Introduction
Liver cancer is the fourth leading cause of cancer
mortality worldwide (1,2). Despite significant progress over the
past decades, the clinical outcomes of patients with liver cancer
remain poor and an in-depth understanding of the molecular
pathogenesis of liver cancer could facilitate the development of
novel diagnostic and therapeutic techniques.
Adenosine is an endogenous nucleoside that controls
many physiological processes through interactions with adenosine
receptors, such as A1, A2A, A2B, and A3 (3). Adenosine A2 receptors have two
segmented isoforms; high-affinity A2A receptor which is highly
concentrated in the striatum, and relatively low-affinity A2B
receptors which present throughout the brain (4). Generally, adenosines can be found
eluted into the extracellular matrix of ordinary solid organs under
low-oxygen conditions (5). However,
in cancerous organs, the signaling pathway generated between
adenosine and its receptor has been shown to coordinate adenosine
accumulation and the increased levels of adenosine in the
extracellular fluid of solid tumors stimulate cancer cell
proliferation and tumor angiogenesis (6). The relationship between the tumor
progression and adenosine A2B receptor expression has been
investigated in many types of tumors including bladder (7), breast (8), colon (9), and prostate (10) cancers. While some researchers have
shown that A2B is highly expressed in cancerous tissues in patients
with HCC (11), the regulatory
pathways and the function of A2B in liver cancer remain
undiscovered.
The adenosine A2B receptor is reported to be
transcriptionally induced by tumor necrosis factor-alpha (TNF-α)
(12), interferon-gamma (IFN-γ)
(13), and hypoxia-inducible
factor-1α (HIF-1α) (14,15). HIF-1α is an oxygen-regulated subunit
of HIF-1, a heterodimeric transcription factor consisting of α- and
β-subunits. Intra-tumoral hypoxia is the major cause of increased
HIF-1 activity in human HCC (16),
and HIF-1α protein stabilization in cancer cells leading to the
upregulation of multiple HIF-1 target genes that are required for
HCC tumor growth, as demonstrated by both genetic and pharmacologic
loss-of-function studies (17).
HIF-1α mediated expression of adenosine A2B receptor activations in
hypoxia has reported in endothelial cells (15), acute lung injury (18), and breast cancer (19). In addition to this, many researchers
suggested that hypoxia is one factor for inducing A2B expressions,
however, direct HIF-1α mediated A2B increment during tumor
progression, especially in liver cancer has not been investigated
yet. Therefore, we designed experiments to verify whether
transcriptional regulation pathways control the endogenous hepatic
adenosine signaling during a low-oxygen condition of liver cancer
cells. Furthermore, we also investigated the potential HIF-1α
binding regions in the A2B promoter genes. We believe our study
will contribute to the development of new therapeutic targets to
treat liver cancers.
Materials and methods
Cell culture and hypoxic
conditions
We purchased human liver cancer cell lines,
including HepG2 (KCLB no. 88065; passage 21), the hepatoblastoma
cell line (20), and the
hepatocellular carcinoma (HCC) cell lines SK-Hep1 (KCLB no. 30052;
passage 49) and SNU-449 (KCLB no. 00449; passage 28) from the
Korean Cell Line Bank (KCLB; Seoul, Republic of Korea). HepG2 and
SK-Hep1 cells were maintained in Dulbecco's modified Eagle's medium
(DMEM) high-glucose (Hyclone; Thermo Fisher Scientific, Inc.),
supplemented with 10% fetal bovine serum (FBS; Mediatech) and 100
U/ml penicillin/streptomycin (HyClone). The HCC SNU-449 cells were
maintained in RPMI-1640 (Invitrogen; Thermo Fisher Scientific,
Inc.), supplemented with 10% FBS and 100 U/ml
penicillin/streptomycin. We cultured all the cells in a standard
humidified incubator at 37°C in an atmosphere of 5% CO2.
To induce hypoxic conditions, we placed cells in a hypoxia
incubator (MCO-18M; Sanyo) filled with a mixture of 5%
CO2, 94.5% N2, and 0.5% O2 gas.
Authentication of the cell lines was done using short tandem repeat
(STR) profiling by the Korean Cell Line Bank (Seoul National
University College of Medicine, Seoul, Republic of Korea;
http://cellbank.snu.ac.kr/) with proper
STR references. The cell lines were tested for mycoplasma
contamination before use and were negative for mycoplasma.
Establishment of A2B luciferase
constructs
We used a pGL3 basic luciferase vector as a control
plasmid and performed luciferase assays with previously designed
constructs (15). The full-length
construct (1095 bp) had a hypoxia-response element (HRE). The other
truncated construct (477 bp) lacked an HRE. Additionally, we used a
mutant form of the 1095 bp plasmid with a modified sequence
(ACGTG was
altered to AATCG).
A2B reporter assay
To confirm the transcriptional activity of the
adenosine A2B receptor, we used HepG2 cells as an easily
transfectable cellular model. First, to investigate the A2B
promoter region, we used HepG2 cells to measure the reporter gene
activity. We co-transfected cells with 2 µg of A2B-Luc
promoter-reporter and 0.02 µg Renilla reporter vector for 24 h
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. Next, we exposed the cells to hypoxic conditions for
up to 12 h. To prepare whole protein lysates, we used 1× passive
lysis buffer (cat no. E1910; Promega). To perform the luciferase
assay, we followed the protocol of the Dual-Luciferase Reporter
Assay (Promega).
RNA interference
Small interfering RNAs (siRNA) specific to either
HIF-1α (HIF-1α-siRNA), A2B (A2B-siRNA), or scrambled sequences
(scr-siRNA) were prepared by Bioneer Corporation. We used 0.5 µg of
siRNA for transfections using Lipofectamine® 2000
reagent (Invitrogen). The sequences of siRNAs were: Scrambled-siRNA
(scr-siRNA), sense, 5′-CCUACGCCACCAAUUUCGU(dTdT)-3′, antisense,
5′-ACGAAAUUGGUGGCGUAGG(dTdT)-3′; HIF-1α-siRNA, sense,
5′-GUGGUUGGAUCUAACACUA(dTdT)-3′, antisense,
5′-UAGUGUUAGAUCCAACCAC(dTdT)-3′; A2B-siRNA, sense,
5′-GAGACUUCCGCUACACUUU(dTdT)-3′, antisense,
5′-AAAGUGUAGCGGAAGUCUC(dTdT)-3′.
