Introduction
Interstitial lung disease (ILD), also known as
diffuse parenchymal lung disease, is common in the respiratory
system accounting for 14–16% of respiratory diseases (1,2). The
incidence rate of the disease has increased with industrialization
(3), and the cause of this disease
may be related to air pollution and viral infection, according to
the study of Salisbury et al (4). With less obvious specific symptoms in
the early stage, the disease is usually ignored by patients who
therefore miss the best treatment time (5). Lesions of ILD with complex onset are
mainly in the alveolar wall and the surrounding tissue of the
alveoli, so patients may suffer from pulmonary fibrosis if not
treated in time. Moreover, ILD even causes respiratory failure with
disease progression, posing a serious threat to the patient's life
(6,7). Therefore, it is important to choose an
effective treatment plan with few adverse reactions.
At present, ILD is symptomatically treated with
antibiotics and glucocorticoids (8).
Prednisone inhibits the aggregation of macrophages and leukocytes,
and has anti-inflammatory response and anti-stress reaction
(9). Cyclophosphamide blocks B-cell
proliferation and inhibits the antibody production, as well as
complements immunoadsorption due to its long action time, thereby
ensuring good efficacy (10).
According to a study by Reece et al (11), prednisone alone improves renal
function in the treatment of multiple myeloma, however its total
effective rate is lower than that of prednisone combined with
cyclophosphamide. As a tumor necrosis factor, widely present in
alveoli and histocytes, and an important factor in immune
mediation, TNF-α produced by macrophages and neutrophils is
abundantly expressed in the presence of pneumonia and kills
abnormal cells, which induces the release of other inflammatory
factors (12).
Currently, there are few studies on cyclophosphamide
combined with hormones for the treatment of ILD. Therefore, in the
present study, a retrospective analysis was performed on the
medical records of patients with ILD, and prednisone alone was
compared with cyclophosphamide combined with prednisone in terms of
efficacy, adverse reactions and TNF-α expression levels, before and
after treatment, in order to provide a reference for the clinical
treatment of ILD.
Patients and methods
Clinical information
A prospective analysis was performed on 198 patients
with ILD in Jinan Central Hospital Affiliated to Shandong
University (Jinan, China) from January 2010 to December 2017. In
total, 131 males and 67 females, aged 21–70 years, were included,
with an average age of 57.34±4.54 years. Among them, 101 patients
treated with cyclophosphamide combined with prednisone were
assigned in the combined treatment group, and 97 patients treated
with prednisone alone in the control group. Inclusion criteria:
Patients with early and intermediate stages of ILD who were
diagnosed by chest imaging, pulmonary ventilation and diffusion
functions, pathological biopsy; patients in the two groups with
balanced severity; patients of ≤70 years of age; patients with
complete medical records; patients who had not been diagnosed and
treated in other hospitals. Exclusion criteria: Patients allergic
to the drugs of the study; patients with other respiratory
diseases; pregnant or lactating women; patients with acute
gastrointestinal bleeding or other severe diseases; patients with
communication or cognitive disorders. All patients and their
families signed an informed consent form and cooperated with the
medical staff to complete the relevant medical treatment. The study
was approved by the Ethics Committee of Jinan Central Hospital
Affiliated to Shandong University.
Methods
Patients in the control group were treated with
prednisone, 10 mg/time and 3 times/day (Zhejiang Xianju
Pharmaceutical Co., Ltd.; SFDA approval no. H33021207) for 4
consecutive weeks. After that, the dosage was gradually reduced
according to the patient's condition. In the combined treatment
group, the patients were intravenously dripped with
cyclophosphamide for infusion, 4 mg/kg and 1 time/day (Jiangsu
Hengrui Pharmaceutical Co., Ltd.; SFDA approval no. H32020856) for
3 consecutive weeks. After that, the dosage was gradually reduced
according to the patient's condition. Both groups of patients were
treated for 12 weeks.
Spirometer (Jaeger, Ltd.) was used to detect the
forced vital capacity (FVC), the forced expiratory volume in first
second (FEV1), the diffusing capacity of lung for carbon monoxide
(DLCO) and DLCO% before and at 12 weeks after treatment. Fasting
venous blood was extracted and centrifuged at 3,000 × g for 15 min
at 4°C on admission and at 12 weeks after treatment, in order to
determine TNF-α with enzyme-linked immunosorbent assay (ELISA),
following strictly the manufacturer's instructions of TNF-α kit
(Shanghai Yuanmu Biological Technology Co., Ltd.; cat. no.
