Introduction
Cancer-induced bone pain (CIBP), resulting from
metastasis of primary tumors or bone tumors, is one of the most
severe types of chronic pain, and involves a complex molecular
mechanism (1,2). CIBP has been demonstrated to cause
depression, anxiety and many other complications that can
significantly lower quality of life (3). Therefore, investigation into the
potential molecular mechanisms underlying CIBP is necessary for the
development of novel therapeutic targets.
Brain-derived neurotrophic factor (BDNF) levels are
significantly enhanced in the spinal dorsal horn (4) and dorsal root ganglion (DRG) of CIBP
rodent models (5). In addition, our
previous work demonstrated that BDNF participates in the
development and maintenance of behavioral hyperalgesia in the CIBP
rat model (6). However, the
mechanisms of BDNF signaling that underlie CIBP remain poorly
understood. Upregulation of neurotrophin receptor p75
(p75NTR), a low-affinity receptor of BDNF, induces a
variety of cellular events, triggering several potential
proapoptotic cascades. Studies have demonstrated that
p75NTR expression is markedly increased in both the
peripheral (7) and central nervous
systems following noxious stimuli or injury (8). Injury-induced neuropathic pain is
significantly reduced via pharmacological blockade of
p75NTR in DRG neurons of rats with spinal nerve ligation
(7). Mice lacking p75NTR
are insensitive to noxious thermal stimuli (9). Despite the widespread expression of
p75NTR in DRG and the spinal cord, there are few reports
demonstrating that p75NTR is involved in CIBP at the
spinal cord level. Furthermore, investigation into the role of
BDNF/p75NTR in the pathobiology of cancer-related pain
is still at the relatively early stages.
Mammalian target of rapamycin (mTOR) is a
serine/threonine protein kinase that exists in the mammalian
nervous system. There is evidence that widespread dysregulation of
mTOR and its downstream signaling pathways contributes to
neuropathic pain (10–12). The mTOR pathway is activated in DRG
and the spinal dorsal horn in models of CIBP, and intrathecal
injection of rapamycin, a specific inhibitor of mTOR, reduces
mechanical hyperalgesia in CIBP rats (13). Finally, it has been demonstrated that
activation of mTOR and its downstream effectors, such as ribosomal
protein S6 kinase B1 (P70S6K), in DRG and the spinal dorsal horn
serves a role in CIBP (14).
The present study hypothesized that the
BDNF/p75NTR pathway was involved in the development and
maintenance of hyperalgesia in CIBP through mTOR upregulation.
Firstly, it was investigated whether mTOR activation in primary
sensory neurons was caused by the enhanced release of BDNF. It was
then determined whether CIBP increased p75NTR and mTOR
expression in DRG and the spinal dorsal horn in vivo.
Finally, the effect of p75NTR silencing on the
development and maintenance of CIBP was determined.
Materials and methods
Animal study
The study was approved by the Ethics Committee of
Soochow University. A total of 67 healthy adult female
Sprague-Dawley (SD) rats (age, 6–8 weeks; 200±20 g) and 40 healthy
neonatal rats (aged 24–48 h; 6.3±1.4 g; neonatal rats of both sexes
were used) were kept under controlled conditions (20–25°C; relative
humidity 40–60%; 12 h light/dark cycle with food and water ad
libitum). Care and handling of rats complied with guidelines of
the Institutional Animal Care and Use Committee of Soochow
University. All experiments were performed in accordance with the
National Institutes of Health guide for the care and use of
laboratory animals (15) and the
guidelines of the International Association for the Study of Pain
(16).
Primary DRG cell culture
preparation
Rat DRG cultures were prepared as described
previously (17). The neonatal rats
were anesthetized with isoflurane and euthanized by cervical
dislocation, followed by decapitation. DRG were removed under
aseptic conditions and immediately placed in Neurobasal™-A medium
(cat. no. 10888022; Gibco; Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.). DRG were digested with collagenase D (1.8–2.0
mg/ml; cat. no. 11088858001; Roche Diagnostics) and 0.25% trypsin
(cat. no. T4799; Sigma-Aldrich; Merck KGaA) in D-Hanks' solution at
37°C for 20 min. The DRG suspensions were centrifuged at 105 × g
for 5 min at room temperature. The supernatants were discarded and
the DRG were triturated using flame-polished glass pipettes.
Dissociated DRG neurons were cultured in 24-well clusters at 37°C
with 5% CO2 for 24 h, then maintained in Neurobasal-A
medium containing 10% FBS. The neurobasal media was replaced after
24 h.
Cell preparation and CIBP rat
model
The Walker-256 mammary gland carcinoma cell line was
purchased from the American Type Culture Collection and cultured as
previously described (3). A total of
10 SD rats were used for the isolation of ascitic fluid in the
present study. In brief, Walker-256 cells (2×107 cells
in 1 ml D-Hank's balanced salt solution; cat. no. PB180321; Beijing
Solarbio Science & Technology Co., Ltd.) were injected into the
abdominal cavity of SD rats. After 7 days, cancerous ascitic fluid
was collected from the rats. Cells were then diluted to a final
concentration of 2×107 cells/ml with D-Hank's solution.
Establishment of the CIBP rat model was in accordance with our
previous study (18). Animals were
anesthetized via an intraperitoneal injection of sodium
pentobarbital (50 mg/kg) for surgery. Then, 20 µl diluted tumor
cells were slowly injected into the rat tibial bone marrow cavity
with a 23-gauge needle. The sham rat group received 20 µl D-Hank's
solution injected into the tibial bone marrow cavity.
Immunofluorescence staining
For the in vitro experiments, following 48 h
of culture, DRG neurons were treated with exogenous BDNF (20 ng/ml;
cat. no. B-250; Alomone Labs) for another 24 h, with the control
group cultured in medium only (n=5 for each group). DRG neurons
were plated for fluorescent labeling at 1×105 cells/well
on a cover slip coated with poly-l-lysine. Cells were fixed with 4%
paraformaldehyde for 20 min at room temperature followed by three
washes in PBS. A blocking step was performed by incubating the
coverslips in PBS containing 5% donkey serum (cat. no. ab7475;
Abcam) at room temperature for 2 h. For immunofluorescence
staining, DRG neurons were incubated with primary antibody at 4°C
overnight: Rabbit anti-mTOR (cat. no. ab2732; 1:200; Abcam); mouse
anti-RNA binding fox-1 homolog 3 (NeuN; cat. no. MAB377; 1:500; EMD
Millipore); or mouse anti-p75NTR (cat. no. ab61425;
1:100; Abcam). Following primary incubation, samples were then
incubated with Alexa Fluor 488-conjugated secondary antibodies
(cat. no. ab150077; 1:200; Abcam) or Alexa Fluor 594-conjugated
secondary antibodies (cat. no. ab150120; 1:200; Abcam) for 2 h at
room temperature. Immunofluorescent staining for mTOR was primarily
in the cytoplasm, whilst NeuN staining was mainly in the nucleus of
the DRG neurons (11,19). Images were captured using a 20X
objective under a fluorescence microscope (Nikon Corporation) and
analyzed with Image Pro Plus 6.0 software (Media Cybernetics,
Inc.).
For the in vivo experiments, the rats were
divided into the sham group, CIBP group or CIBP +
si-p75NTR group at random (n=4 for each group). Animals
were anesthetized by intraperitoneal injection of sodium
pentobarbital (50 mg/kg) and transcardially perfused with normal
saline followed by 4% paraformaldehyde, as previously described
(20). L4-L6 segments of the spinal
cord and DRG were removed and fixed for 4 h at 4°C, then treated
with 30% sucrose in PBS at 4°C overnight. Transverse spinal cord
(30 µm) and DRG (12 µm) slices were cut in the cryostat. All slices
were blocked with 5% donkey serum at room temperature for 2 h, then
incubated with primary antibody, rabbit anti-mTOR (cat. no. ab2732;
1:200; Abcam) and mouse anti-p75NTR (cat. no. ab61425;
1:100; Abcam), at 4°C overnight. Following primary incubation,
samples were incubated with secondary antibodies harboring Alexa
Fluor 488 (cat. no. ab150077; 1:200; Abcam) or Alexa Fluor 594
(cat. no. ab150120; 1:200; Abcam) for 2 h at room temperature.