HIF-1α plasmid overexpression
We seeded HepG2 cells into 6-well plates at a
density of 1.0×106 cells per well and incubated them for
24 h at 37°C. The cells were transfected with a
HIF-1-pcDNA3.1-expressing plasmid using Lipofectamine®
2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.). We
confirmed HIF-1α overexpression by western blot. The negative
control plasmid pcDNA3.1(+) and the HIF-1-pcDNA3.1-expressing
plasmids were purchased from Addgene. We treated cells with range
from 3 to 300 nM of echinomycin (cat no. 5520; Tocris Bioscience),
a specific inhibitor of HIF-1 DNA binding activity, to achieve
chemical interference of HIF activity.
BrdU assay
Exponentially growing HepG2 cells were digested,
centrifuged, collected, and inoculated into 96-well culture plates
at a density of 5.0×103 cells/well. After culturing for
24 h in DMEM containing 10% FBS, the cells were transfected with
scrambled-siRNA (scr-siRNA) and A2B-siRNA. HepG2 cell proliferation
activity was analyzed using BrdU cell proliferation assay kits (cat
no. 2750; Millipore), 24, 48, 72, and 96 h after siRNA
transfection. The BrdU reagent was added to the culture wells at
each time point, and the plates were incubated at 37°C for an
additional 4 h. The cells were then fixed, and the BrdU levels were
detected colorimetrically following the manufacturer's
instructions. To plot the growth curve, a microplate reader
(Sunrise TECAN, Labx, Canada) was used to measure absorbance at 450
nm. Experiments were repeated four times.
Cell growth and viability test
Cell growth and viability were determined by
performing a 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay in 12 well cell culture plates (NEST
Biotechnology). HepG2 cells were seeded at a density of
5.0×104 per well for 24 h and cultured in DMEM
containing 10% FBS. The cells were then transfected with scr-siRNA
or A2B-siRNA. After incubation for 24, 48, and 72 h, 20 µl of MTT
solution (5 mg/ml in phosphate buffer saline) was added to each
well, and the plates were incubated in the dark for 4 h at 37°C.
Cell growth and viability were expressed as the percentage of
absorbance of MTT-treated cells relative to that of untreated cells
at a wavelength of 540 nm. Measurements for all treatment groups
were taken four times.
Patient tissue samples
We collected five individual human HCC specimens
from patients with HCC, who visited the Asan Medical Center
(Division of Liver Transplantation and Hepatobiliary Surgery;
Seoul, Republic of Korea) between 2014 and 2015 (Table I). None of the patients received any
form of treatment before surgical procedures. We separately
collected small pieces of the tumor and adjacent normal tissues and
snap-froze them into liquid nitrogen. Frozen samples were stored at
−80°C until use. The Institutional Review Board of Asan Medical
Center reviewed and approved the collection and use of patient
specimens (approval no. 2016-0582). All patients who provided
tissue samples agreed to donate their specimens and provided
written informed consents.
 | Table I.Clinical characteristic of five
patients with HCC in the Asan Medical Center. |
Table I.
Clinical characteristic of five
patients with HCC in the Asan Medical Center.
| Patients | P1 | P2 | P3 | P4 | P5 |
|---|
| Grading | Grade III | Grade III | Grade III | Grade III | Grade III |
| Gender | F | M | M | M | F |
| Age (years) | 38 | 54 | 62 | 65 | 68 |
| Risk Factors | HBV | HBV | HBV | HBV | HBV |
| AST (U/l) | 174 | 82 | 133 | 191 | 190 |
| ALT (U/l) | 128 | 72 | 87 | 147 | 137 |
| Albumin (g/dl) | 3.5 | 3.6 | 3.3 | 3.0 | 2.8 |
| Tumor size
(cm) | 3.7×3.3×3.3 | 2.4×1.5×1.0 | 4.5×4.0×3.3 | 11.6×10.8×9.6 | 14.2×10.8×7.5 |
RNA extraction and RT-PCR
We isolated total RNA from human livers and liver
cancer cells using QIAzol reagent (Qiagen). We homogenized the
frozen tissues and liver cancer cell suspensions in QIAzol reagent.
The homogenates were mixed thoroughly after adding chloroform and
were centrifuged at 16,000 × g for 15 min. The aqueous phase was
removed, and the RNA was precipitated with isopropyl alcohol. RNA
was pelleted, washed with 70% ethanol, dried, and eluted using
DEPC-treated water. We used a spectrophotometer Nanodrop 2000
(Nanodrop) to quantify the final concentration.
We quantified transcription of relevant genes using
real-time reverse transcription-polymerase chain reaction (RT-PCR)
in a CFX Connect Real-Time PCR Detection System (Bio-Rad) with 5×
HOT FIREPol EvaGreen qPCR Supermix (Solis BioDyne), according to
the manufacturer's instructions. In brief, the samples were first
denatured at 95°C for 15 min, followed by 40 cycles of denaturation
at 95°C for 15 sec, annealing at 55°C-60°C for 15 sec, and
elongation at 72°C for 20 sec. The data are expressed as the fold
changes in the treatment groups relative to the control level and
were normalized to GAPDH levels using the delta-delta Ct
methods (21). Table II lists the sense and antisense
primer sequences.
 | Table II.Sequences of Primers used in the
study. |
Table II.
Sequences of Primers used in the
study.
| Gene | Primer
sequence |
|---|
| Adora1 | Forward
5′-TGCACTGGCCTGTTCTGTAG-3′ |
|
| Reverse
5′-CTGCCTCTCCCACGTACAAT-3′ |
| Adora2a | Forward
5′-GGAGTTTGCCCCTTCCTAAG-3′ |
|
| Reverse
5′-CTGCTTCCTCAGAACCCAAG-3′ |
| Adora2b | Forward
5′-ATCTCCAGGTATCTTCTC-3′ |
|
| Reverse
5′-GTTGGCATAATCCACACAG-3′ |
| Adora3 | Forward
5′-CCTGGGCATCACAATCCACT-3′ |
|
| Reverse
5′-ACCCTCTTGTATCTGACGGTA-3′ |
| Gapdh | Forward
5′-GAGTCAACGGATTTGGTCGT-3′ |
|
| Reverse
5′-TTGATTTTGGAGGGATCTCG-3′ |
Protein isolation and western
blotting
We extracted protein samples from
~2.0×106 cells or 70 mg of frozen liver tissues. The
samples were homogenized in lysis buffer (RIPA; Biosesang),
quantified with BCA protein assay kit (Thermo Fisher Scientific,
Inc.), and immunoblotted with anti-adenosine A2B receptor antibody
(1:1,000 dilution; ab40002, Abcam) followed by rabbit anti-goat IgG
HRP (1:20,000 dilution; A5420, Sigma-Aldrich); or anti-HIF-1α
antibody (1:1,000 dilution; cat no. 610959, BD Biosciences)
followed by goat anti-mouse IgG HRP (1;5,000; sc-2005, Santa Cruz
Biotechnology). We also probed the nitrocellulose membranes with
actin-peroxidase conjugate (1:20,000 dilution; A3854,
Sigma-Aldrich). The protein signals were detected using an enhanced
chemiluminescence solution (SuperSignal™ West Femto; Thermo Fisher
Scientific, Inc.), and images were obtained using the ImageQuant
LAS 4000 system (GE Healthcare Biosciences).
