Introduction
In the past decade, one of the most noteworthy
discoveries in the field of biology is that non-protein-coding RNAs
(ncRNAs) have crucial roles in human diseases (1). MicroRNAs (miRNAs or miRs) (2), long non-coding RNAs (lncRNAs) (3), transfer RNA-derived small RNA (4) and circular RNAs (5) have been indicated to be up- or
downregulated in multiple types of cancer and to be associated with
the prognosis of affected patients. Among these ncRNAs, lncRNAs
have attracted an increased amount of attention. lncRNAs have
important roles in regulating cancer cell apoptosis (6), metastasis (7), autophagy (8), angiogenesis (9) and epithelial-to-mesenchymal transition
(EMT) (10). lncRNAs are able to
interact with proteins. For instance, lncRNA EPIC1 binds to MYC to
promote the expression of cell cycle regulators (11). lncRNAs have been demonstrated to
function as miRNA sponges and to be involved in
post-transcriptional regulation of gene expression. For instance,
lncRNA activated by transforming growth factor-β competitively
sponges miR-200 to activate the expression of zinc finger E-box
binding homeobox 1 (ZEB1) and ZEB2 during EMT (12). lncRNAs have also been indicated to
interact with chromatin. For instance, nuclear enriched abundant
transcript 1 (NEAT1) is a regulator of nuclear paraspeckles, which
has been reported to bind to the promoters of a series of targets
in prostate cancer (13). However,
the functions of the majority of lncRNAs in cancer have remained
elusive.
Non-small cell lung cancer (NSCLC) constitutes ~80%
of all lung cancer cases and is the most common type of human lung
cancer. There is evidence to indicate that ncRNAs have key roles in
the tumorigenesis and progression of lung cancer. lncRNAs may
function as either oncogenes or tumor suppressors in lung cancer.
For instance, lncRNA MIR4435-2 host gene (HG) has been reported to
promote lung cancer progression by activating β-catenin signaling
(14). lncRNA myocardial infarction
associated transcript has been indicated to promote NSCLC
proliferation and metastasis by inducing matrix metalloproteinase 9
expression (15). MIR22HG has a
tumor-suppressive role in lung cancer by regulating Y-box binding
protein 1, MET and p21 (16).
However, lncRNA-low expression in tumor (LET) inhibits NSCLC cell
migration and invasion (17). Growth
arrest specific 5-antisense 1 (AS1) has also been indicated to
suppress NSCLC metastasis by reducing ZEB1, N-cadherin, vimentin
and/or Snail1 (18). Furthermore,
certain lncRNAs have been reported to be potential biomarkers for
NSCLC. For instance, LET is downregulated in NSCLC and low
expression of LET is associated with a more favorable prognosis
(19). The exploration of the
functions of lncRNAs in NSCLC may provide potential diagnostic and
prognostic markers for NSCLC.
Transducer of ERBB2, 1 (TOB1) is a well-known tumor
suppressor, which exerts its roles by negatively regulating the
activity of receptor tyrosine-kinase ERBB2 (20). TOB1 was indicated to be downregulated
in multiple human cancer types, including liver cancer (21), breast cancer (22) and lung cancer (23). For instance, a previous study
reported that overexpression of TOB1 significantly suppresses lung
cancer cell proliferation and metastasis (23). TOB1 was demonstrated to suppress
cancer cell proliferation and metastasis via a series of downstream
regulators, including cyclin D1, AKT signaling, BCL-2, BCL-XL and
SMAD4 (22,24–26).
LncRNA TOB1-AS1 originates from the TOB1 gene cluster. A previous
study suggested that TOB1-AS1 acts as a tumor suppressor in
cervical cancer (27). TOB1-AS1
inhibits cervical cancer cell proliferation, cell cycle and
metastasis through interacting with miR-27b (27). However, the roles of TOB1-AS1 in lung
cancer have remained largely elusive.
In the present study, lncRNA TOB1-AS1 was identified
to be downregulated in NSCLC samples by analyzing public datasets.
Bioinformatics analysis and experimental validation were performed
to reveal the potential functions of TOB1-AS1 in NSCLC cells. The
results of the present study demonstrate that TOB1-AS1 may be a
potential biomarker for NSCLC.
Materials and methods
Cell lines and transfection
A549 and H1299 cells were obtained from the American
Type Culture Collection. The A549 and H1299 cells were cultured in
RPMI-1640 medium (HyClone; GE Healthcare) containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.). The NSCLC
cells were cultured at 37°C in a humidified incubator with 5%
CO2. Small interfering RNA (siRNA) targeting TOB1-AS1
(si-TOB1-AS1) and non-specific control siRNA (si-NC) were
synthesized by GenePharma Co., Ltd. and transfected into cells by
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.). The sequences of si-TOB1-AS1 were as follows: si-RNA1,
5′-GCACCCGATTAATTGAATA-3′; si-RNA2, 5′-GCGACTCGGATCCGTTTAT-3′;
si-NC, 5′-TTCTCCGAACGTGTCACGT-3′. The knockdown efficacy was
determined by reverse transcription-quantitative (RT-q) PCR. Cells
were harvested 24 h following transfection for use in the
subsequent experiments.
RT-qPCR
For detection of the subcellular localization of
TOB1-AS1 in NSCLC cells, cytoplasmic and nuclear extracts were
prepared using NE-PER™ nuclear and cytoplasmic extraction reagents
(Pierce; Thermo Fisher Scientific, Inc.). Then, total cytoplasmic
and nuclear RNA were extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). Subsequently, complementary (c)DNA
was synthesized using the RevertAid First Strand cDNA Synthesis kit
(Promega Corporation) in accordance with the manufacturer's
protocol. qPCR was performed using iQ™ SYBR-Green Supermix (Bio-Rad
Laboratories, Inc.). The following primers were used for qPCR:
TOB1-AS1 forward, 5′-GCCAGGCCTAGAAGCTTTTG-3′ and reverse,
5′-TCTTCCCACCCCTTCTCCTA-3′; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′, dynein cytoplasmic 2 light
intermediate chain 1 (DYNC2LI1) forward, 5′-TGAACCACCAAAACCAACCT-3′
and reverse, 5′-TGGTGTGTTGTGCCCTTTTG-3′; E4F transcription factor 1
(E4F1) forward, 5′-GTTGCTGGGCCAGGAGG-3′ and reverse,
5′-GATGTCTGCTGCCAGAGAGG-3′; TSPY-like 4 (TSPYL4) forward,
5′-CTGGCTGTGTCCTGATACCC-3′ and reverse, 5′-CCAATTTGGGTTGATGGCCG-3′;
component of oligomeric Golgi complex 7 (COG7) forward,
5′-GAATGCAACTTGCTGCCGAA-3′ and reverse, 5′-AGCTTGGCAGAAATCACAGC-3′,
inositol hexakisphosphate kinase 2 (IP6K2) forward,
5′-AGACCCCTAAGGACTGGGTG-3′ and reverse,
5′-ACAAGACTTCAGATTTCTTTAGCCA-3′; Deltex E3 Ubiquitin Ligase 3
(DTX3) forward, 5′-TTCCATGGGCTTTCCAGGTC-3′ and reverse,
5′-GCCATTCTGGACAGGACGAA-3′. The 2−ΔΔCq method was used
to calculate the relative expression levels (28).
Cell migration and invasion assay
The cell migration and invasion assays were
performed as previously described (29).