Images were captured with a 20X objective under a fluorescence
microscope (Nikon Corporation) and analyzed with Image Pro Plus 6.0
software (Media Cybernetics, Inc.).
Intrathecal catheter
According to a method described previously (18,21,22),
intrathecal catheters were administered 5 days before the
establishment of the CIBP rat model. Animals were anesthetized by
intraperitoneal injection of sodium pentobarbital (50 mg/kg) and
polyethylene catheters (PE-10 tube; Smiths Medical) were inserted
into the subarachnoid space of the spinal cord between the L4 and
L5 spinous processes. Correct positioning was confirmed by the tail
flick response immediately following insertion of the catheter.
Following housing for 4 days, animals that were observed to have
motor dysfunction were excluded from the experiment (22).
Administration of small interfering
RNA (siRNA)
siRNA sequences targeting the P75NTR gene
(GenBank: NM_012610; http://www.ncbi.nlm.nih.gov/genbank/) were purchased
from Shanghai GenePharma Co., Ltd. and designed as follows: Sense
sequence, 5′-AACGCTTGATGCCCTTTTAGC-3′ and antisense sequence,
5′-AATCGCATGCGTTCCATTTCG-3′. The negative sequences, as mismatch
controls, were as follows: Sense sequence,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense sequence,
5′-ACGUGACACGUUCGGAGAATT-3′. Following transfection with
siRNA/mismatch using Lipofectamine™ 2000 (also used as the vehicle
treatment) (Invitrogen; Thermo Fisher Scientific, Inc.) for 24 h or
72 h, the cultured DRG neurons were harvested and used for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
In vivo administration was used to investigate whether
intrathecal p75NTR-targeting siRNA
(si-p75NTR) could attenuate the mechanical pain of CIBP
rats. siRNA was then modified with one stabilizing
2′-fluorodeoxyuridine substitution at the 3′-end of the sense
strand. A total of 10 µl si-p75NTR/mismatch siRNA/in
vivo-jetPEI™ (cat. no. 201-10G; Polyplus transfection; Tamar
Laboratory Supplies, Ltd.; defined as the vehicle) was injected
into the lumbar region of the spinal cord through the intrathecal
catheter. Three days after injection of carcinoma cells, the
si-p75NTR or corresponding negative controls were injected to the
rats once daily for three consecutive days, from days 4 to 6.
RT-qPCR
CO2 euthanasia followed by physical
examination was performed on the animals before collecting the
tissues. Total RNA from primary cultured DRG cells, L4-6 spinal
cord segments and DRG in vivo (n=5 for each group) were
extracted using TRIzol® reagent (cat. no. 15596026;
Invitrogen; Thermo Fisher Scientific, Inc.) as previously described
(23). Absorbance at 260 and 280 nm
was measured for RNA quantification and quality control. RNA
samples were reverse transcribed using the RevertAid First Strand
cDNA Synthesis kit, according to the manufacturer's protocol (cat.
no. K1622; Thermo Fisher Scientific, Inc.). qPCR was performed
using SYBR Green PCR MasterMix (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The 20 µl reaction volume contained 2 µl cDNA, 1
µl of each pair of primers and 13 µl SYBR Green MasterMix and 3 µl
ddH2O. Thermal cycling was performed as follows: 95°C
for 5 min, 40 cycles of 95°C for 10 sec, 58°C for 15 sec and 72°C
for 20 sec. Relative expression levels of each gene were quantified
using the 2−ΔΔCq method and normalized to β-actin. All
experiments were repeated three times for reproducibility. The
primer sequences were as follows: mTOR (cat. no. NM_019906)
forward, 5′-AGAACCTGGCTCAAGTACGC-3′ and reverse,
5′-AGGATGGTCAAGTTGCCGAG-3′; p75NTR (cat. no. NM_012610) forward,
5′-AGGGATGGCGTGACTTTC-3′ and reverse, 5′-GTTGGCTTCAGGCTTATGC-3′;
and β-actin (cat. no. NM_031144) forward,
5′-TCTATCCTGGCCTCACTGTC-3′ and reverse,
5′-AACGCAGCTCAGTAACAGTCC-3′.
Behavioral test
The rats were divided into the sham, CIBP, CIBP +
si-p75NTR, CIBP + mismatch RNA and CIBP + vehicle groups
(n=8 for each group). All behavioral tests were performed between
9:00 a.m. and 6:00 p.m. To quantify the mechanical sensitivity of
the hind paw, the animals were housed in individual plastic cages
for at least 30 min until calm. For mechanical allodynia, von Frey
filaments were used to measure the 50% likelihood of paw withdrawal
thresholds (PWTs) via the up-and-down method, as described
previously (18,24). Spontaneous pain was assessed by
ambulatory score in the tumor-injected hind limb, as described
previously (24–26). Limb use during spontaneous ambulation
was scored on a scale of 0–4: 0, normal use; 1, slight limping; 2,
pronounced limping; 3, partial non-use of the limb; and 4, complete
lack of limb use. All behavioral tests were performed by an
investigator who was blinded to the experimental design.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 5.01. All data are expressed as the mean ± SEM from
4–6 repeats as previously described (27,28).
One-way analysis of variance (ANOVA) followed by the Bonferroni
post hoc test was used for the analysis of the RT-qPCR and
immunofluorescence data. Two-way ANOVA with repeated measures,
followed by the Bonferroni post hoc test, was used for PWT and the
ambulatory pain score. Student's t-test was used if only two groups
were being analyzed. P<0.05 was considered to indicate a
statistically significant difference.
