Introduction
Obstructive jaundice (OJ) is a common disease in
clinical surgery. It can cause damage to various tissues and organs
in the body with the most serious leading to hepatic injury.
Studies have demonstrated that the accumulation of inflammatory
factors in liver tissues and abnormal serum biochemical indexes are
important causes of hepatic injury (1–3).
However, the specific mechanism of hepatic injury caused by OJ
remains not fully elucidated. An abnormal increase in hepatocyte
apoptosis is currently considered to be the pathogenesis of hepatic
atrophy caused by OJ (4,5). It has been reported that, due to
endotoxin stimulation during OJ, Kupffer cells secrete a large
number of inflammatory factors, such as tumor necrosis factor-α
(TNF-α) and interleukin-6 (IL-6), which induce hepatocyte apoptosis
(6,7). Hepatocyte apoptosis is a complex
process involving multiple factors. Therefore, it is of great
significance to better understand the mechanism of hepatocyte
apoptosis for the prevention and treatment of hepatic injury caused
by OJ.
Dexmedetomidine (Dex) is a new type of highly
selective adrenergic receptor agonist that has been widely used in
the clinic. Its molecular formula is
C13H16N2 containing methyl and
imidazole functional groups. Dex can bind the presynaptic
α2 adrenoceptor, activate the negative feedback loop of
the sympathetic response and reduce the release of norepinephrine,
thereby inhibiting the sympathetic reflex and stress response, and
maintaining homeostasis (8). It has
been demonstrated that Dex can inhibit the inflammatory reaction
induced by endotoxemia whilst also having a protective effect on
organ tissues (9). Zhang et
al (10) reported that Dex
inhibited the release of inflammatory cytokines such as IL-6 and
TNF-α, and alleviated local and systemic inflammatory reactions.
Dex protects the lung via increasing the expression levels of
protein kinase B (Akt) in acute lung injury tissues (11). The phosphoinositide 3-kinase
(PI3K)/Akt signaling pathway is widely present in cells, and is
also one of the important pathways involved in the regulation of
cell apoptosis. However, to the best of our knowledge, the effect
and mechanism of Dex on hepatocyte apoptosis in an OJ rat model has
not been reported. Therefore, the present study investigated an OJ
rat model treated with Dex to observe the liver tissue inflammatory
reaction and hepatocyte apoptosis. The present findings provided
the theoretical and experimental basis for Dex in treating hepatic
injury caused by OJ.
Materials and methods
Experimental animals
A total of 30 healthy male 8-week-old Sprague Dawley
(SD) rats (Shanghai SLAC Laboratory Animal Co., Ltd.) weighing
250±20 g each were raised in the Animal Experimental Center of the
Affiliated Hospital of Inner Mongolia Medical University. The
temperature of the housing area was 21±2°C with a relative humidity
of 30–70% and light-dark cycle of 12/12 h. Rats were fed three
times a day. Rats were fasted for 12 h before surgery and had free
access to water. The Animal Experimental Ethics Committee of the
Affiliated Hospital of Inner Mongolia Medical University approved
this research.
Establishment of the OJ rat model
All rats were fasted for 12 h prior to surgery with
free access to water. The rats were anesthetized by intraperitoneal
injection of 2% sodium pentobarbital (30 mg/kg). The abdominal
cavity was ascended through the midline incision under aseptic
operations, and the bile duct was found in the hepatoduodenal
ligament. The bile duct was double ligated with 5-0 silk thread at
a distance of 0.8 cm from the hilum, and the incision was sutured
layer by layer.
Experimental grouping
The SD rats were randomly divided into 3 groups:
Sham group, bile duct ligation (BDL) group and BDL+Dex group, with
10 rats in each group. In the sham group, the bile ducts of the
rats were isolated only and BDL was not implemented. The rats in
BDL group underwent surgery to establish the OJ model. After
successful construction, the rats were injected with saline via the
tail vein. The rats in BDL+Dex group underwent surgery to establish
the OJ model. After successful construction, the rats were injected
with 100 µg/kg Dex via the tail vein once a day. After 1 week of
intervention, the SD rats were anesthetized with intraperitoneal
injection of 2% pentobarbital (30 mg/kg), and then sacrificed by
spinal dislocations for subsequent experimentation.
Observation indexes
The serum liver function indexes, IL-6 and TNF-α
expression levels, liver pathological changes, hepatocyte apoptosis
rate, Akt expression levels, phosphorylation of Akt (p-Akt;
Thr308), caspase-3 and cleaved-caspase-3 protein expression in
liver tissues were compared among groups.