Statistical analysis
We performed all statistical analyses using the
GraphPad Prism 6.0 software (GraphPad Software). All other data are
presented as the mean ± SD. For western blot analyses, we repeated
each experiment three times. We used a one-way analysis of variance
(ANOVA) followed by Bonferroni's multiple comparison test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Hypoxia triggered A2B induction is
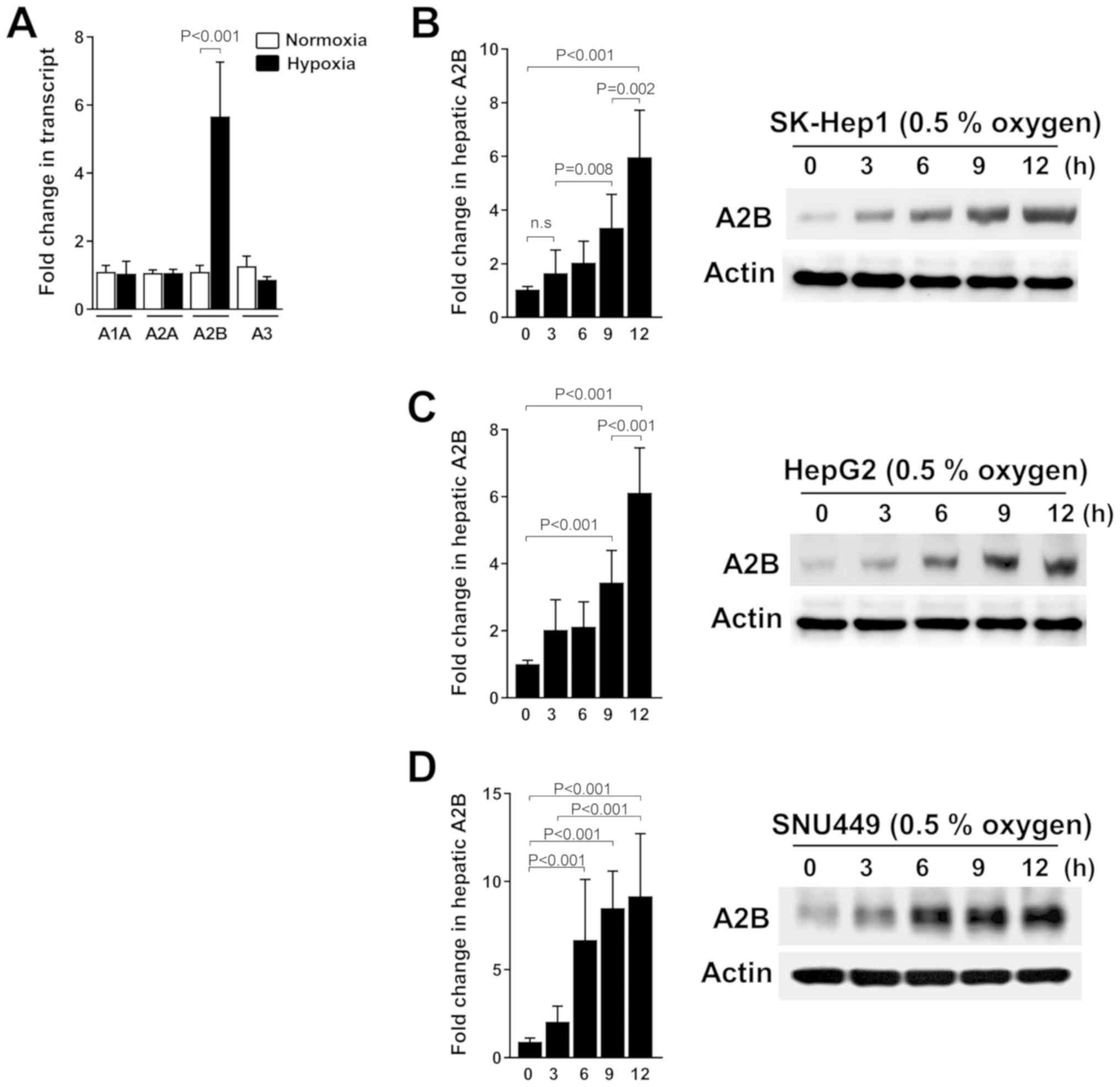
time-dependent in liver cancer cells
In this study, we investigated whether A2B is
upregulated under hypoxic conditions or not. Thus, we grew three
liver cancer cell lines, SK-Hep1, HepG2, and SNU449, under hypoxic
conditions. We consistently detected the significant expression of
only A2B mRNA levels compared to the expression of other receptor
genes (A1, A2A, and A3) under low-oxygen conditions (Fig. 1A). As shown, the A2B mRNA level and
protein expressions were expressed in a time-dependent manner
during hypoxia in SK-Hep1 cells (Fig.
1B). Additionally, A2B is upregulated under hypoxic conditions
in HepG2 (Fig. 1C) and SNU449 cells
(Fig. 1D). These data reveal a
selective induction of A2B during hypoxic conditions.