Cell proliferation assay
The Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) was used to examine cell proliferation. All
procedures were performed according to the manufacturer's
protocols. The absorbance at 450 nm was measured and the absorbance
at 615 nm was used as a reference using a microplate reader (BioTek
Instruments, Inc.).
Public database analysis
In the present study, the expression levels of
TOB1-AS1 in lung adenocarcinoma (LUAD), lung squamous cell
carcinoma (LUSC) and normal lung tissues were evaluated by using
Gene Expression Profiling Interactive Analysis (GEPIA) databases
(http://gepia.cancer-pku.cn/index.html) (30). Informed consent had been obtained
from all lung cancer patients (31).
LncATLAS dataset (http://lncatlas.crg.eu/) was used to evaluate the
subcellular localization of TOB1-AS1 in A549, HeLa and K562 cells
(32).
Kaplan-Meier plotter database
analysis
The Kaplan-Meier plotter online database (http://kmplot.com/analysis/index.php?p=service&cancer=lung)
(33) was used to assess the
prognostic significance of TOB1-AS1 expression in lung cancer. In
order to evaluate the prognostic value of TOB1-AS1, the patient
samples were divided into two groups: High expression and low
expression, on the basis of the median expression of TOB1-AS1 (high
expression, top 50%; low expression, bottom 50%). The Kaplan-Meier
survival plots were obtained by entering the survival time [overall
survival (OS) and post-progression survival (PPS; PPS was recorded
as the time from tumor progression until death or was censored on
the date of the last follow-up consultation)] (34) of patients with NSCLC. The P-values of
the log-rank test and hazard ratio with 95% confidence intervals
were also calculated.
Construction of ceRNA networks
First, interactions between miRNA and mRNA, and
between miRNA and lncRNA were obtained from StarBase v2.0
(http://starbase.sysu.edu.cn/) (35) and Targetscan v7.1 database
(http://www.targetscan.org/vert_71/)
(36), which were used to
comprehensively analyze miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale crosslinking
immunoprecipitation-sequencing data. Subsequently, the ceRNA
networks were generated using Cytoscape Software (version 3.6.0;
http://www.cytoscape.org) (37).
Statistical analysis
Data analysis was performed using SPSS 24.0 software
(IBM Corp.) and figures were created using GraphPad Prism 7
software (GraphPad Software, Inc.). Statistical comparisons between
groups of normalized data were performed using the Student's t-test
according to the test conditions. One-way analysis of variance
followed by the Newman-Keuls post-hoc test was used to identify the
differences among multiple groups. P<0.05 was considered to
indicate a statistically significant difference with a 95%
confidence level.
Results
TOB1-AS1 is downregulated in
NSCLC
The expression levels of TOB1-AS1 in NSCLC and
normal tissues have so far remained elusive. The public dataset
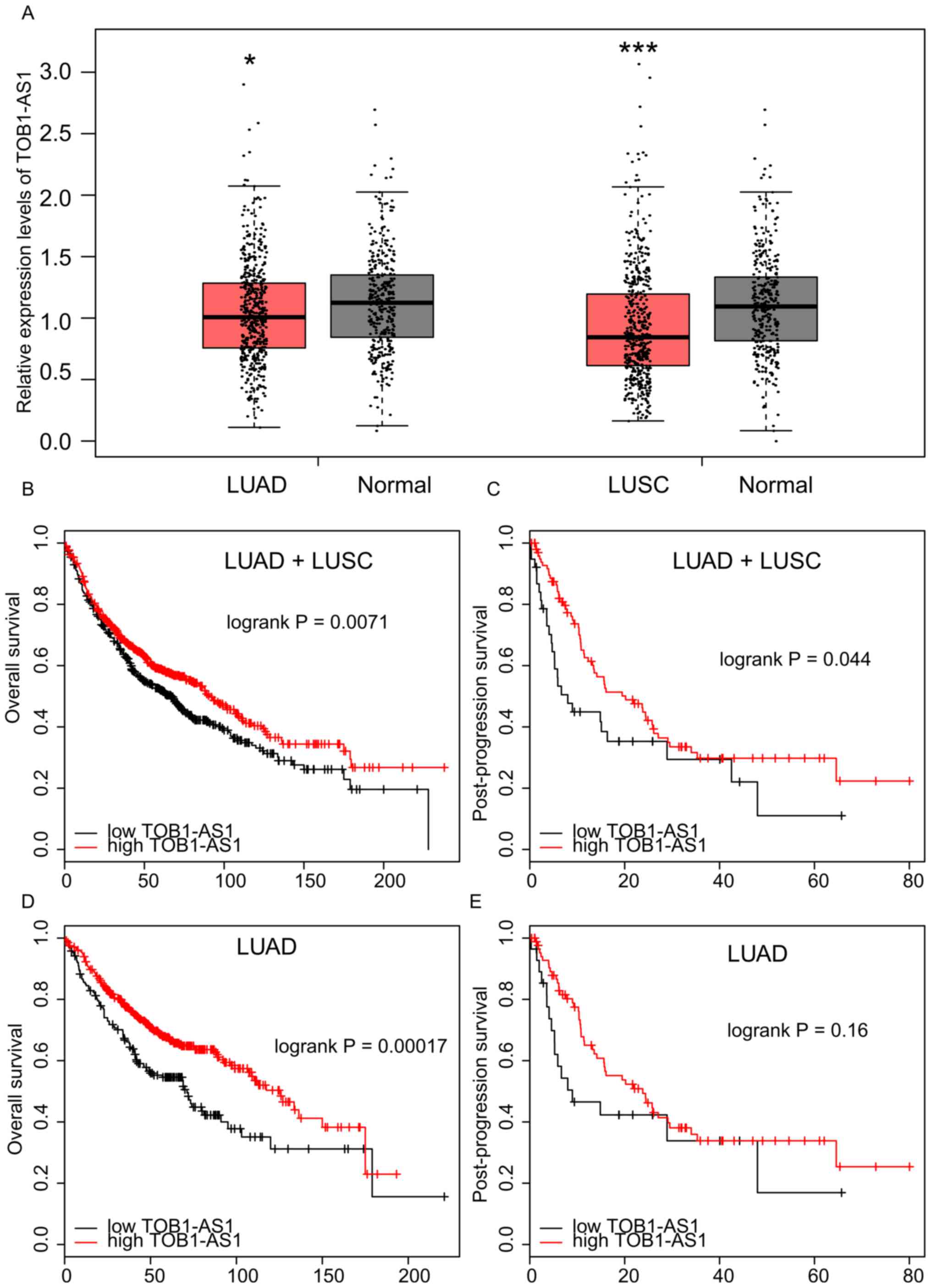
GEPIA was used in the present study. As presented in Fig. 1A, the results revealed that TOB1-AS1
was significantly downregulated in LUAD and lung squamous cell
carcinoma (LUSC) compared with the matched normal samples.
Higher expression of TOB1-AS1 is
associated with a better prognosis in NSCLC
The association between TOB1-AS1 expression and OS
time or PPS time in NSCLC was analyzed using the Kaplan-Meier
Plotter database. As presented in Fig.
1B and C, it was observed that NSCLC patients with a higher
expression of TOB1-AS1 had a longer OS and PPS time than those with
low expression. Furthermore, a higher expression of TOB1-AS1 in
LUAD was associated with a longer OS time (Fig. 1D). However, no significant
associations were observed between TOB1-AS1 expression and PPS time
in the LUAD patients (Fig. 1E) or
between TOB1-AS1 expression and OS or PPS time in the LUSC samples
(data not shown).