YM-QP10200). The St. George's Respiratory Questionnaire (SGRQ)
score was used to evaluate patients' quality of life with a total
score of 100 points. The higher the score, the better the activity
was. Changes in indicator levels and the incidence rate of adverse
reactions were recorded and compared between the two groups.
Criteria for efficacy evaluation
The clinical efficacy on ILD was evaluated based on
chest CT before and after treatment, referring to relevant criteria
(13). Complete remission (CR):
Target lesions partially disappeared, and the pleural edge was
regular. Partial remission (PR): Lesions had ground-glass
opacities, with reduced stripes and reticular shadows. Stable
disease (SD): No significant changes in target lesions. Progressive
disease (PD): Lesions had ground-glass opacities, with increased
stripes and reticular shadows, or lesions had honeycomb opacities.
The clinical total effective rate = (CR + PR)/(total number of
cases) ×100%.
Statistical analysis
SPSS 17.4 software (Beijing NDTimes Technology Co.,
Ltd.) was used for statistical analysis. Enumeration data were
expressed as n (%) and tested by Chi-square test. Measurement data
were expressed as the mean ± standard deviation, and t-test was
used for the differences between two groups. Paired t-test was used
for comparison of the data before and after treatment. Data among
multiple groups were compared with ANOVA and Dunnett's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of clinical
information
There were no significant differences between the
two groups in terms of sex, age, dyspnea, mucopurulent sputum,
anorexia, weakness, arthralgia in limbs, fever or alveolitis
(P>0.05). Thus, the groups were comparable (Table I).
 | Table I.Basic patient information of the
combined-treatment group and the control group [n (%)]. |
Table I.
Basic patient information of the
combined-treatment group and the control group [n (%)].
| Characteristics | Combined treatment
group (n=101) | Control group
(n=97) | χ2 | P-value |
|---|
| Sex |
|
| 0.125 | 0.724 |
| Male | 68 (67.33) | 63 (64.95) |
|
|
|
Female | 33 (32.67) | 34 (35.05) |
|
|
| Age (years) |
|
| 0.061 | 0.805 |
|
<45 | 35 (34.65) | 32 (32.99) |
|
|
| ≥45 | 66 (65.35) | 65 (67.01) |
|
|
| Dyspnea |
|
| 0.432 | 0.511 |
| Yes | 78 (77.23) | 71 (73.20) |
|
|
| No | 23 (22.77) | 26 (26.80) |
|
|
| Mucous purulent
sputum |
|
| 0.116 | 0.733 |
| Yes | 72 (71.29) | 67 (69.07) |
|
|
| No | 29 (28.71) | 30 (30.93) |
|
|
| Anorexia |
|
| 1.011 | 0.315 |
| Yes | 64 (63.37) | 68 (70.10) |
|
|
| No | 37 (36.63) | 29 (29.90) |
|
|
| Weakness |
|
| 0.322 | 0.980 |
| Yes | 73 (72.28) | 76 (78.35) |
|
|
| No | 28 (27.72) | 21 (21.65) |
|
|
| Arthralgia in
limbs |
|
| 1.175 | 0.278 |
| Yes | 69 (68.32) | 73 (75.26) |
|
|
| No | 32 (31.68) | 24 (24.74) |
|
|
| Fever |
|
| 0.615 | 0.433 |
| Yes | 58 (57.43) | 61 (62.89) |
|
|
| No | 43 (42.57) | 36 (37.11) |
|
|
| Cell type in alveolar
structure |
|
| 0.807 | 0.369 |
|
Neutrophil-type pulmonary
fibrosis | 54 (53.47) | 58 (59.79) |
|
|
|
Lymphocyte-type pulmonary
fibrosis | 47 (46.53) | 39 (40.21) |
|
|
Comparison of lung function indices
before and after treatment
Before treatment, there were no statistically
significant differences between the two groups in FVC, FEV1, DLCO
or DLCO% (P>0.05). After treatment, the patients in the combined
treatment group had significantly higher FVC and FEV1 compared with
the control group, however significantly lower DLCO and DLCO%
(P<0.05). In the combined treatment and control groups, the
patients after treatment had higher FVC and FEV1, but lower DLCO
and DLCO%, compared with before treatment (P<0.05) (Table II and Fig. 1).