HIF-1α directly regulated the
expression of A2B by binding to the HRE in the A2B promoter
region
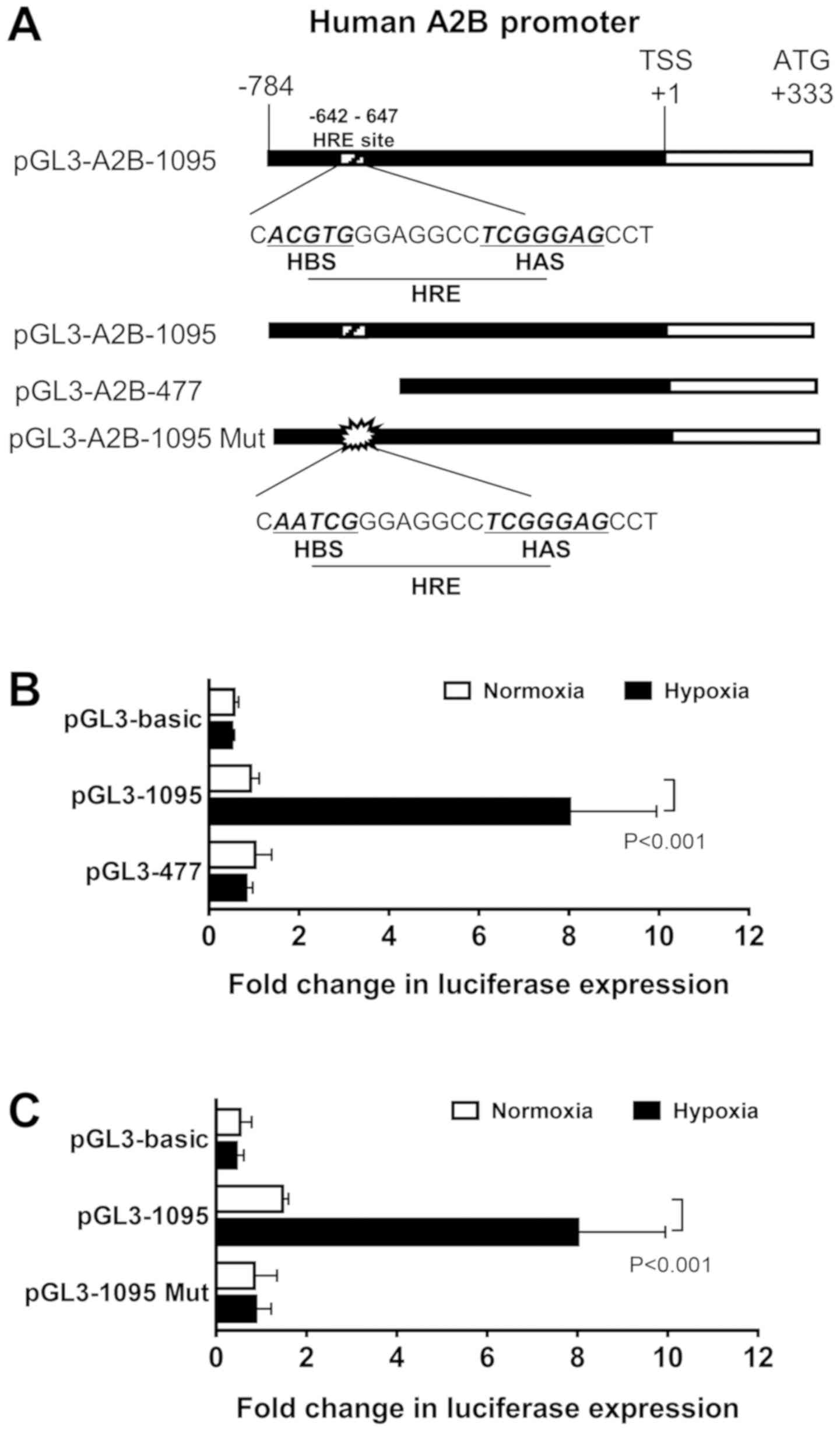
To verify if A2B is transcriptionally induced by
HIF-1α, we first searched for the human A2B promoter region, which
has an HRE, containing −642 to −647 bp, at the 5′-CACGTGG-3′
sequence and its typical HIF-1α ancillary HAS site (5′-CGGGGAG-3′)
at −546 to −541 (Fig. 2A). To
investigate the function of the HIF-1α binding site, we used two
modified constructs, a full-length promoter construct (1095 bp) and
a truncated construct (477 bp) of HIF-1α (Fig. 2B). As shown in the results, the
full-length HRE construct-transfected cells had significantly
higher activity than the truncated construct. Next, to define
whether the full-length construct was acted upon by HIF-1α, we
performed luciferase assays with site-directed mutagenesis
constructs. The mutant form of this construct had an HRE
AATCG change
from ACGTG
(Fig. 2C). The mutant construct
(1095 Mut) exhibited low luciferase assay activity levels, as
hypothesized. These results confirmed the A2B promoter region has
an HRE site in it.
HIF-1α upregulates A2B expression
during hypoxia
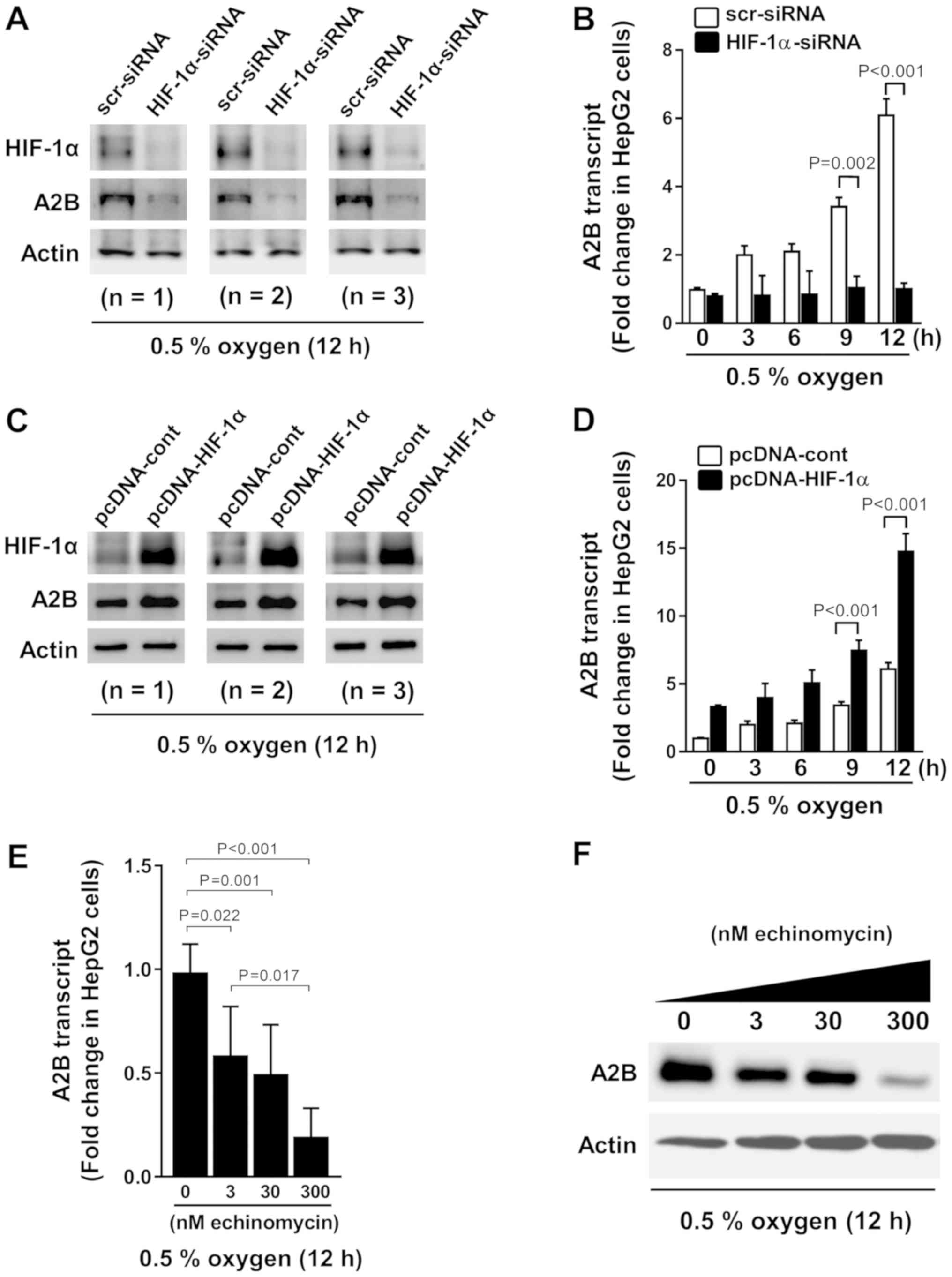
To confirm the HRE function within the promoter
region of A2B, we performed experiments with HIF-1α siRNA and
HIF-1α pcDNA plasmids. We transfected HIF-1α siRNA for 12 h
(Fig. 3A) and then exposed the cells
to hypoxia for different durations of time (Fig. 3B). HIF-1α siRNA-transfected cells
displayed low A2B expression levels while HIF-1α is being absent.
