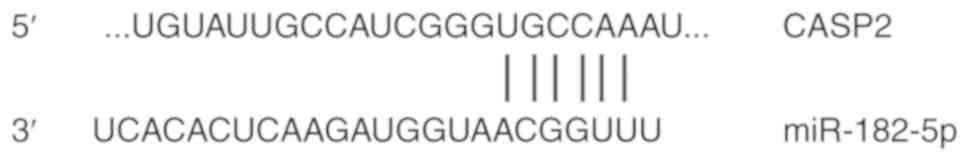Introduction
Lung cancer has the highest morbidity and mortality
rates among all malignant tumors worldwide (1). Lung cancer accounts for ~13% of all new
cancer cases each year (2,3). In China, ~600,000 people succumb to
lung cancer every year (4,5), and therefore early prevention and
diagnosis of lung cancer is important. The current treatment
strategy for lung cancer is mainly surgery and chemoradiotherapy;
targeted therapy is an emerging treatment strategy (6).
MicroRNAs (miRNA or miR) is a class of small,
non-encoding RNA molecules (7).
miRNAs serve important roles in several biological and pathological
processes by promoting mRNA degradation or inhibiting mRNA
translation, to negatively regulate target gene expression
(8). Certain miRNA molecules can
function as oncogenes or tumor-suppressors and, together with their
target genes, can serve important roles in the occurrence and
development of several types of cancer (7). Previous studies identified the
important role of miRNAs in non-small cell lung cancer (NSCLC), as
several aberrantly expressed miRNAs were identified in NSCLC tissue
samples (9,10). Previous studies demonstrated that
miR-182-5p may serve different roles in different types of cancer,
including renal cell carcinoma and liver cancer (11,12).
However, the functional role of miR-182-5p in NSCLC remains
unknown.
Caspase 2 (CASP2) may function in stress-induced
cell death pathways, cell cycle maintenance and tumor suppression
(13). CASP2 can induce apoptosis,
and overexpression of CASP2 was previously demonstrated in several
types of cells (14,15). CASP2 is regulated by several miRNAs,
such as miR-183 in ovarian cancer (16). In the case of a calorie-restricted
diet, CASP2 in liver cells has been shown to be precisely regulated
by miR-125a-5p, which delays aging (17). The aforementioned studies shown that
the regulation of CASP2 by miRNAs has become one of the important
factors affecting the expression of CASP2.
In the current study, bioinformatics analysis was
used to identify CASP2 as a potential target gene of miR-182-5p,
however the association between CASP2 and miR-182-5p has not yet
been reported. The current study demonstrated that miR-182-5p
expression is upregulated, while CASP2 expression is downregulated
in NSCLC tissue and peripheral blood samples from patients with
NSCLC. Therefore, miR-182-5p may regulate NSCLC cell proliferation
and apoptosis via direct interaction with CASP2.
Materials and methods
Patient samples
NSCLC tissue and adjacent normal tissue samples were
collected from 33 patients (male, n=21 and female, n=12; age range,
36–68 years; median age, 51 years) who had undergone surgical
resection surgery at Department of Pathology and Pathophysiology,
Zibo Zhoucun People's Hospital Affiliated to Qilu Medical
University (Zibo, China) between December 2013 and December 2017.
None of the patients received any prior treatment with hormones,
Chinese medicines or chemoradiotherapy. In addition, 26 healthy
control patients (male, n=16 and female, n=10; age range, 35–72
years; median age, 52 years) who had undergone physical
examinations at Zibo Zhoucun People's Hospital Affiliated to Qilu
Medical University between December 2013 and December 2017 were
included in the study. Peripheral blood was collected from patients
with NSCLC and healthy controls. The current study was approved by
the Ethics Committee of Qilu Medical University and written
informed consent was obtained from all patients or their
families.
Cell culture and transfection
The NSCLC cell line H1299 was purchased from the
Cell Bank of the Chinese Academy of Sciences (Shanghai, China), and
cultured in high-glucose Dulbecco's modified Eagle's medium (DMEM;
Hyclone; GE Healthcare Life Sciences, Little Chalfont, UK)
supplemented with 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences), 100 IU/ml penicillin and 100 IU/ml
streptomycin. Cells were maintained at 37°C in a 5% CO2
incubator with 70% humidity. The cells were passaged every 4 days,
and log-phase cells were collected for further experimentation.
H1299 cells were seeded into 24-well plates at a
density of 3×105 cells/well and cultured in
antibiotic-free DMEM supplemented with 10% FBS until 70% confluent.
In a vial, 1 µl miR-182-5p inhibitor (20 pmol/ml;
5′-AAACCGUUACCAUCUUGAGUGUGA-3′) with CASP2 small interfering
(si)RNA (siCASP2; forward, 5′-ACAGCUGUUGUUGAGCGAAdTdT-3′; reverse,
5′-UUCGCUCAACAACAGCUGUdTdT-3′) or siNC (forward,
5′-UUCUCCGAACGUGUCACGUdTdT-3′; reverse,
5′-ACGUGACACGUUCGGAGAAdTdT-3′; both 20 pmol/µl; Sangon Biotech Co.,
Ltd., Shanghai, China) were mixed with 50 µl Opti-MEM medium
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). In a second
vial, 1 µl Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.) was mixed with 50 µl Opti-MEM medium. Following 5
min incubation at room temperature, the two vials were combined and
incubated for a further 20 min at room temperature. The
transfection mixture was subsequently added to cells and following
6 h incubation at 37°C, the medium was replaced with F12/DMEM
supplemented with 10% FBS. Following 48-h transfection, cells were
collected and used in further experimentation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Tissue samples (100 mg) were ground into powder in
liquid nitrogen. Total RNA was extracted from powdered tissue
samples, plasma (100 µl) or cells (3×106) using 1 ml
TRIzol® reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol and RNA quality and
concentration was detected by ultraviolet spectrophotometry
(NanoDrop™ 2000; Thermo Fisher Scientific, Inc.). To detect the
mRNA expression level of CASP2, total RNA (1 µg) was reverse
transcribed into cDNA using the TIANScript II cDNA First Strand
Synthesis kit (Tiangen Biotech Co., Ltd., Beijing, China). qPCR was
subsequently performed using the SuperReal PreMix (SYBR Green) kit
(Tiangen Biotech Co., Ltd.) with the following primer pairs: CASP2
forward, 5′-GCAAACCTCAGGGAAACATTC-3′ and reverse,
5′-TGTCGGCATACTGTTTCAGCA-3′; and GAPDH forward,
5′-AGGAGCGAGACCCCACTAACAT-3′ and reverse,
5′-GTGATGGCATGGACTGTGGT-3′. The following thermocycling conditions
were used for the qPCR: Initial denaturation at 95°C for 5 min; 46
cycles of 95°C for 20 sec, 55°C for 20 sec and 72°C for 30 sec
which was performed using an iQ5 Real-Time PCR system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). CASP2 mRNA levels were
quantified using the 2−ΔΔCq method and normalized to the
internal reference gene GAPDH (18).