TOB1-AS1 is localized in the cytoplasm
and nucleus
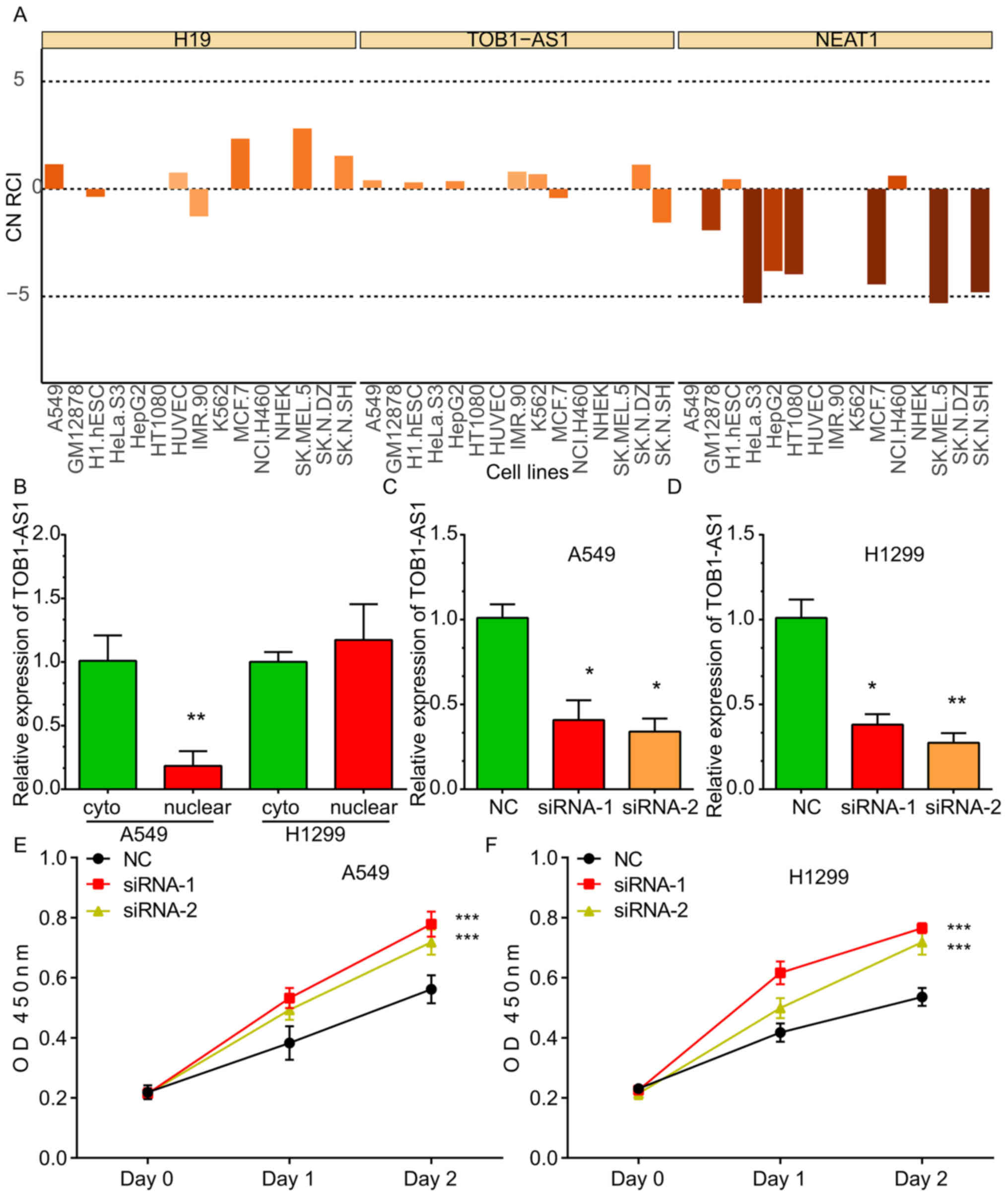
By analyzing the LncATLAS dataset, it was indicated
that TOB1-AS1 was localized in the cytoplasm and nucleus of the
majority of the human cancer cell lines, including A549, HeLa and
K562 cells. The expression pattern of H19 located in the cytoplasm
and that of NEAT1 located in the nuclei of cells were used as
controls (Fig. 2A). Furthermore,
RT-qPCR was used to detect the subcellular location of TOB1-AS1. As
presented in Fig. 2, the TOB1-AS1
levels in the cytoplasm were higher compared with the nucleus in
A549 cells, whereas TOB1-AS1 levels in the cytoplasm were
comparable with that in the nucleus in H1299 cell lines (Fig. 2B).
In order to explore the molecular roles of TOB1-AS1
in NSCLC, loss-of-function assays were performed in the A549 and
H1299 cells. For this, two siRNAs against TOB1-AS1 were designed,
which effectively reduced the expression of TOB1-AS1 in the A549
(Fig. 2C) and H1299 (Fig. 2D) cells.
TOB1-AS1 knockdown promotes NSCLC cell
proliferation
To investigate the effects of TOB1-AS1 knockdown on
the proliferation of NSCLC cells, CCK-8 assays were performed. The
results revealed that the proliferation rate of the cells
transfected with si-TOB1-AS1 was significantly upregulated compared
with that in the control group of A549 (Fig. 2E) and H1299 cells (Fig. 2F).
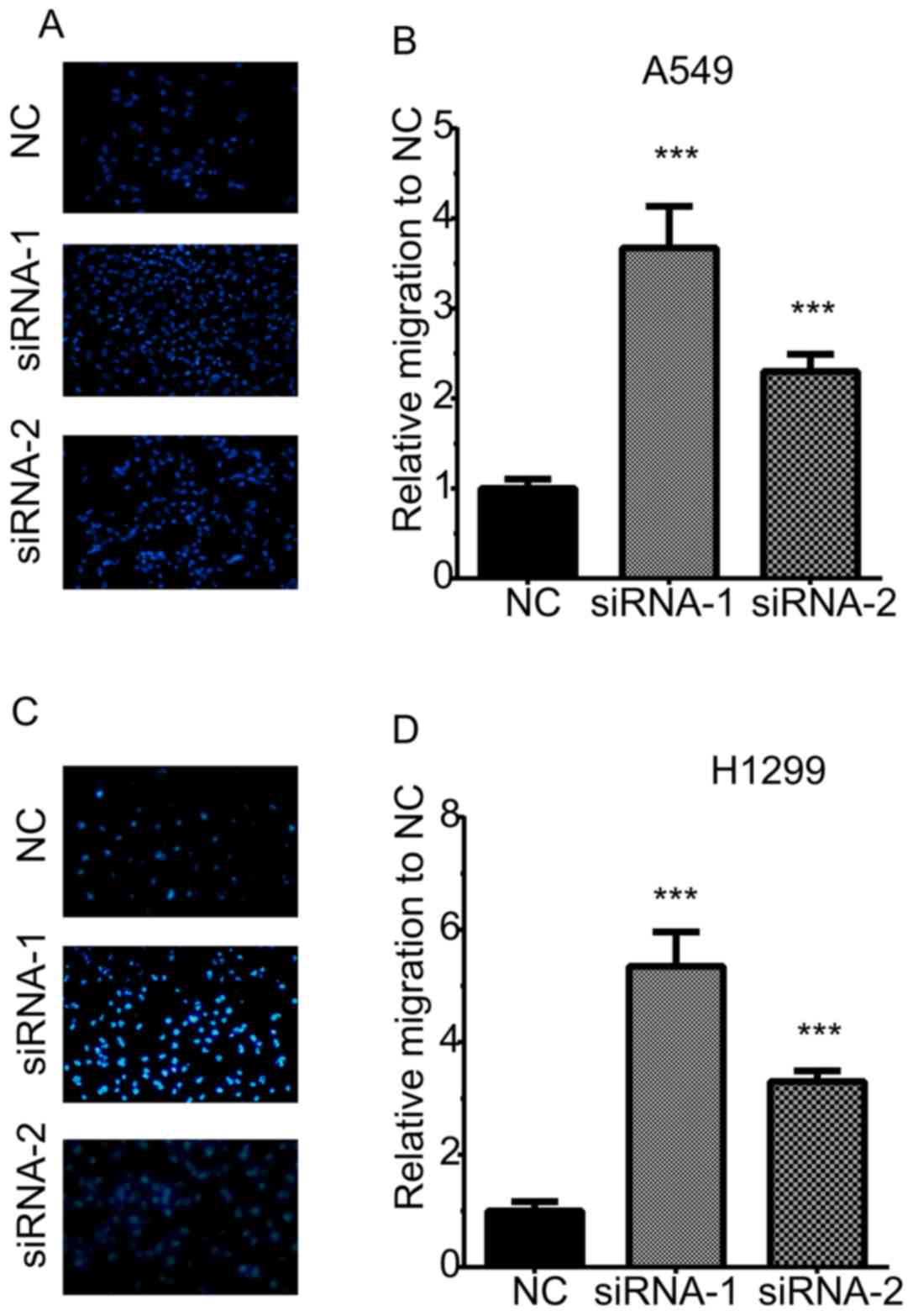
TOB1-AS1 knockdown promotes NSCLC cell
migration and invasion
A Transwell assay was then performed to determine
the effects of TOB1-AS1 knockdown on cell migration (Fig. 3). The results revealed that the
numbers of migrating cells in the TOB1-AS1 knockdown group were
significantly increased by 3.67- and 2.3-fold of those in the
control group of A549 cells (Fig.
