Introduction
Acute pancreatitis (AP) is a common acute abdominal
disease characterized by pancreatic inflammation, ranging from mild
edema to severe tissue necrosis (1).
Severe AP (SAP) is the most serious type of AP, due to its high
morbidity and mortality (2). In
recent years, the incidence of pancreatitis has increased
worldwide. However, the pathophysiological mechanism behind AP is
currently not fully understood. The protection of damaged
pancreatic cells remains an important treatment for AP (3). Previous studies have focused on
developing drug therapies for AP, such as drugs that inhibit
pancreatic secretions, suppress acid secretion or have
anti-inflammatory effects (4–6).
However, for severe necrotizing pancreatitis, drug treatment is
ineffective and mortality remains high. At present, researchers are
investigating the molecular mechanisms and developing gene
therapies for the treatment of AP or SAP (7–9).
Pim-3 proto-oncogene, serine/threonine kinase
(Pim-3) is a highly conserved serine/threonine kinase, which was
originally considered to be a depolarization-inducing gene with
numerous biological activities (10). Studies have reported that Pim-3 is
highly expressed in a variety of endoderm-derived cancers, such as
hepatocellular carcinoma, pancreatic cancer and colon cancer
(11–14). Pim-3 promotes cell proliferation and
prevents apoptosis through the phosphorylation and inactivation of
the apoptosis-promoting protein, BAD (15). In addition, it has been confirmed
that Pim-3 expression is significantly upregulated in abnormally
proliferative pancreata, and the apoptosis of pancreatic cells is
significantly enhanced after Pim-3 gene knockout (13). Recent studies have shown that Pim-3
exerts protective effects on fulminant hepatic failure, intestinal
mucosal damage and lipopolysaccharide (LPS)-stimulated hepatic
stellate cell injury (16–18).
Based on the aforementioned evidence, it was
speculated that Pim-3 has a beneficial effect on pancreatic acinar
cell injury. The purpose of the present study was to investigate
the protective effect of Pim-3 on pancreatic cell injury induced by
LPS and to investigate its potential mechanism.
Materials and methods
Materials and reagents
Rat pancreatic acinar AR42J cells were obtained from
the American Type Culture Collection. Ham's F12 medium and LPS were
purchased from Sigma-Aldrich; Merck KGaA. FBS was purchased from
Gibco; Thermo Fisher Scientific, Inc. Vacant vector plasmid
p-enhanced green fluorescent protein (pEGFP)-N2 and the recombinant
plasmid pEGFP-N2/Pim-3 were provided generously by Jiangxi
Provincial Key Laboratory of Molecular Medicine; these plasmids
were described in previous studies (16,17).
Liposome (Lipofectamine® 2000) and TRIzol®
were purchased from Invitrogen; Thermo Fisher Scientific, Inc. G418
and MTT were purchased from Beijing Solarbio Science &
Technology Co., Ltd. Annexin V-FITC was purchased from Invitrogen;
Thermo Fisher Scientific, Inc. Propidium iodide (PI) was purchased
from Sigma-Aldrich; Merck KGaA. DMSO was purchased from Amresco,
LLC. Reverse transcription (RT) kit (M-MLV kit) was purchased from
Fermentas; Thermo Fisher Scientific, Inc. PCR primers were
synthesized by Generay Biotech Co., Ltd. 2X Taq PCR Master Mix was
purchased from Tiangen Biotech Co., Ltd. Total protein extraction
kit was purchased from Sangon Biotech Co., Ltd. BCA assay kit was
purchased from Sigma-Aldrich; Merck KGaA. Anti-Pim-3 (cat. no.
4165; 1:1,000) primary antibody was purchased from Cell Signaling
Technology. Primary antibodies against interleukin (IL)-6 (cat. no.
sc-57315; 1:1,000), IL-1β (cat. no. sc-4592; 1:1,000), tumor
necrosis factor (TNF)-α (cat. no. sc-12744; 1:1,000), intercellular
adhesion molecule (ICAM)-1 (cat. no. sc-8439; 1:1,000), Occludin
(cat. no. sc-133256; 1:1,000) and β-actin (cat. no. sc-81178;
1:1,000) were purchased from Santa Cruz Biotechnology, Inc.
Horseradish peroxidase-conjugated secondary antibodies were
purchased from OriGene Technologies, Inc.
Cell culture
AR42J cells were maintained in Ham's F12 medium
containing 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin and
400 µg/ml G418. The cells were routinely plated at a density of
1×105 cells/ml in 6-well cluster dishes and incubated in
a humidified incubator at 37°C with 95% atmospheric air and 5%
CO2.
Transfection and LPS treatment
Prior to plasmid transfection, 1×105
AR42J cells were seeded onto a 35 mm-dish in 2 ml Ham's F-12 medium
supplemented with 10% FBS, without antibiotics, and cultured to 90%
confluence at 37°C with 95% air and 5% CO2. The cells
were subsequently transfected with 4 µg/ml pEGFP-N2 or
pEGFP-N2/Pim-3 in the presence of Lipofectamine® 2000 at
room temperature. In the control group, culture medium alone was
added to the cells. After 4 h, the medium was refreshed with Ham's
F12 medium containing 10% FBS, and the cells were incubated for 48
h at 37°C with 95% air and 5% CO2. GFP was observed
under a fluorescence microscope. The proliferation of AR42J cells
treated with various concentrations of LPS (24, 36 and 48 h) was
analyzed, and the 2 µg/ml dose was selected for subsequent
experiments. After plasmid transfection, AR42J cells were divided
into six groups for subsequent experiments: i) Control group,
untransfected AR42J cells without LPS treatment; ii) LPS group,
untransfected AR42J cells treated with 2 µg/ml LPS; iii) pEGFP-N2
group, AR42J cells transfected with empty vector pEGFP-N2; iv)
pEGFP-N2 + LPS group, AR42J cells transfected with empty vector
pEGFP-N2 and treated with 2 µg/ml LPS; v) pEGFP-N2/Pim-3 group,
AR42J cells transfected with recombinant plasmid pEGFP-N2/Pim-3;
and vi) pEGFP-N2/Pim-3 + LPS group, AR42J cells transfected with
recombinant plasmid pEGFP-N2/Pim-3 and treated with 2 µg/ml
LPS.
MTT assay
AR42J cells in each group were treated with LPS for
24, 36 and 48 h at 37°C with 95% air and 5% CO2, and
then 1×104 cells/well were inoculated in a 96-well
culture plate. The cells were washed with PBS and incubated with 5
mg/ml MTT in 20 µl PBS at 37°C for 4 h. Subsequently, 150 µl DMSO
was added to stop the reaction. The absorbance was measured at 492
nm using a spectrophotometer after 10 min. Each independent
experiment was performed three times and data are presented as mean
± SD.