Results
Treatment with exogenous BDNF
upregulates mTOR and NeuN expression in cultured primary DRG
neurons
To investigate whether the mTOR activation in
primary sensory neurons was caused by enhanced release of BDNF from
microglia, cultured primary DRG neurons were incubated with
exogenous BDNF (20 ng/ml) for 24 h, with the dose of BDNF the same
as that used in a previous study (29). Following culture for 48 h, DRG
neurons of the BDNF group were treated with exogenous BDNF for 24 h
whilst the control group was cultured in medium only (without
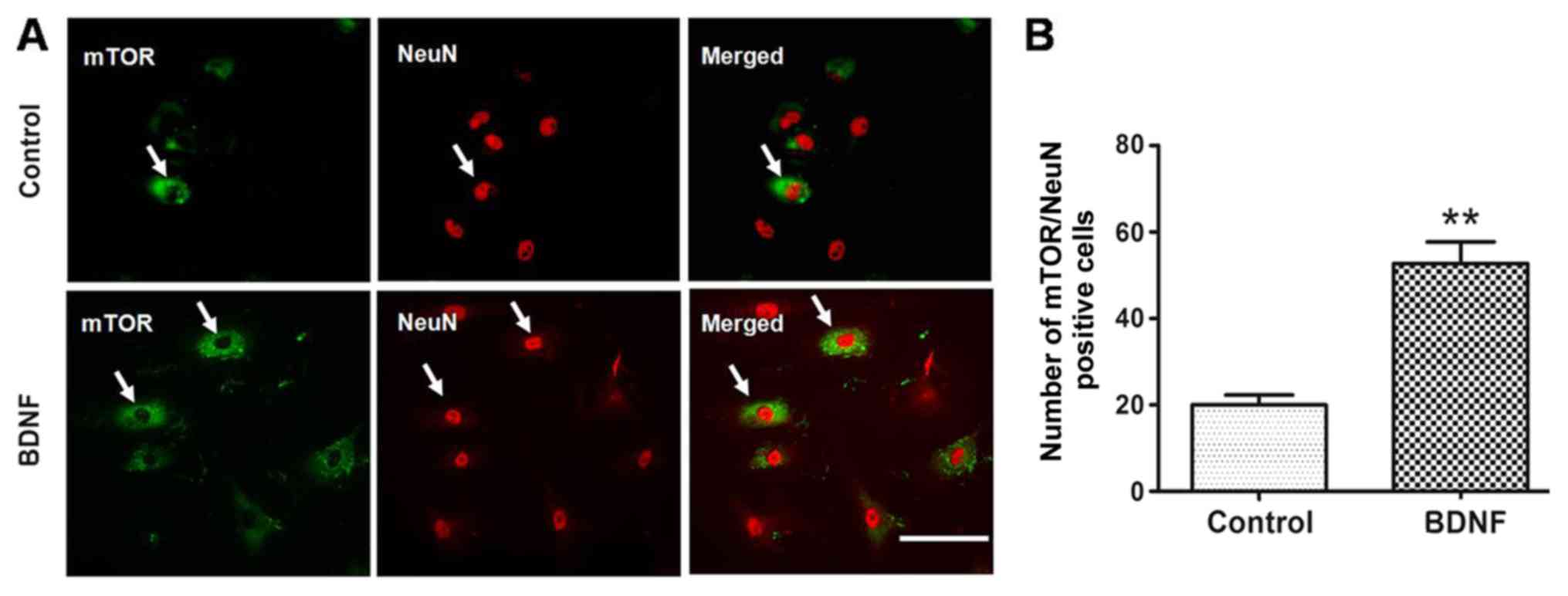
BDNF). DRG neurons were stained for mTOR (Fig. 1A; green; cytoplasm) and NeuN
(Fig. 1A; red; nucleus). When
compared with the control group, BDNF treatment increased the
coexpression of mTOR and NeuN (Fig.
1B; P<0.01). In the control group, 19.93±0.02% of
NeuN-positive neurons in cultured primary DRG neurons were positive
for mTOR, compared with 51.10±0.05% in the BDNF group (Fig. 1B). This result indicated that
treatment with BDNF upregulated mTOR in the DRG neurons.
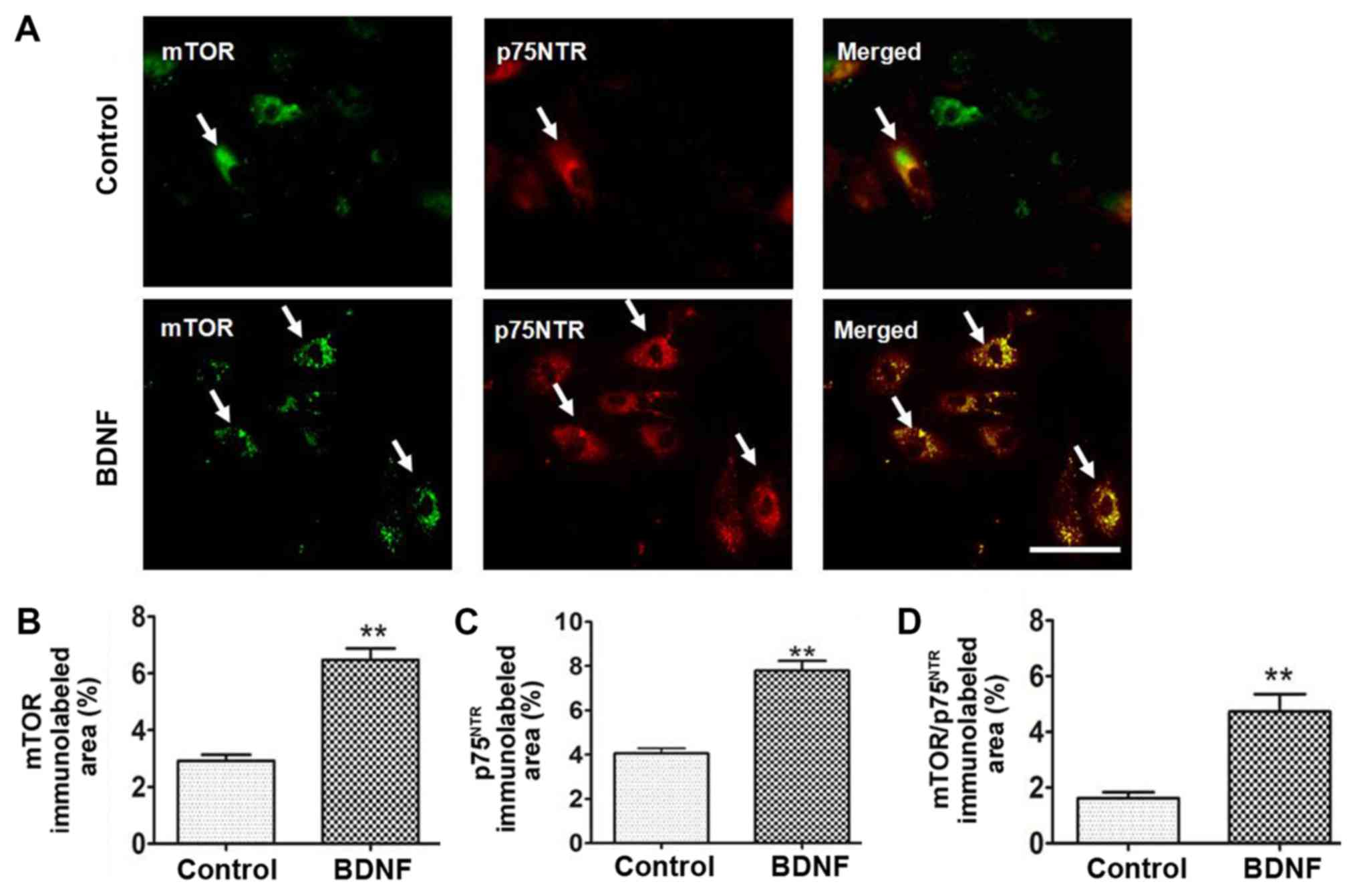
Treatment with exogenous BDNF
upregulates the coexpression of mTOR and p75NTR in
cultured primary DRG neurons
The effect of BDNF (20 ng/ml) treatment on mTOR and
p75NTR expression in DRG neurons was then investigated.
DRG neurons were stained for mTOR (Fig.
2A; green; cytoplasm) and p75NTR (Fig. 2A; red; cytoplasm). The area of mTOR
immunoreactivity in the control group was 2.92±0.21% and in BDNF
group was 6.48±0.41% (Fig. 2B). The
area of p75NTR immunoreactivity in the control group was
4.07±0.22% and in BDNF group was 7.79±0.44% (Fig. 2C). The area of mTOR and
p75NTR coexpression immunoreactivity was significantly
increased in the BDNF group (Fig.
2D; P<0.01). The area of coexpression in the control group
was 1.62±0.21% compared with 4.73±0.62% in the BDNF group.