3A,B). Furthermore, in H1299 cells, TOB1-AS1 knockdown promoted
cell migration by 5.3- and 3.3-fold of that in the control group
(Fig. 3C,D).
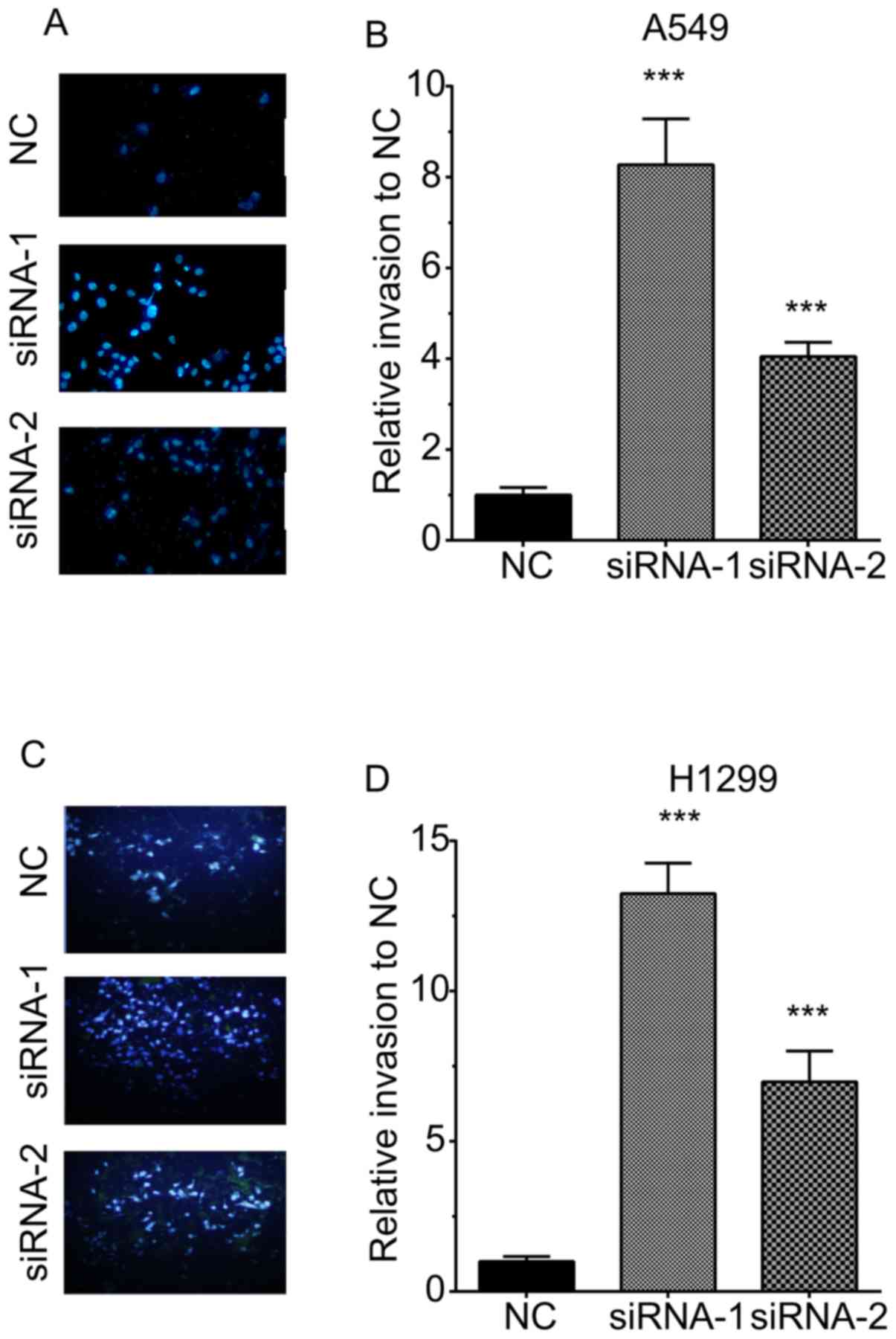
The invasive ability of H1299 cells was also
detected by quantifying the cells transgressing through Matrigel in
a Transwell chamber. As illustrated in Fig. 3, TOB1-AS1 knockdown promoted cell
invasion. The numbers of invasive cells were increased by 8.3- and
4.1-fold in A549 cells transfected with si-TOB1-AS1 compared with
those in the negative control group (Fig. 4A,B). The numbers of invasive cells
were increased by 13.25- and 6.98-fold in H1299 cells transfected
with si-TOB1-AS1-1 and si-TOB1-AS1-2, respectively, in comparison
with those in the negative control group (Fig. 4C,D).
Construction of TOB1-AS1-mediated
ceRNA network in NSCLC
In order to elucidate the mechanisms of action of
TOB1-AS1 regarding NSCLC progression, a TOB1-AS1-mediated ceRNA
network in NSCLC was constructed. The co-expressed genes with a
Pearson's score of >0.4 were selected as targets of TOB1-AS1.
The Starbase and Targetscan databases were used to predict the
potential miRNAs mediating the regulatory interaction between
TOB1-AS1 and its targets. As presented in Fig. 5, a total of 5 miRNAs [Homo
sapiens (hsa)-miR-27a-3p, hsa-miR-23a-3p, hsa-miR-23b-3p,
hsa-miR-27b-3p and hsa-miR-23c] and 6 mRNAs (DYNC2LI1, E4F1,
TSPYL4, COG7, IP6K2 and DTX3) were included in this network
(Fig. 5A). In addition, the
expression levels of these 6 mRNAs were measured after the
silencing of TOB1-AS1 in A549 cells. The results indicated that
knockdown of TOB1-AS1 using siRNA-1 significantly suppressed the
expression of DYNC2LI1, E4F1, TSPYL4, COG7 and IP6K2 (Fig. 5B). By analyzing the GEPIA dataset,
the expression of TOB1-AS1 in NSCLC was found to be positively
correlated with DTX3, TSPYL4, E4F1, IP6K2, COG7 and DYNC2LI1
(Fig. 5C-H).