To detect the expression level of miR-182-5p, miRNA was reverse
transcribed into cDNA using the miRcute miRNA cDNA First Strand
Synthesis kit (Tiangen Biotech Co., Ltd.). qPCR was performed using
the miRcute miRNA qPCR Detection kit (Tiangen Biotech Co., Ltd.)
and the following primer pairs: miR-182-5p forward,
5′-ACACTCCAGCTGGGTTTGGCAATGGTAGAACT-3′ and reverse,
5′-TGGTGTCGTGGAGTCG-3′; and U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and
reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The following thermocycling
conditions were used for qPCR: Initial denaturation at 95°C for 5
min; 46 cycles of 95°C for 10 sec, 56°C for 25 sec and 72°C for 30
sec which was performed using an iQ5 Real-Time PCR system (Bio-Rad
Laboratories, Inc.). miR-182-5p expression levels were quantified
using the 2−ΔΔCq method and normalized using U6 as an
internal reference (18,19). Each experiment was performed in
triplicate.
MTT assay
Following transfection, cells were seeded into
96-well plates at a density of 2×103 cells/well and
cultured for 24, 48 and 72 h. Following incubation, 20 µl MTT (5
g/l; cat. no. JRDC000003; JRDUN Biotechnology, Co., Ltd., Shanghai,
China) was added to each well and further incubated for 4 h at
37°C. Each condition was assessed in triplicate. Following
incubation, the culture medium was aspirated and dimethyl sulfoxide
(150 µl/well) was added to dissolve the purple formazan crystals.
The absorbance of each well was measured at 490 nm using a
microplate reader (Bio-Rad Laboratories, Inc.) and cell
proliferation curves were plotted. Each experiment was performed in
triplicate.
Flow cytometry
Following transfection, cells (1×106)
from each group were washed twice with pre-cooled PBS twice and
cell apoptosis was analyzed by flow cytometry using the FITC
Annexin V Apoptosis Detection Kit I (BD Biosciences, Franklin
Lakes, NJ, USA), according to the manufacturer's protocol. Annexin
V-positive staining were early apoptotic cells, those with
propidium iodide-positive staining were necrotic cells and those
with double positive staining were late apoptotic cells. Each
experiment was performed in triplicate. CFlow Plus software
(v1.0.264.15; BD Biosciences) was used to analysis the results.
Bioinformatics analysis
Bioinformatics prediction is a powerful tool for the
study of miRNAs. Bioinformatics analysis was performed using
miRanda (www.microrna.org/microrna/home.do), TargetScan
(www.targetscan.org), PiTa (genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (bibiserv.techfak.uni-bielefeld.de/rnahybrid) and
PICTA (pictar.mdc-berlin.de) to predict
potential target genes of miR-182-5p.
Western blot analysis
Prior to lysis, tissue samples were ground into
powder in liquid nitrogen, and cells were trypsinized and
collected. Subsequently, total protein was extracted from tissue
samples or cells using pre-cooled radioimmunoprecipitation assay
lysis buffer (600 µl; 50 mM Tris-base, 1 mM EDTA, 150 mM NaCl, 0.1%
sodium dodecyl sulfate, 1% TritonX-100, 1% sodium deoxycholate;
Beyotime Institute of Biotechnology, Haimen, China) for 30 min on
ice. The lysates were centrifuged at 10,000 × g for 10 min at 4°C
and the supernatant was used to quantify protein concentration
using a bicinchoninic acid protein concentration determination kit
(cat. no. RTP7102; Real-Times Biotechnology Co., Ltd., Beijing,
China). The samples were mixed with 5X SDS loading buffer prior to
denaturation in a water bath at 100°C for 5 min. Following
denaturation, 20 µg protein/lane was separated at 100 V via
SDS-PAGE on a 10% gel. The separated proteins were transferred onto
polyvinylidene difluoride membranes (100 V, 2 h) and blocked with
5% skimmed milk at room temperature for 1 h. The membranes were
incubated with primary antibodies: Rabbit anti-human CASP2
(1:1,000; cat. no. ab182657) or β-actin (1:5,000; cat. no.
ab129348; both Abcam, Cambridge, UK) overnight at 4°C. The
membranes were washed three times for 15 min with PBS containing
Tween®−20. The membranes were incubated with goat
anti-rabbit horseradish peroxidase (HRP)-conjugated secondary
antibody (1:3,000; cat. no. ab6721; Abcam) for 1 h at room
temperature. The membranes were washed three times for 15 min with
PBS containing Tween®−20. Protein bands were developed
using the ECL Western Blotting Substrate kit (cat. no. ab65623;
Abcam). Protein expression was quantified using Image Lab v3.0
software (Bio-Rad Laboratories, Inc.) with β-actin as the loading
control. Each experiment was performed in triplicate.
ELISA
Peripheral blood was centrifuged at 1,000 × g for 10
min at 4°C. The CASP2 Human ELISA kit (cat. no. KA2636; Abnova,
Taipei, Taiwan) was used for the quantitative detection and
measurement of CASP2 in serum. Briefly, standards (50 µl) and
samples (10 µl serum and 40 µl diluent) were added to predefined
wells and blank wells were left empty. In each well, for
HRP-labeled conjugates (100 µl) were added to each well and the
plates were sealed and incubated at 37°C for 1 h. Plates were
washed 5 times and substrates A (50 µl) and B (50 µl) were added to
each well and incubated at 37°C for 15 min. Following incubation,
stop solution (50 µl) was added to each well and the absorbance was
measured within 15 min at 450 nm using a Multiskan FC (Thermo
Fisher Scientific, Inc.). Each experiment was performed in
triplicate.