Serum liver function indexes
Following 1 week of intervention, 2 ml of blood was
taken from the tail vein of rats from each group, and the serum was
separated via centrifugation at 1,500 × g for 10 min. The indexes
of alanine aminotransferase (ALT), aspartate aminotransferase (AST)
and total bilirubin (TBIL) were detected by iMagic automatic
biochemical analyzer (China Shenzhen Kubel Biotechnology Co.,
Ltd.). Data were analyzed by iMagic Manager (version 1.0; China
Shenzhen Kubel Biotechnology Co., Ltd.).
Expression levels of serum
inflammatory cytokines IL-6 and TNF-α measured with ELISA
Following 1 week of intervention, 2 ml of blood was
taken from the tail vein of rats from each group. The serum was
separated from the blood by centrifuging the blood at 4°C and 1,000
× g for 5 min. The contents of IL-6 (cat. no. PR6000B) and TNF-α
(cat. no. PRTA00) were detected by enzyme-linked immunosorbent
assay (ELISA) supplied by R&D Systems, Inc. and the operation
procedure was strictly in accordance with the manufacturer's
protocol.
Liver pathological changes following
immunostaining
Following anesthesia, the livers from each group of
rats were exposed along the original incision, then part of the
liver tissue was removed and quickly stored in liquid nitrogen for
later use. A selected portion of liver tissues was fixed with 4%
paraformaldehyde solution at 4°C for 12 h, and routine hematoxylin
and eosin staining was applied after paraffin-embedded 5-µm-thick
section. The sections were stained with hematoxylin for 3 min and
eosin for 2 min at room temperature. The pathological structure of
the liver tissues of each group was examined under a light
microscope (magnification, ×200).
Hepatocyte apoptosis rate using flow
cytometry
Under aseptic operation, the shredded liver tissues
were completely digested in 0.3% type I collagenase digestive
solution. The cells were then collected into a centrifuge tube
using a 200 µm cell strainer and prepared into a single cell
suspension with the concentration adjusted to lx106
cell/ml. The cell suspension of 100 µl was collected and 5 µl
Annexin V-fluorescein isothiocyanate and 10 µl propidium iodide
solution from FITC Annexin V Apoptosis Detection Kit I (cat. no.
556547; both BD Biosciences) were added. It was mixed evenly then
incubated at room temperature for 15 min in the dark. Then 400 µl
of PBS was added into the mixture, and the rate of hepatocyte
apoptosis was measured via FACS Canto II flow cytometry (BD
Biosciences). The hepatocyte apoptosis rate in each group was
analyzed by Accuri™ C6 software (version 6.2; BD Biosciences).
Expression levels of Akt, p-Akt
(Thr308), caspase-3 and cleaved-caspase-3 protein in liver tissues
measured by western blot analysis
The liver tissues were ground and lysed, and total
protein was extracted from the liver tissues collected from each
group of rats with the RIPA buffer (Sigma-Aldrich) containing 1%
sodium deoxycholate and 0.1% SDS. The protein concentration was
determined using the bicinchoninic acid protein concentration assay
kit (cat. no. P0012-1; Beyotime Institute of Biotechnology). The
protein samples were separated liver tissues were ground and lysed,
Then electrophoresis separation was performed on protein samples
(30 µg/lane) using SDS-PAGE gel (5% spacer gel and 10% separation
gel). The protein was transferred to polyvinylidene fluoride
membrane via the enzyme-linked immunoelectrotransfer blot method.
The membranes were blocked using Τris-buffered saline-T solution
containing 5% skimmed milk powder at room temperature for 2 h then
incubated with primary antibodies against Akt (1:400; cat. no.
sc-5298; Santa Cruz Biotechnology, Inc.), p-Akt (1:500; cat. no.
sc-33437; Santa Cruz Biotechnology, Inc.), cleaved-caspase-3
(1:700; cat. no. 9661S Cell Signaling Technology, Inc.), caspase-3
(1:500; cat. no. sc-271759; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. Following washes with TBST, the membrane was
incubated with a horseradish peroxidase-labeled goat anti-rabbit
immunoglobulin G secondary antibody (1:600; cat. no. A0208;
Beyotime Institute of Biotechnology) and incubated at 4°C for 2 h;
then the membrane was visualized using hypersensitive
electrogenerated chemiluminescence reagent (Beyotime Institute of
Biotechnology). ImageJ software (version 1.8.0; National Institutes
of Health) was used for quantitative analysis of the bands. GAPDH
was used as an internal reference to compare the optical density
values of the target bands in each group.
Statistical analysis
All data were analyzed using SPSS v.20.0 statistical
software (IBM Corp.) The count data were expressed as a percentage
and the chi-square test was used for comparisons amongst groups.
The measurement data were expressed as mean ± standard deviation.