Detection of apoptosis
After 48 h of LPS treatment, the AR42J cells in each
group were trypsinized and collected by centrifugation at 37°C for
5 min at a speed of 1,000 × g. The cells were washed twice with
PBS. At least 1×105 cells were re-suspended in 500 µl
binding buffer (Invitrogen; Thermo Fisher Scientific, Inc.).
Annexin V-FITC (5 µl) and 5 µl PI were added to the cell
suspension. Finally, the cells were incubated in the dark at room
temperature for 15 min. A FACSCalibur flow cytometer (BD
Biosciences) was used to detect the apoptotic rate of cells,
including the early apoptotic rate (right lower quadrant) and the
late apoptotic rate (right upper quadrant). BD CellQuest™ Pro
software version 5.1 (BD Biosciences) was used to analyze the
data.
Cell cycle assay
After 48 h of LPS treatment, AR42J cells in each
group were digested with trypsin and collected by centrifugation at
37°C for 5 min at a speed of 1,000 × g. The supernatant was
subsequently discarded, and the cells were washed 3 times with PBS.
The cells were incubated with propidium iodide (PI) stain buffer
for 10 min and then treated with 200 µl of PBS, 200 µl of RNase A
and 200 µl of PI for 10 min at 4°C in the dark. Finally, the
distribution of cells in each of the cell cycle phases were
analyzed using a FACSCalibur flow cytometer (BD Biosciences). Data
were acquired and analyzed using the ModFit™ software (version 4.1;
Verity Software House, Inc.).
Wound healing assay
Following transfection, AR42J cells were seeded at
4×105 per well in 12-well plates and incubated at 37°C
to reach 100% confluence. A longitudinal scratch of equal width was
created by scratching the cell surface with a 1 ml pipette tip. The
floating cells were washed away with PBS. Subsequently, the cells
were incubated with Ham's F-12 with 2% FBS and 2 µg/ml LPS for 48 h
at 37°C. The wound healing was observed under a light microscope,
and the image of the closed area was taken immediately after
injury. The ability of cells to close the wound was calculated by
measuring the average distance between the wound edge of the
scratched space and calculating the percentage closure compared to
the starting width through the equation: (0–48 h)/0 h ×100. Each
independent experiment was performed three times and data are
presented as mean ± SD.
RT-PCR
After 48 h of LPS treatment, total RNA was extracted
from AR42J cells in each group using TRIzol®, according
to the manufacturer's protocol. A total of 2 µg RNA was used as the
template to synthesize first-strand cDNA using a M-MLV RT kit. The
reverse transcription temperature protocol used was 37°C for 15 min
and 85°C for 30 sec. The PCR primer sequences were designed using
Primer Premier 5.0 software (PREMIER Biosoft; Table I). PCR was performed with 500 ng
cDNA, 1 µl sense primer, 1 µl antisense primer and 12.5 µl 2X Taq
PCR MasterMix; ddH2O was added to create a final volume
of 25 µl. PCR was performed using the following thermal cycling
conditions: Pre-denaturation at 94°C for 5 min; 35 cycles of
denaturation at 94°C for 45 sec, primer annealing at 55°C for 45
sec, primer extension at 72°C for 55 sec; and a final extension at
72°C for 7 min. PCR products were electrophoresed on a 1.5% agarose
gel and stained with ethidium bromide and subjected to
densitometric scanning, and the bands were semi-quantified with NIH
Image 1.62 (National Institutes of Health). The mRNA expression
levels were normalized to β-actin.
 | Table I.Primer sequences used for PCR. |
Table I.
Primer sequences used for PCR.
| Gene | Primer
sequences | Product size,
bp |
|---|
| Pim-3 | Forward:
5′-CACTGACTTTGATGGCACCC-3′ | 770 |
|
| Reverse:
5′-ATGCCCAGACGAAGACCAG-3′ |
|
| IL-6 | Forward:
5′-GTAGCCGCCCCACACAGACAGCC-3′ | 174 |
|
| Reverse:
5′-GCCATCTTTGGAAGGTTCAGG-3′ |
|
| IL-1β | Forward:
5′-CCTTCTTTTCCTTCATCTTTG-3′ | 372 |
|
| Reverse:
5′-ACCGCTTTTCCATCTTCTTCT-3′ |
|
| TNF-α | Forward:
5′-CTGGGCAGCGTTTATTCT-3′ | 249 |
|
| Reverse:
5′-TTGCTTCTTCCCTGTTCC-3′ |
|
| ICAM-1 | Forward:
5′-TCAAACGGGAGATGAATGG-3′ | 230 |
|
| Reverse:
5′-CCTCCTCCTGAGCCTTCTG-3′ |
|
| Occludin | Forward:
5′-CTGTCTATGCTCGTCATCG-3′ | 294 |
|
| Reverse:
5′-CATTCCCGATCTAATGACG-3′ |
|
| β-actin | Forward:
5′-AGAGGGAAATCGTGCGTGAC-3′ | 445 |
|
| Reverse:
5′-TGGAAGGTGGACAGTGAGGC-3′ |
|
Western blot analysis
After 48 h of LPS treatment, total protein was
extracted using a total protein extraction kit and the protein
concentration was measured using the BCA assay kit, according to
the manufacturer's protocol. A total of 30 µg protein was loaded on
a 10% SDS-polyacrylamide gel, electrophoresed and transferred onto
a nitrocellulose membrane. Membranes were blocked in 5% non-fat
milk for 1 h at room temperature. The membranes were probed with
Pim-3, IL-6, IL-1β, TNF-α, ICAM-1, Occludin or β-actin antibodies
overnight at 4°C, followed by incubation with horseradish
peroxidase-conjugated secondary antibodies at room temperature for
2 h. Antibody probes on the membranes were detected using an ECL
substrate kit (Shanghai Xuanling Biotechnology Co., Ltd.) and
exposed to X-ray film. Semi-quantification of bands was carried out
by scanning the films. The densities of the protein bands were
quantified using ImageJ 1.8.0 software (National Institutes of
Health). β-actin was used as a loading control.
Statistical analysis
All experiments in this study were repeated at least
three times, and the final data are presented as mean ± SD.