Dual-luciferase reporter assay
The wild-type (WT) and mutant 3′-untranslated region
(UTR) of CASP2 containing the seed regions of miR-182-5p were
chemically synthesized in vitro and cloned into the
pMIR-REPORT luciferase reporter plasmid (Promega Corporation,
Madison, WI, USA) between the Spe-1 and Hind III
restriction sites. 293T cells (Cell Bank, Chinese Academy of
Sciences, Shanghai, China) were subsequently co-transfected with
agomiR-182-5p (100 nM; forward, 5′-UUUGGCAAUGGUAGAACUCACACU-3′;
reverse, 3′-AAACCGUUACCAUCAAGAGUGUGA-5′; Sangon Biotech Co., Ltd.)
and the WT or mutant 3′-UTR CASP2 luciferase reporter plasmids (0.8
µg). 293T cells were transfected with agomiR-negative control (NC;
forward, 5′-UUCUCCGAACGUGUCACGUTT-3′; reverse,
3′-TTAAGAGGCUUGCACAGUGCA-5′; Sangon Biotech Co., Ltd.) as a
control. Following 24-h transfection, cells were lysed and
luciferase activities were measured using the Dual-Luciferase
Reporter Assay system (Promega Corporation) according to the
manufacturer's protocol and luciferase activity was detected using
a GloMax 20/20 luminometer (Promega Corporation). Firefly
luciferase activity was normalized to Renilla luciferase
activity and each experiment was performed in triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation.
All statistical analyses were performed using SPSS statistical
software (version 20.0; IBM Corp., Armonk, NY, USA). Data were
tested for normality and statistical analysis among multiple groups
was analyzed by one-way analysis of variance followed by
Student-Newman-Keuls test. Comparison between NSCLC and adjacent
normal tissue samples from patients with NSCLC was performed using
a paired Student's t-test, while comparison between the
experimental and control groups was carried out using an unpaired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
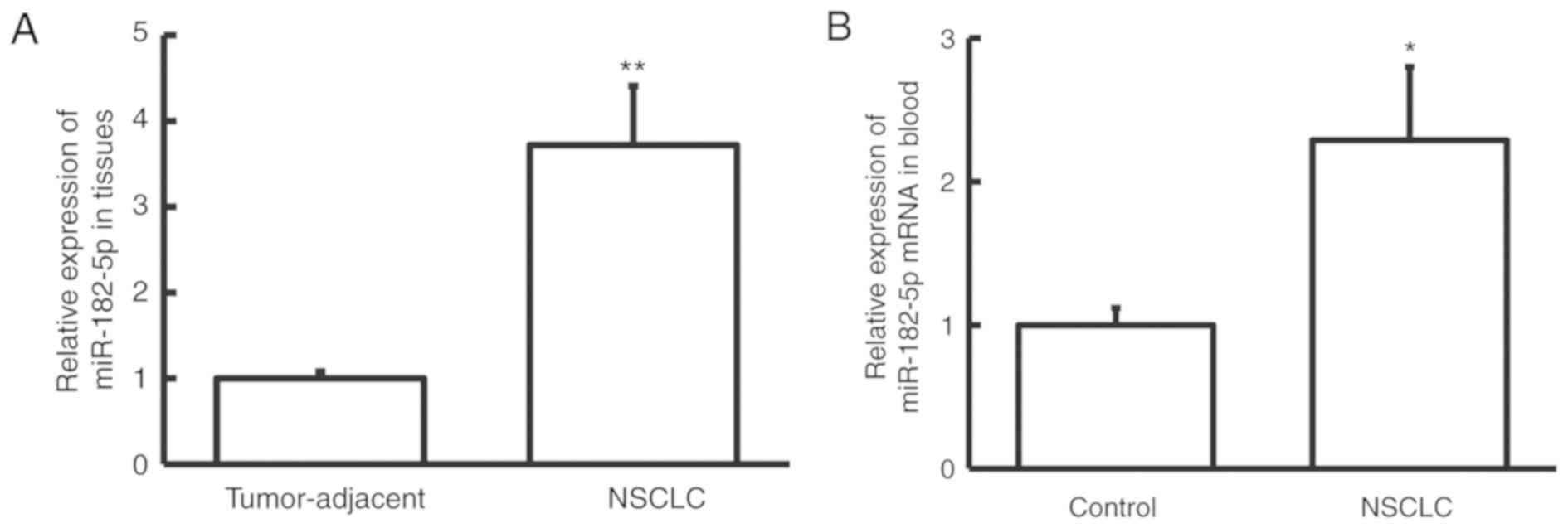
miR-182-5p expression is upregulated
in NSCLC
To examine the expression pattern of miR-182-5p in
NSCLC, the miR-182-5p expression level was determined by RT-qPCR.
The miR-182-5p expression level was significantly increased in
NSCLC tissue samples compared with adjacent normal tissue samples
(P<0.01; Fig. 1A). Similarly, the
miR-182-5p expression level was significantly increased in
peripheral blood samples from patients with NSCLC compared with
healthy controls (P<0.05; Fig.
1B). These results suggest that miR-182-5p expression is
upregulated in NSCLC, and therefore miR-182-5p may exert its
biological function in NSCLC.