The comparison between two groups was performed by independent
sample t-test, and the comparison of three groups was analyzed by
one-way analysis of variance with Bonferroni method as post hoc
test. P<0.05 was considered to indicate statistical
significance.
Results
Dex treatment decreases the serum
levels of ALT, AST and TBIL following BDL
Compared with the sham group, the serum levels of
ALT, AST and TBIL in the BDL and BDL+Dex group increased
significantly (P<0.001; Table I).
However, compared with the BDL group, the serum levels of ALT, AST
and TBIL in the BDL+Dex group decreased significantly (P<0.001;
Table I).
 | Table I.Comparison of liver function in each
group. |
Table I.
Comparison of liver function in each
group.
| Group | ALT (U/l) | AST (U/l) | TBIL (µmol/l) |
|---|
| Sham group | 43.92±17.56 | 138.72±32.57 | 11.24±2.43 |
| BDL group |
794.46±118.23a |
1,358.61±225.86a |
119.25±20.18a |
| BDL+Dex group |
271.31±97.85a,b |
537.91±180.42a,b |
64.07±12.29a,b |
| F-test | 25.063 | 28.725 | 31.284 |
| P-value | 0.000 | 0.000 | 0.000 |
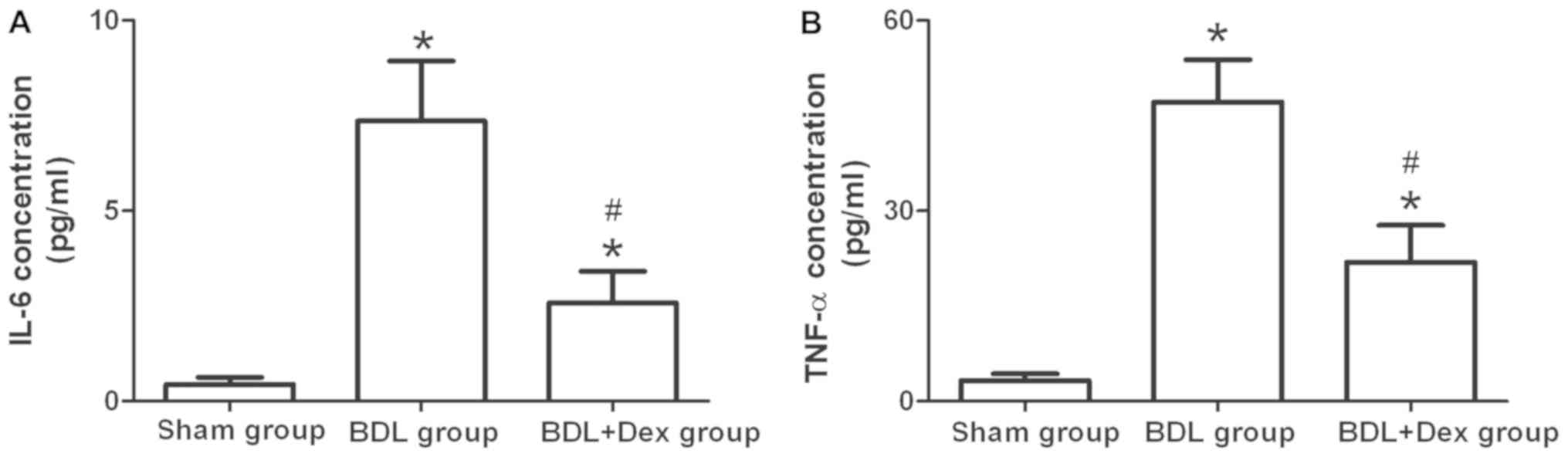
Dex treatment decreases IL-6 and TNF-α
levels
The expression levels of serum IL-6 and TNF-α in the
rats of the BDL+Dex group were significantly lower compared with
the BDL group (P<0.05; Fig. 1).
The expression levels of IL-6 and TNF-α in rats of the BDL group
and the BDL+Dex group were significantly higher compared with the
sham group (P<0.05; Fig. 1).