Statistical significance was evaluated using one-way ANOVA among ≥3
groups and LSD t-test post hoc test between 2 groups, using SPSS
19.0 (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
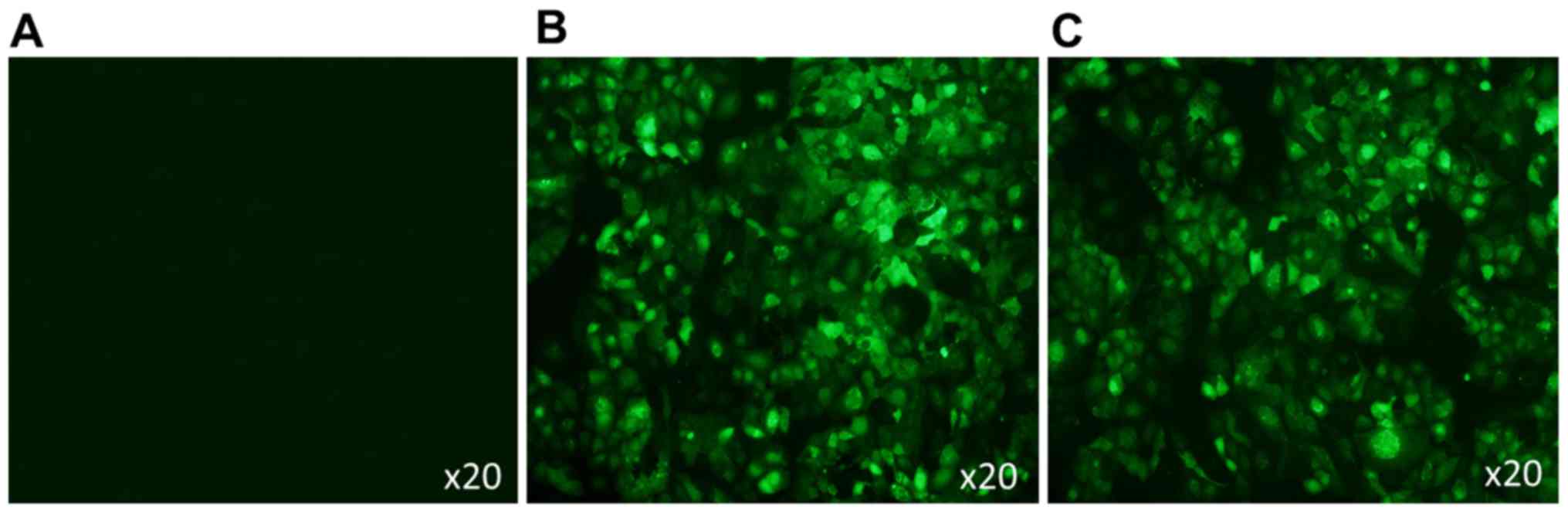
pEGFP-N2 and pEGFP-N2/Pim-3 plasmids
were successfully transfected into AR42J cells
In the presence of Lipofectamine® 2000,
pEGFP-N2 or pEGFP-N2/Pim-3 plasmids were transiently transfected
into the AR42J cells. After 48 h, these cells were observed using a
fluorescence microscope. As shown in Fig. 1, the cells were successfully
transfected with pEGFP-N2 and pEGFP-N2/Pim-3 as shown by
EGFP+ green fluorescence.
LPS-treated AR42J cells exhibit a
dose-dependent reduction in cell proliferation
As shown in Fig. 2A,
AR42J cell proliferation was measured following treatment with 1, 2
and 4 µg/ml LPS using MTT assays. A concentration of 1 µg/ml LPS
had little effect on the proliferation of AR42J cells, and there
was no significantly difference compared to the control group. A
concentration of 4 µg/ml LPS had a fatal effect on the AR42J cells
and the cell viability was considered too poor to continue with
this treatment. A concentration of 2 µg/ml LPS had a pronounced
effect on the proliferation of AR42J cells and cell viability
recovered significantly after 48 h of LPS treatment. Notably, 2
µg/ml LPS was the most suitable concentration and selected for
subsequent experiments.
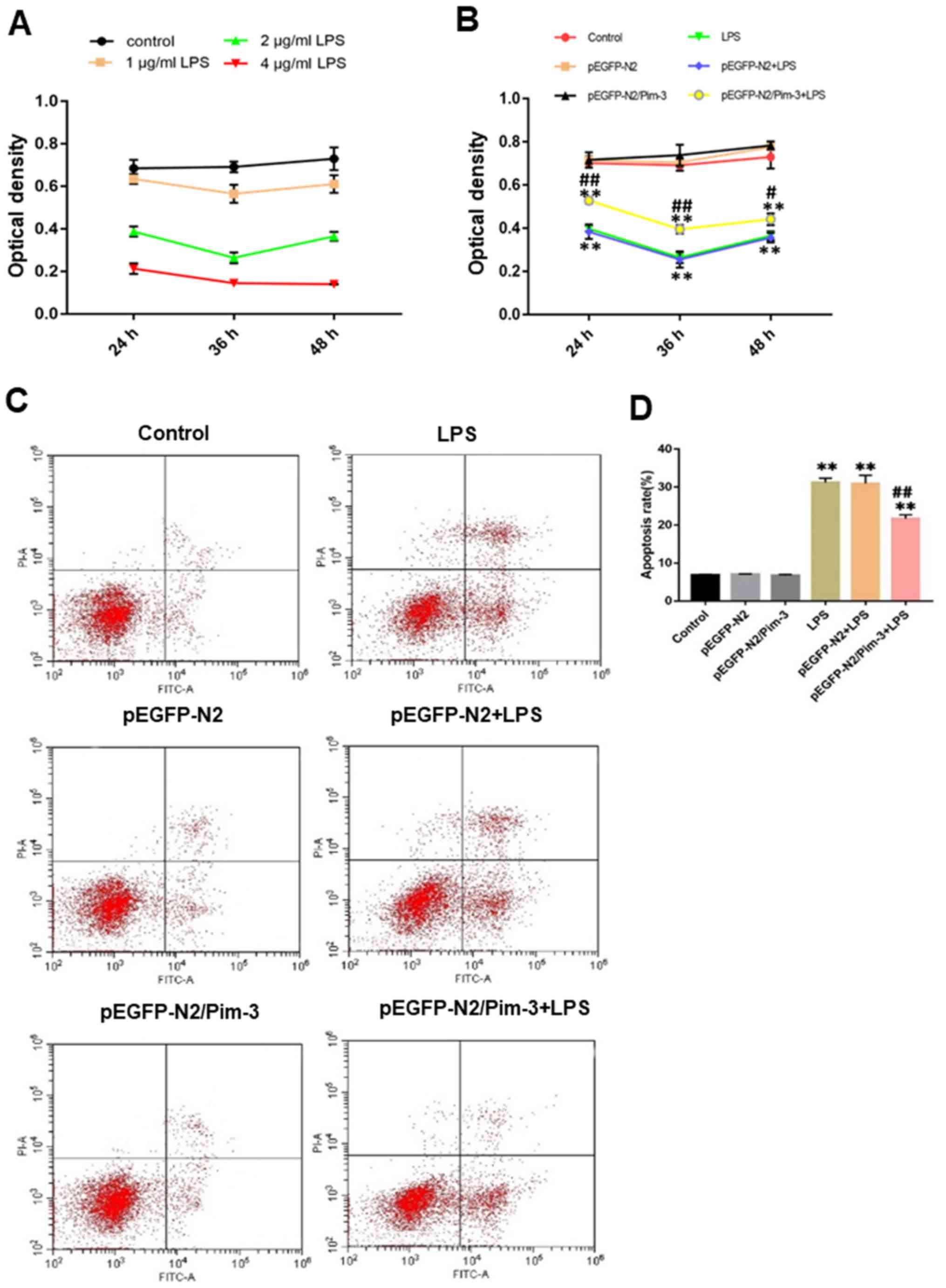 | Figure 2.Effect of Pim-3 on AR42J cell growth
following LPS treatment. (A) AR42J cells were treated with various
concentrations of LPS and proliferation was measured using an MTT
assay (optical density increases with cell proliferation). (B)
After transfection for 48 h, AR42J cells were treated with LPS for
24, 36 and 48 h. Proliferation was then measured using an MTT
assay. (C) After transfection for 48 h, AR42J cells were treated
with LPS for 48 h and the cells were subjected to combined staining
with FITC-Annexin V and PI. Cell apoptosis was determined by flow
cytometry and (D) apoptosis rates were compared. (E) After
transfection for 48 h, AR42J cells were treated with LPS for 48 h.