In contrast, the cells overexpressing HIF-1α exhibited high A2B
expression levels (Fig. 3C) and
hypoxia time dependency (Fig. 3D).
Moreover, we treated cells with echinomycin (a cell-permeable
inhibitor of HIF-1α-mediated gene transcription) and observed a
dose-dependent decrease in the A2B mRNA and protein expression
levels during low-oxygen conditions (Fig. 3E and F). Our results suggest HIF-1α
is a transcriptional regulator of A2B.
Silencing of A2B in liver cancer cell
suppressed cell growth and proliferation
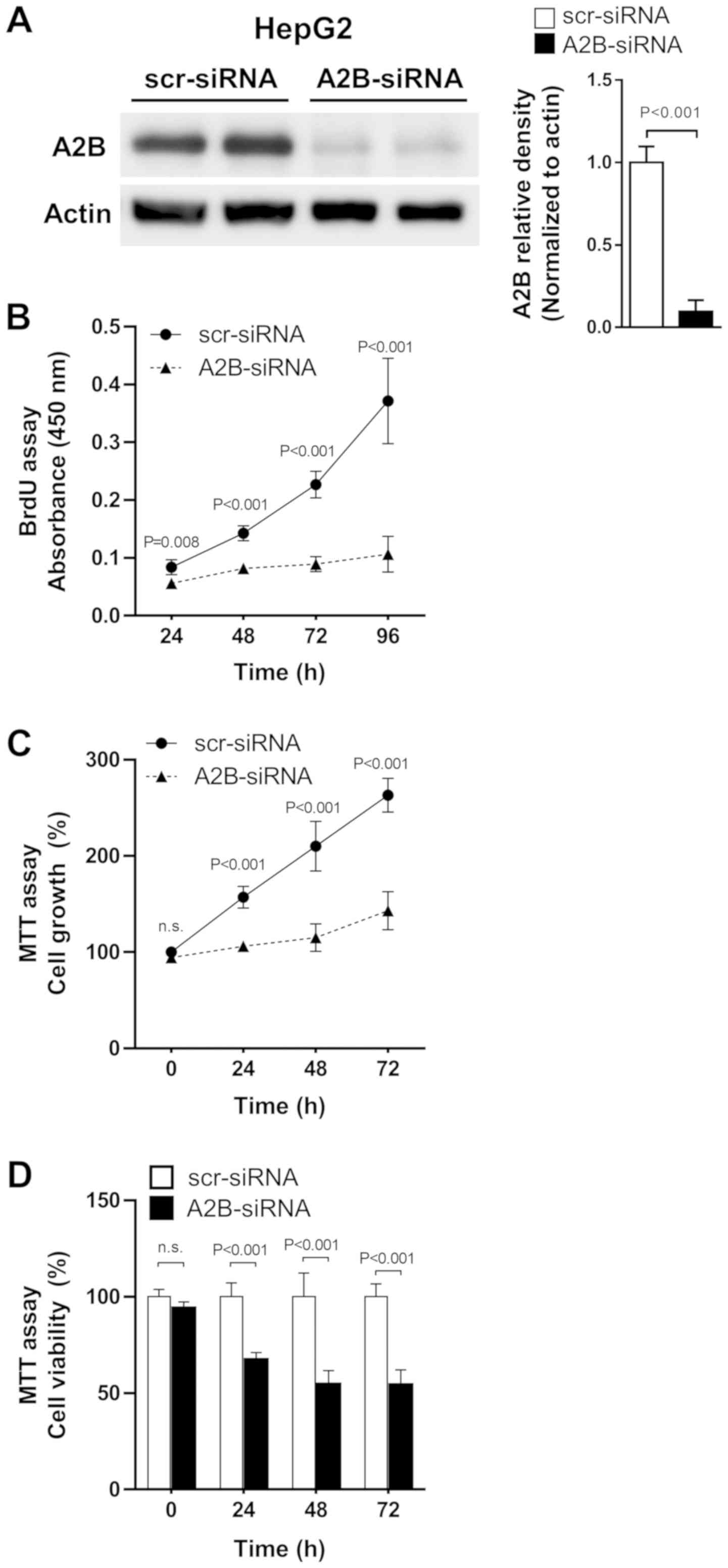
As increased expression of adenosine A2B was
observed in liver cancer cells, we tested whether the direct
knock-down of A2B expression could affect cell growth and
proliferation in liver cancer cells. We transfected HepG2 (the
hepatoblastoma cell line) cells with A2B-siRNA and maintained them
for up to 96 h. The A2B-siRNA effectively inhibited A2B expressions
compared to scr-siRNA (Fig. 4A).
Treatment with A2B-siRNA for 24 to 96 h significantly (P<0.001)
decreased cell proliferation, as revealed by the BrdU assay
(Fig. 4B). Similarly, the MTT assay
also showed that cell growth was greatly decreased when cells were
treated with A2B-siRNA (Fig. 4C).