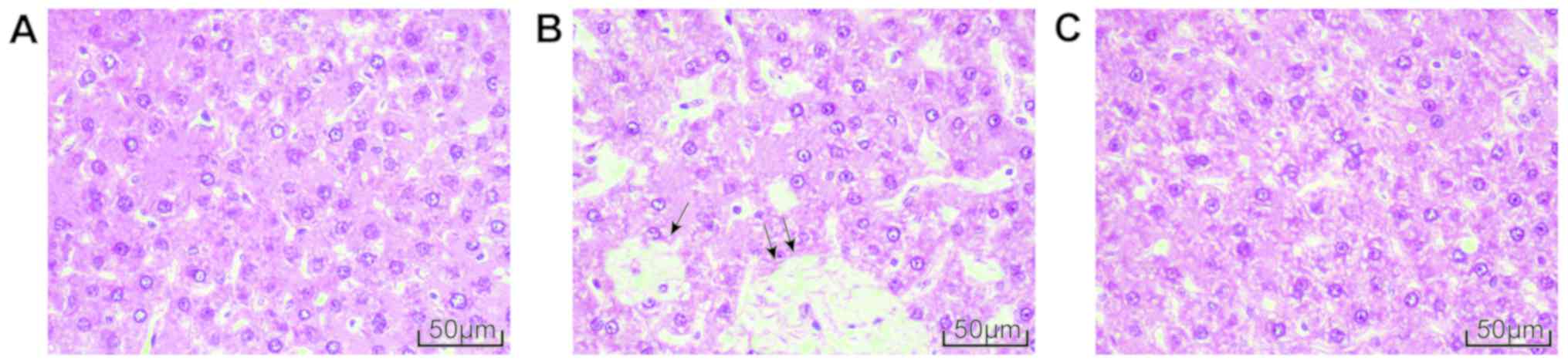
Dex treatment attenuates liver
pathological changes following BDL
Optical microscopy demonstrated that there were no
abnormal changes in the morphology of hepatocytes in the sham group
with the structures of the hepatic lobule, portal area and central
venous well defined (Fig. 2A). The
hepatocytes of BDL group were significantly degenerated and
necrotic with the hepatic lobule structure damaged and
pseudo-lobule formed. Inflammatory cells had also infiltrated the
structure (Fig. 2B). The liver
pathological changes in the BDL+Dex group were intermediate between
the aforementioned two groups, as the damage to hepatocytes was
improved, the arrangement of the hepatic cord was relatively neat
and the structure of hepatic lobule was relatively clear. Only a
small number of inflammatory cells had infiltrated the structure
(Fig. 2C).
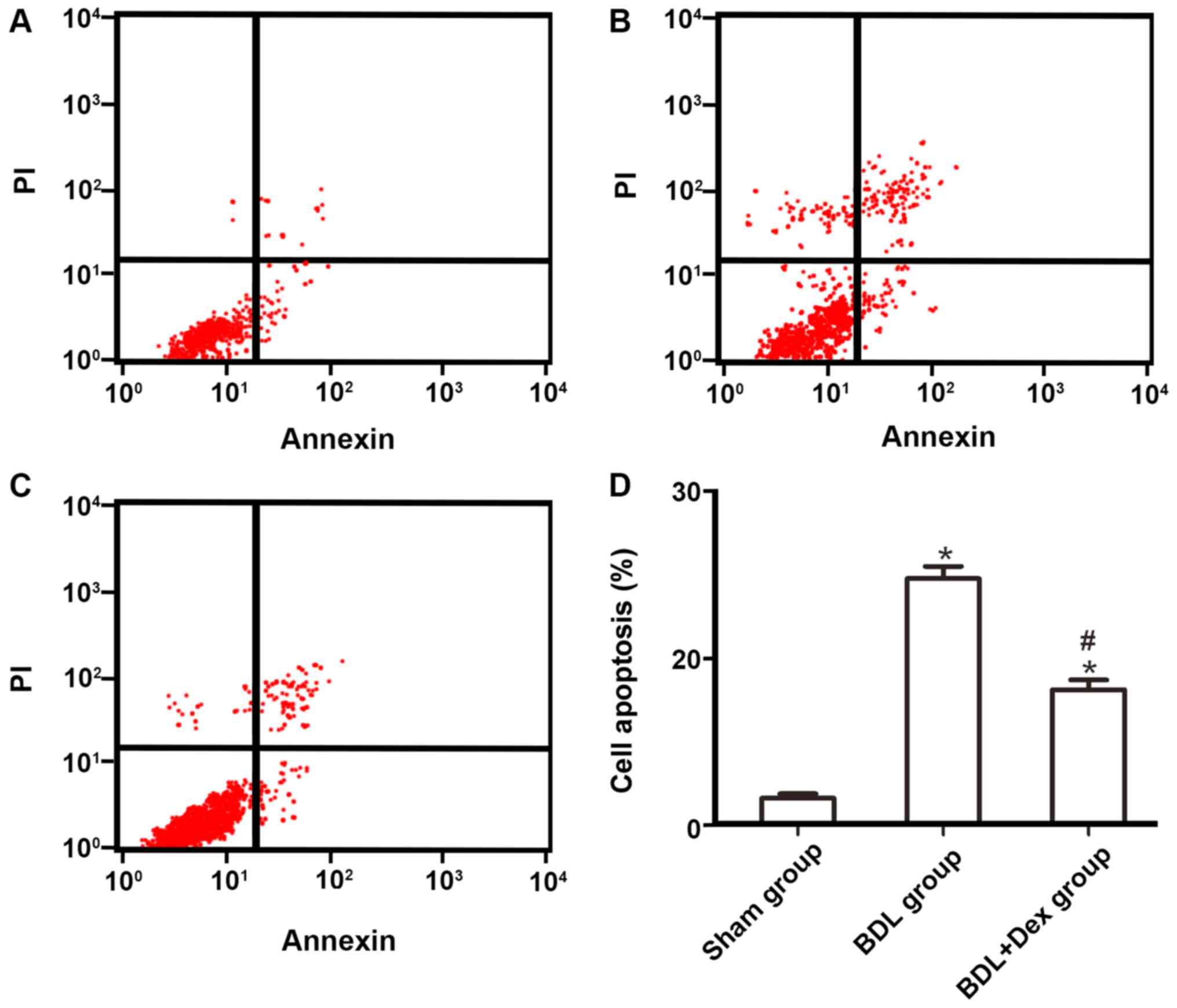
Dex treatment decreases apoptosis rate
of hepatocytes following BDL
Flow cytometry demonstrated that the apoptosis rate
of hepatocytes in the sham group was 3.25±0.54%. Compared with the
sham group, the apoptosis rate of hepatocytes in the BDL group and
BDL+Dex group increased significantly (P<0.05; Fig. 3A-C). However, compared with the BDL
group (29.54±1.48%), the apoptosis rate of hepatocytes in the
BDL+Dex group (16.24±1.18%) decreased significantly (P<0.05;
Fig. 3).
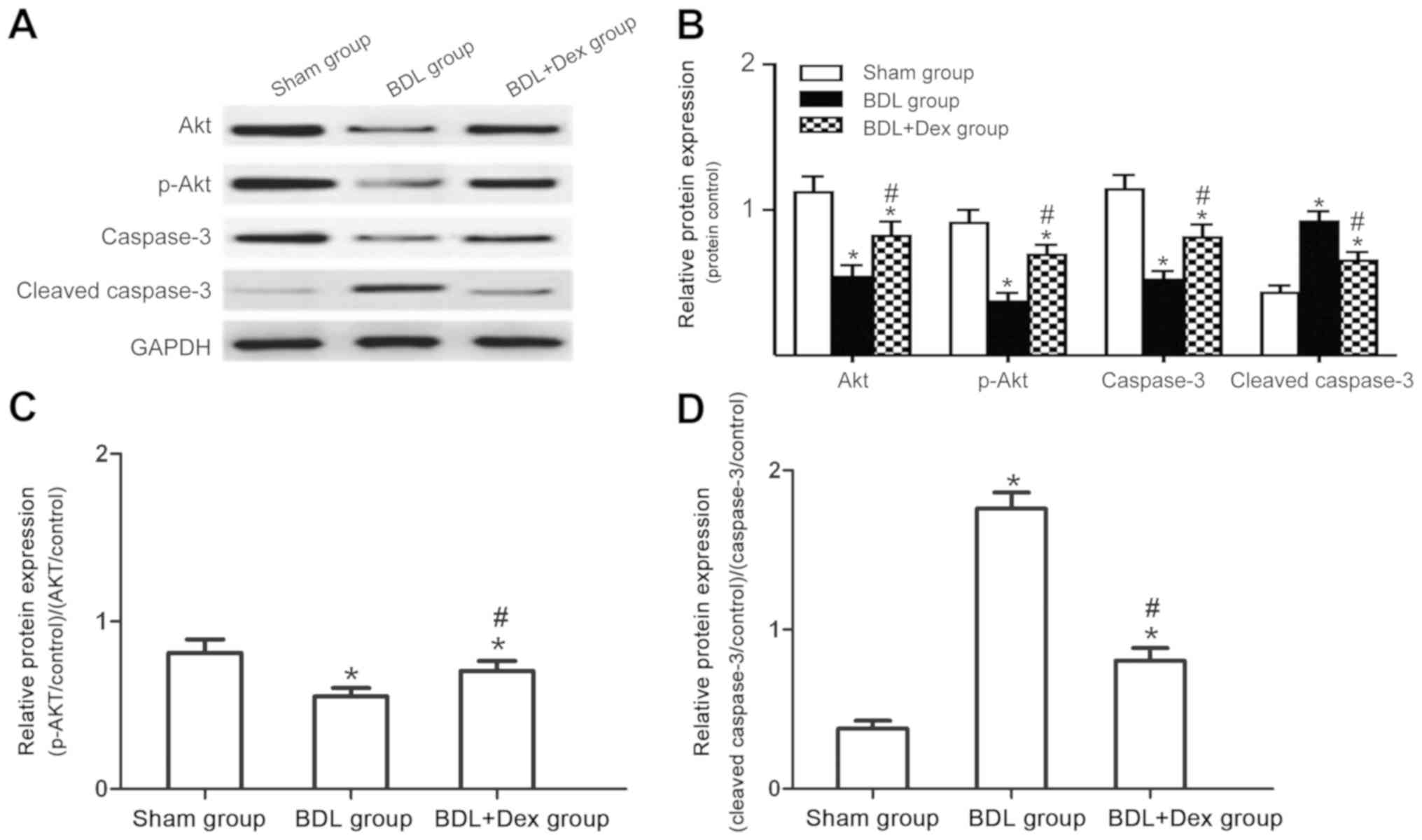
Comparisons of Akt, p-Akt, caspase-3
and cleaved caspase-3 protein expression in liver tissues of
rats
The levels of Akt, caspase-3 and cleaved caspase-3