The cells were then treated with 40 µg/ml PI. The distribution of
the cell cycle phases was determined using a flow cytometer and (F)
the percentage of cells in G1, S and G2 are displayed. Data are
expressed as the mean ± SD, n=12 for all groups in (A) and (B) or
n=6 for all groups in (C, D, and E). **P<0.01 vs. the control
group, pEGFP-N2 group and pEGFP-N2/Pim-3 group.
#P<0.05 vs. the LPS group and pEGFP-N2 + LPS group.
##P<0.01 vs. the LPS group and pEGFP-N2 + LPS group.
EGFP, enhanced green fluorescent protein; LPS, lipopolysaccharide;
PI, propidium iodide; Pim-3, Pim-3 proto-oncogene, serine/threonine
kinase. |
Pim-3 increases the proliferation of
impaired AR42J cells
As shown in Table II
and Fig. 2B, the optical density
(OD) of the pEGFP-N2/Pim-3 + LPS group was higher than that in the
LPS group and the pEGFP-N2 + LPS group (LPS for 24 and 36 h,
P<0.01; LPS for 48 h, P<0.05). The OD value in the LPS group
was significantly lower than that in the control group (P<0.01).
There was no significant difference in OD value between the control
group, the pEGFP-N2 group and the pEGFP-N2/Pim-3 group (P>0.05).
These results indicated that Pim-3 increased the proliferation of
injured pancreatic cells, but had no effect on normal pancreatic
cells.
 | Table II.Effects of Pim-3 on AR42J cell
proliferation following LPS treatment. |
Table II.
Effects of Pim-3 on AR42J cell
proliferation following LPS treatment.
|
| Optical
density |
|---|
|
|
|
|---|
| Treatment
group | 24 h | 36 h | 48 h |
|---|
| Control | 0.7023±0.0114 | 0.6924±0.0145 | 0.7303±0.0308 |
| pEGFP-N2 | 0.7158±0.0103 | 0.7053±0.0115 | 0.7765±0.0045 |
| pEGFP-N2/Pim-3 | 0.7169±0.0204 | 0.7391±0.0276 | 0.7837±0.0105 |
| LPS |
0.3983±0.0074a |
0.2642±0.0148a |
0.3654±0.0119a |
| pEGFP-N2 + LPS |
0.3841±0.0193a |
0.2554±0.0215a |
0.3565±0.0127a |
| pEGFP-N2/Pim-3 +
LPS |
0.5284±0.0086a,b |
0.3954±0.0115a,b |
0.4417±0.0157a,c |
Pim-3 inhibits the apoptosis of AR42J
cells following LPS treatment
The total apoptosis rate of AR42J cells in the
pEGFP-N2/Pim-3 + LPS group was significantly lower than that in the
LPS group and the pEGFP-N2 + LPS group (P<0.01). The total
apoptosis rate in the LPS group was higher than that in the control
group (P<0.01). There was no significant difference in the total
apoptosis rate between the control group, the pEGFP-N2 group and
the pEGFP-N2/Pim-3 group (P>0.05; Fig. 2C and D).
Pim-3 can regulate cell cycle
progression of AR42J cells after LPS treatment
The percentage of AR42J cells in G1 phase in the
pEGFP-N2/Pim-3 + LPS group decreased significantly, whereas the
proportion of cells in the S and G2 phases increased compared with
in the LPS group and the pEGFP-N2 + LPS group (P<0.05, P<0.05
and P<0.01, respectively). The proportion of cells in the G1
phase in the LPS group was significantly increased, whereas the
proportion of cells in the S and G2 phases was decreased compared
with in the control group (P<0.01). There was no significant
difference in the proportions of cells in the various cell cycle
phases between the control group, the pEGFP-N2 group and the
pEGFP-N2/Pim-3 group (P>0.05) (Fig.
2E and F).