Additionally, the relative cell viabilities were significantly
(P<0.001) lowered after transfection of cells with A2B-siRNA
(Fig. 4D). As A2B deficient liver
cancer cells show consistent decrease in cell growth and
proliferation, we could conclude that A2B takes an important role
in liver cancer cell proliferation.
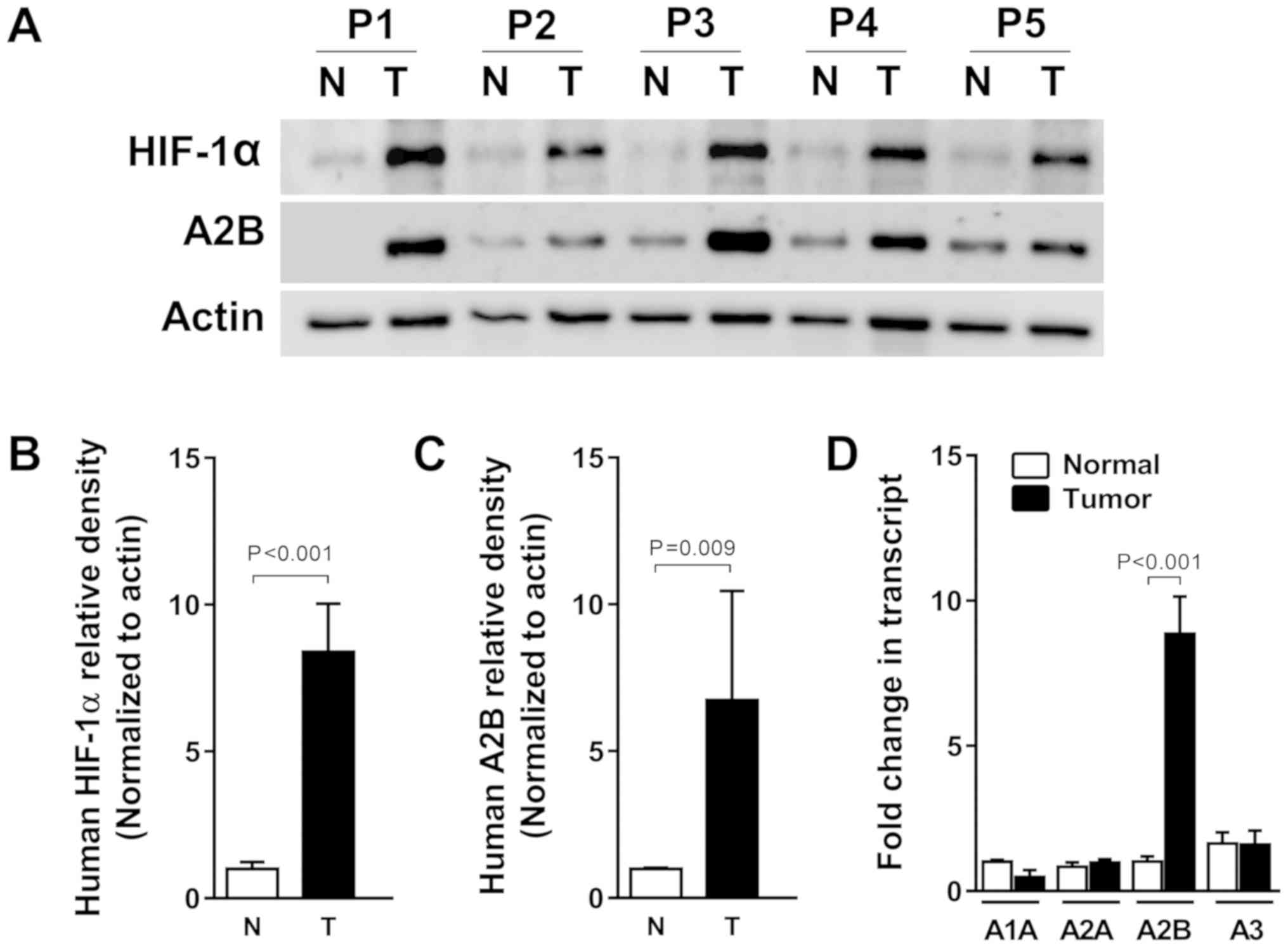
HIF-1α levels are associated with A2B
transcript and protein levels in human liver cancer specimens
Solid tumors, including liver cancer, usually
experience hypoxic conditions due to the fast growth (22). To confirm the relationship between
adenosine A2B receptor expression and human liver cancer, we tested
A2B induction from both tumor tissues and the adjacent normal
tissues came from identical individuals diagnosed as a liver
cancer. As expected, HIF-1α protein expression was high in the five
human liver cancer specimens compared to its expression in normal
tissues. A2B protein expression levels were also increased,
suggesting that HIF-1α correlates with A2B in tumors, according to
the fold changes of HIF-1α and A2B densitometry results (Fig. 5A-C). Collectively, HIF-1α, as a
master transcription factor, can bind to the A2B promoter region
and induce expression of A2B, thereby promoting tumor growth and
survival. Moreover, we also confirmed the higher level of A2B gene
expression in the HCC specimens by qRT-PCR. By contrast, we found
no associations between the expression levels of mRNAs of A1, A2A,
or A3 between normal and HCC specimens (Fig. 5D).
Discussion
In this study, we defined the various expression
patterns of adenosine receptor isoforms from three different liver
cancer cell lines and human liver cancer specimens. We found that
the A2B expression patterns were persistently higher than those of
A1, A2A, and A3 receptor subtypes in liver cancer cell lines when
maintained under low-oxygen conditions. Those patterns were also
observed in human liver cancers. Under the hypothesis that
hypoxia-mediated HIF-1α activation leads to liver cancer cell
proliferation by activating adenosine A2B receptors, we followed
transcription factor binding assays utilizing A2B promoter
constructs and constructs with site-directed mutagenesis and have
identified HIF-1α as the key regulator of A2B-induction during
hypoxic conditions. We also investigated loss- and gain-of-function
using HIF-1α siRNA and overexpressing vectors. We found that the
expression levels of A2B were modulated HIF-1α-dependently. In
addition to this, echinomycin treatment dose-dependently inhibited
A2B expressions under hypoxia, which indicate transcriptional
induction of A2B by HIF-1α. We also showed that active deprivation
of A2B expression by siRNA suppresses cell growth and proliferation
of the hepatoblastoma cell line, HepG2. Those results indicate the
activation of hepatic A2B signals during low oxygen condition is
related to liver cancer cell proliferation. Consistent with our
in vitro results, human liver cancer specimens showed
elevated levels of HIF-1α along with A2B in both transcript and
protein levels. These data implicate HIF-1α in the regulation of
adenosine-elicited cancer growth in liver.