in each group were expressed relative to the GAPDH control, while
the levels of p-Akt in each group were calculated relative to the
total Akt. Compared with the sham group, Akt and p-Akt expression
decreased in the BDL and BDL+Dex group, whilst the expression of
caspase-3 protein decreased significantly and the expression of
cleaved caspase-3 protein increased significantly (P<0.05;
Fig. 4). Compared with the BDL
group, the Akt and p-Akt expressions in the BDL+Dex group
increased, whilst the expression of caspase-3 protein increased
significantly and levels of cleaved caspase-3 protein decreased
significantly (P<0.05; Fig.
4).
Discussion
OJ is a common pathogenesis of many bile duct
diseases and a relatively prevalent surgical disease; however, its
aetiology is complicated. OJ causes accumulation of inflammatory
factors such as TNF-α and IL-6 in liver tissues, and also
infiltration of inflammatory cells such as neutrophils. This
results in elevated hepatic function markers such as AST, ALT and
TBIL, eventually leading to hepatic parenchyma injury and a
decrease in hepatocytes (12,13). It
has been demonstrated that a decrease of hepatocytes during OJ
development is mainly caused by apoptosis due to cholestasis,
mitochondrial dysfunction, reactive oxygen species and certain
cytokines that can affect the process of apoptosis, but the
specific mechanism is not clear (14–16).
Therefore, relieving biliary obstruction, alleviating the
inflammatory reaction and inhibiting hepatocyte apoptosis are key
to reducing hepatic damage in patients.
Dex is a novel highly selective adrenergic receptor