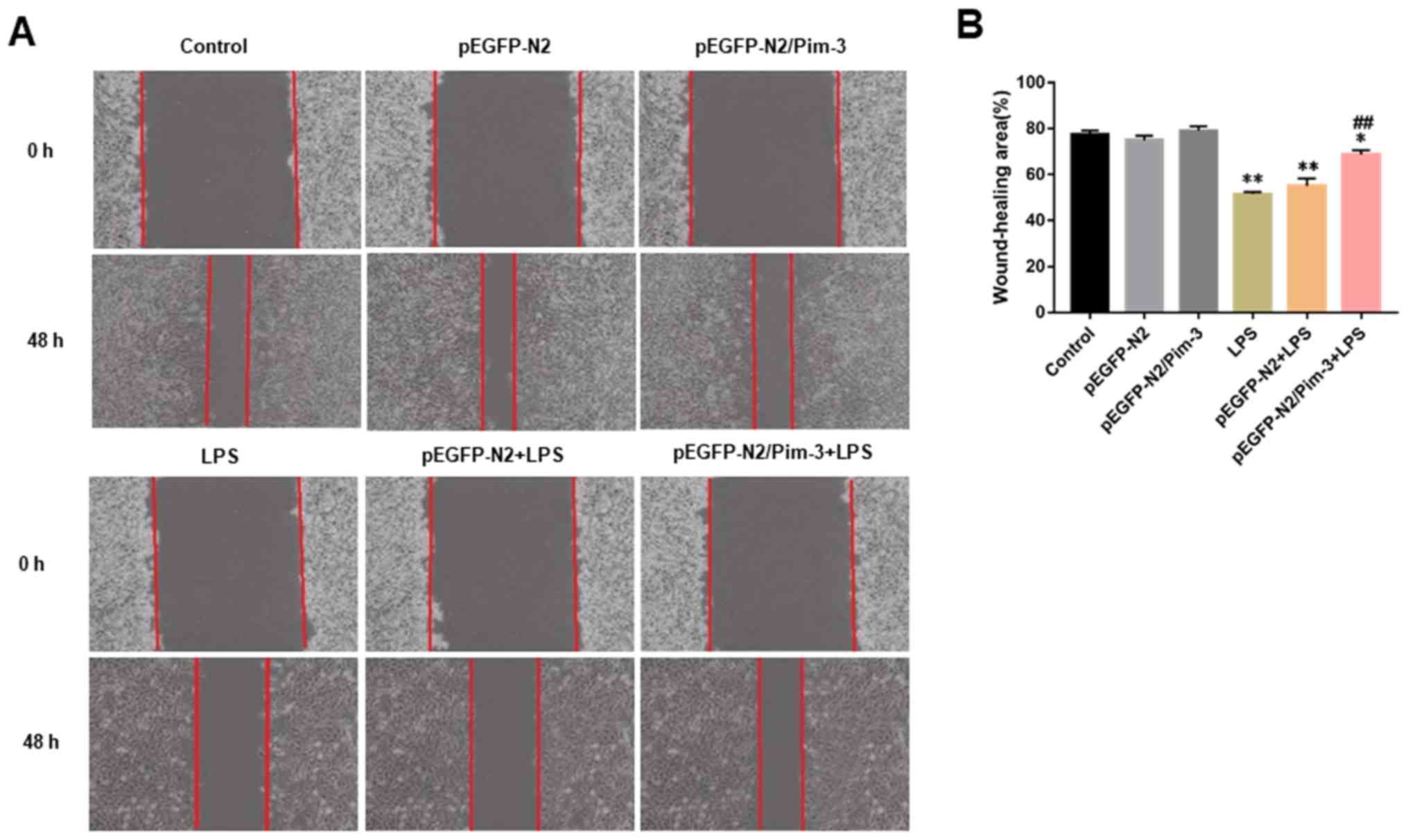
Pim-3 promotes impaired AR42J cell
migration
As shown in Fig. 3A and
B, LPS significantly inhibited the migration of AR42J cells
(LPS group vs. control group, 51.53±0.49% vs. 77.52±0.97%,
P<0.01). Whereas, the migration of AR42J cells in the
pEGFP-N2/Pim-3 + LPS group was markedly increased compared with in
the LPS group and the pEGFP-N2 + LPS group (pEGFP-N2/Pim-3 + LPS
group vs. LPS group or pEGFP-N2 + LPS group, 68.68±1.11% vs.
51.53±0.49% or 55.12±1.87%, respectively, P<0.01). There was no
significant difference in cell migration between the control group,
the pEGFP-N2 group and the pEGFP-N2/Pim-3 group (P>0.05).
Pim-3 regulates IL-6, IL-1β, TNF-α,
ICAM-1 and Occludin mRNA expression in the impaired AR42J
cells
The relative mRNA expression levels of Pim-3 in the
pEGFP-N2/Pim-3 group and the pEGFP-N2/Pim-3 + LPS group were
markedly elevated compared with in the control group, the pEGFP-N2
group, the LPS group and the pEGFP-N2 + LPS group (P<0.01). The
mRNA expression levels of IL-6, IL-1β, TNF-α and ICAM-1 in the LPS
group were significantly increased compared with in the control
group, whereas Occludin mRNA expression was significantly decreased
(P<0.01). However, the mRNA expression levels of IL-6, IL-1β,
TNF-α, and ICAM-1 in the pEGFP-N2/Pim-3 + LPS group were
significantly decreased compared with in the LPS group and the
pEGFP-N2 + LPS group (P<0.01, P<0.01, P<0.05 and
P<0.01, respectively), whereas the expression levels of Occludin
mRNA were increased (P<0.05). There was no significant
difference between the mRNA expression levels of all of the
measured transcripts between the control group, the pEGFP-N2 group
and the pEGFP-N2/Pim-3 group (P>0.05) (Fig. 4A and B).
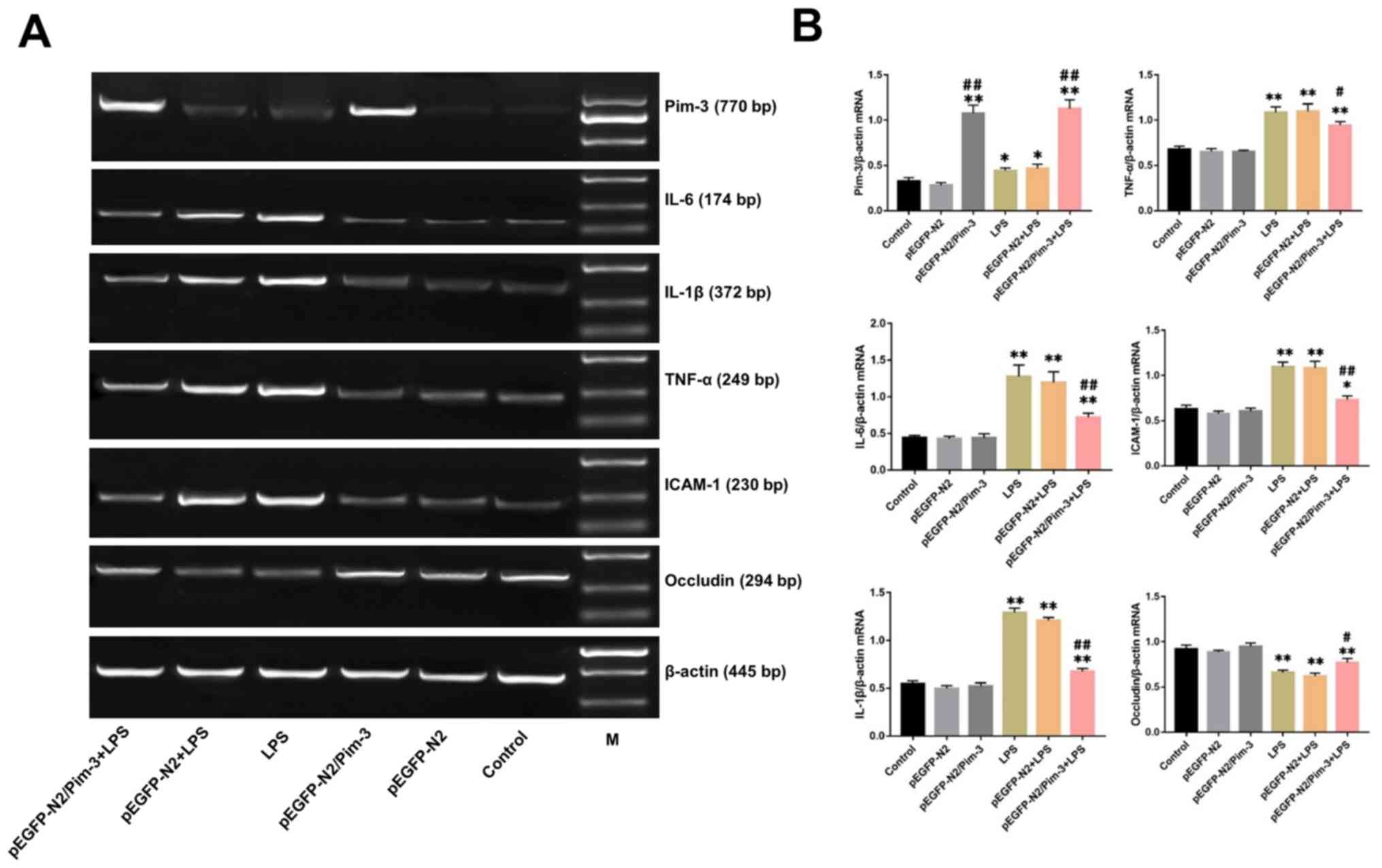 | Figure 4.Changes in mRNA and protein
expression levels of Pim-3, IL-6, IL-1β, TNF-α, ICAM-1 and Occludin
in AR42J cells following LPS treatment. After transfection for 48
h, AR42J cells were treated with LPS for 48 h. (A) PCR bands
demonstrating the relative mRNA expression levels of Pim-3, IL-6,
IL-1β, TNF-α, ICAM-1 and Occludin. (B) Bands were semi-quantified
to compare changes in expression levels. (C) Relative protein
expression levels of Pim-3, IL-6, IL-1β, TNF-α, ICAM-1 and
Occludin. (D) Bands were semi-quantified to compare changes in
expression levels. Control group (no treatment); pEGFP-N2 group (48
h after pEGFP-N2 transfection); pEGFP-N2/Pim-3 group (48 h after
pEGFP-N2/Pim-3 transfection); LPS group (48 h of LPS treatment);
pEGFP-N2 + LPS group (48 h after pEGFP-N2 transfection and LPS
treatment for 48 h); pEGFP-N2/Pim-3 + LPS group (48 h after
pEGFP-N2/Pim-3 transfection and LPS treatment for 48 h); M, DL2000
DNA marker. Data are expressed as the mean ± SD, n=6. *P<0.05
vs. the control group, pEGFP-N2 group and pEGFP-N2/Pim-3 group.
**P<0.01 vs. the control group, pEGFP-N2 group and
pEGFP-N2/Pim-3 group. #P<0.05 vs. the LPS group and
pEGFP-N2 + LPS group. ##P<0.01 vs. the LPS group and
pEGFP-N2 + LPS group. EGFP, enhanced green fluorescent protein;