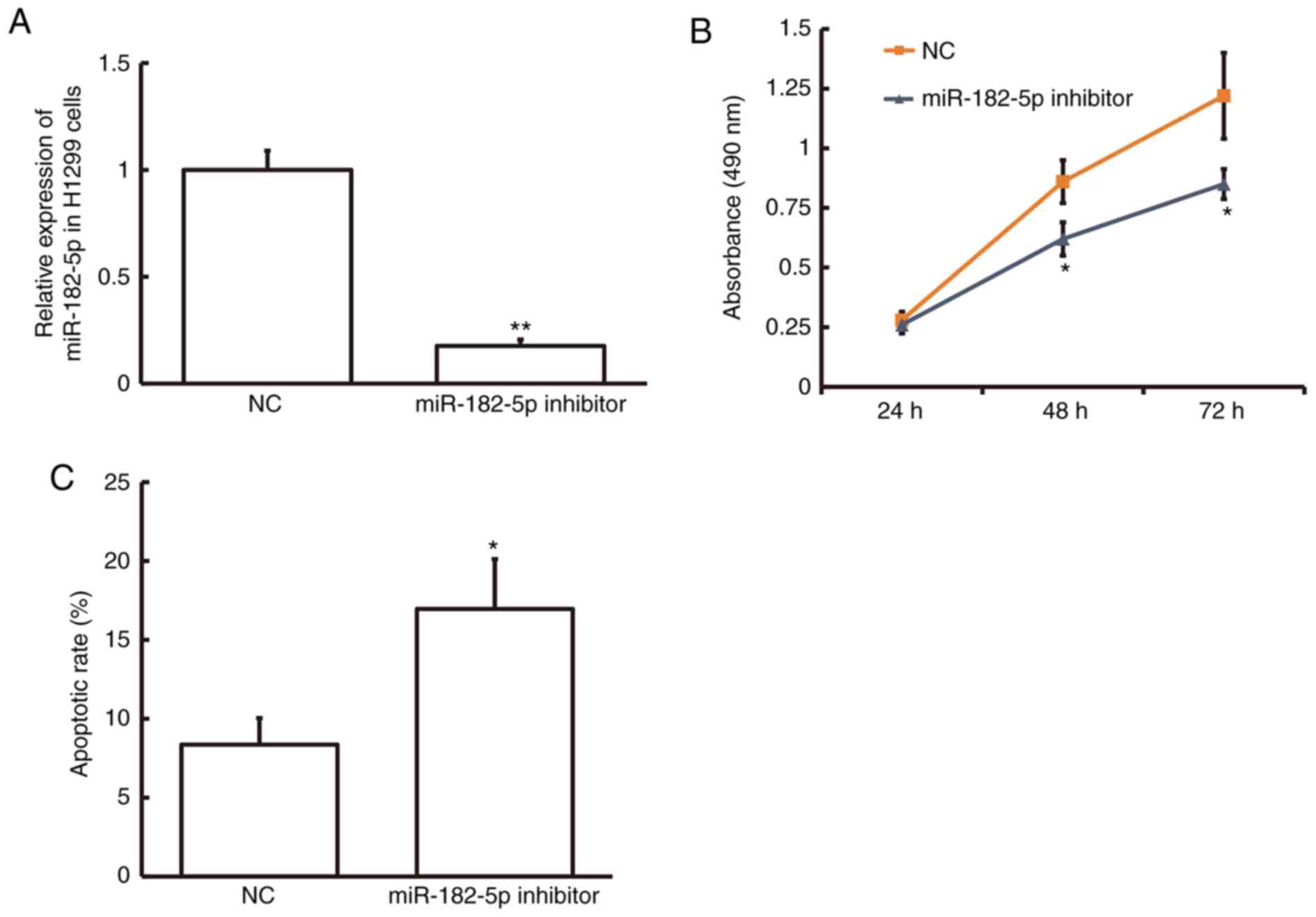
Inhibition of miR-182-5p expression
suppresses cell proliferation and promotes cell apoptosis in NSCLC
cells
To examine the effect of miR-182-5p on NSCLC cell
proliferation, the MTT assay was performed in the NSCLC cell line
H1299 following transfection with miR-182-5p inhibitor. The
miR-182-5p level was significantly decreased in H1299 cells
following transfection with miR-182-5p inhibitor compared with the
NC (P<0.01; Fig. 2A). MTT assay
demonstrated that cell proliferation was significantly decreased in
H1299 cells following 48 and 72-h transfection with miR-182-5p
inhibitor compared with the NC (P<0.05; Fig. 2B). Flow cytometry demonstrated that
the rate of apoptosis was significantly increased in H1299 cells
following transfection with miR-182-5p inhibitor compared with the
NC (P<0.05; Fig. 2C). These
results suggest that inhibition of miR-182-5p expression suppresses
cell proliferation and promotes cell apoptosis in NSCLC cells and
therefore miR-182-5p may serve a role in NSCLC progression.
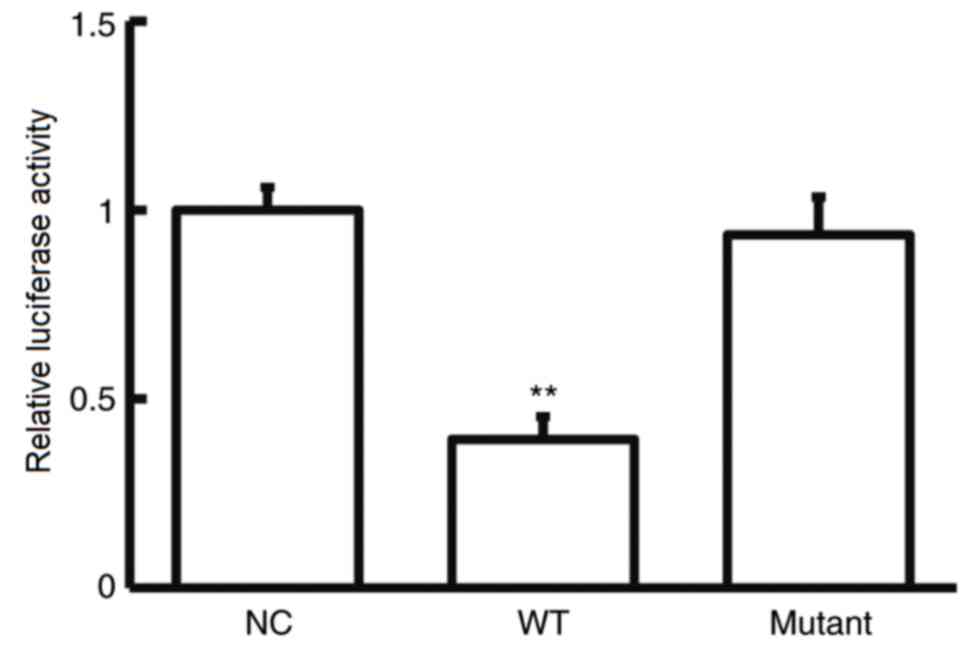
CASP2 is a direct target gene of
miR-182-5p in NSCLC cells
To further investigate the role of miR-182-5p in
NSCLC, potential targets of miR-182-5p were examined.