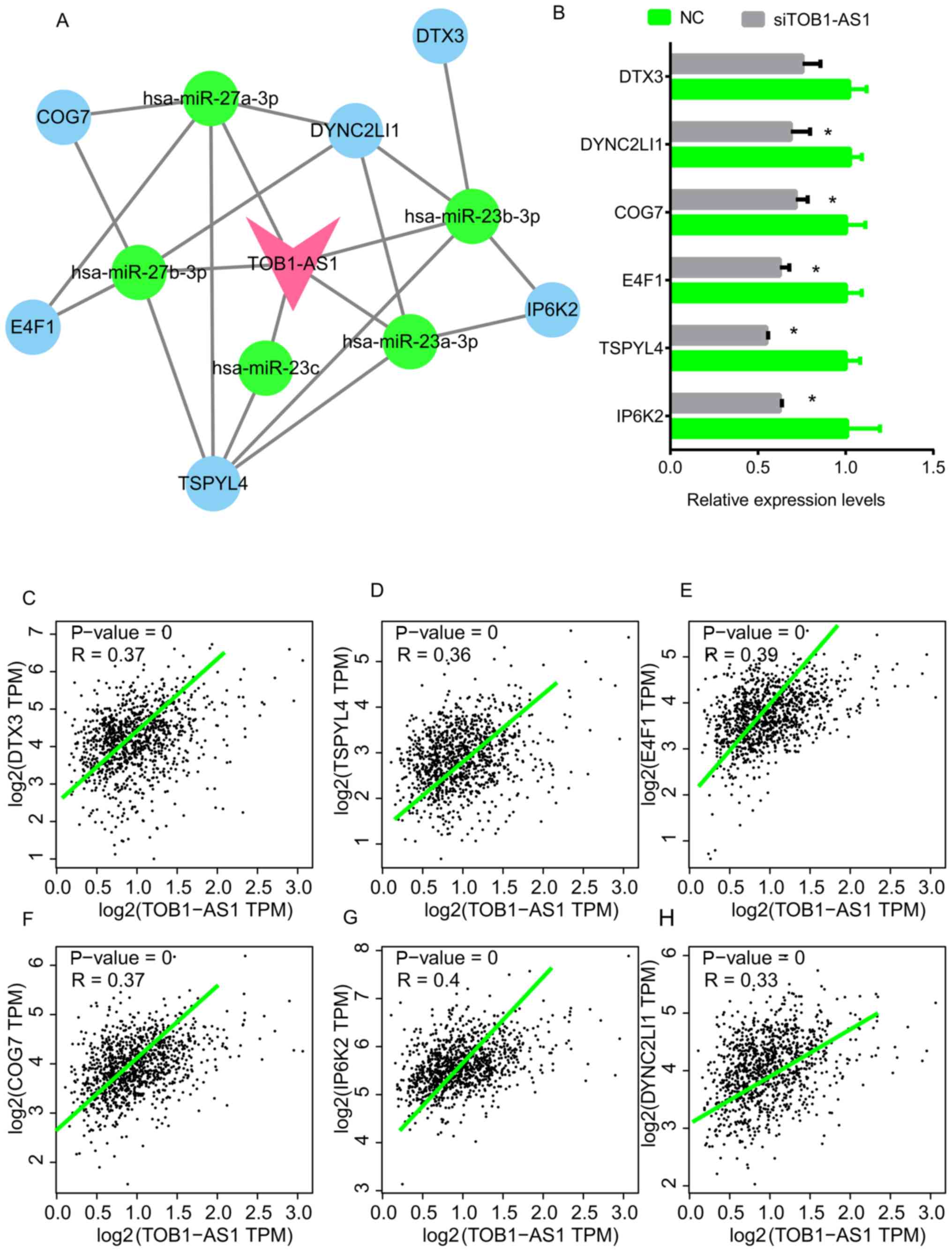 | Figure 5.TOB1-AS1-mediated ceRNA network in
NSCLC and key genes. (A) A TOB1-AS1 mediated ceRNA network in NSCLC
was constructed, pink node represents TOB1-AS1; blue nodes
represent mRNAs; green nodes represent miRNAs. (B) The expression
levels of DTX3, TSPYL4, E4F1, IP6K2, COG7 and DYNC2LI1 in A549
cells were detected after the knockdown of TOB1-AS1. *P<0.05 vs.
NC. (C-H) The expression of TOB1-AS1 in NSCLC was positively
correlated with (C) DTX3, (D) TSPYL4, (E) E4F1, (F) IP6K2, (G) COG7
and (H) DYNC2LI1 by using the GEPIA dataset. NSCLC, non-small cell
lung cancer; TOB1-AS1, transducer of ERBB2, 1-antisense 1; siRNA,
small interfering RNA; NC, negative control; ceRNA, competing
endogenous RNA; hsa, Homo sapiens; miR, microRNA; DYNC2LI1,
dynein cytoplasmic 2 light intermediate chain 1; E4F1, E4F
transcription factor 1; TSPYL4, TSPY-like 4; COG7, component of
oligomeric Golgi complex 7; IP6K2, inositol hexakisphosphate kinase
2; DTX3, deltex E3 ubiquitin ligase 3; TPM, transcript per
million. |
Dysregulation of TOB1-AS1 targets in
NSCLC is associated with prognosis
The association between the expression levels of
ceRNAs and OS time in NSCLC was then analyzed in the TCGA dataset
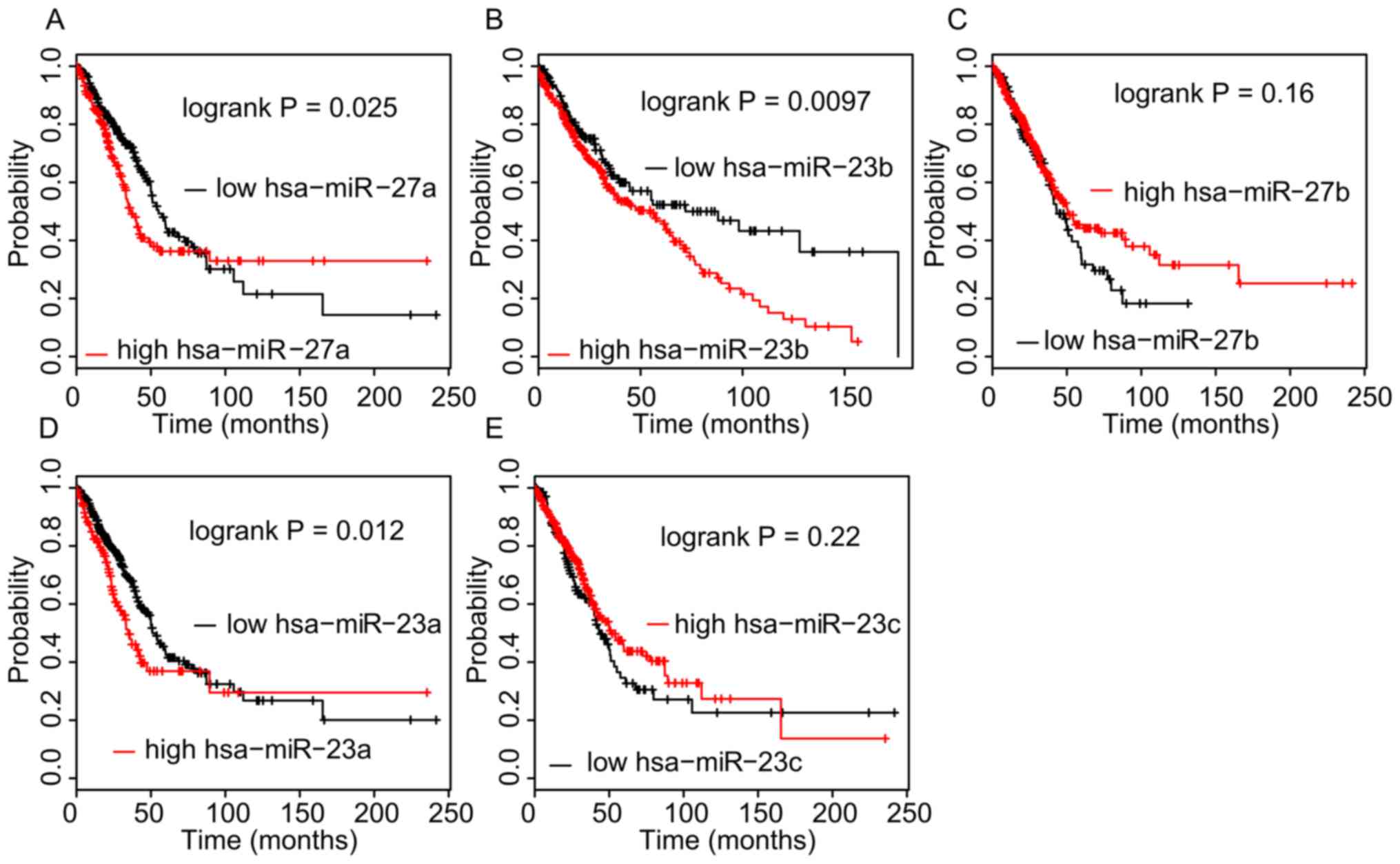
using the Kaplan-Meier method. As presented in Fig. 6, a higher expression of
hsa-miR-27a-3p (P=0.025), hsa-miR-23a-3p (P=0.012) and
hsa-miR-23b-3p (P=0.0097) in NSCLC tissues was significantly