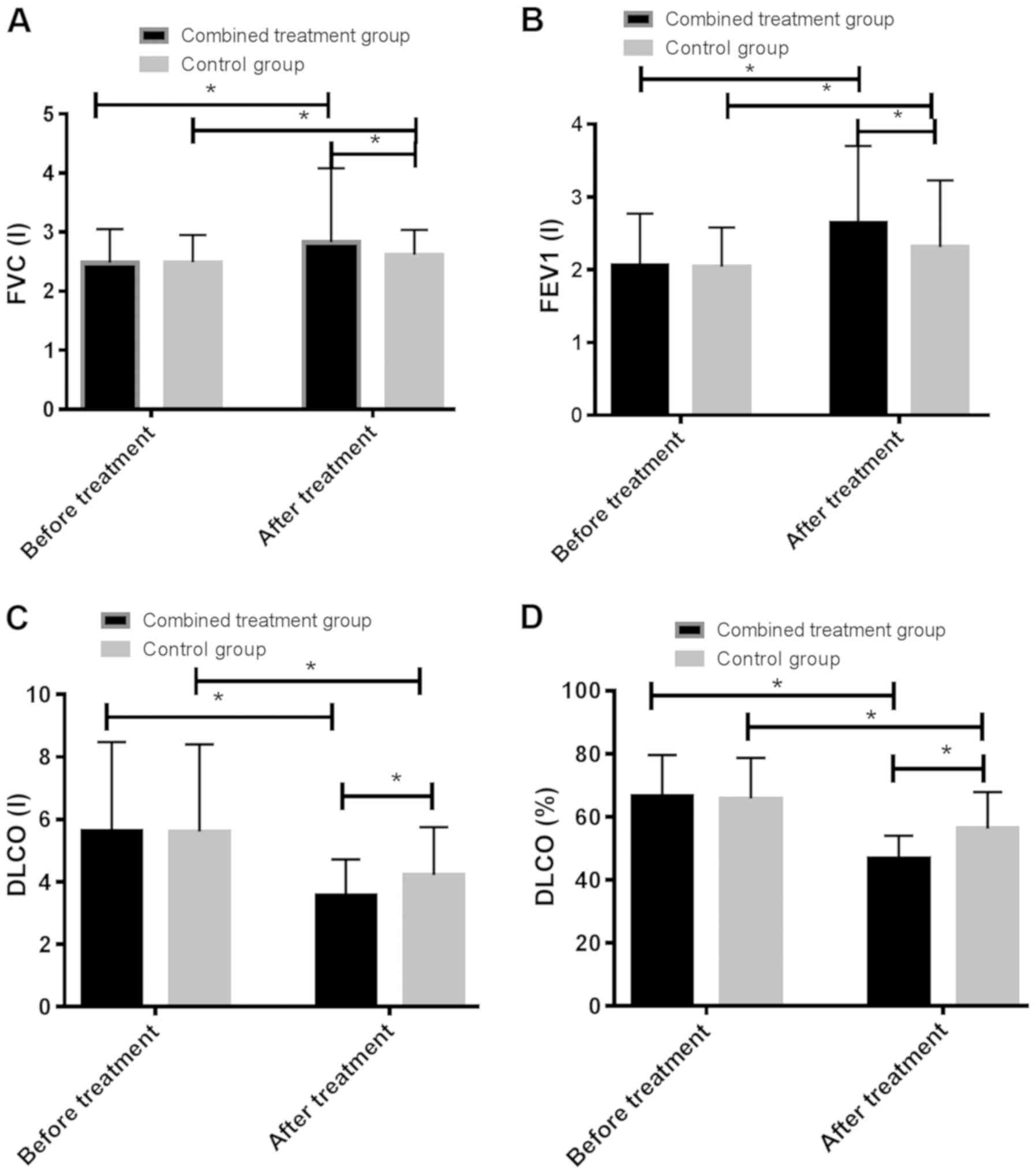 | Figure 1.Comparison of lung function indicators
before and after treatment in the combined treatment group and the
control group. (A) After treatment, the FVC index of the combined
treatment group was significantly higher than that of the control
group, and the FVC index after treatment in the two groups was
higher than that before treatment. (B) After treatment, the FEV1
index of the combined treatment group was significantly higher than
that of the control group. In the two groups, the FEV1 index after
treatment was higher than that before treatment. (C) After
treatment, the DLCO index of the combined treatment group was
significantly lower than that of the control group, and the DLCO
index after treatment in the two groups was lower than that before
treatment. (D) The DLCO% index of the combined treatment group was
significantly lower than that of the control group, and the DLCO%
index after treatment in the two groups was lower than before
treatment. *P<0.05. FVC, forced vital capacity; FEV1, forced
expiratory volume in first second; DLCO, diffusing capacity of lung
for carbon monoxide |
 | Table II.Comparison of lung function before and
after treatment between the combined treatment group and the
control group. |
Table II.