Bioinformatics analysis showed that CASP2 was identified as a
potential target gene of miR-182-5p (Fig. 3). The dual-luciferase reporter assay
was used verify the interaction between miR-182-5p and the 3′-UTR
of CASP2. The current study demonstrated that agomiR-182-5p
overexpression significantly decreased pMIR-REPORT-WT luciferase
activity compared with the NC (P<0.05; Fig. 4). By contrast, co-transfection with
agomiR-182-5p and the pMIR-REPORT-mutant luciferase reporter
plasmid had no significant effect on luciferase activity. These
results indicate that miR-182-5p can directly bind with the 3′-UTR
of CASP2.
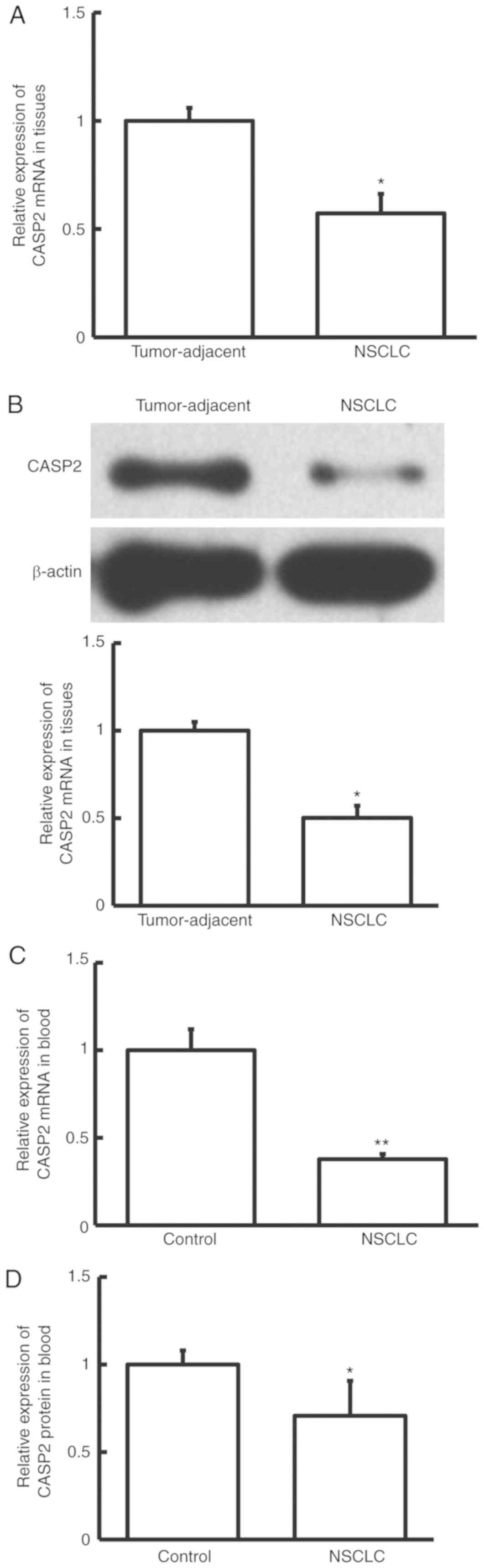
CASP2 expression is downregulated in
NSCLC tissue and peripheral blood samples from patients with
NSCLC
To investigate CASP2 expression in NSCLC, the mRNA
and protein expression levels of CASP2 were determined by RT-qPCR,
and western blotting and ELISA, respectively. The mRNA and protein
expression levels of CASP2 were significantly decreased in NSCLC
tissue samples compared with adjacent normal tissue samples from
patients with NSCLC (P<0.05; Fig. 5A
and B). Similarly, RT-qPCR and ELISA demonstrated that the mRNA
and protein expression levels of CASP2 were significantly decreased
in peripheral blood samples from patients with NSCLC compared with
healthy controls (P<0.01 and P<0.05, respectively; Fig. 5C and D). These results suggest that
CASP2 expression is downregulated in patients with NSCLC.
miR-182-5p regulates NSCLC cell
proliferation and apoptosis through regulation of CASP2
expression
To investigate the underlying mechanism of
miR-182-5p on NSCLC cell proliferation, the MTT assay was performed
in the NSCLC cell line H1299 following transfection with miR-182-5p
inhibitor with or without CASP2. Initially, transfection efficiency
was examined in H1299 cells. The mRNA and protein expression levels
of CASP2 were significantly decreased following transfection with
CASP2 siRNA compared with siNC (P<0.05; Fig. 6A and B). In addition, inhibition of
miR-182-5p significantly increased CASP2 mRNA and protein
expression levels compared with NC (P<0.01; Fig. 6C and D). Furthermore, co-transfection
with miR-182-5p inhibitor and CASP2 siRNA significantly decreased
CASP2 mRNA and protein expression levels compared with miR-182-5p
inhibitor alone (P<0.05; Fig. 6C and
D). MTT assay demonstrated that cell proliferation was
significantly decreased in H1299 cells following 48-and 72-h
transfection with miR-182-5p inhibitor compared with the NC
(P<0.05; Fig. 6E); however, cell
proliferation was significantly increased following 72-h
co-transfection with CASP2 siRNA compared with miR-182-5p inhibitor
alone (P<0.05; Fig. 6E). Flow
cytometry demonstrated that the rate of apoptosis was significantly
increased in H1299 cells following transfection with miR-182-5p
inhibitor compared with the NC (P<0.01; Fig. 6F), however the rate of apoptosis was
significantly decreased following co-transfection with CASP2 siRNA
compared with miR-182-5p inhibitor alone (P<0.05; Fig. 6F). Taken together, these results
suggest that miR-182-5p may regulate H1299 cell proliferation and
apoptosis by directly binding with the 3′-UTR of CASP2 to regulate
CASP2 expression.