associated with a shorter OS time. However, analysis of the
Kaplan-Meier Plotter database indicated that a higher expression of
DYNC2LI1, E4F1, TSPYL4, and COG7 in NSCLC was associated with a
longer OS time (Fig. 7).
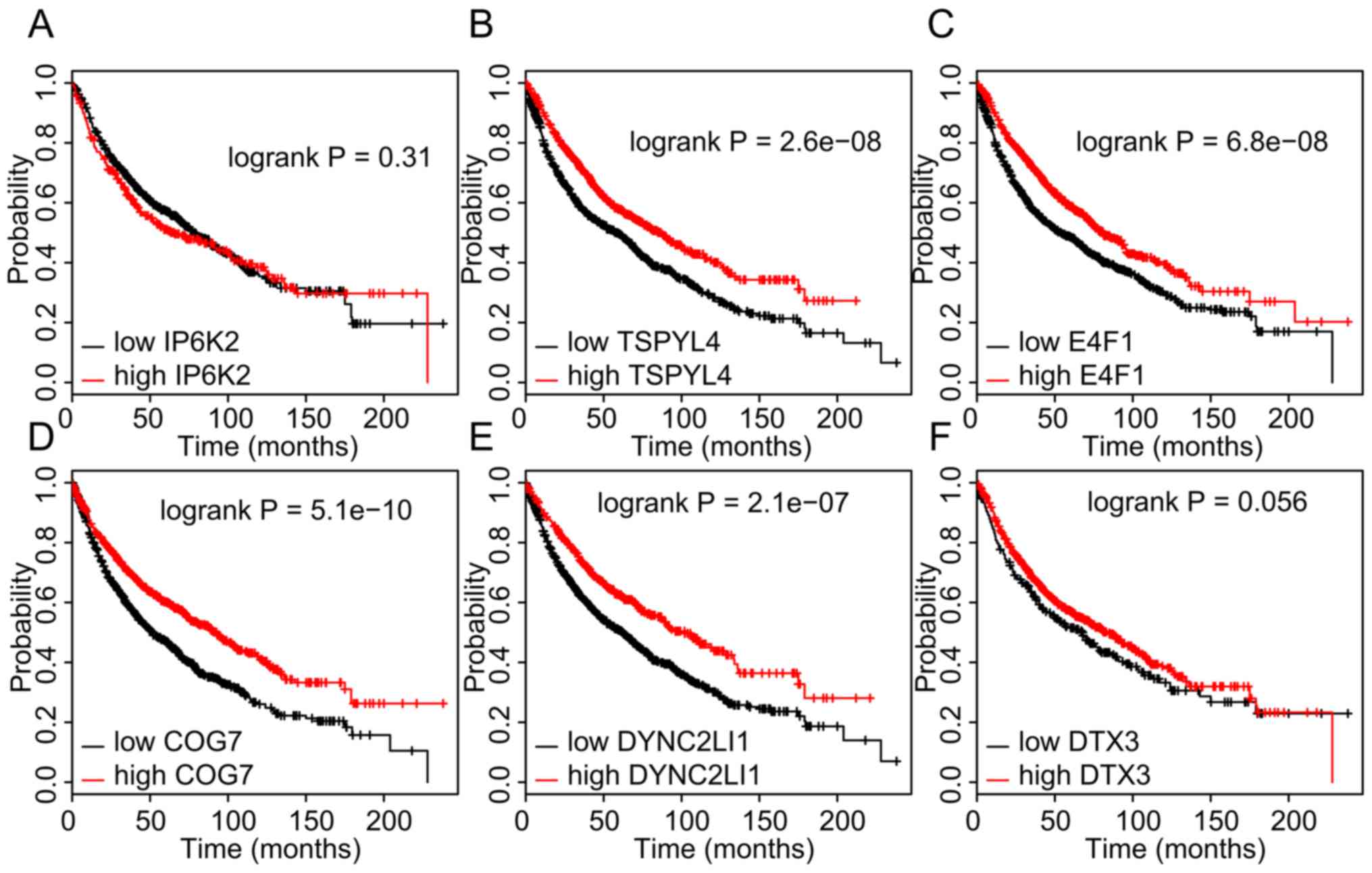 | Figure 7.Higher expression of DYNC2LI1, E4F1,
TSPYL4, and COG7 in non-small cell lung cancer samples is
associated with longer OS time. (A-F) Kaplan-Meier analysis of the
dataset from The Cancer Genome Atlas was performed to assess the
influence of high/low expression of (A) IP6K2, (B) TSPYL4, (C)
E4F1, (D) COG7, (E) DYNC2LI1 and (F) DTX3 on OS. P<0.05 was
considered to indicate a statistically significant difference with
a 95% confidence level. OS, overall survival; DYNC2LI1, dynein
cytoplasmic 2 light intermediate chain 1; E4F1, E4F transcription
factor 1; TSPYL4, TSPY-like 4; COG7, component of oligomeric Golgi
complex 7; IP6K2, inositol hexakisphosphate kinase 2; DTX3, deltex
E3 ubiquitin ligase 3. |
Discussion
The mechanisms underlying NSCLC have remained to be
fully elucidated. Recent studies have demonstrated that lncRNAs
have crucial roles in regulating NSCLC-associated pathways. For
instance, p53 inducible cancer associated RNA transcript 1
suppresses NSCLC proliferation and invasion by targeting the AKT1
signaling pathway (38). Small
nucleolar RNA host gene 1 promotes NSCLC progression by activating
Wnt/β-catenin signaling (39).