We have previously studied the HIF-1α stabilization
during hypoxic conditions in liver cell lines (23) and identified the protective role of
HIF-1α stabilization mediated by hypoxia-induced inhibition of
succinate dehydrogenase, concomitant increases in glycolytic
capacity, and improved tricarboxylic acid (TCA) cycle function in
alveolar epithelium (24). Also the
pharmacologic studies with HIF activator or inhibitor treatment
implicated HIF-1α-stabilization increases in liver cancer
proliferation (25).
On the other hand, studies have shown the key roles
of adenosine in HCC cell proliferation (26). Adenosine also upregulates endothelial
cell proliferation by associating with A2B in porcine and rat
arterial endothelial cells (27). In
retinal endothelial cells, activated A2B can initiate
neovascularization through increased angiogenic growth factor
expression (28). In contrast,
adenosine inhibits the growth of cardiac fibroblasts and aortic and
vascular smooth muscle cells through the activation of A2B, in
addition to increasing the proliferation of peripheral
micro-vessels, but exerts the opposite effect on cardiac tissue and
capillaries (29). Thus, A2B can
promote cancer proliferation and expansion by initiating
neovascularization around cancer masses (30).
We showed that A2B is induced under hypoxic
conditions. In general, solid cancers, such as breast, lung, colon,
and prostate cancers undergo similar low-oxygen growth conditions
(31); moreover, to maintain
homeostasis under low-oxygen supply conditions, specific genes need
to be systematically activated and expressed or inactivated.
It was previously reported that bleomycin, a
chemical irritant which induce HIF-1α, can mediate A2B receptor
expression while inducing adenosine accumulation in acute lung
injury (32). In addition, HIF-1α
mediated expression of A2B in endothelial cells (15), lung injury (18), breast cancer (19), and dendritic cells (33) has been reported. Nevertheless, the
hypoxia mediated A2B signaling mechanism promoting liver cancer
cell proliferation is yet to be elucidated.
Other researchers have suggested that adenosine
receptors are highly controlled and, thus, adenosine responses
might be regulated by the surface expression of adenosine
receptors. Consistent with our study, Eltzschig et al
reported that microarray analyses of cDNA derived from endothelial
cells subjected to various periods of hypoxia revealed significant
changes in the adenosine receptor's profiles, wherein the prominent
phenotypic change favored A2B expression with concomitant
downregulation of A1 and A2 subtypes (34). In another study, Feoktistov and
Biaggioni suggested that hypoxia increases A2B expression levels
releasing vascular endothelial growth factor through decreasing
adenosine (35). Human endothelial
and smooth muscle cells have also been shown to express A2B
(36). Moreover, hypoxia reduces
matrix metalloproteinase-9 (MMP-9) production by human
monocyte-derived dendritic cells and requires the activation of
adenosine A2B via the cAMP/protein kinase (PKA) signaling pathway
(37,38). Collectively, these data suggest that
A2B increases HIF-1α dependently under hypoxic conditions and might
promote liver cancer cell proliferation.
In summary, we investigated the expressions of A2B
and HIF-1α in liver cancer cells to find an association suggesting
that under hypoxic conditions, HIF-1α induces A2B expression to
maintain tumor proliferation. Our results show that the upregulated
A2B plays a critical role in liver cancer cell growth, suggesting
A2B as a potential target for drug design; thus, an effective A2B
inhibitor might be useful for treating liver cancers.
Acknowledgements
The authors would like to thank Dr Holger K.
Eltzschig (University of Texas Health Science Center at Houston,
TX, USA) for providing the human A2B-Luc reporter construct.
Funding
The present study was supported by Asan Institute
for Life Sciences (grant no. 15-662 and 18-IT0622), the National
Research Foundation of Korea (grant no. NRF-2015K1A4A3046807), the
Basic Science Research Program through the National Research
Foundation of Korea funded by the Ministry of Education (grant no.
NRF-2017R1D1A1B04032429) and the Yuhan Corporation (grant no.
2015-0908) and a grant of the Korea Health Technology R&D
Project through the Korea Health Industry Development Institute
(KHIDI), funded by the Ministry of Health & Welfare, Republic
of Korea (grant no. HI15C0972).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JHK and ET designed the study. JL and JK conducted
the experiments. JHK, GWS, YIY, SH, GCP and SGL performed human
specimen handling. YHJ, VAK, BJK and NK contributed to the
interpretation of the results. GCP and ET were involved in
manuscript writing and analyzing the data. SGL, GCP and ET
supervised the work. All authors provided critical feedback and
conducted the research, analyzed the data and prepared the
manuscript.
Ethics approval and consent to
participate
The Institutional Review Board (IRB) of Asan Medical
Center (Seoul, Republic of Korea) reviewed and approved the
collection and use of patient specimens (approval no. 2016-0582).
All patients who provided tissue samples agreed to donate their
specimens and provided written informed consents.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
HIF-1α
|
hypoxia inducible factor-1α
|
|
HRE
|
hypoxia response element
|
|
A2B
|
adenosine A2B receptor
|
References
|
1
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Collis MG and Hourani SM: Adenosine
receptor subtypes. Trends Pharmacol Sci. 14:360–366. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haskó G, Pacher P, Sylvester Vizi E and
Illes P: Adenosine receptor signaling in the brain immune system.
Trends Pharmacol Sci. 26:511–516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haskó G, Linden J, Cronstein B and Pacher
P: Adenosine receptors: Therapeutic aspects for inflammatory and
immune diseases. Nat Rev Drug Discov. 7:759–770. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blay J, White TD and Hoskin DW: The
extracellular fluid of solid carcinomas contains immunosuppressive
concentrations of adenosine. Cancer Res. 57:2602–2605.
1997.PubMed/NCBI
|
|
7
|
Cekic C, Sag D, Li Y, Theodorescu D,
Strieter RM and Linden J: Adenosine A2B receptor blockade slows
growth of bladder and breast tumors. J Immunol. 188:198–205. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Panjehpour M, Castro M and Klotz KN: Human
breast cancer cell line MDA-MB-231 expresses endogenous A2B
adenosine receptors mediating a Ca2+ signal. Br J Pharmacol.