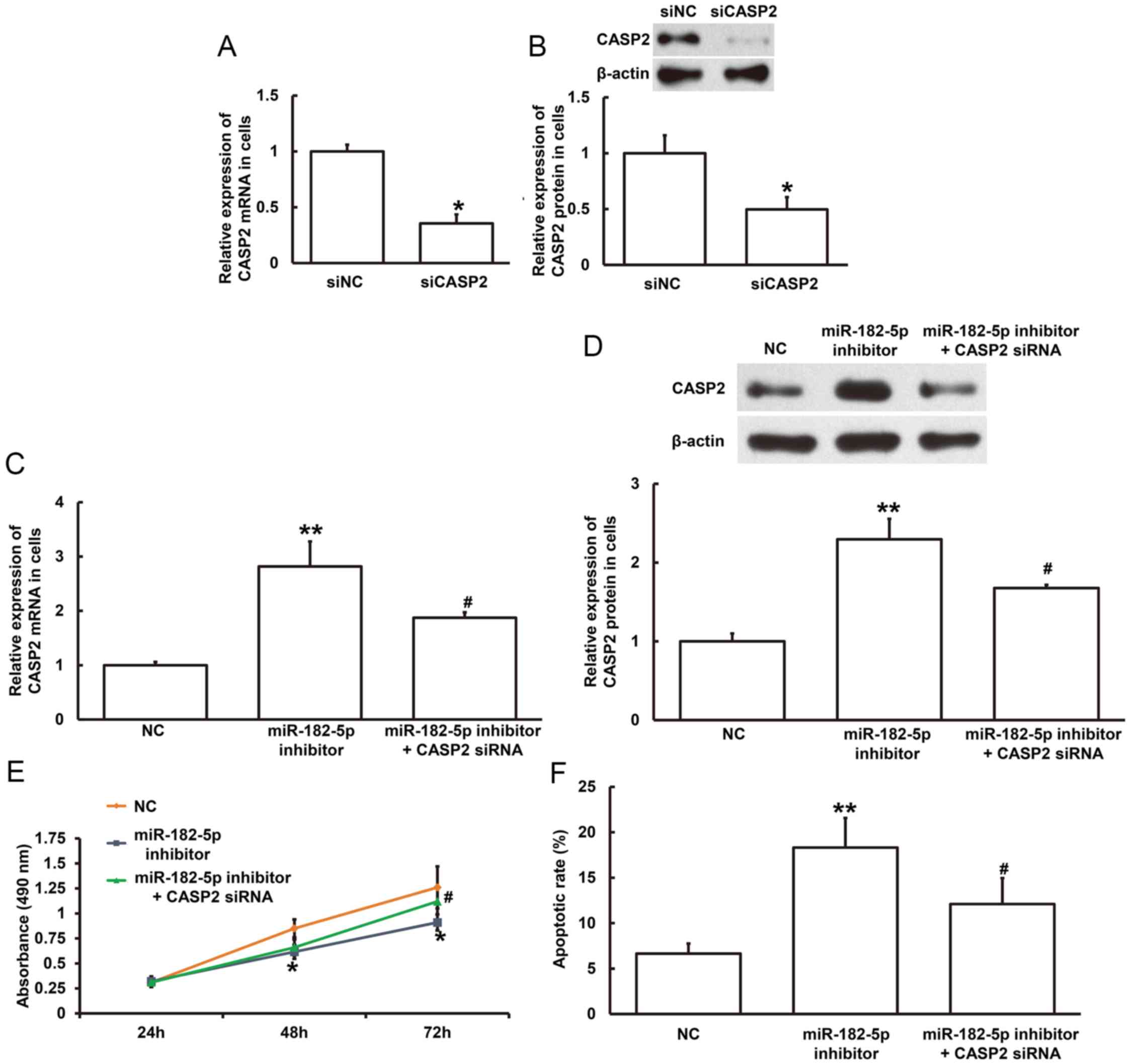 | Figure 6.Effect of miR-182-5p on CASP2
expression and NSCLC cell proliferation. The relative (A) mRNA and
(B) protein expression level of CASP2 in H1299 cells following
transfection with siNC or CASP2 siRNA. *P<0.05 compared with
siNC group. The relative (C) mRNA and (D) protein expression level
of CASP2 in H1299 cells following transfection with NC, miR-182-5p
inhibitor or miR-182-5p inhibitor and CASP2 siRNA. **P<0.01 vs.
NC; #P<0.05 vs. miR-182-5p inhibitor. (E) MTT assay was used to
examine cell proliferation of H1299 cells transfected with NC,
miR-182-5p inhibitor or miR-182-5p inhibitor and CASP2 siRNA for
24, 48 and 72 h. *P<0.05 vs. NC; #P<0.05 vs. miR-182-5p
inhibitor. (F) Flow cytometry was used to detect apoptosis in H1299
cells transfected with NC, miR-182-5p inhibitor or miR-182-5p
inhibitor and CASP2 siRNA. **P<0.01 vs. NC; #P<0.05 vs.
miR-182-5p inhibitor. miR, microRNA; CASP2, caspase 2; NSCLC,
non-small cell lung cancer; siNC, negative control siRNA; siRNA,
small interfering RNA; NC, negative control. |
Discussion
Lung cancer can be divided into two histologic
classes: small-cell lung cancer (SCLC) and NSCLC (20,21).
NSCLC accounts for ~80–85% of all lung cancer cases, with a 5-year
survival rate of 16% (22). In the
early (occult) stage, lung cancer is difficult to diagnose and most
patients with lung cancer are diagnosed with late stage lung cancer
(23). Although surgery and/or
chemoradiotherapy can prolong the survival time of patients with
lung cancer, prognosis remains poor (24). In recent years, the development of
molecular targeted drugs has improved the survival rates of
patients with advanced lung cancer, however due to the relatively
small subset of NSCLC patients and high drug resistance, the
application of molecular targeted drugs has had limited success
(25–28). It is therefore important to
investigate the molecular mechanisms underlying the occurrence and
development of lung cancer, and identify novel diagnostic and
therapeutic targets with high specificity.
Several studies have demonstrated the important
roles of miRNAs in the initiation and progression of NSCLC
(22,29,30). In
addition, previous studies revealed that miR-182-5p may serve
different roles in different types of cancer, including renal cell
carcinoma, neuroblastoma, liver cancer, ovarian cancer, prostate
cancer and bladder cancer (11,12,31–37). The
expression of miR-182-5p is associated with cell proliferation and
prognosis in renal cell carcinoma (11,32), as
well as cell differentiation and apoptosis in neuroblastoma
(31). In prostate cancer, the
expression of miR-182-5p is associated with cancer cell
proliferation and invasion (33). In
addition, the expression of miR-182-5p is known to have a
carcinogenic effect in bladder cancer (36). In the present study, miR-182-5p
expression was upregulated in tumor tissue and peripheral blood
samples from patients with NSCLC and the MTT assay demonstrated
that inhibition of miR-182-5p decreased NSCLC cell proliferation.