However, the functions of the majority of lncRNAs in NSCLC remain
elusive. To the best of our knowledge, the present study was the
first to demonstrate that lncRNA TOB1-AS1 expression was reduced in
NSCLC samples compared with that in normal tissues. Higher
expression levels of TOB1-AS1 were associated with a better
prognosis in NSCLC. These analyses indicated that TOB1-AS1 may
serve as a novel biomarker for NSCLC.
TOB1-AS1 is a recently discovered lncRNA. A study on
cervical cancer demonstrated that TOB1-AS1 functions as a tumor
suppressor (27). TOB1-AS1 inhibits
cervical cancer cell proliferation, the cell cycle and metastasis.
Regarding possible mechanisms, TOB1-AS1 was reported to interact
with miR-27b to degrade its expression (27). The results of the present study were
consistent with those of previous studies. The present results
indicated that TOB1-AS1 was localized in the cytoplasm and nucleus.
Knockdown of TOB1-AS1 induced cell proliferation. Furthermore,
silencing of TOB1-AS1 promoted A549 and H1299 cell migration and
invasion. These results suggest that TOB1-AS1 functions as a tumor
suppressor in NSCLC. Of note, siRNA-1 and siRNA-2 appeared to carry
the same transfection efficiency despite siRNA-1 being more
effective in inhibiting migration/invasion. This may have been due
to off-target effects of the siRNAs. In spite of this, the present
results indicate that knockdown of TOB1-AS1 promoted NSCLC
proliferation and migration. With the development of the clustered
regularly interspaced short palindromic repeat and associated
nuclease Cas9 method (40), knockout
assays may be a more powerful tool to explore the potential roles
of lncRNAs in human cancers and to overcome off-target effects.
The mechanisms of action of TOB1-AS1 in NSCLC remain
to be fully elucidated. To gain further insight, a
TOB1-AS1-mediated ceRNA network in NSCLC was constructed in the
present study, including hsa-miR-27a-3p, hsa-miR-23a-3p,
hsa-miR-23b-3p, hsa-miR-27b-3p, hsa-miR-23c, DYNC2LI1, E4F1,
TSPYL4, COG7, IP6K2 and DTX3. Further analysis revealed that these
TOB1-AS1 targets were also dysregulated and associated with the
prognosis of NSCLC. miR-27a has been reported to regulate cell
proliferation, migration and invasion in NSCLC (41–43),
while miR-23a was indicated to promote NSCLC metastasis (44). miR-27b was demonstrated to inhibit
NSCLC growth and invasion by targeting LIM domain kinase 1
(45). E4F1 is an ubiquitin E3
ligase for p53 and regulates p53 transcription functions (46). These studies, together with the
present results, suggest that TOB1-AS1 and its targets have crucial
roles in NSCLC.
Of note, the present study had several limitations.
First, gain-of-function assays should be further performed to
validate the roles of TOB1-AS1 in NSCLC. TOB1-AS1 is a novel
lncRNA. Despite the RNA sequence provided in the NCBI database, the
accurate 5'sequence and 3'sequence still require to be validated
using 5′race and 3′race assays. Furthermore, in vivo assays
should be performed to further validate the suppressive roles of
TOB1-AS1 in mouse models (47). Such
a study may provide useful information to understand the molecular
functions of TOB1-AS1 in NSCLC.
In conclusion, the present study demonstrated for
the first time, at least to the best of our knowledge, that
TOB1-AS1 has a tumor-suppressive role in NSCLC. Knockdown of
TOB1-AS1 significantly induced NSCLC migration, invasion and
proliferation. Furthermore, microarray and bioinformatics analyses
were performed to explore the underlying mechanisms of the
regulation of NSCLC metastasis by TOB1-AS1. It was also revealed
that TOB1-AS1 in NSCLC samples was associated with a longer OS
time. Despite the loss-of-functions assays, the roles of TOB1-AS1
on NSCLC still require further validation. However, the present
study clearly demonstrated that TOB1-AS1 may serve as a novel
biomarker for NSCLC.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81373621 and 81774166), the
Excellent Academic Leaders of Health and Family Planning System in
Shanghai (grant no. 2017BR044), Clinical Science and Technology
Innovation Project of the Science and Technology Commission of
Shanghai Municipality (grant no. 16401970800) and was sponsored by
the Shanghai Sailing Program (grant no. 17YF1419700).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JHT and LSL designed the study. WJS and HL performed
experiments and wrote the manuscript. ZJQ and FFQ performed
bioinformatics analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kopp F and Mendell JT: Functional
classification and experimental dissection of long Noncoding RNAs.
Cell. 172:393–407. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pekarsky Y and Croce CM: Noncoding RNA
genes in cancer pathogenesis. Adv Biol Regul. 71:219–223. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shang Q, Yang Z, Jia R and Ge S: The novel
roles of circRNAs in human cancer. Mol Cancer. 18:62019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mao C, Wang X, Liu Y, Wang M, Yan B, Jiang
Y, Shi Y, Shen Y, Liu X, Lai W, et al: A G3BP1-interacting lncRNA
promotes ferroptosis and apoptosis in cancer via nuclear
sequestration of p53. Cancer Res. 78:3484–3496. 2018.PubMed/NCBI
|
|
7
|
Deng SJ, Chen HY, Ye Z, Deng SC, Zhu S,
Zeng Z, He C, Liu ML, Huang K, Zhong JX, et al: Hypoxia-induced
LncRNA-BX111 promotes metastasis and progression of pancreatic
cancer through regulating ZEB1 transcription. Oncogene.
37:5811–5828. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu CY, Zhang YH, Li RB, Zhou LY, An T,
Zhang RC, Zhai M, Huang Y, Yan KW, Dong YH, et al: LncRNA CAIF
inhibits autophagy and attenuates myocardial infarction by blocking
p53-mediated myocardin transcription. Nat Commun. 9:292018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao J, Du P, Cui P, Qin Y, Hu C, Wu J,
Zhou Z, Zhang W, Qin L and Huang G: LncRNA PVT1 promotes
angiogenesis via activating the STAT3/VEGFA axis in gastric cancer.
Oncogene. 37:4094–4109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang WC, Fu WM, Wong CW, Wang Y, Wang WM,
Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF and Waye MM: The lncRNA
H19 promotes epithelial to mesenchymal transition by functioning as
miRNA sponges in colorectal cancer. Oncotarget. 6:22513–22525.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z, Yang B, Zhang M, Guo W, Wu Z, Wang
Y, Jia L, Li S; Cancer Genome Atlas Research Network, ; Xie W and
Yang D: lncRNA epigenetic landscape analysis identifies EPIC1 as an
oncogenic lncRNA that Interacts with MYC and promotes cell-cycle
progression in cancer. Cancer Cell. 33:706–720.e9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chakravarty D, Sboner A, Nair SS,
Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K,
Kossai M, et al: The oestrogen receptor alpha-regulated lncRNA
NEAT1 is a critical modulator of prostate cancer. Nat Commun.