Comparison of lung function before and
after treatment between the combined treatment group and the
control group.
| Group | FVC (l) | FEV1 (l) | DLCO (l) | DLCO (%) |
|---|
| Combined treatment
group (n=101) |
| Before
treatment | 2.48±0.57 | 2.06±0.71 | 5.64±2.84 |
66.51±13.12 |
| After
treatment | 2.83±1.25 | 2.64±1.06 | 3.57±1.15 | 46.67±7.34 |
| t | 2.560 |
4.569 |
6.790 | 13.260 |
|
P-value | 0.011 | <0.001 | <0.001 | <0.001 |
| Control group
(n=97) |
| Before
treatment |
2.49±0.46a |
2.04±0.54a |
5.61±2.79a |
65.82±12.89a |
| After
treatment |
2.62±0.42b |
2.31±0.92b |
4.22±1.53b |
56.25±11.63b |
| t | 2.055 |
2.493 |
4.302 |
4.294 |
|
P-value | 0.041 |
0.014 | <0.001 | <0.001 |
Comparison of SGRQ score before and
after treatment
Before treatment, there was no statistically
significant difference between the two groups in SGRQ score
(P>0.05). However, after treatment the SGRQ score in the
combined treatment group was significantly higher than that in the
control group (P<0.05). In the combined treatment and control
groups, SGRQ scores before treatment were higher than those after
treatment (P<0.05) (Table
III).
 | Table III.Comparison of SGRQ score before and
after treatment between the combined treatment group and the
control group. |
Table III.
Comparison of SGRQ score before and
after treatment between the combined treatment group and the
control group.
| Group | Combined treatment
group (n=101) | Control group
(n=97) | t | P-value |
|---|
| Before
treatment | 62.38±13.27 |
61.75±12.86 | 0.339 |
0.735 |
| After
treatment | 46.84±11.81 | 35.51±9.57 | 7.399 | <0.001 |
| t |
8.792 | 16.12 |
|
|
|
P-value | <0.001 |
<0.001 |
|
|
Comparison of efficacy before and
after treatment
There were no statistically significant differences
in patients with PR or PD between the combined treatment and
control groups (both P>0.05). Compared with the control group,
the combined treatment group had significantly more patients with
CR and significantly higher total effective rate, but less patients
with SD (P<0.05) (Table IV).
 | Table IV.Comparison of treatment efficacy
before and after treatment between the combined treatment group and
the control group [n (%)]. |
Table IV.
Comparison of treatment efficacy
before and after treatment between the combined treatment group and
the control group [n (%)].
| Treatment
outcome | Combined treatment
group (n=101) | Control group
(n=97) | χ2 | P-value |
|---|
| CR | 51 (50.50) | 35 (36.08) | 4.183 | 0.041 |
| PR | 35 (34.65) | 33 (34.02) | 0.009 | 0.925 |
| SD | 14 (13.86) | 25 (25.77) | 4.439 | 0.035 |
| PD | 1
(1.0) | 4
(4.12) | 1.974 | 0.160 |
| Total effective
rate | 86 (85.15) | 68 (70.10) | 6.480 | 0.011 |
Comparison of adverse reactions before
and after treatment
The combined treatment group had less patients with
gastrointestinal reactions, hyperglycemia and chemical cystitis
than the control group (P<0.05) (Table V).
 | Table V.Comparison of adverse reactions
before and after treatment between the combined treatment group and
the control group [n (%)]. |
Table V.