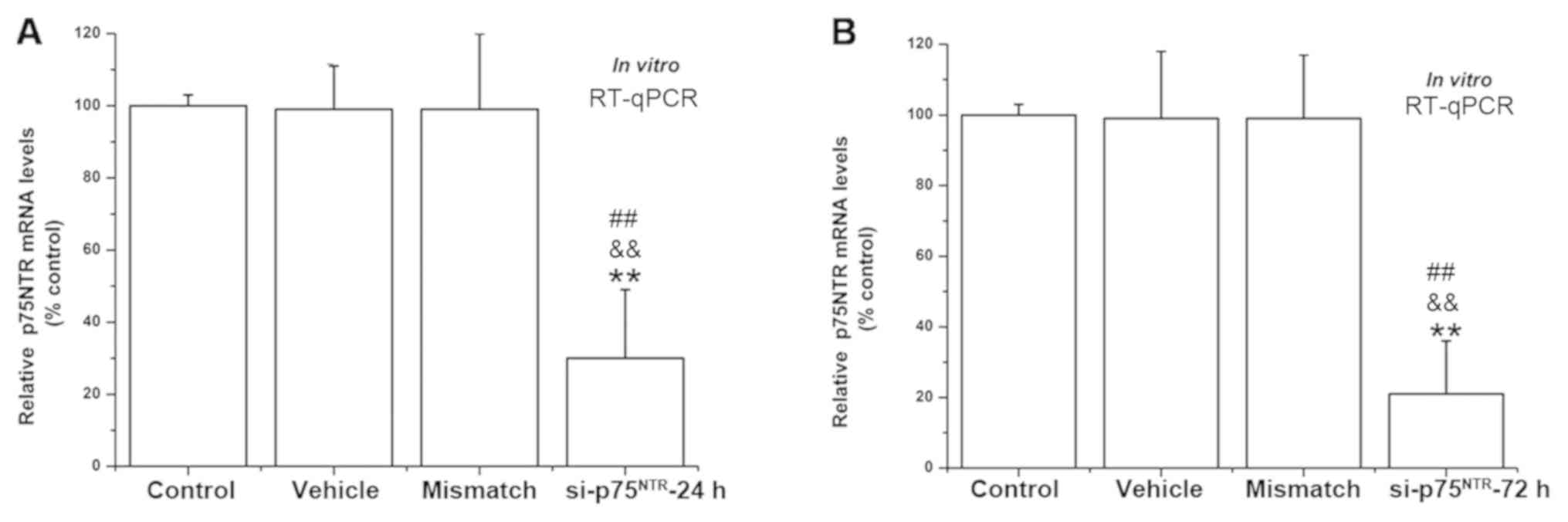
Identification of siRNA for knockdown
of p75NTR in cultured primary DRG neurons
RT-qPCR was used to check the transfection
efficiency of the siRNA sequences in the primary DRG neurons in
vitro. Following transfection for 24 h or 72 h,
p75NTR mRNA levels in cultured primary DRG neurons
transfected with si-p75NTR −24 h (Fig. 3A) or −72 h (Fig. 3B) were significantly reduced when
compared with the control, vehicle or mismatch group.
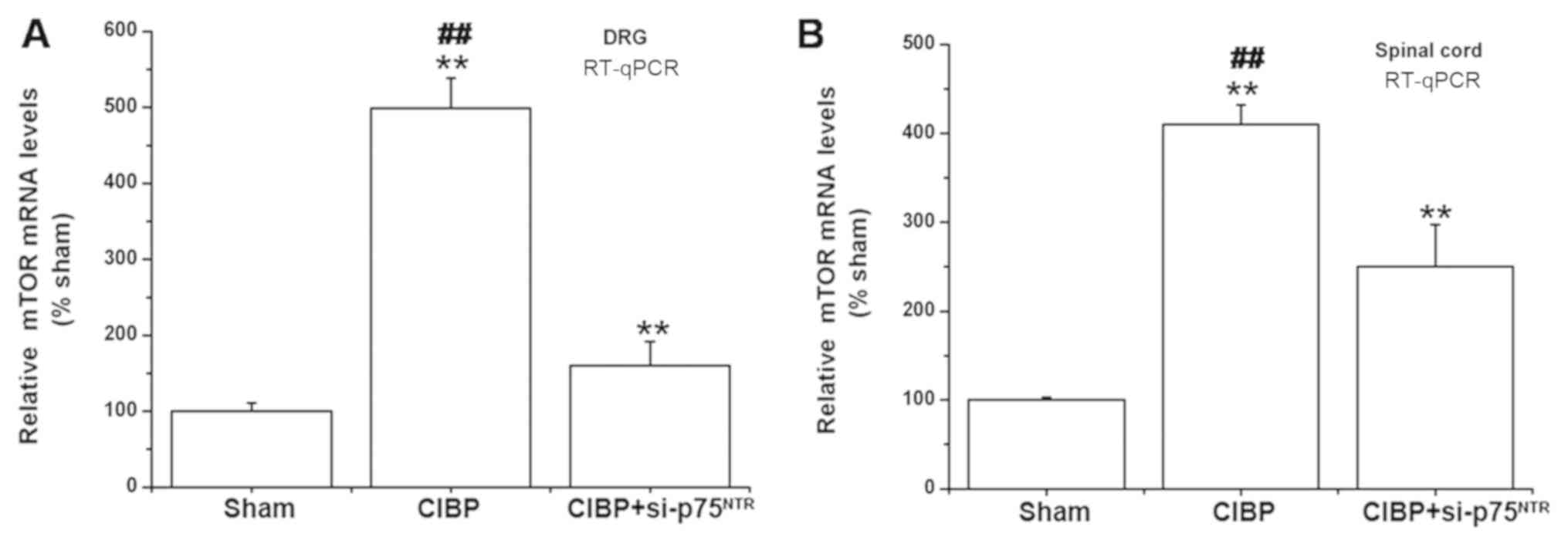
Treatment with si-p75NTR
decreases mTOR mRNA expression in the DRG and spinal cords of CIBP
rats
The present study subsequently investigated whether
the injection of si-p75NTR reversed mTOR mRNA expression
in the model of CIBP rats. The sham group rats received 20 µl
D-Hanks' solution injection into the tibial bone marrow cavity. The
CIBP group rats were injected with 20 µl diluted tumor cells into
the rat bone marrow cavity. The CIBP+si-p75NTR group
rats were injected with si-p75NTR daily for 3
consecutive days from day 4 to day 6 post-tumor cell injection. It
was determined that si-p75NTR treatment markedly
suppressed mTOR mRNA expression in DRG of CIBP+
si-p75NTR group rats at day 9 (Fig. 4A). Similarly, mTOR expression levels
were significantly decreased in spinal cords of CIBP+
si-p75NTR group rats (Fig.
4B).