agonist that displays strong intrinsic activity, short elimination
half-life and distribution half-life (17). Recent studies have demonstrated that
Dex has protective effects on the heart, kidney, central nervous
system, respiratory system and small intestine (18,19).
Presently, the protective effects of Dex on hepatic injury have
received increased attention. It has been reported that Dex can
significantly stabilize hemodynamics and improve the quality of
recovery in patients with OJ during anesthesia (20). Research into the effects of Dex in OJ
is mainly focused on animal experimentation. For example, it has
been identified that intraperitoneal injection of Dex can
significantly reduce the oxidative stress indexes in a rat model of
hepatic ischemia-reperfusion whilst increasing the antioxidant
capacity (21). Kucuk et al
(22) reported that Dex could
restore heat shock protein 60 and tumor protein p53 in hepatocytes
of rats with ischemia-reperfusion injury and protect the liver from
injury by regulating the expression of apoptosis-related proteins.
The results of the present study demonstrated that Dex could not
only significantly improve the pathological morphology of liver
tissues and the changes of hepatic function parameters ALT, AST and
TBIL, but also inhibit the expression levels of serum inflammatory
cytokines IL-6 and TNF-α in rats with OJ. The present findings
indicated that Dex had a protective effect on hepatic injury caused
by OJ through reducing the levels of serum inflammatory cytokines
IL-6 and TNF-α, which is in agreement with the studies of Venn
et al (23) and Wang et
al (24).
