are centrally engaged in maintaining the homeostatic balance of the
ECM, and the recruitment and release of inflammatory cytokines can
stimulate the proliferation of fibroblasts and the synthesis of ECM
(28). A previous study has reported
that the levels of IL-1β and IL-6 were upregulated in BALF in
BLM-induced lung fibrosis in vivo (29). In addition, BLM administration
stimulated the mRNA expression of profibrotic cytokines IL-13 and
IL-4, and the alternatively activated macrophages (M2) markers
including, arginase 1, resistin-like a, C-C motif chemokine (Ccl)17
and Ccl24 in cells collected from BALF in a BLM-induced lung
fibrosis mouse model (30). The
expression of IL-6, IL-8, TNF-α and TGF-β was upregulated in
BLM-treated rat lung tissues (31).
These results indicated that the expression of inflammatory
cytokines and fibrogenic mediators was significantly elevated in
BALF in BLM-induced lung fibrosis. Previous studies have reported
that IL-6 has pro-fibrotic effects both in vitro and in
vivo, and that the inhibition of IL-6 could attenuate PF in
animal models through the STAT3 signaling pathway (32,33).
IL-1β has an indirect pro-fibrotic effect in silica-induced lung
fibrosis in C57BL/6 mice or in fibroblasts (34–36).
TNF-α is a pro-inflammatory cytokine, which can cause fibrosing
alveolitis in mice (37). A previous
study has reported that TGF-β1 could induce proliferation of WI38
fibroblasts (38). Therefore, in the
current study, WI38 cells were incubated with BALF from BLM-treated
mice to induce fibrosis in vitro. The results of the present
study demonstrated that the administration of pirfenidone
significantly decreased the levels of pro-inflammatory cytokines
and mRNA levels of type I collagen in mice with BLM-induced IPF.
These results indicated that the antifibrotic effect of pirfenidone
may be partly mediated by anti-inflammatory mechanisms.
CB2R is mainly expressed in the immune system
(39). A previous study has reported
that CB2R expression was detected in cultured hepatic
myofibroblasts and in activated hepatic stellate cells which
triggered a potent antifibrogenic effect. To the best of our
knowledge, Julien et al (12)
was the first to demonstrate that CB2R is highly upregulated in the
cirrhotic liver, predominantly in hepatic fibrogenic cells. Michler
et al (40) reported that
activation of CB2R could reduce inflammation in acute experimental
pancreatitis through a p38-dependent signaling pathway. In a
previous study, the expression levels of CB2R were increased in
rats with acute and chronic cystitis (41). Furthermore, it was reported that
endocannabinoids could reduce ulcerative colitis-associated
inflammation through binding to CB2R (42). CB2R was previously reported to be
activated in liver fibrosis (12).
TGF-β1 could induce mouse lung fibroblasts and increase the levels
of CB2R, while CB2R agonist JWH133 decreased TGF-β1-induced
pulmonary fibrosis (13). In
addition, celastrol alleviates renal fibrosis by upregulating
cannabinoid receptor 2 expression (43). These results indicated that CB2R may
be upregulated in pulmonary fibrosis and activation of CB2R may
reduce pulmonary fibrosis. In addition, a previous study has
demonstrated that treatment with pirfenidone for 2 years could
decrease fibrosis and cytokine levels such as IL-6, as well as
enhance CB2R gene expression, in patients with chronic hepatitis
(14). Therefore, it may be
hypothesized that pirfenidone plays a protective role against
pulmonary fibrosis by upregulating CB2R expression. The present
results revealed that CB2R expression was upregulated in
BLM-induced IPF of mouse model, whilst pirfenidone significantly
increased the expression of CB2R in mice with BLM-induced IPF and
in BALF-treated WI38 cells, which could be reversed by the CB2R
antagonist SR144528, suggesting that SR144528 could block CB2R
endogenous activity and inhibit its upregulated expression caused
by pirfenidone. CB2R agonist JWH-015 could activate CB2R, along
with an upregulated CB2R expression, while pirfenidone also
increased CB2R expression. CB2R antagonist SR144528 effectively
inhibited pirfenidone-induced upregulated CB2R expression,
suggesting that pirfenidone may increase CB2R expression by
activating CB2R endogenous activity. In addition, the
anti-inflammatory effects of pirfenidone in BALF-treated WI38 cells
could also be reversed by the CB2R antagonist SR144528. The present
results indicated that the activation of CB2R may be involved in
the antifibrotic effects of pirfenidone.