Treatment with si-p75NTR
reverses upregulated coexpression of mTOR and p75NTR in DRG of CIBP
rats
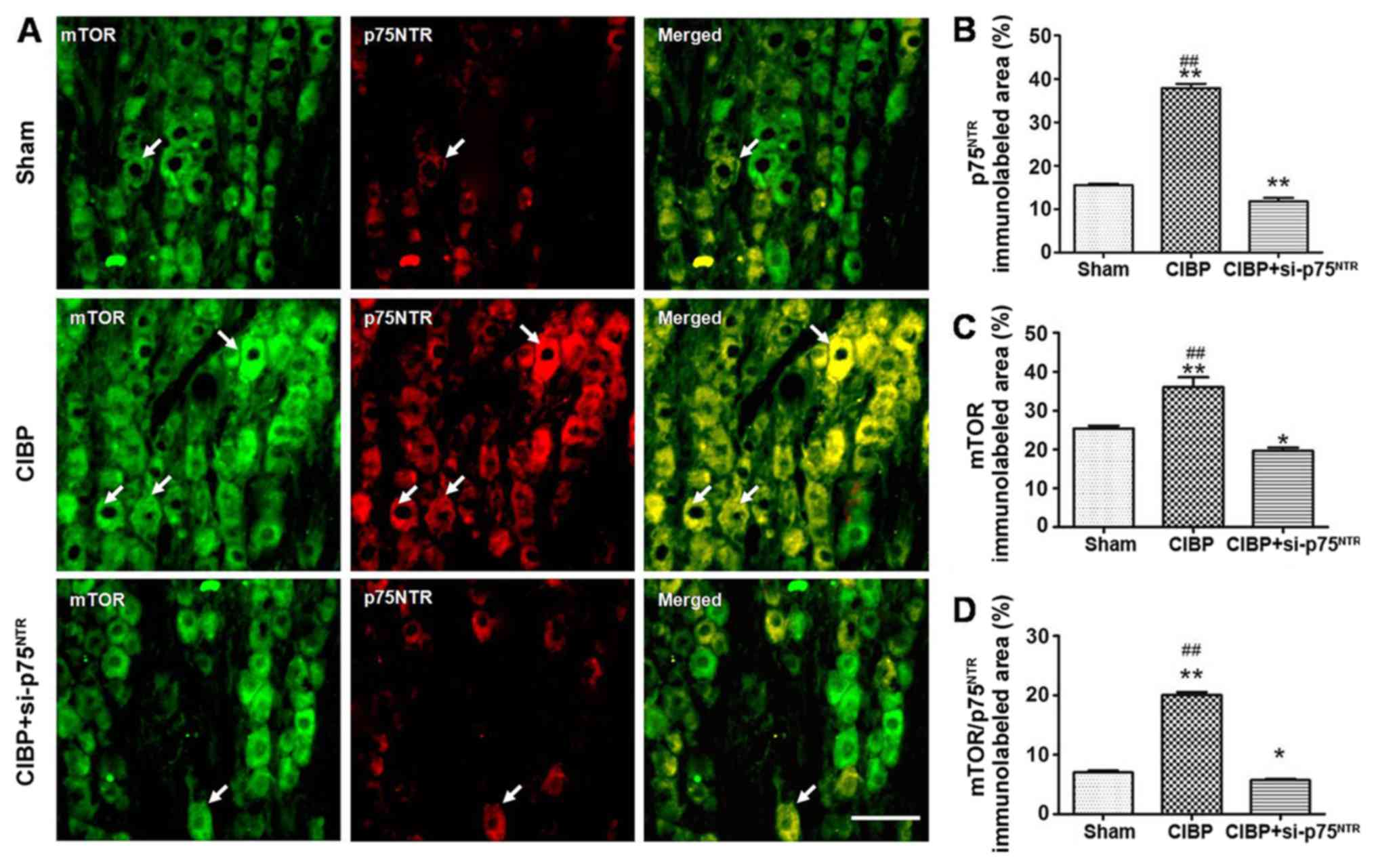
The results indicated that mTOR and
p75NTR may participate in the development of chronic
pain, including CIBP. It was then investigated whether
p75NTR contributed to CIBP by activating mTOR in the
DRG. The results demonstrated that the area of p75NTR
immunoreactivity in the CIBP group was increased in the DRG slices
when compared with that in sham rats. However, following injection
with si-p75NTR, the area of p75NTR
immunoreactivity in the CIBP+si-p75NTR groups was
significantly decreased when compared with that in CIBP rats
(Fig. 5A). The area of
p75NTR immunoreactivity in the sham group was
15.25±0.46%, 37.86±0.98% in the CIBP group and 11.75±0.82% in
CIBP+si-p75NTR groups (Fig.
5B). When compared with the CIBP group, si-p75NTR
treatment markedly suppressed mTOR immunoreactivity in
CIBP+si-p75NTR group rats following injection with tumor
cells (Fig. 5A). The area of mTOR
immunoreactivity in the sham group was 25.40±0.40%, 36.06±2.54% in
the CIBP group and 19.68±0.75 in CIBP+si-p75NTR group
(Fig. 5C). In addition, it was
identified that treatment with si-p75NTR reversed the
area of coexpression of mTOR and p75NTR in DRG of CIBP
rats. The coexpression area of mTOR and p75NTR
immunoreactivity in the sham group was 7.04±0.29%, in the CIBP
group it was 20.03±0.51%, and in the CIBP+si-p75NTR
group it was 5.75±0.21% (Fig.
5D).
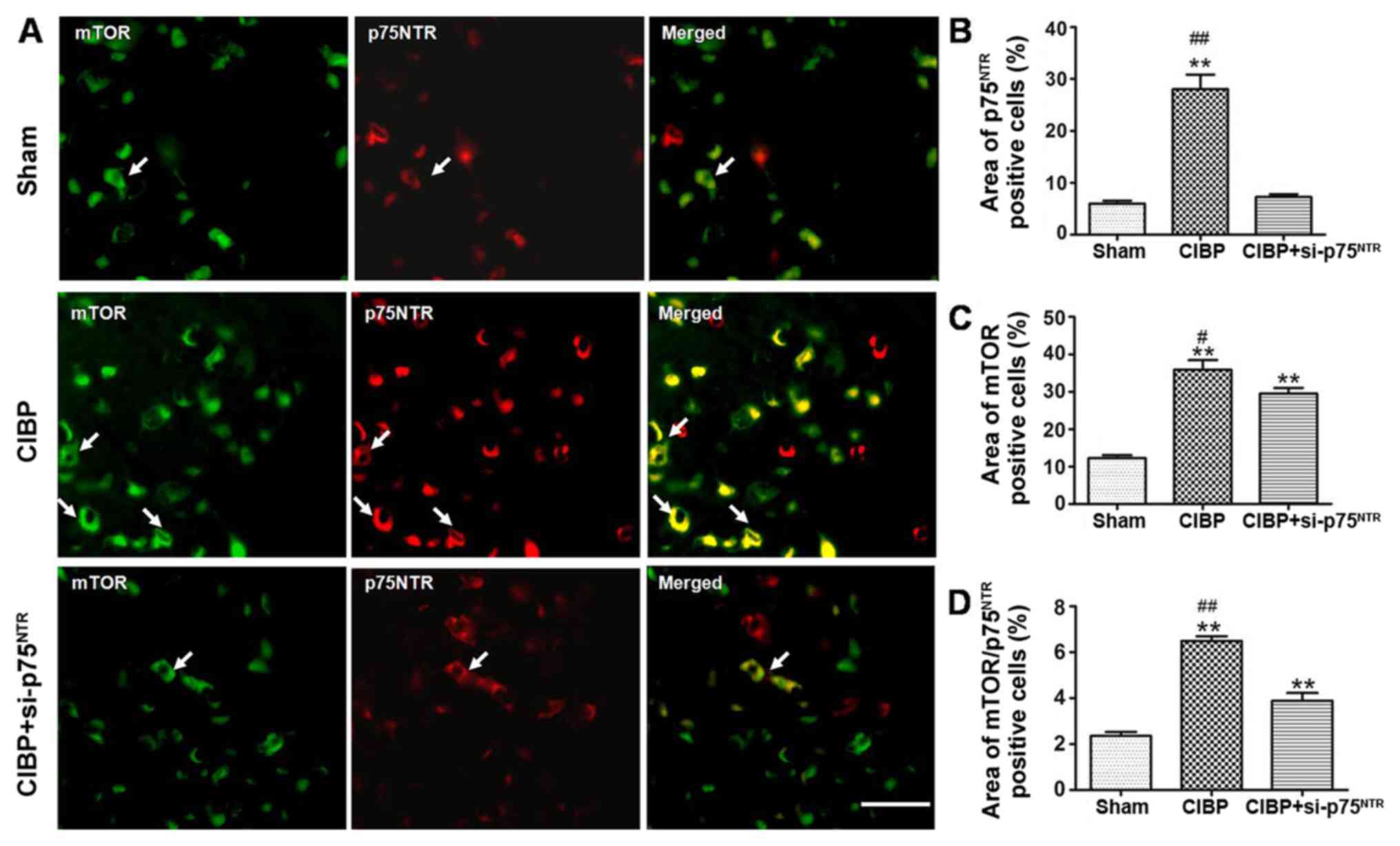
Treatment with si-p75NTR
reverses mTOR and p75NTR upregulation in the spinal dorsal horn of
CIBP rats
The rats were divided into the sham, CIBP or
CIBP+si-p75NTR groups at random (n=4). The results
demonstrated that the area of p75NTR immunoreactivity in
the CIBP group was significantly increased in the spinal dorsal
horn slices when compared with that in sham rats. However,
following injection with siRNA-p75NTR, the area of
p75NTR immunoreactivity in the CIBP+si-p75NTR
group was significantly decreased when compared with that in CIBP
rats (Fig. 6A). The area of
p75NTR immunoreactivity in the sham group was 6.0±0.52%,
in CIBP group it was 28.02±2.82%, and in the si-p75NTR
group it was 7.29±0.55% (Fig. 6B).