These results suggest that miR-182-5p may be involved in NSCLC
progression.
Bioinformatics analysis was used to identify CASP2
as a potential target gene of miR-182-5p. CASP2 serves important
roles in stress-induced apoptosis, and CASP2 activation occurs in
response to apoptosis-stimulating factors, which include tumor
necrosis factor-α (38,39), Fas (40) and growth factor deficiency (41,42). In
the current study, CASP2 expression is downregulated in tumor
tissue and peripheral blood samples from patients with NSCLC, which
suggests that CASP2-mediated apoptosis may be involved in the
pathogenesis of NSCLC. In addition, the current study demonstrated
that miR-182-5p may regulate NSCLC cell proliferation and apoptosis
via the regulation of CASP2 expression, as decreased cell
proliferation induced by the inhibition of miR-182-5p was partially
restored by CASP2 siRNA, possibly due to transfection efficiency
and cell type (18). The
dual-luciferase reporter assay demonstrated that miR-182-5p
interacts directly with the 3′-UTR of CASP2 mRNA to regulate CASP2
expression.
In the current study, both tumor tissue and
peripheral blood samples were examined, and the results
demonstrated the potential diagnostic application of blood sample
collection when screening patients for NSCLC (31,32). The
present study demonstrated that upregulated miR-182-5p expression
in tumor tissue and peripheral blood samples from patients with
NSCLC may be associated with the downregulation of CASP2
expression. In addition, miR-182-5p may regulate NSCLC
proliferation via CASP2, however future studies are required to
further clarify the association between miR-182-5p and CASP2 in
NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Science and Technology Project of Shandong Education Department
(grant no. J17KB088).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY, YD and MX designed the study. LY, YD, ZS, HC,
XL, QW PG and YQ performed the experiments. LY, YD, ZS and HC
analyzed the data. All authors collaborated to interpret the
results and prepare the manuscript. All authors read and approved
the final manuscript.
Ethical approval and consent to
participate
The present study was approved by the Ethics
Committee of Qilu Medical University (Zibo, China). Written
informed consent was obtained from all patients or their
families.
Patient consent for publication
Written informed consents for publication of any
associated data and accompanying images were obtained from all
patients or their parents, guardians or next of kin.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang CG, Lee HJ, Kim SH and Lee EO:
Zerumbone suppresses osteopontin-induced cell invasion through
inhibiting the FAK/AKT/ROCK pathway in human non-small cell lung
cancer A549 Cells. J Nat Prod. 79:156–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu YJ, Du Y and Fan Y: Long noncoding RNAs
in lung cancer: What we know in 2015. Clin Transl Oncol.
18:660–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: Challenges and interventions. Chest.
143:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Q and Zhou Q: The challenges of lung
cancer in China. J Cancer Res Ther. 9 (Suppl 2):S65–S66. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakanishi K, Mizuno T, Sakakura N, Kuroda
H, Shimizu J, Hida T, Yatabe Y and Sakao Y: Salvage surgery for
small cell lung cancer after chemoradiotherapy. Jpn J Clin Oncol.
1:389–392. 2019. View Article : Google Scholar
|
|
7
|
Gamazon ER, Trendowski MR, Wen Y, Wing C,
Delaney SM, Huh W, Wong S, Cox NJ and Dolan ME: Gene and MicroRNA
perturbations of cellular response to pemetrexed implicate
biological networks and enable imputation of response in lung
adenocarcinoma. Sci Rep. 8:7332018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inoue K: MicroRNA function in animal
development. Tanpakushitsu Kakusan Koso. 52:197–204. 2007.(In
Japanese). PubMed/NCBI
|
|
9
|
Liu G, Li YI and Gao X: Overexpression of
microRNA-133b sensitizes non-small cell lung cancer cells to
irradiation through the inhibition of glycolysis. Oncol Lett.
11:2903–2908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang LP, Zhu ZT and He CY: Expression of
miRNA-26b in the diagnosis and prognosis of patients with
non-small-cell lung cancer. Future Oncol. 12:1105–1115. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan Y, Li H, Ma X, Go Y, Bao X, Du Q, Ma
M, Liu K, Yao Y, Huang Q, et al: Dicer suppresses the malignant
phenotype in VHL-deficient clear cell renal cell carcinoma by
inhibiting HIF-2α. Oncotarget. 7:18280–18294. 2016.PubMed/NCBI
|
|
12
|
Assal RA, El Tayebi HM, Hosny KA, Esmat G
and Abdelaziz AI: A pleiotropic effect of the single clustered
hepatic metastamiRs miR-96-5p and miR-182-5p on insulin-like growth
factor II, insulin-like growth factor-1 receptor and insulin-like
growth factor-binding protein-3 in hepatocellular carcinoma. Mol
Med Rep. 12:645–650. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dorstyn L, Puccini J, Wilson CH, Shalini
S, Nicola M, Moore S and Kumar S: Caspase-2 deficiency promotes
aberrant DNA-damage response and genetic instability. Cell Death
Differ. 19:1288–1298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Liu C, Wang J, Zhang Y and Chen L:
Iodine-131 induces apoptosis in human cardiac muscle cells through
the p53/Bax/caspase-3 and PIDD/caspase-2/tBID/cytochrome
c/caspase-3 signaling pathway. Oncol Rep. 38:1579–1586. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jang TH, Lee SJ, Woo CH, Lee KJ, Jeon JH,
Lee DS, Choi K, Kim IG, Kim YW, Lee TJ and Park HH: Inhibition of
genotoxic stress induced apoptosis by novel TAT-fused peptides
targeting PIDDosome. Biochem Pharmacol. 83:218–227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Dou Y and Sheng M: Inhibition of
microRNA-383 has tumor suppressive effect in human epithelial
ovarian cancer through the action on caspase-2 gene. Biomed
Pharmacother. 83:1286–1294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Makwana K, Patel SA, Velingkaar N, Ebron
JS, Shukla GC and Kondratov RVKV: Aging and calorie restriction
regulate the expression of miR-125a-5p and its target genes Stat3,
Casp2 and Stard13. Aging (Albany NY). 9:1825–1843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xia D, Li X, Niu Q, Liu X, Xu W, Ma C, Gu
H, Liu Z, Shi L, Tian X, et al: MicroRNA-185 suppresses pancreatic
cell proliferation by targeting transcriptional coactivator with
PDZ-binding motif in pancreatic cancer. Exp Ther Med. 15:657–666.