Comparison of adverse reactions
before and after treatment between the combined treatment group and
the control group [n (%)].
| Adverse
reaction | Combined treatment
group (n=101) | Control group
(n=97) | χ2 | P-value |
|---|
| Gastrointestinal
reaction | 6 (5.94) | 14 (14.43) | 3.930 | 0.047 |
| Elevated blood
glucose | 2 (1.98) | 8
(8.25) | 4.053 | 0.044 |
| Chemical
cystitis | 1 (1.0) | 6
(6.19) | 3.916 | 0.048 |
| Others | 4 (3.96) | 12 (12.37) | 4.712 | 0.030 |
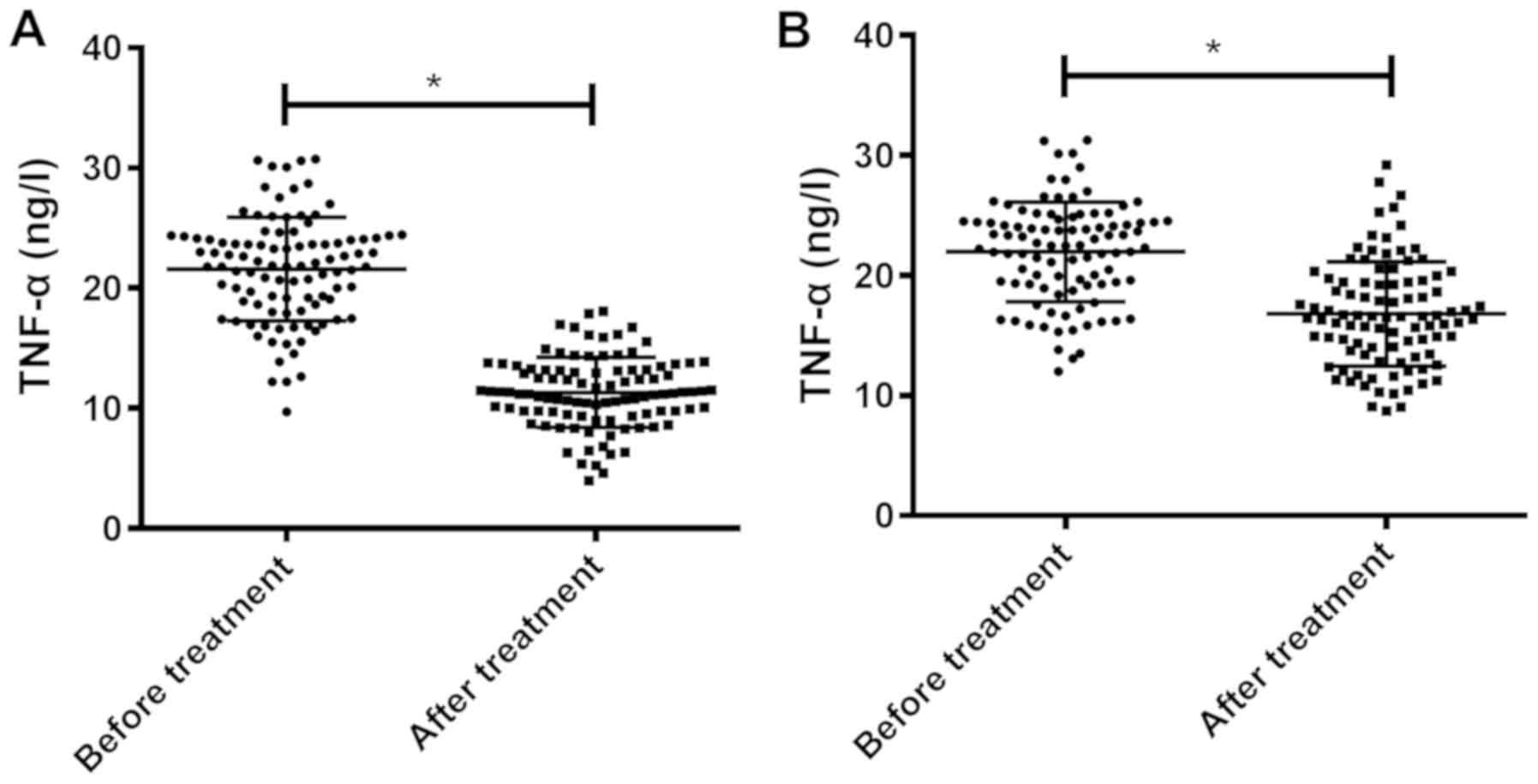
Comparison of TNF-α expression level
before and after treatment
In the combined treatment group, TNF-α expression
levels before and after treatment were 21.83±4.22 and 11.56±3.26
ng/l, respectively, and in the control group were 22.14±4.53 and
16.13±4.15 ng/l, respectively. Before treatment, there was no
statistically significant difference between the two groups in
TNF-α expression level (P>0.05). However, the TNF-α expression
in the combined treatment group after treatment was significantly
lower than that in the control group (P<0.05). In the combined
treatment and control groups, TNF-α expression levels before
treatment were higher than those after treatment (P<0.05)
(Table VI and Fig. 2).
 | Table VI.Changes in blood TNF-α levels in
patients before and after treatment in the combined treatment group
and the control group (ng/l). |
Table VI.