When compared with the CIBP group, si-p75NTR treatment
significantly suppressed mTOR immunoreactivity in
CIBP+si-p75NTR group rats following tumor cell
injection. The area of mTOR immunoreactivity in the sham group was
12.28±0.78%, in the CIBP group it was 35.91±2.53%, and in the
CIBP+si-p75NTR group it was 29.53±1.48% (Fig. 6C). In addition, it was identified
that treatment with siRNA-p75NTR reversed the effect on
the area of mTOR and p75NTR coexpression in the spinal
dorsal horn of CIBP rats. The coexpression area of mTOR and
p75NTR immunoreactivity in the sham group was
2.36±0.17%, in the CIBP group it was 6.49±0.20%, and in the
CIBP+si-p75NTR group it was 3.89±0.34% (Fig. 6D).
Treatment with si-p75NTR
attenuates hyperalgesia in CIBP rats
Finally, the present study investigated whether
blocking p75NTR upregulation in DRG and dorsal horns via
intrathecal injection of si-p75NTR decreased the
behavioral hyperalgesia of CIBP rats. Compared with the sham group
rats, the 50% PWT of CIBP group rats was significantly decreased
between days 6 and 18. In the CIBP+si-p75NTR group, the
PWT was significantly higher compared with that in the CIBP, CIBP +
mismatch RNA and CIBP + vehicle group rats (Fig. 7A). By contrast, the ambulatory scores
of CIBP + si-p75NTR group rats were significantly lower
than those observed in the CIBP, CIBP + mismatch RNA and CIBP +
vehicle group rats (Fig. 7B).
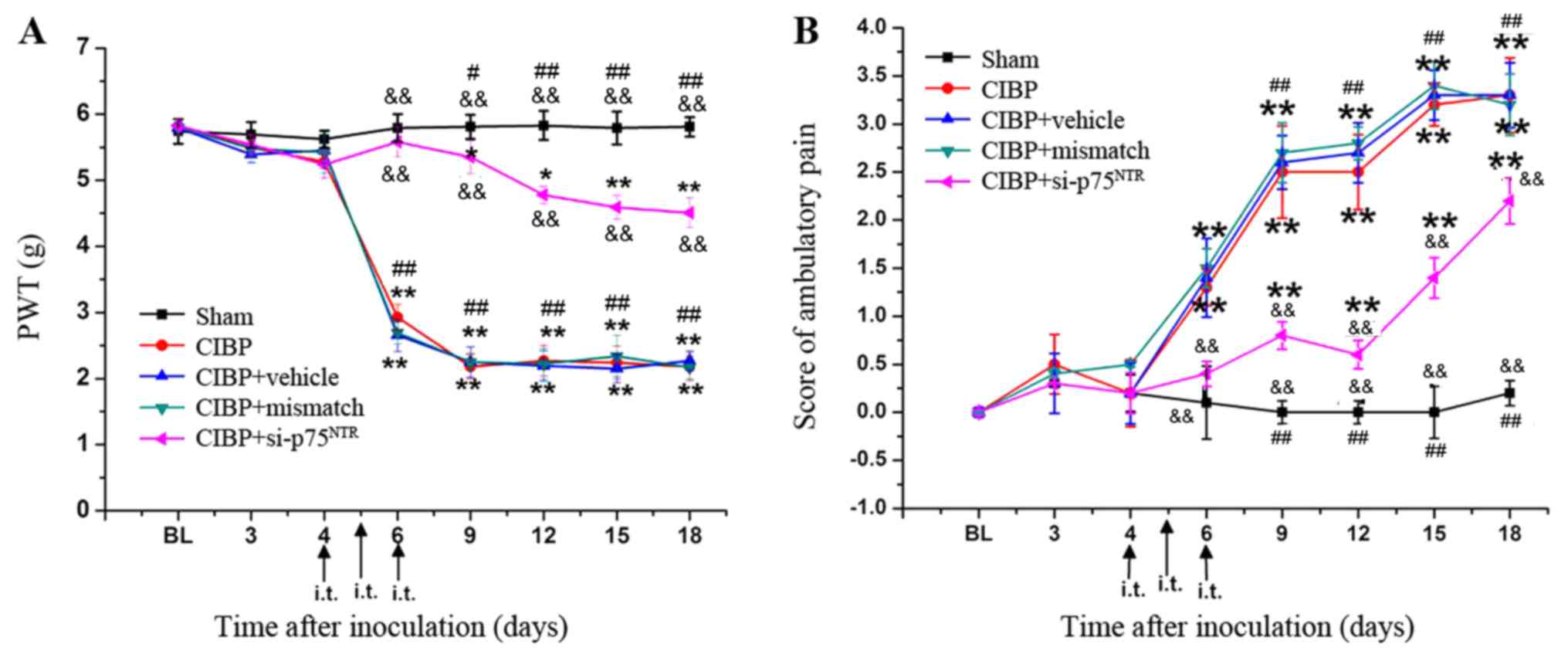 | Figure 7.Treatment with si-p75NTR
attenuates hyperalgesia in CIBP rats. (A) Following injection with
si-p75NTR, the PWTs of CIBP+si-p75NTR group
rats were significantly higher compared with those in the CIBP,
CIBP+mismatch or CIBP+vehicle groups. (B) Following injection with
si-p75NTR, the ambulatory scores of the
CIBP+siRNA-p75NTR group were significantly lower than
those observed in the CIBP, CIBP+mismatch and CIBP+vehicle groups.
No significant differences were observed between CIBP+mismatch,
CIBP+vehicle rats and CIBP group rats. *P<0.05 and **P<0.01
vs. sham group; &&P<0.01 vs. CIBP group;
#P<0.05 and ##P<0.01 vs.
CIBP+si-p75NTR group. si, small interfering;
p75NTR, neurotrophin receptor p75; CIBP, cancer-induced
bone pain; PWT, paw withdrawal thresholds; i.t., intrathecal
injection. |
Discussion
CIBP is a growing health concern due to the severity
of the pain, the limited efficacy of current drug treatments and
the unacceptable side effects for cancer patients (30). In order to mimic the clinical pain of
patients with cancer-related pain, studies have injected cancer
tumor cells into the bone to produce hyperalgesia in animal models
(3,18). Emerging evidence has indicated that
the BDNF tropomyosin receptor kinase B (TrkB) complex is trafficked
to the cell for downstream signaling pathway transduction, and then
participates in the development of neuropathic pain (31–33).