LPS, lipopolysaccharide; ICAM, intercellular adhesion molecule; IL,
interleukin; Pim-3, Pim-3 proto-oncogene, serine/threonine kinase;
TNF, tumor necrosis factor. |
Pim-3 regulates IL-6, IL-1β, TNF-α,
ICAM-1 and Occludin protein expression in the impaired AR42J
cells
As shown in Fig. 4C and
D, the relative protein expression levels of Pim-3 in the
pEGFP-N2/Pim-3 group and pEGFP-N2/Pim-3 + LPS group were
significantly increased compared with in the control group, the
pEGFP-N2 group, the LPS group and the pEGFP-N2 + LPS group
(P<0.01). The protein expression levels of IL-6, IL-1β, TNF-α,
and ICAM-1 were markedly elevated in the LPS group compared with in
the control group, whereas Occludin protein expression was reduced
after LPS treatment (P<0.01). Conversely, the relative protein
expression levels of IL-6, IL-1β, TNF-α and ICAM-1 in the
pEGFP-N2/Pim-3 + LPS group were markedly reduced compared with the
LPS group and the pEGFP-N2 + LPS group (P<0.01, P<0.05,
P<0.01 and P<0.01, respectively), whereas Occludin protein
expression was increased (P<0.05). There was no significant
difference in protein expression levels of IL-6, IL-1β, TNF-α,
ICAM-1 and Occludin between the control group, the pEGFP-N2 group
and the pEGFP-N2/Pim-3 group (P>0.05).
Discussion
AP is a common acute abdominal malignancy. A
previous study found that ~30% of patients with AP will experience
severe attacks, which are associated with a high mortality rate
(19). AP manifests as localized
inflammation, which is amplified by the action of various
inflammatory mediators, such as cytokines, reactive oxygen species,
chemokines, leukocyte adhesion molecules and lipids, resulting in
pancreatic inflammation (20–22). The
present study also showed that the expression levels of IL-6,
IL-1β, TNF-α, and ICAM-1 in AR42J cells stimulated by LPS were
markedly higher than in the control group. Although the
pathogenesis behind AP or SAP has been extensively studied, the
exact mechanism of action has not been fully elucidated. Because
the pathogenesis is still unclear, AP presents a common clinical
challenge (23).
Pim-3 is a serine/threonine kinase, which is
involved in numerous cellular processes, including cell
proliferation, survival and apoptosis (24). Pim-3 is overexpressed in cancerous
tissue of endoderm-derived organs, such as the liver, pancreas,
stomach and colon (12–14,25).
Pim-3 can regulate the proliferation, survival, angiogenesis and
apoptosis of pancreatic cancer cells (26). Furthermore, downregulating Pim-3
expression causes pancreatic cancer cells to become more sensitive
to chemotherapy and radiotherapy (27,28).
Recent studies have demonstrated that Pim-3 has protective effects
on fulminant hepatic failure, damaged intestinal mucosa and
LPS-stimulated hepatic stellate cells (16–18). In
this present study, it was found that Pim-3 could prevent the
apoptosis of pancreatic acinar cells injured by LPS and promote the
survival of pancreatic acinar cells. In addition, Pim-3 regulated
the cell cycle of pancreatic acinar cells and enhanced their motor
ability, appearing to protect the damaged AR42J cells.