Dex can exert cell survival effects by activating
the PI3K/Akt signaling pathway (25). The caspase enzyme family is one of
the important downstream effectors of the PI3K/Akt pathway. The
process of apoptosis involves the cascade amplification reaction
process of irreversible limited hydrolytic substrate of caspases,
with caspase-3 the most critical downstream apoptotic protease. It
has been reported that the activation of PI3K/Akt leads to the
phosphorylation of the proapoptotic protein Bcl-2-associated death
promoter in nerve cells, the upregulation of the Inhibitior of
apoptotis proteins levels, and the inhibition of the endogenous
apoptotic pathway regulated by the Caspase cascade signal pathway,
eventually exerting a cell protective effect (26). A study has demonstrated that 100
ng/ml Dex can induce apoptosis of inflammatory cells by activating
the caspase cascade reaction of neutrophils and other inflammatory
cells, whilst 1 ng/ml Dex has no significant effect on apoptosis of
neutrophils (27), indicating that
Dex has dose dependent effects on cell apoptosis. The results of
the present study determined that compared with the BDL group, the
hepatocyte apoptosis rate in the OJ rat model following Dex
treatment was significantly lower. The expression levels of Akt and
p-Akt in liver tissues of the BDL+Dex group were significantly
increased, the expression of caspase-3 protein was significantly
higher, and the expression levels of cleaved caspase-3 were
significantly lower compared with the OJ rat model group. Findings
suggested that Dex activated the PI3K/Akt signaling pathway,
inhibited the activation level of caspase-3, and reduced the
apoptosis of hepatocytes.
In summary, OJ induced hepatocyte apoptosis in rats.
Dex significantly improved the OJ-induced injury of hepatic tissues
and reduced hepatocyte apoptosis. It was hypothesized that Dex had
a protective role potentially via activating the PI3K/Akt signaling
pathway.
Acknowledgements
Not applicable.
Funding
This work was supported by the Medical and Health
Research Project of the Health and Family Planning Commission of
Inner Mongolia Autonomous Region (grant. no. 201701070), and Fund
Project of Inner Mongolia Science and Technology Department [grant.
no. 2015MS(LH)0809].
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX was the guarantor for the integrity of the study
and contributed to study concepts, study design, definition of
intellectual content, statistical analysis, manuscript preparation
and manuscript editing. CG collected and analyzed the data,
prepared the figures and also contributed substantially to
manuscript revision. CG and LS were responsible for the production
of animal models of obstructive jaundice. YL and LS drafted the
manuscript. YL also collated and analyzed the current literature on
this subject, and was responsible for the breeding and laboratory
work of rats. LS constructed the animal models of obstructive
jaundice and collected the data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Inner Mongolia Medical
University (Inner Mongolia, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li X, Li J, Ou YJ, Zhu XX, Yin XY, Zhu YX
and Tang D: Hepatoprotective effect of ulinastatin in a rat model
of major hepatectomy after obstructive jaundice. Dig Dis Sci.
60:1680–1689. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pavlidis ET and Pavlidis TE:
Pathophysiological consequences of obstructive jaundice and
perioperative management. Hepatobiliary Pancreat Dis Int. 17:17–21.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Unal Y, Tuncal S, Kosmaz K, Kucuk B,
Kismet K, Cavusoglu T, Celepli P, Senes M, Yildiz S and Hucumenoglu
S: The effect of calcium dobesilate on liver damage in experimental
obstructive jaundice. J Invest Surg. 32:238–244. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Q, Li BS, Song YJ, Hu MG, Lu JY, Gao
A, Sun XJ, Guo XM and Liu R: Hydrogen-rich saline protects against
mitochondrial dysfunction and apoptosis in mice with obstructive
jaundice. Mol Med Rep. 13:3588–3596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fang D, He Y and Luan Z: Simvastatin
augments activation of liver regeneration through attenuating
transforming growth factor-beta1 induced-apoptosis in obstructive
jaundice rats. Exp Ther Med. 14:4839–4845. 2017.PubMed/NCBI
|
|
6
|
Monteiro MEL, Xavier AR and Azeredo VB:
Diet and liver apoptosis in rats: A particular metabolic pathway.
Nutr Hosp. 34:463–468. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Zhang Y, Bai R, Wang M and Du S:
Baicalin attenuates alcoholic liver injury through modulation of
hepatic oxidative stress, Inflammation and Sonic hedgehog pathway
in rats. Cell Physiol Biochem. 39:1129–1140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Z, Ding T and Ma CG: Dexmedetomidine
(DEX) protects against hepatic ischemia/reperfusion (I/R) injury by
suppressing inflammation and oxidative stress in NLRC5 deficient
mice. Biochem Biophys Res Commun. 493:1143–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang ZX, Huang CY, Hua YP, Huang WQ, Deng
LH and Liu KX: Dexmedetomidine reduces intestinal and hepatic
injury after hepatectomy with inflow occlusion under general
anaesthesia: A randomized controlled trial. Br J Anaesth.
112:1055–1064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Wang J, Qian W, Zhao J, Sun L,
Qian Y and Xiao H: Dexmedetomidine inhibits tumor necrosis
factor-alpha and interleukin-6 in lipopolysaccharide-stimulated
astrocytes by suppression of c-Jun N-terminal kinases.