Chodroff et al (34)
demonstrated that BDNF signaling constitutes a novel mechanism
whereby oral squamous cell carcinoma induces pain. Identification
of the key role of TrkB signaling in oral cancer pain may serve as
a novel target for drug development. Recent research has
demonstrated that a population of positive-TrkB sensory neurons are
both necessary and sufficient for producing pain from only light
touch following nerve injury in mice (35). These results highlighted the
potential value of BDNF TrkB as a therapeutic target. However,
another report indicated that blocking of all three Trk receptors
markedly inhibited sprouting and neuroma formation in sensory nerve
fibers and reversed CIBP behavior by 50–60% (20). It is likely that BDNF might
contribute to the development and progression of CIBP by regulating
other signaling pathways which need to be further explored. Yao
et al (36) demonstrated that
the neurotrophin receptors p75NTR in the DRG and spinal
cords were significantly increased in asparaginyl
endopeptidase-induced CIBP. However, this study did not further
investigate whether p75NTR contributed to the
hypersensitivity of CIBP. The present study determined that the
hyperalgesia in CIBP was significantly attenuated through
inhibition of the BDNF/p75NTR pathway. Between days 6
and 12, the effect reached a >80% reduction compared with the
sham group, which indicated its potency. However, the molecular
mechanisms underlying CIBP are complex and remain to be fully
understood. The reversion appeared to be attenuated after day 12,
indicating that other factors may play a core role at that
point.
A previous study determined that treatment with mTOR
inhibitors blocks the BDNF-increased metastasis of neuroblastoma
(37). In order to investigate
whether BDNF contributed to CIBP by upregulating mTOR, the present
study used exogenous BDNF to imitate the over-secretion of BDNF by
microglia. It was determined that BDNF treatment increased mTOR
expression in primary cultured DRG neurons. In addition, it was
demonstrated that mTOR mRNA levels were significantly increased in
the DRG neurons and spinal dorsal horn of CIBP rats. These results
indicated that mTOR activated through the BDNF signaling pathway
participated in regulating behavioral hyperalgesia in the CIBP rat
model. Research has clarified that CIBP-induced mechanical
allodynia and thermal hyperalgesia are markedly attenuated when
mTOR is inhibited by intrathecal injection of rapamycin (14,37). The
activation of mTOR further triggers the phosphorylation of
downstream pathways, such as P70S6K and eukaryotic translation
initiation factor 4E binding protein 1, to regulate mRNA
translation and protein synthesis (10,14,38).
Preclinical and clinical studies have demonstrated that
N-methyl-D-aspartate (NMDA) receptor inhibition produces a
significant analgesic effect on CIBP, suggesting that NMDA
receptors are required for the induction of hypersensitivity
(14,29). These results are consistent with the
authors' previous study, which demonstrated that BDNF-activated
NMDA receptors in the spinal cord or DRG were involved in the
development of central sensitization and behavioral
hypersensitivity in CIBP rats (18).
Shih et al (14) further
demonstrated that NMDA receptors participate in the activation of
mTOR and its downstream signaling pathway in the spinal dorsal horn
in a CIBP model (13,14). A recent study demonstrated that
BDNF/mTOR signaling could activate the NMDA receptor, which is
required for inflammatory pain-related aversion in the rostral
anterior cingulate cortex of rats (39). Therefore, the present study
speculated that BDNF/p75NTR may be involved in the
regulation of hyperalgesia by activating mTOR-NMDA signaling in the
CIBP model. However further investigation is required in future
work.
In conclusion, the present study identified the
critical function of BDNF/p75NTR by upregulating mTOR
expression in the DRG and spinal cord of CIBP rats. mTOR expression
was significantly reduced in the DRG and spinal cords following
p75NTR silencing, and behavioral hyperalgesia in CIBP
rats was attenuated. The present findings suggested that
BDNF/p75NTR/mTOR signaling may serve a major role in the
development of CIBP; therefore, potent and specific inhibitors of
p75NTR could be developed into novel drugs for treating
CIBP.
Acknowledgements
The authors would like to thank Dr Yong-gang Li
(Department of Radiology, The First Affiliated Hospital of Soochow
University) for providing technical support for data analysis and
revising the manuscript critically for important intellectual
content.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81471136, 81701098 and
81671743), Jiangsu Provincial Medial Youth Talent (grant no.
QNRC2016740), Jiangsu Provincial Medical Innovation Team (grant no.
CXTDA2017043), Suzhou Citizenship Technology (grant no. SYS201738)
and Priority Academic Program Development of Jiangsu Higher
Education Institutions.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XWM performed experiments, analyzed data, prepared
figures and drafted the manuscript. XHJ performed experiments,
analyzed data and drafted the manuscript. XW performed experiments
and analyzed data. LNW designed and supervised the experiments and
edited the manuscript. JPY analyzed data and edited the manuscript.
FHJ analyzed data and edited the manuscript.
Ethics approval and consent to
participate
Experimental protocols were approved by the
Institutional Animal Care and Use Committee of Soochow University.
All experiments were performed in accordance with the National
Institutes of Health guide for the Care and Use of Laboratory
Animals and the guidelines of the International Association for the
Study of Pain.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Falk S and Dickenson AH: Pain and
nociception: Mechanisms of cancer-induced bone pain. J Clin Oncol.
32:1647–1654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu S, Wang C, Han Y, Song C, Hu X and Liu
Y: Sigma-1 receptor antagonist BD1047 reduces mechanical allodynia
in a rat model of bone cancer pain through the inhibition of spinal
NR1 phosphorylation and microglia activation. Mediators Inflamm.
2015:2650562015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu L, Gao XJ, Ren CG, Hu JH, Liu XW,
Zhang P, Zhang ZW and Fu ZJ: Monocyte chemoattractant protein-1
contributes to morphine tolerance in rats with cancer-induced bone
pain. Exp Ther Med. 13:461–466. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bao Y, Hou W, Liu R, Gao Y, Kong X, Yang
L, Shi Z, Li W, Zheng H, Jiang S, et al: PAR2-mediated upregulation
of BDNF contributes to central sensitization in bone cancer pain.
Mol Pain. 10:282014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomotsuka N, Kaku R, Obata N, Matsuoka Y,
Kanzaki H, Taniguchi A, Muto N, Omiya H, Itano Y, Sato T, et al:
Up-regulation of brain-derived neurotrophic factor in the dorsal
root ganglion of the rat bone cancer pain model. J Pain Res.
7:415–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang LN, Yang JP, Ji FH, Wang XY, Zuo JL,
Xu QN, Jia XM, Zhou J, Ren CG and Li W: The role of brain-derived
neurotrophic factor in pain facilitation and spinal mechanism in
rat model of bone cancer pain. Zhonghua Yi Xue Za Zhi.
91:1188–1192. 2011.(In Chinese). PubMed/NCBI
|
|
7
|
Obata K, Katsura H, Sakurai J, Kobayashi
K, Yamanaka H, Dai Y, Fukuoka T and Noguchi K: Suppression of the
p75 neurotrophin receptor in uninjured sensory neurons reduces
neuropathic pain after nerve injury. J Neurosci. 26:11974–11986.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuura Y, Iwakura N, Ohtori S, Suzuki T,
Kuniyoshi K, Murakami K, Hiwatari R, Hashimoto K, Okamoto S,
Shibayama M, et al: The effect of anti-NGF receptor (p75
Neurotrophin Receptor) antibodies on nociceptive behavior and
activation of spinal microglia in the rat brachial plexus avulsion
model. Spine (Phila Pa 1976). 38:E332–E338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee KF, Li E, Huber LJ, Landis SC, Sharpe
AH, Chao MV and Jaenisch R: Targeted mutation of the gene encoding
the low affinity NGF receptor p75 leads to deficits in the
peripheral sensory nervous system. Cell. 69:737–749. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Li X, Huang B and Ma S: Blocking
mammalian target of rapamycin (mTOR) improves neuropathic pain
evoked by spinal cord injury. Transl Neurosci. 7:50–55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lutz BM, Nia S, Xiong M, Tao YX and Bekker
A: MTOR, a new potential target for chronic pain and opioid-induced
tolerance and hyperalgesia. Mol Pain. 11:322015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lisi L, Aceto P, Navarra P and Dello Russo
C: MTOR kinase: A possible pharmacological target in the management
of chronic pain. Biomed Res Int. 2015:3942572015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Z, Wu S, Wu X, Zhong J, Lv A, Jiao J
and Chen Z: Blocking mammalian target of rapamycin alleviates bone
cancer pain and morphine tolerance via micro-opioid receptor. Int J
Cancer. 138:2013–2020. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shih MH, Kao SC, Wang W, Yaster M and Tao
YX: Spinal cord NMDA receptor-mediated activation of mammalian
target of rapamycin is required for the development and maintenance
of bone cancer-induced pain hypersensitivities in rats. J Pain.