Changes in blood TNF-α levels in
patients before and after treatment in the combined treatment group
and the control group (ng/l).
| Group | Combined treatment
group (n=101) | Control group
(n=97) | t | P-value |
|---|
| Before
treatment | 21.83±4.22 | 22.14±4.53 | 0.498 | 0.619 |
| After
treatment | 11.56±3.26 | 16.13±4.15 | 8.635 | <0.001 |
| t |
4.079 |
4.264 |
|
|
| P-value | <0.001 | <0.001 |
|
|
Discussion
Heterogeneous ILD has complex causes, so its
pathogenesis remains unclear (14).
The disease has no special symptoms in the early stage, so it is
diagnosed through etiology, pathological manifestations and imaging
features. As a result, most patients are in the advanced stage of
irreversible pulmonary fibrosis when diagnosed. In the advanced
stage of ILD, inflammation spreads to blood vessels and the
interstitium, destroys the lung tissue and leads to pulmonary
fibrosis, which damages the lung function, increases the difficulty
of treatment and causes patient death (15). With the advancement of modern
medicine, ILD is controlled but difficult to cure, with high
incidence and mortality rates and a long treatment cycle (16). Therefore, timely drug treatment is
the key to control the deterioration of the disease. ILD is
currently treated based on anti-pulmonary fibrosis and
anti-inflammation.
In the present study, a prospective analysis was
performed on 198 patients with ILD in Jinan Central Hospital
Affiliated to Shandong University from January 2010 to December
2017. Patients in the combined treatment and control groups were
compared in terms of efficacy, adverse reactions and TNF-α
expression level, before and after treatment. The results of lung
function tests, efficacy and SGRQ score before and after treatment
in the combined treatment group were better than those in the
control group. Anti-inflammatory and anti-allergic prednisone
regulates protein biosynthesis and metabolism, reduces connective
tissue proliferation and inflammatory exudation, and inhibits
histamine formation and release (17). The inflammatory state of advanced ILD
is less obvious, however, the lung becomes gradually fibrotic with
disease progression, so anti-fibrotic therapy is necessary for the
patients (18). According to a study
(19), the efficacy of hormones is
not significant on systemic sclerosis-associated ILD, so the
disease is currently treated with prednisone combined with
cyclophosphamide. Cyclophosphamide treats autoimmune diseases, and
restricts the transformation of viruses into immunoblasts through
non-specifically killing small lymphocytes (20,21).
According to a study by Mok (22),
cyclophosphamide combined with prednisone in the treatment of lupus
nephritis was shown to have a high total effective rate, suggesting
that the combination treatment improves the patient results of lung
function tests and quality of life, which further supports the
results of this study. In the present study, patients with
gastrointestinal reactions, hyperglycemia and chemical cystitis in
the combined treatment group were less than those in the control
group. Prednisone leads to hyperglycemia through promoting protein
to convert into sugar, and gastrointestinal reactions and other
adverse reactions through promoting gastric secretion (23). Cyclophosphamide interferes with the
production of DNA and RNA, and cross-links with the former, thereby
inhibiting the immune response, proliferation and division of
immune lymphocytes, and blocking immune complex deposition, so as
to treat diseases. Due to fewer adverse reactions, cyclophosphamide
has been widely used in the treatment of lymphatic systemic and
autoimmune diseases (24). According
to a study by Mulvenna et al (25), non-small cell lung cancer weakens
lung function, and the high incidence rate of adverse reactions
after treatment with prednisone reduces the immune function of the
body, and therefore adverse reactions occur easily. In comparison
of TNF-α expression levels before treatment, there was no
statistically significant difference between the two groups,
whereas after treatment, TNF-α expression level was significantly
lower in the combined treatment group than that in the control
group. TNF-α mediates the expression of inflammatory factors,
aggravates inflammatory responses and proliferates fibroblasts. A
large amount of collagen secretion causes the occurrence and
development of pulmonary fibrosis, which plays a key role in
respiratory diseases. Therefore, the combined treatment reduces
TNF-α expression level (26).