Proinflammatory cytokines, including IL-6, IL-1β and
TNF-α, are considered to serve a prominent role in the occurrence
and progression of AP or SAP. Previous studies have reported that
IL-6 has a crucial role in the early stages of inflammation, and
TNF-α, another important inflammatory factor, can mediate the
initiation and progression of AP (29–31).
IL-1β and TNF-α serve an important role in the pathogenesis of AP
or SAP, leading to inflammation, edema and necrosis (32). Adhesion molecules are thought to be
involved in activating leukocyte rolling, binding to vascular
tissue and initiating inflammatory cascade (33). ICAM-1 is an important adhesion
molecule in regulating leukocyte adhesion (34). During inflammation, ICAM-1 binds to
integrin subunit αL and integrin subunit β2, two integrins
expressed by leukocytes, promoting cell adhesion and
transepithelial migration (35). It
has been reported that ICAM-1 expression is elevated during AP and
its expression levels are closely related to the severity of the
disease (36,37). Tight junctions are the major apical
structures of epithelial and endothelial cells, playing an
important role in the cell barrier by forming intercellular
contacts and physically blocking paracellular pathways (38,39).
Occludin is an important structural protein in the tight junctions
of endothelial cells. Occludin forms a tight extracellular zipper
by bringing opposing outer membrane surfaces of cells in contact to
regulate barrier function. It can also bind to various molecules
and induce signaling pathways, allowing Occludin to participate in
the regulation of tight junction formation (40). In caerulein-induced AP rats, the loss
of Occludin precedes the elevation of serum lipase and amylase, and
is linked with an increase in extracellular permeability,
suggesting that Occludin may serve an important role in pancreatic
barrier function (41,42). The present study also revealed that
the expression levels of IL-6, IL-1β, TNF-α and ICAM-1 were
markedly increased in pancreatic acinar cells after LPS
stimulation, whereas the expression of Occludin was significantly
reduced after LPS intervention. These results also showed that the
transfection of exogenous Pim-3 into the AR42J cells prior to LPS
treatment, not only significantly reduced the expression of
pro-inflammatory cytokines, such as IL-6, IL-1β, TNF-α, and ICAM-1,
but also promoted the expression of Occludin, thus abating the
pancreatic injury mediated by LPS.
In summary, the present study revealed that
exogenous Pim-3 could alleviate pancreatic acinar cell injury
induced by LPS by reducing the expression of IL-6, IL-1β, TNF-α and
ICAM-1, and promoting Occludin expression. Pim-3 may function to
protect damaged pancreatic acinar cells through regulating the
inflammatory responses in the microenvironment. As such, Pim-3 has
a great potential for use as a therapeutic gene for treating AP or
SAP.
Acknowledgements
The authors would like to thank Dr Xia Gan, Dr
Li-Hong Gan, Dr Ya-Qing Huang and Dr Ling Yao (Department of
Gastroenterology, Third Affiliated Hospital of Nanchang University)
for their helpful suggestions regarding the writing of this
manuscript.
Funding
This study was supported by the Health and Family
Planning Commission Science and Technology Plan of Jiangxi Province
(grant no. 20184002).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJ and NF designed the experiments. JJ, SL, YZC,
JBQ, BL and LZ performed the experiments. JJ contributed to the
analysis of the data and wrote the manuscript. NF corrected the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lankisch PG, Apte M and Banks PA: Acute
pancreatitis. Lancet. 386:85–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zerem E: Treatment of severe acute
pancreatitis and its complications. World J Gastroenterol.
20:13879–13892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeong YK, Lee S, Lim JW and Kim H:
Docosahexaenoic acid inhibits cerulein-induced acute pancreatitis
in rats. Nutrients. 9:E7442017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang G, Liu Y, Zhou SF, Qiu P, Xu L, Wen
P, Wen J and Xiao X: Effect of somatostatin, ulinastatin and
gabexate on the treatment of severe acute pancreatitis. Am J Med
Sci. 351:506–512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murata A, Ohtani M, Muramatsu K and
Matsuda S: Effects of proton pump inhibitor on outcomes of patients
with severe acutepancreatitis based on a national administrative
database. Pancreatology. 15:491–496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nesseler N, Ross JT and Mallédant Y:
Infected necrotizing pancreatitis: Antibiotic administration
remains the first step. Lancet. 391:2501–2502. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gorskii VA, Agapov MA, Khoreva MV, Petrov
VA, Kravchenko AY and Battaev AI: Effect of lornoxicam therapy on
expression of TLR2 and TLR4 mRNA during systemic complications of
acute pancreatitis. Bull Exp Bio Med. 158:13–15. 2014. View Article : Google Scholar
|
|
8
|
Sherman MH, Yu RT, Engle DD, Ding N,
Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S,
et al: Vitamin D receptor-mediated stromal reprogramming suppresses
pancreatitis and enhances pancreatic cancer therapy. Cell.
159:80–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hua J, He ZG, Qian DH, Lin SP, Gong J,
Meng HB, Yang TS, Sun W, Xu B, Zhou B and Song ZS: Angiopoietin-1
gene- modified human mesenchymal stem cells promote angiogenesis
and reduce acute pancreatitis in rats. Int J Clin Exp Pathol.
7:3580–3595. 2014.PubMed/NCBI
|
|
10
|
Liu J, Qu X, Shao L, Hu Y, Yu X, Lan P,
Guo Q, Han Q, Zhang J and Zhang C: Pim-3 enhances melanoma cell
migration and invasion by promoting STAT3 phosphorylation. Cancer
Biol Ther. 19:160–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Narlik-Grassow M, Blanco-Aparicio C and
Carnero A: The PIM family of Serine/Threonine kinases in cancer.
Med Res Rev. 34:136–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujii C, Nakamoto Y, Lu P, Tsuneyama K,
Popivanova BK, Kaneko S and Mukaida N: Aberrant expression of
serine/threonine kinase Pim-3 in hepatocellular carcinoma
development and its role in the proliferation of human hepatoma
cell lines. Int J Cancer. 114:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li YY, Popivanova BK, Nagai Y, Ishikura H,
Fujii C and Mukaida N: Pim-3, a proto-oncogene with
serine/threonine kinase activity, is aberrantly expressed in human
pancreatic cancer and phosphorylates bad to block bad-mediated
apoptosis in human pancreatic cancer cell lines. Cancer Res.