2018.PubMed/NCBI
|
|
19
|
He X, Ping J and Wen D: MicroRNA-186
regulates the invasion and metastasis of bladder cancer via
vascular endothelial growth factor C. Exp Ther Med. 14:3253–3258.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oser MG, Niederst MJ, Sequist LV and
Engelman JA: Transformation from non-small-cell lung cancer to
small-cell lung cancer: Molecular drivers and cells of origin.
Lancet Oncol. 16:e165–e172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen G, Umelo IA, Lv S, Teugels E, Fostier
K, Kronenberger P, Dewaele A, Sadones J, Geers C and De Greve J:
miR-146a inhibits cell growth, cell migration and induces apoptosis
in non-small cell lung cancer cells. PLoS One. 8:e603172013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hofman P: The challenges of evaluating
predictive biomarkers using small biopsy tissue samples and liquid
biopsies from non-small cell lung cancer patients. J Thorac Dis. 11
(Suppl 1):S57–S64. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Castanon E, Martin P, Rolfo C, Fusco JP,
Ceniceros L, Legaspi J, Santisteban M and Gil-Bazo I: Epidermal
growth factor receptor targeting in non-small cell lung cancer:
Revisiting different strategies against the same target. Curr Drug
Targets. 15:1273–1283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen G, Kronenberger P, Teugels E, Umelo
IA and De Greve J: Effect of siRNAs targeting the EGFR T790M
mutation in a non-small cell lung cancer cell line resistant to
EGFR tyrosine kinase inhibitors and combination with various
agents. Biochem Biophys Res Commun. 431:623–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen G, Kronenberger P, Teugels E, Umelo
IA and De Greve J: Targeting the epidermal growth factor receptor
in non-small cell lung cancer cells: The effect of combining RNA
interference with tyrosine kinase inhibitors or cetuximab. BMC Med.
10:282012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen G, Noor A, Kronenberger P, Teugels E,
Umelo IA and De Greve J: Synergistic effect of afatinib with
su11274 in non-small cell lung cancer cells resistant to gefitinib
or erlotinib. PLoS One. 8:e597082013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lan D, Zhang X, He R, Tang R, Li P, He Q
and Chen G: MiR-133a is downregulated in non-small cell lung
cancer: A study of clinical significance. Eur J Med Res. 20:502015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Li P, Rong M, He R, Hou X, Xie Y
and Chen G: MicroRNA-141 is a biomarker for progression of squamous
cell carcinoma and adenocarcinoma of the lung: Clinical analysis of
125 patients. Tohoku J Exp Med. 235:161–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rihani A, Van Goethem A, Ongenaert M, De
Brouwer S, Volders PJ, Agarwal S, De Preter K, Mestdagh P, Shohet
J, Speleman F, et al: Genome wide expression profiling of p53
regulated miRNAs in neuroblastoma. Sci Rep. 5:90272015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen L, Ma H, Hu H, Gao L, Wang X, Ma J,
Gao Q, Liu B, Zhou G and Liang C: Special role of Foxp3 for the
specifically altered microRNAs in Regulatory T cells of HCC
patients. BMC Cancer. 14:4892014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu X, Wu J, Li S, Hu Z, Xu X, Zhu Y, Liang
Z, Wang X, Lin Y, Mao Y, et al: Downregulation of microRNA-182-5p
contributes to renal cell carcinoma proliferation via activating
the AKT/FOXO3a signaling pathway. Mol Cancer. 13:1092014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang L, Zhu MJ, Ren AM, Wu HF, Han WM, Tan
RY and Tu RQ: A ten-microRNA signature identified from a
genome-wide microRNA expression profiling in human epithelial
ovarian cancer. PLoS One. 9:e964722014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hirata H, Ueno K, Shahryari V, Deng G,
Tanaka Y, Tabatabai ZL, Hinoda Y and Dahiya R: MicroRNA-182-5p
promotes cell invasion and proliferation by down regulating FOXF2,
RECK and MTSS1 genes in human prostate cancer. PLoS One.
8:e555022013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hirata H, Ueno K, Shahryari V, Tanaka Y,
Tabatabai ZL, Hinoda Y and Dahiya R: Oncogenic miRNA-182-5p targets
Smad4 and RECK in human bladder cancer. PLoS One. 7:e510562012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsuchiyama K, Ito H, Taga M, Naganuma S,
Oshinoya Y, Nagano K, Yokoyama O and Itoh H: Expression of
microRNAs associated with Gleason grading system in prostate
cancer: miR-182-5p is a useful marker for high grade prostate
cancer. Prostate. 73:827–834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suzuki Y and Farbman AI: Tumor necrosis
factor-alpha-induced apoptosis in olfactory epithelium in vitro:
Possible roles of caspase 1 (ICE), caspase 2 (ICH-1), and caspase 3
(CPP32). Exp Neurol. 165:35–45. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lv XX, Yu XH, Wang HD, Yan YX, Wang YP, Lu
DX, Qi RB, Hu CF and Li HM: Berberine inhibits
norepinephrine-induced apoptosis in neonatal rat cardiomyocytes via
inhibiting ROS-TNF-α-caspase signaling pathway. Chin J Integr Med.
19:424–431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Droin N, Bichat F, Rebe C, Wotawa A,
Sordet O, Hammann A, Bertrand R and Solary E: Involvement of
caspase-2 long isoform in Fas-mediated cell death of human leukemic
cells. Blood. 97:1835–1844. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jean YY, Ribe EM, Pero ME, Moskalenko M,
Iqbal Z, Marks LJ, Greene LA and Troy CM: Caspase-2 is essential
for c-Jun transcriptional activation and Bim induction in neuron
death. Biochem J. 455:15–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Troy CM, Rabacchi SA, Hohl JB, Angelastro
JM, Greene LA and Shelanski ML: Death in the balance: Alternative
participation of the caspase-2 and −9 pathways in neuronal death
induced by nerve growth factor deprivation. J Neurosci.
21:5007–5016. 2001. View Article : Google Scholar : PubMed/NCBI
|