In this investigation, due to the small number of
patients with ILD in Jinan Central Hospital Affiliated to Shandong
University, the sample size is small, so there may be contingency
in the results. Therefore, a longer-term follow-up survey will be
conducted in the future.
In conclusion, cyclophosphamide combined with
prednisone is effective and safe in the treatment of ILD, without
severe adverse reactions and reducing the TNF-α expression level,
and therefore is worthy of clinical promotion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL performed ELISA and wrote the manuscript. XC and
YQ collected and interpreted the general data of the patients and
were responsible for the statistical analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Jinan Central Hospital Affiliated to Shandong University (Jinan,
China). Patients who participated in this research, signed an
informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hagmeyer L, Theegarten D, Wohlschläger J,
Treml M, Matthes S, Priegnitz C and Randerath WJ: The role of
transbronchial cryobiopsy and surgical lung biopsy in the
diagnostic algorithm of interstitial lung disease. Clin Respir J.
10:589–595. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anthimopoulos M, Christodoulidis S, Ebner
L, Christe A and Mougiakakou S: Lung pattern classification for
interstitial lung diseases using a deep convolutional neural
network. IEEE Trans Med Imaging. 35:1207–1216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Douglas WW, Tazelaar HD, Hartman TE,
Hartman RP, Decker PA, Schroeder DR and Ryu JH:
Polymyositis-dermatomyositis-associated interstitial lung disease.
Am J Respir Crit Care Med. 164:1182–1185. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salisbury ML, Xia M, Murray S, Bartholmai
BJ, Kazerooni EA, Meldrum CA, Martinez FJ and Flaherty KR:
Predictors of idiopathic pulmonary fibrosis in absence of
radiologic honeycombing: A cross sectional analysis in ILD patients
undergoing lung tissue sampling. Respir Med. 118:88–95. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johannson KA, Marcoux VS, Ronksley PE and
Ryerson CJ: Diagnostic yield and complications of transbronchial
lung cryobiopsy for interstitial lung disease. A systematic review
and metaanalysis. Ann Am Thorac Soc. 13:1828–1838. 2016.PubMed/NCBI
|
|
6
|
Solomon JJ, Chung JH, Cosgrove GP,
Demoruelle MK, Fernandez-Perez ER, Fischer A, Frankel SK, Hobbs SB,
Huie TJ, Ketzer J, et al: Predictors of mortality in rheumatoid
arthritis-associated interstitial lung disease. Eur Respir J.
47:588–596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mathai SC and Danoff SK: Management of
interstitial lung disease associated with connective tissue
disease. BMJ. 352:h68192016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wallace B, Vummidi D and Khanna D:
Management of connective tissue diseases associated interstitial
lung disease: A review of the published literature. Curr Opin
Rheumatol. 28:236–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park SI, Felipe CR, Pinheiro-Machado PG,
Garcia R, Fernandes FB, Casarini DE, Tedesco-Silva H Jr and
Medina-Pestana JO: Tacro-limus pharmacokinetic drug interactions:
Effect of prednisone, mycophenolic acid or sirolimus. Fundam Clin
Pharmacol. 23:137–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eichhorst B, Fink AM, Bahlo J, Busch R,
Kovacs G, Maurer C, Lange E, Köppler H, Kiehl M, Sökler M, et al
international group of investigators; German CLL Study Group
(GCLLSG), : First-line chemoimmunotherapy with bendamustine and
rituximab versus fludarabine, cyclophosphamide, and rituximab in
patients with advanced chronic lymphocytic leukaemia (CLL10): An
international, open-label, randomised, phase 3, non-inferiority
trial. Lancet Oncol. 17:928–942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reece DE, Trieu Y, Masih-Khan E, Atenafu
EG, Chen C, Prica A, Tiedemann R, Trudel S and Kukreti V:
Cyclophosphamide and bortezomib with prednisone or dexamethasone
for the treatment of relapsed and refractory multiple myeloma. Clin
Lymphoma Myeloma Leuk. 16:387–394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He QQ, He X, Wang YP, Zou Y, Xia QJ, Xiong
LL, Luo CZ, Hu XS, Liu J and Wang TH: Transplantation of bone
marrow- derived mesenchymal stem cells (BMSCs) improves brain
ischemia-induced pulmonary injury in rats associated to TNF-α
expression. Behav Brain Funct. 12:92016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryerson CJ, Cayou C, Topp F, Hilling L,
Camp PG, Wilcox PG, Khalil N, Collard HR and Garvey C: Pulmonary
rehabilitation improves long-term outcomes in interstitial lung
disease: A prospective cohort study. Respir Med. 108:203–210. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trudzinski FC, Kaestner F, Schäfers HJ,
Fähndrich S, Seiler F, Böhmer P, Linn O, Kaiser R, Haake H, Langer
F, et al: Outcome of patients with interstitial lung disease
treated with extracorporeal membrane oxygenation for acute
respiratory failure. Am J Respir Crit Care Med. 193:527–533. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Volkmann ER and Tashkin DP: Treatment of
systemic sclerosis-related interstitial lung disease: A review of
existing and emerging therapies. Ann Am Thorac Soc. 13:2045–2056.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hutchinson JP, Fogarty AW, McKeever TM and
Hubbard RB: In-hospital mortality after surgical lung biopsy for
interstitial lung disease in the United States. 2000 to 2011. Am J
Respir Crit Care Med. 193:1161–1167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen H, Zhong JM, Yi ZS, Zha J, Chen Y and
Cai LY: Immunological mechanism of prednisone in the treatment of
infantile spasm. Zhongguo Dang Dai Er Ke Za Zhi. 19:1044–1050.