145:211–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma DF, Kondo T, Nakazawa T, Niu DF,
Mochizuki K, Kawasaki T, Yamane T and Katoh R: Hypoxia-inducible
adenosine A2B receptor modulates proliferation of colon carcinoma
cells. Hum Pathol. 41:1550–1557. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vecchio EA, Tan CY, Gregory KJ,
Christopoulos A, White PJ and May LT: Ligand-independent adenosine
A2B receptor constitutive activity as a promoter of prostate cancer
cell proliferation. J Pharmacol Exp Ther. 357:36–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiang HJ, Liu ZC, Wang DS, Chen Y, Yang YL
and Dou KF: Adenosine A(2b) receptor is highly expressed in human
hepatocellular carcinoma. Hepatol Res. 36:56–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kolachala V, Asamoah V, Wang L, Obertone
TS, Ziegler TR, Merlin D and Sitaraman SV: TNF-alpha upregulates
adenosine 2b (A2b) receptor expression and signaling in intestinal
epithelial cells: A basis for A2bR overexpression in colitis. Cell
Mol Life Sci. 62:2647–2657. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xaus J, Mirabet M, Lloberas J, Soler C,
Lluis C, Franco R and Celada A: IFN-gamma up-regulates the A2B
adenosine receptor expression in macrophages: A mechanism of
macrophage deactivation. J Immunol. 162:3607–3614. 1999.PubMed/NCBI
|
|
14
|
Eltzschig HK, Ibla JC, Furuta GT, Leonard
MO, Jacobson KA, Enjyoji K, Robson SC and Colgan SP: Coordinated
adenine nucleotide phosphohydrolysis and nucleoside signaling in
posthypoxic endothelium: Role of ectonucleotidases and adenosine
A2B receptors. J Exp Med. 198:783–796. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong T, Westerman KA, Faigle M, Eltzschig
HK and Colgan SP: HIF-dependent induction of adenosine A2B receptor
in hypoxia. FASEB J. 20:2242–2250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stolze IP, Mole DR and Ratcliffe PJ:
Regulation of HIF: Prolyl hydroxylases. Novartis Found Symp.
272:15–36. 2006.PubMed/NCBI
|
|
17
|
Semenza GL: HIF-1: Upstream and downstream
of cancer metabolism. Curr Opin Genet Dev. 20:51–56. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eckle T, Kewley EM, Brodsky KS, Tak E,
Bonney S, Gobel M, Anderson D, Glover LE, Riegel AK, Colgan SP and
Eltzschig HK: Identification of hypoxia-inducible factor HIF-1A as
transcriptional regulator of the A2B adenosine receptor during
acute lung injury. J Immunol. 192:1249–1256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lan J, Lu H, Samanta D, Salman S, Lu Y and
Semenza GL: Hypoxia-inducible factor 1-dependent expression of
adenosine receptor 2B promotes breast cancer stem cell enrichment.
Proc Natl Acad Sci USA. 115:E9640–E9648. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: Hep G2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
21
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C (T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tak E, Lee S, Lee J, Rashid MA, Kim YW,
Park JH, Park WS, Shokat KM, Ha J and Kim SS: Human carbonyl
reductase 1 upregulated by hypoxia renders resistance to apoptosis
in hepatocellular carcinoma cells. J Hepatol. 54:328–339. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eckle T, Brodsky K, Bonney M, Packard T,
Han J, Borchers CH, Mariani TJ, Kominsky DJ, Mittelbronn M and
Eltzschig HK: HIF1A reduces acute lung injury by optimizing
carbohydrate metabolism in the alveolar epithelium. PLoS Biol.
11:e10016652013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Selak MA, Armour SM, MacKenzie ED,
Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB
and Gottlieb E: Succinate links TCA cycle dysfunction to
oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer
Cell. 7:77–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tak E, Jun DY, Kim SH, Park GC, Lee J,
Hwang S, Song GW and Lee SG: Upregulation of P2Y2 nucleotide
receptor in human hepatocellular carcinoma cells. J Int Med Res.
44:1234–1247. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dubey RK, Gillespie DG and Jackson EK:
A(2B) adenosine receptors stimulate growth of porcine and rat
arterial endothelial cells. Hypertension. 39:530–535. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grant MB, Tarnuzzer RW, Caballero S, Ozeck
MJ, Davis MI, Spoerri PE, Feoktistov I, Biaggioni I, Shryock JC and
Belardinelli L: Adenosine receptor activation induces vascular
endothelial growth factor in human retinal endothelial cells. Circ
Res. 85:699–706. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mustafa SJ, Morrison RR, Teng B and Pelleg
A: Adenosine receptors and the heart: Role in regulation of
coronary blood flow and cardiac electrophysiology. Adenosine
Receptors in Health and Disease. Springer; pp. 161–188. 2009,
View Article : Google Scholar
|
|
30
|
Merighi S, Mirandola P, Varani K, Gessi S,
Leung E, Baraldi PG, Tabrizi MA and Borea PA: A glance at adenosine
receptors: Novel target for antitumor therapy. Pharmacol Ther.
100:31–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hockel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Volmer JB, Thompson LF and Blackburn MR:
Ecto-5′-nucleotidase (CD73)-mediated adenosine production is tissue
protective in a model of bleomycin-induced lung injury. J Immunol.
176:4449–4458. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang M, Ma C, Liu S, Shao Q, Gao W, Song
B, Sun J, Xie Q, Zhang Y, Feng A, et al: HIF-dependent induction of
adenosine receptor A2b skews human dendritic cells to a
Th2-stimulating phenotype under hypoxia. Immunol Cell Biol.
88:165–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eltzschig HK, Eckle T, Mager A, Küper N,
Karcher C, Weissmüller T, Boengler K, Schulz R, Robson SC and
Colgan SP: ATP release from activated neutrophils occurs via
connexin 43 and modulates adenosine-dependent endothelial cell
function. Circ Res. 99:1100–1108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feoktistov I and Biaggioni I:
Pharmacological characterization of adenosine A2B receptors:
Studies in human mast cells co-expressing A2A and A2B adenosine
receptor subtypes. Biochem Pharmacol. 55:627–633. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Igata M, Motoshima H, Tsuruzoe K, Kojima
K, Matsumura T, Kondo T, Taguchi T, Nakamaru K, Yano M, Kukidome D,
et al: Adenosine monophosphate-activated protein kinase suppresses
vascular smooth muscle cell proliferation through the inhibition of
cell cycle progression. Circ Res. 97:837–844. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao P, Li XG, Yang M, Shao Q, Wang D, Liu
S, Song H, Song B, Zhang Y and Qu X: Hypoxia suppresses the
production of MMP-9 by human monocyte-derived dendritic cells and
requires activation of adenosine receptor A2b via cAMP/PKA
signaling pathway. Mol Immunol. 45:2187–2195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen H, Koupenova M, Yang D, Sume SS,
Trackman PC and Ravid K: Regulation of MMP-9 expression by the A2b
adenosine receptor and its dependency on TNF-α signaling. Exp
Hematol. 39:525–530. 2011. View Article : Google Scholar : PubMed/NCBI
|