In conclusion, pirfenidone significantly attenuated
BLM-induced lung fibrosis in mice, which may be mediated by CB2R
activation. However, the present report is a preliminary study,
since it did not elucidate whether the activation of CB2R is the
direct effect of pirfenidone or the result of a secondary effect.
In addition, the present study did not determine the location of
CB2R in lung tissues. Thus, further studies are required to
elucidate the role and underlying mechanism of CB2R.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL wrote the manuscript, and analyzed and
interpreted the data. GS searched the literature, collected the
data, designed the study and revised the manuscript.
Ethics approval and consent to
participate
The animal protocols were approved by the Baodi
Clinical College of Tianjin Medical University Animal Care
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al: An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary
fibrosis: Evidence-based guidelines for diagnosis and management.
Am J Respir Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pardo A and Selman M: Idiopathic pulmonary
fibrosis: New insights in its pathogenesis. Int J Biochem Cell
Biol. 34:1534–1538. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lynch JR III, Saggar R, Weigt SS, Zisman
DA and White ES: Usual interstitial pneumonia. Semin Respir Crit
Care Med. 27:634–651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Antoniou KM, Wuyts W, Wijsenbeek M and
Wells AU: Medical therapy in idiopathic pulmonary fibrosis. Semin
Respir Crit Care Med. 37:368–377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taguchi Y, Ebina M, Hashimoto S, Ogura T,
Azuma A, Taniguchi H, Kondoh Y, Suga M, Takahashi H, Nakata K, et
al: Efficacy of pirfenidone and disease severity of idiopathic
pulmonary fibrosis: Extended analysis of phase III trial in Japan.
Respir Investig. 53:279–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iyer SN, Gurujeyalakshmi G and Giri SN:
Effects of pirfenidone on transforming growth factor-beta gene
expression at the transcriptional level in bleomycin hamster model
of lung fibrosis. J Pharmacol Exp Ther. 291:367–373.
1999.PubMed/NCBI
|
|
7
|
Iyer SN, Gurujeyalakshmi G and Giri SN:
Effects of pirfenidone on procollagen gene expression at the
transcriptional level in bleomycin hamster model of lung fibrosis.
J Pharmacol Exp Ther. 289:211–218. 1999.PubMed/NCBI
|
|
8
|
Oku H, Shimizu T, Kawabata T, Nagira M,
Hikita I, Ueyama A, Matsushima S, Torii M and Arimura A:
Antifibrotic action of pirfenidone and prednisolone: Different
effects on pulmonary cytokines and growth factors in
bleomycin-induced murine pulmonary fibrosis. Eur J Pharmacol.
590:400–408. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pertwee RG: Pharmacology of cannabinoid
receptor ligands. Curr Med Chem. 6:635–664. 1999.PubMed/NCBI
|
|
10
|
Zoratti C, Kipmen-Korgun D, Osibow K,
Malli R and Graier WF: Anandamide initiates Ca (2+) signaling via
CB2 receptor linked to phospholipase C in calf pulmonary
endothelial cells. Br J Pharmacol. 140:1351–1362. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piomelli D, Giuffrida A, Calignano A and
Rodríguez de Fonseca F: The endocannabinoid system as a target for
therapeutic drugs. Trends Pharmacol Sci. 21:218–224. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Julien B, Grenard P, Teixeira-Clerc F, Van
Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A and Lotersztajn S:
Antifibrogenic role of the cannabinoid receptor CB2 in the liver.
Gastroenterology. 128:742–755. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Q, Zheng Y, Dong X, Wang L and Jiang
CG: Activation of cannabinoid receptor type 2 by JWH133 alleviates
bleomycin-induced pulmonary fibrosis in mice. Oncotarget.