Inflammation. 37:942–949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng L, Li L, Lu S, Li K, Su Z, Wang Y,
Fan X, Li X and Zhao G: The protective effect of dexmedetomidine on
LPS-induced acute lung injury through the HMGB1-mediated
TLR4/NF-kappaB and PI3K/Akt/mTOR pathways. Mol Immunol. 94:7–17.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tag CG, Weiskirchen S, Hittatiya K, Tacke
F, Tolba RH and Weiskirchen R: Induction of experimental
obstructive cholestasis in mice. Lab Anim. 49 (Suppl 1):S70–S80.
2015. View Article : Google Scholar
|
|
13
|
Taniguchi T, Kidani Y, Kanakura H,
Takemoto Y and Yamamoto K: Effects of dexmedetomidine on mortality
rate and inflammatory responses to endotoxin-induced shock in rats.
Crit Care Med. 32:1322–1326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maillette de Buy Wenniger L and Beuers U:
Bile salts and cholestasis. Dig Liver Dis. 42:409–418. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sokol RJ, Dahl R, Devereaux MW, Yerushalmi
B, Kobak GE and Gumpricht E: Human hepatic mitochondria generate
reactive oxygen species and undergo the permeability transition in
response to hydrophobic bile acids. J Pediatr Gastroenterol Nutr.
41:235–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masud A, Mohapatra A, Lakhani SA,
Ferrandino A, Hakem R and Flavell RA: Endoplasmic reticulum
stress-induced death of mouse embryonic fibroblasts requires the
intrinsic pathway of apoptosis. J Biol Chem. 282:14132–14139. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lv M, Zeng H, He Y, Zhang J and Tan G:
Dexmedetomidine promotes liver regeneration in mice after 70%
partial hepatectomy by suppressing NLRP3 inflammasome not
TLR4/NFkappaB. Int Immunopharmacol. 54:46–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang L, Hu M, Lu Y, Cao Y, Chang Y and
Dai Z: The protective effects of dexmedetomidine on ischemic brain
injury: A meta-analysis. J Clin Anesth. 40:25–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong Z, Ma L, Zhong YL, Li J, Lv J and Xie
YB: Myocardial protective effects of dexmedetomidine in patients
undergoing cardiac surgery: A meta-analysis and systematic review.
Exp Ther Med. 13:2355–2361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L and Yu WF: Obstructive jaundice and
perioperative management. Acta Anaesthesiol Taiwan. 52:22–29. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tufek A, Tokgoz O, Aliosmanoglu I,
Alabalik U, Evliyaoglu O, Ciftci T, Guzel A and Yildirim ZB: The
protective effects of dexmedetomidine on the liver and remote
organs against hepatic ischemia reperfusion injury in rats. Int J
Surg. 11:96–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kucuk A, Yaylak F, Cavunt-Bayraktar A,
Tosun M, Arslan M, Comu FM and Kavutcu M: The protective effects of
dexmedetomidine on hepatic ischemia reperfusion injury. Bratisl Lek
Listy. 115:680–684. 2014.PubMed/NCBI
|
|
23
|
Venn RM, Bryant A, Hall GM and Grounds RM:
Effects of dexmedetomidine on adrenocortical function, and the
cardiovascular, endocrine and inflammatory responses in
post-operative patients needing sedation in the intensive care
unit. Br J Anaesth. 86:650–656. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Hu B, Zou Y, Bo L, Wang J, Li J
and Luo Y: Dexmedetomidine premedication attenuates concanavalin
A-induced hepatitis in mice. J Toxicol Sci. 39:755–764. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu YM, Wang CC, Chen L, Qian LB, Ma LL,
Yu J, Zhu MH, Wen CY, Yu LN and Yan M: Both PI3K/Akt and ERK1/2
pathways participate in the protection by dexmedetomidine against
transient focal cerebral ischemia/reperfusion injury in rats. Brain
Res. 1494:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin XF, Wang S, Shen M, Wen X, Han XR, Wu
JC, Tang GZ, Wu DM, Lu J and Zheng YL: Effects of rehabilitation
training on apoptosis of nerve cells and the recovery of neural and
motor functions in rats with ischemic stroke through the PI3K/Akt
and Nrf2/ARE signaling pathways. Brain Res Bull. 134:236–245. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kishikawa H, Kobayashi K, Takemori K,
Okabe T, Ito K and Sakamoto A: The effects of dexmedetomidine on
human neutrophil apoptosis. Biomed Res. 29:189–194. 2008.
View Article : Google Scholar : PubMed/NCBI
|