2017.(In Chinese). PubMed/NCBI
|
|
18
|
Witt LJ, Demchuk C, Curran JJ and Strek
ME: Benefit of adjunctive tacrolimus in connective tissue
disease-interstitial lung disease. Pulm Pharmacol Ther. 36:46–52.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kundu S, Paul S, Hariprasath K, Agarwal R,
Ghosh S and Biswas D: Effect of sequential intravenous pulse
cyclophosphamide-azathioprine in systemic sclerosis-interstitial
lung disease: An open-label study. Indian J Chest Dis Allied Sci.
58:7–10. 2016.PubMed/NCBI
|
|
20
|
Yunyun F, Yu C, Panpan Z, Hua C, Di W,
Lidan Z, Linyi P, Li W, Qingjun W, Xuan Z, et al: Efficacy of
cyclophosphamide treatment for immunoglobulin G4-related disease
with addition of glucocorticoids. Sci Rep. 7:61952017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Daillère R, Vétizou M, Waldschmitt N,
Yamazaki T, Isnard C, Poirier-Colame V, Duong CPM, Flament C,
Lepage P, Roberti MP, et al: Enterococcus hirae and
Barnesiella intestinihominis facilitate
cyclophosphamide-induced therapeutic immunomodulatory effects.
Immunity. 45:931–943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mok CC: Con: Cyclophosphamide for the
treatment of lupus nephritis. Nephrol Dial Transplant.
31:1053–1057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gerards MC, Venema GE, Patberg KW, Kross
M, Potter van Loon BJ, Hageman IMG, Snijders D, Brandjes DPM,
Hoekstra JBL, Vriesendorp TM, et al: Dapagliflozin for
prednisone-induced hyperglycaemia in acute exacerbation of chronic
obstructive pulmonary disease. Diabetes Obes Metab. 20:1306–1310.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tashkin DP, Roth MD, Clements PJ, Furst
DE, Khanna D, Kleerup EC, Goldin J, Arriola E, Volkmann ER, Kafaja
S, et al Sclerodema Lung Study II Investigators, : Mycophenolate
mofetil versus oral cyclophosphamide in scleroderma-related
interstitial lung disease (SLS II): A randomised controlled,
double-blind, parallel group trial. Lancet Respir Med. 4:708–719.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mulvenna P, Nankivell M, Barton R,
Faivre-Finn C, Wilson P, McColl E, Moore B, Brisbane I, Ardron D,
Holt T, et al: Dexamethasone and supportive care with or without
whole brain radiotherapy in treating patients with non-small cell
lung cancer with brain metastases unsuitable for resection or
stereotactic radiotherapy (QUARTZ): Results from a phase 3,
non-inferiority, randomised trial. Lancet. 388:2004–2014. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang AX, Lu LW, Liu WJ and Huang M:
Plasma inflammatory cytokine IL-4, IL-8, IL-10, and TNF-α levels
correlate with pulmonary function in patients with asthma - chronic
obstructive pulmonary disease (COPD) overlap syndrome. Med Sci
Monit. 22:2800–2808. 2016. View Article : Google Scholar : PubMed/NCBI
|