66:6741–6747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Popivanova BK, Li YY, Zheng H, Omura K,
Fujii C, Tsuneyama K and Mukaida N: Proto-oncogene, Pim-3 with
serine/threonine kinase activity, is aberrantly expressed in human
colon cancer cells and can prevent Bad-mediated apoptosis. Cancer
Sci. 98:321–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mukaida N, Wang YY and Li YY: Roles of
Pim-3, a novel survival kinase, in tumorigenesis. Cancer Sci.
102:1437–1442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu LM, Zhang JX, Wang XP, Guo HX, Deng H
and Luo J: Pim-3 protects against hepatic failure in
D-galactosamine (D-GalN)-sensitized rats. Eur J Clin Invest.
40:127–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JY, Shen XW and Zhang JX: Protective
role of Pim-3 gene in intestinal mucosa damaged by burn or
lipopolysaccharide. Zhonghua Yi Xue Za Zhi. 87:2960–2964. 2007.(In
Chinese). PubMed/NCBI
|
|
18
|
Liu LH, Lai QN, Chen JY, Zhang JX and
Cheng B: Overexpression of pim-3 and protective role in
lipopolysaccharide-stimulated hepatic stellate cells. World J
Gastroenterol. 21:8858–8867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai F, Cui N, Ma H, Wang X, Qiao G and Liu
D: Interleukin-10-1082A/G polymorphism is associated with the
development of acute pancreatitis in a Chinese population. Int J
Clin Exp Pathol. 8:15170–15176. 2015.PubMed/NCBI
|
|
20
|
Simsek O, Kocael A, Kocael P, Orhan A,
Cengiz M, Balci H, Ulualp K and Uzun H: Inflammatory mediators in
the diagnosis and treatment of acute pancreatitis: Pentraxin3,
procalcitonin and myeloperoxidase. Arch Med Sci. 14:288–296. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ge N, Xia Q, Yang ZH, Ding QF and Zeng Z:
Vascular endothelial injury and apoptosis in rats with severe acute
pancreatitis. Gastroenterol Res Pract. 2015:2350172015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pan YL: The effects of glycyrrhizin on
acute pancreatitis in mice. Eur Rev Med Pharmacol Sci.
18:3943–3947. 2014.PubMed/NCBI
|
|
23
|
Banks PA: Acute pancreatitis: Landmark
studies, management decisions, and the future. Pancreas.
45:633–640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li YY and Mukaida N: Pathophysiological
roles of Pim-3 kinase in pancreatic cancer development and
progression. World J Gastroenterol. 20:9392–9404. 2014.PubMed/NCBI
|
|
25
|
Zheng HC, Tsuneyama K, Takahashi H, Miwa
S, Sugiyama T, Popivanova BK, Fujii C, Nomoto K, Mukaida N and
Takano Y: Aberrant Pim-3 expression is involved in gastric
adenoma-adenocarcinoma sequence and cancer progression. J Cancer
Res Clin Oncol. 134:481–488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang C, Li HY, Liu B, Huang S, Wu L and Li
YY: Pim-3 promotes the growth of human pancreatic cancer in the
orthotopic nude mouse model through vascular endothelium growth
factor. J Surg Res. 185:595–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liang C, Yu XJ, Guo XZ, Sun MH, Wang Z,
Song Y, Ni QX, Li HY, Mukaida N and Li YY: MicroRNA-33a-mediated
downregulation of Pim-3 kinase expression renders human pancreatic
cancer cells sensitivity to gemcitabine. Oncotarget. 6:14440–14455.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen XY, Wang Z, Li B, Zhang YJ and Li YY:
Pim-3 contributes to radioresistance through regulation of the cell
cycle and DNA damage repair in pancreatic cancer cells. Biochem
Biophys Res Commun. 473:296–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gregoric P, Sijacki A, Stankovic S,
Radenkovic D, Ivancevic N, Karamarkovic A, Popovic N, Karadzic B,
Stijak L, Stefanovic B, et al: SIRS score on admission and initial
concentration of IL-6 as severe acute pancreatitis outcome
predictors. Hepatogastroenterology. 57:349–353. 2010.PubMed/NCBI
|
|
30
|
De Waele JJ and Blot S: The value of IL-6
in predicting the severity of acute pancreatitis. J Clin
Gastroenterol. 41:5342007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sternby H, Hartman H, Johansen D,
Thorlacius H and Regnér S: IL-6 and CRP are superior in early
differentiation between mild and non-mild acute pancreatitis.
Pancreatology. 17:550–554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vonlaufen A, Apte MV, Imhof BA and
Frossard JL: The role of inflammatory and parenchymal cells in
acute pancreatitis. J Pathol. 213:239–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rubant SA, Ludwig RJ, Diehl S, Hardt K,
Kaufmann R, Pfeilschifter JM and Boehncke WH: Dimethylfumarate
reduces leukocyte rolling in vivo through modulation of adhesion
molecule expression. J Invest Dermatol. 128:326–331. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dabrowski A, Osada J, Dabrowska MI,
Wereszczynska-Siemiatkowska U and Siemiatkowski A: Increased
expression of the intercellular adhesion molecule-1 (ICAM-1) on
peripheral blood neutrophils in acute pancreatitis. Adv Med Sci.
59:102–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun W, Watanabe Y and Wang ZQ: Expression
and significance of ICAM-1 and its counter receptors LFA-1 and
Mac-1 in experimental acute pancreatitis of rats. World J
Gastroenterol. 12:5005–5009. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu HH and Jiang LL: Serum inter-cellular
adhesion molecule 1 is an early marker of diagnosis and prediction
of severe acute pancreatitis. World J Gastroenterol. 18:2554–2560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Staubli SM, Oertli D and Nebiker CA:
Laboratory markers predicting severity of acute pancreatitis. Crit
Rev Clin Lab Sci. 52:273–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shin K, Fogg VC and Margolis B: Tight
junctions and cell polarity. Annu Rev Cell Dev Biol. 22:207–235.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oliveira SS and Morgado-Díaz JA: Claudins:
Multifunctional players in epithelial tight junctions and their
role in cancer. Cell Mol Life Sci. 64:17–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kojima T and Sawada N: Regulation of tight
junctions in human normal pancreatic duct epithelial cells and
cancer cells. Ann NY Acad Sci. 1257:85–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xia XM, Li BK, Xing SM and Ruan HL: Emodin
promoted pancreatic claudin-5 and occludin expression in
experimental acute pancreatitis rats. World J Gastroenterol.
18:2132–2139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen D, Li L, Yan J, Yang X, You Y, Zhou Y
and Ling X: The loss of αSNAP downregulates the expression of
occludin in the intestinal epithelial cell of acute pancreatitis
model. Pancreatology. 14:347–355. 2014. View Article : Google Scholar : PubMed/NCBI
|