5:53832014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian H, Chen L, Huang J, Wang X, Ma S, Cui
F, Luo L, Ling L, Luo K and Zheng G: The lncRNA MIR4435-2HG
promotes lung cancer progression by activating β-catenin
signalling. J Mol Med (Berl). 96:753–764. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai IL, Yang CA, Lin PC, Chan WL, Lee YT,
Yen JC, Chang YS and Chang JG: Long noncoding RNA MIAT promotes
non-small cell lung cancer proliferation and metastasis through
MMP9 activation. Oncotarget. 8:98148–98162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su W, Feng S, Chen X, Yang X, Mao R, Guo
C, Wang Z, Thomas DG, Lin J, Reddy RM, et al: Silencing of long
noncoding RNA MIR22HG triggers cell Survival/Death signaling via
oncogenes YBX1, MET, and p21 in lung cancer. Cancer Res.
78:3207–3219. 2018.PubMed/NCBI
|
|
17
|
Li S, Zhao H, Li J, Zhang A and Wang H:
Downregulation of long non-coding RNA LET predicts poor prognosis
and increases Notch signaling in non-small cell lung cancer.
Oncotarget. 9:1156–1168. 2017.PubMed/NCBI
|
|
18
|
Wu Y, Lyu H, Liu H, Shi X, Song Y and Liu
B: Downregulation of the long noncoding RNA GAS5-AS1 contributes to
tumor metastasis in non-small cell lung cancer. Sci Rep.
6:310932016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun GG, Lu YF, Cheng YJ and Hu WN: The
expression of BTG1 is downregulated in NSCLC and possibly
associated with tumor metastasis. Tumour Biol. 35:2949–2957. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee HS, Kundu J, Kim RN and Shin YK:
Transducer of ERBB2.1 (TOB1) as a Tumor suppressor: A mechanistic
perspective. Int J Mol Sci. 16:29815–29828. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ho KJ, Do NL, Otu HH, Dib MJ, Ren X,
Enjyoji K, Robson SC, Terwilliger EF and Karp SJ: Tob1 is a
constitutively expressed repressor of liver regeneration. J Exp
Med. 207:1197–1208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang YW, Nasto RE, Varghese R, Jablonski
SA, Serebriiskii IG, Surana R, Calvert VS, Bebu I, Murray J, Jin L,
et al: Acquisition of estrogen independence induces TOB1-related
mechanisms supporting breast cancer cell proliferation. Oncogene.
35:1643–1656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiao Y, Sun KK, Zhao L, Xu JY, Wang LL and
Fan SJ: Suppression of human lung cancer cell proliferation and
metastasis in vitro by the transducer of ErbB-2.1 (TOB1). Acta
Pharmacol Sin. 33:250–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu D, Zhou W, Wang S, Zhou Z, Wang S and
Chen L: Tob1 enhances radiosensitivity of breast cancer cells
involving the JNK and p38 pathways. Cell Biol Int. 39:1425–1430.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schulze-Topphoff U, Casazza S,
Varrin-Doyer M, Pekarek K, Sobel RA, Hauser SL, Oksenberg JR,
Zamvil SS and Baranzini SE: Tob1 plays a critical role in the
activation of encephalitogenic T cells in CNS autoimmunity. J Exp
Med. 210:1301–1309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun KK, Zhong N, Yang Y, Zhao L and Jiao
Y: Enhanced radiosensitivity of NSCLC cells by transducer of
erbB2.1 (TOB1) through modulation of the MAPK/ERK pathway. Oncol
Rep. 29:2385–2391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao J, Li Z, Yang Z, Xue H, Chang H, Zhang
X, Li T and Guo K: Long noncoding RNA TOB1-AS1, an epigenetically
silenced gene, functioned as a novel tumor suppressor by sponging
miR-27b in cervical cancer. Am J Cancer Res. 8:1483–1498.
2018.PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yi C, Wan X, Zhang Y, Fu F, Zhao C, Qin R,
Wu H, Li Y and Huang Y: SNORA42 enhances prostate cancer cell
viability, migration and EMT and is correlated with prostate cancer
poor prognosis. Int J Biochem Cell Biol. 102:138–150. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mas-Ponte D, Carlevaro-Fita J, Palumbo E,
Hermoso Pulido T, Guigo R and Johnson R: LncATLAS database for
subcellular localisation of long noncoding RNAs. RNA. 23:1080–1087.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Győrffy B, Surowiak P, Budczies J and
Lánczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Imai H, Mori K, Wakuda K, Ono A, Akamatsu
H, Shukuya T, Taira T, Kenmotsu H, Naito T, Kaira K, et al:
Progression-free survival, post-progression survival, and tumor
response as surrogate markers for overall survival in patients with
extensive small cell lung cancer. Ann Thoracic Med. 10:61–66.
2015.
|
|
35
|
Zhou KR, Liu S, Cai L, Bin L, et al:
ENCORI: The encyclopedia of RNA interactomes.
|
|
36
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015.doi: 10.7554/eLife.05005. View Article : Google Scholar
|
|
37
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang C, Su C, Song Q, Dong F, Yu S and
Huo J: LncRNA PICART1 suppressed non-small cell lung cancer cells
proliferation and invasion by targeting AKT1 signaling pathway. Am
J Transl Res. 10:4193–4201. 2018.PubMed/NCBI
|
|
39
|
Cui Y, Zhang F, Zhu C, Geng L, Tian T and
Liu H: Upregulated lncRNA SNHG1 contributes to progression of
non-small cell lung cancer through inhibition of miR-101-3p and
activation of Wnt/β-catenin signaling pathway. Oncotarget.
8:17785–17794. 2017.PubMed/NCBI
|
|
40
|
Shalem O, Sanjana NE and Zhang F:
High-throughput functional genomics using CRISPR-Cas9. Nat Revi
Genet. 16:299–311. 2015. View Article : Google Scholar
|
|
41
|
Chae DK, Ban E, Yoo YS, Kim EE, Baik JH
and Song EJ: MIR-27a regulates the TGF-β signaling pathway by
targeting SMAD2 and SMAD4 in lung cancer. Mol Carcinog.
56:1992–1998. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang Y, Zang A, Jia Y, Shang Y, Zhang Z,
Ge K, Zhang J, Fan W and Wang B: Genistein inhibits A549 human lung
cancer cell proliferation via miR-27a and MET signaling. Oncol
Lett. 12:2189–2193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miao Y, Li J, Qiu X, Li Y, Wang Z and Luan
Y: miR-27a regulates the self renewal of the H446 small cell lung
cancer cell line in vitro. Oncol Rep. 29:161–168. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cao M, Li Y, Lu H, Meng Q, Wang L, Cai L
and Dong X: MiR-23a-mediated migration/invasion is rescued by its
target, IRS-1, in non-small cell lung cancer cells. J Cancer Res
Clin Oncol. 140:1661–1670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sun Y, Xu T, Cao YW and Ding XQ: Antitumor
effect of miR-27b-3p on lung cancer cells via targeting Fzd7. Eur
Rev Med Pharmacol Sci. 21:4113–4123. 2017.PubMed/NCBI
|
|
46
|
Caramel J, Lacroix M, Le Cam L and Sardet
C: E4F1 connects the Bmi1-ARF-p53 pathway to epidermal stem
cell-dependent skin homeostasis. Cell Cycle. 10:866–867. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang Liu and Liu G: lncRNA BANCR
suppresses cell viability and invasion and promotes apoptosis in
non-small-cell lung cancer cells in vitro and in vivo. Cancer Manag
Res. 11:3565–3574. 2019. View Article : Google Scholar : PubMed/NCBI
|