8:103486–103498. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flores-Contreras L, Sandoval-Rodriguez AS,
Mena-Enriquez MG, Lucano-Landeros S, Arellano-Olivera I,
Alvarez-Álvarez A, Sanchez-Parada MG and Armendáriz-Borunda J:
Treatment with pirfenidone for two years decreases fibrosis,
cytokine levels and enhances CB2 gene expression in patients with
chronic hepatitis C. BMC Gastroenterol. 14:1312014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals, . Guide for the care and
use of laboratory animals8th. National Academies Press; Washington:
2011
|
|
16
|
Liu Y, Lu F, Kang L, Wang Z and Wang Y:
Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice
by regulating Nrf2/Bach1 equilibrium. BMC Pulm Med. 17:632017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Phillips RJ, Burdick MD, Hong K, Lutz MA,
Murray LA, Xue YY, Belperio JA, Keane MP and Strieter RM:
Circulating fibrocytes traffic to the lungs in response to CXCL12
and mediate fibrosis. J Clin Invest. 114:438–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song JS, Kang CM, Kang HH, Yoon HK, Kim
YK, Kim KH, Moon HS and Park SH: Inhibitory effect of CXC chemokine
receptor 4 antagonist AMD3100 on bleomycin induced murine pulmonary
fibrosis. Exp Mol Med. 42:465–472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishida Y, Kimura A, Kondo T, Hayashi T,
Ueno M, Takakura N, Matsushima K and Mukaida N: Essential roles of
the CC chemokine ligand 3-CC chemokine receptor 5 axis in
bleomycin-induced pulmonary fibrosis through regulation of
macrophage and fibrocyte infiltration. Am J Pathol. 170:843–854.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song P, Zheng JX, Xu J, Liu JZ, Wu LY and
Liu C: β-catenin induces A549 alveolar epithelial cell mesenchymal
transition during pulmonary fibrosis. Mol Med Rep. 11:2703–2710.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Montecucco F, Burger F, Mach F and
Steffens S: CB2 cannabinoid receptor agonist JWH-015 modulates
human monocyte migration through defined intracellular signaling
pathways. Am J Physiol Heart Circ Physiol. 294:H1145–H1155. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lancaster L, Albera C, Bradford WZ,
Costabel U, du Bois RM, Fagan EA, Fishman RS, Glaspole I, Glassberg
MK, King TE Jr, et al: Safety of pirfenidone in patients with
idiopathic pulmonary fibrosis: Integrated analysis of cumulative
data from 5 clinical trials. BMJ Open Respir Res. 3:e0001052016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inomata M, Kamio K, Azuma A, Matsuda K,
Kokuho N, Miura Y, Hayashi H, Nei T, Fujita K, Saito Y and Gemma A:
Pirfenidone inhibits fibrocyte accumulation in the lungs in
bleomycin-induced murine pulmonary fibrosis. Respir Res. 15:162014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schaefer CJ, Ruhrmund DW, Pan L, Seiwert
SD and Kossen K: Antifibrotic activities of pirfenidone in animal
models. Eur Respir Rev. 20:85–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sgalla G, Biffi A and Richeldi L:
Idopathic pulmonary fibrosis: Diagnosis, epidemiology and natural
history. Respirology. 21:427–437. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Foucquier J and Guedj M: Analysis of drug
combinations: Current methodological landscape. Pharmacol Res
Perspect. 3:e001492015. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lebda MA, Sadek KM, Abouzed TK, Tohamy HG
and El-Sayed YS: Melatonin mitigates Thioacetamide-induced hepatic
fibrosis via antioxidant activity and modulation of Proinflammatory
cytokines and Fibrogenic genes. Life Sci. 192:136–143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aumiller V, Balsara N, Wilhelm J, Gunther
A and Konigshoff M: WNT/β-catenin signaling induces IL-1β
expression by alveolar epithelial cells in pulmonary fibrosis. Am J
Respir Cell Mol Biol. 49:96–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao J, Okamoto Y, Asano Y, Ishimaru K,
Aki S, Yoshioka K, Takuwa N, Wada T, Inagaki Y, Takahashi C, et al:
Sphingosine-1-phosphate receptor-2 facilitates pulmonary fibrosis
through potentiating IL-13 pathway in macrophages. PLoS One.