13:338–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory AnimalsNational Academies Press (US); Washington, DC:
1996
|
|
16
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan B, Tang WH, Lu LJ, Zhou Y, Zhu HY,
Zhou YL, Zhang HH, Hu CY and Xu GY: TLR4 upregulates CBS expression
through NF-kappaB activation in a rat model of irritable bowel
syndrome with chronic visceral hypersensitivity. World J
Gastroenterol. 21:8615–8628. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang LN, Yang JP, Ji FH, Zhan Y, Jin XH,
Xu QN, Wang XY and Zuo JL: Brain-derived neurotrophic factor
modulates N-methyl-D-aspartate receptor activation in a rat model
of cancer-induced bone pain. J Neurosci Res. 90:1249–1260. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao C, Lv C, Li H, Du S, Liu X, Li Z, Xin
W and Zhang W: Geniposide protects primary cortical neurons against
oligomeric Abeta1-42-induced neurotoxicity through a mitochondrial
pathway. PLoS One. 11:e01525512016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghilardi JR, Freeman KT, Jimenez-Andrade
JM, Mantyh WG, Bloom AP, Kuskowski MA and Mantyh PW: Administration
of a tropomyosin receptor kinase inhibitor attenuates
sarcoma-induced nerve sprouting, neuroma formation and bone cancer
pain. Mol Pain. 6:872010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y, Li H, Li TT, Luo H, Gu XY, Lü N,
Ji RR and Zhang YQ: Delayed activation of spinal microglia
contributes to the maintenance of bone cancer pain in female wistar
rats via P2X7 receptor and IL-18. J Neurosci. 35:7950–7963. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Milligan ED, Hinde JL, Mehmert KK, Maier
SF and Watkins LR: A method for increasing the viability of the
external portion of lumbar catheters placed in the spinal
subarachnoid space of rats. J Neurosci Methods. 90:81–86. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song C, Liu D, Yang S, Cheng L, Xing E and
Chen Z: Sericin enhances the insulin-PI3K/AKT signaling pathway in
the liver of a type 2 diabetes rat model. Exp Ther Med.
16:3345–3352. 2018.PubMed/NCBI
|
|
24
|
Zhou YQ, Chen SP, Liu DQ, Manyande A,
Zhang W, Yang SB, Xiong BR, Fu QC, Song ZP, Rittner H, et al: The
role of spinal GABAB receptors in cancer-induced Bone Pain in Rats.
J Pain. 18:933–946. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lan LS, Ping YJ, Na WL, Miao J, Cheng QQ,
Ni MZ, Lei L, Fang LC, Guang RC, Jin Z and Wei L: Down-regulation
of Toll-like receptor 4 gene expression by short interfering RNA
attenuates bone cancer pain in a rat model. Mol Pain. 6:22010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luger NM, Sabino MA, Schwei MJ, Mach DB,
Pomonis JD, Keyser CP, Rahtbun M, Clohisy DR, Honore P, Yaksh TL
and Mantyh PW: Efficacy of systemic morphine suggests a fundamental
difference in the mechanisms that generate bone cancer vs
inflammatory pain. Pain. 99:397–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Appel CK, Gallego-Pedersen S, Andersen L,
Blancheflor Kristensen S, Ding M, Falk S, Sayilekshmy M,
Gabel-Jensen C and Heegaard AM: The src family kinase inhibitor
dasatinib delays pain-related behaviour and conserves bone in a rat
model of cancer-induced bone pain. Sci Rep. 7:47922017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shenoy PA, Kuo A, Khan N, Gorham L,
Nicholson JR, Corradini L, Vetter I and Smith MT: The somatostatin
receptor-4 agonist J-2156 alleviates mechanical hypersensitivity in
a rat model of breast cancer induced bone pain. Front Pharmacol.
9:4952018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen W, Walwyn W, Ennes HS, Kim H,
McRoberts JA and Marvizon JC: BDNF released during neuropathic pain
potentiates NMDA receptors in primary afferent terminals. Eur J
Neurosci. 39:1439–1454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hang LH, Li SN, Dan X, Shu WW, Luo H and
Shao DH: Involvement of spinal CCR5/PKCgamma signaling pathway in
the maintenance of cancer-induced bone pain. Neurochem Res.
42:563–571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X, Zhang L, Zhan Y, Li D, Zhang Y,
Wang G and Zhang M: Contribution of BDNF/TrkB signalling in the
rACC to the development of pain-related aversion via activation of
ERK in rats with spared nerve injury. Brain Res. 1671:111–120.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khan N, Gordon R, Woodruff TM and Smith
MT: Antiallodynic effects of alpha lipoic acid in an optimized
RR-EAE mouse model of MS-neuropathic pain are accompanied by
attenuation of upregulated BDNF-TrkB-ERK signaling in the dorsal
horn of the spinal cord. Pharmacol Res Perspect. 3:e001372015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hildebrand ME, Xu J, Dedek A, Li Y, Sengar
AS, Beggs S, Lombroso PJ and Salter MW: Potentiation of synaptic
gluN2B NMDAR currents by fyn kinase is gated through BDNF-mediated
disinhibition in spinal pain processing. Cell Rep. 17:2753–2765.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chodroff L, Bendele M, Valenzuela V, Henry
M and Ruparel S: Express: BDNF signaling contributes to oral cancer
pain in a preclinical orthotopic rodent model. Mol Pain.
12:1744806916666412016. View Article : Google Scholar
|
|
35
|
Fang X, Yang C, Li S, Zhan G, Zhang J,
Huang N, Du X, Xu H, Hashimoto K and Luo A: Brain-derived
neurotrophic factor-TrkB signaling in the medial prefrontal cortex
plays a role in the anhedonia-like phenotype after spared nerve
injury. Eur Archf Psychiatry Clin Neurosci. 7:102018.
|
|
36
|
Yao P, Ding Y, Han Z, Mu Y, Hong T, Zhu Y
and Li H: Suppression of asparaginyl endopeptidase attenuates
breast cancer-induced bone pain through inhibition of neurotrophin
receptors. Mol Pain. 13:17448069177081272017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hua Z, Gu X, Dong Y, Tan F, Liu Z, Thiele
CJ and Li Z: PI3K and MAPK pathways mediate the BDNF/TrkB-increased
metastasis in neuroblastoma. Tumour Biol. 17:2016.
|
|
38
|
Zhuang F, Li M, Gao X, Wang Y, Wang D, Ma
X, Ma T and Gu S: The antidepressant-like effect of alarin is
related to TrkB-mTOR signaling and synaptic plasticity. Behav Brain
Res. 313:158–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Y, Ji F, Wang G, He D, Yang L and
Zhang M: BDNF activates mTOR to upregulate NR2B expression in the
rostral anterior cingulate cortex required for inflammatory
pain-related aversion in rats. Neurochem Res. 43:681–691. 2018.
View Article : Google Scholar : PubMed/NCBI
|