13:e1976042018.
|
|
31
|
Dong X, Li X, Li M, Chen M, Fan Q and Wei
W: Inhibitory effects of thalidomide on bleomycin-induced pulmonary
fibrosis in rats via regulation of thioredoxin reductase and
inflammations. Am J Transl Res. 9:4390–4401. 2017.PubMed/NCBI
|
|
32
|
Le TT, Karmouty-Quintana H, Melicoff E, Le
TT, Weng T, Chen NY, Pedroza M, Zhou Y, Davies J, Philip K, et al:
Blockade of IL-6 trans signaling attenuates pulmonary fibrosis. J
Immunol. 193:3755–3768. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
O'Donoghue RJ, Knight DA, Richards CD,
Prêle CM, Lau HL, Jarnicki AG, Jones J, Bozinovski S, Vlahos R,
Thiem S, et al: Genetic partitioning of interleukin-6 signalling in
mice dissociates Stat3 from Smad3-mediated lung fibrosis. EMBO Mol
Med. 4:939–951. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Yan JW, Wang YJ, Wan YN, Wang BX,
Tao JH, Chen B, Li BZ, Yang GJ and Wang J: Association of
interleukin 1 family with systemic sclerosis. Inflammation.
37:1213–1220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mia MM, Boersema M and Bank RA:
Interleukin-1β attenuates myofibroblast formation and extracellular
matrix production in dermal and lung fibroblasts exposed to
transforming growth factor-β1. PLoS One. 9:e915592014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guo J, Gu N, Chen J, Shi T, Zhou Y, Rong
Y, Zhou T, Yang W, Cui X and Chen W: Neutralization of
interleukin-1 beta attenuates silica-induced lung inflammation and
fibrosis in C57BL/6 mice. Arch Toxicol. 87:1963–1973. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyazaki Y, Araki K, Vesin C, Garcia I,
Kapanci Y, Whitsett JA, Piguet PF and Vassalli P: Expression of a
tumor necrosis factor-alpha transgene in murine lung causes
lymphocytic and fibrosing alveolitis. A mouse model of progressive
pulmonary fibrosis. J Clin Invest. 96:250–259. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang X, Wang W, Yuan H, Sun J, Li L, Wu
X, Luo J and Gu Y: Sunitinib, a small-molecule kinase inhibitor,
attenuates bleomycin-induced pulmonary fibrosis in mice. Tohoku J
Exp Med. 239:251–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cabral GA, Raborn ES, Griffin L, Dennis J
and Marciano-Cabral F: CB2 receptors in the brain: Role in central
immune function. Br J Pharmacol. 153:240–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Michler T, Storr M, Kramer J, Ochs S, Malo
A, Reu S, Göke B and Schäfer C: Activation of cannabinoid receptor
2 reduces inflammation in acute experimental pancreatitis via
intra-acinar activation of p38 and MK2-dependent mechanisms. Am J
Physiol Gastrointest Liver Physiol. 304:181–192. 2013. View Article : Google Scholar
|
|
41
|
Merriam FV, Wang ZY, Guerios SD and
Bjorling DE: Cannabinoid receptor 2 is increased in acutely and
chronically inflamed bladder of rats. Neurosci Lett. 445:130–134.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Marquéz L, Suárez J, Iglesias M,
Bermudez-Silva FJ, Rodríguez de Fonseca F and Andreu M: Ulcerative
colitis induces changes on the expression of the endocannabinoid
system in the human colonic tissue. PLoS One. 4:e68932009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang M, Cao X, Zhang K, Li Y, Zheng QY, Li
GQ, He QH, Li SJ, Xu GL and Zhang KQ: Celastrol alleviates renal
fibrosis by upregulating cannabinoid receptor 2 expression. Cell
Death Dis. 9:6012018. View Article : Google Scholar : PubMed/NCBI
|