Introduction
Cirrhosis is diffuse liver damage induced by various
chronic hepatitis diseases for a long time or under the influence
of repeated actions. With the aggravation of the disease course,
portal hypertension will occur in patients leading to esophageal
and gastric varices (1–4). Esophageal and gastric varices are the
common causes of gastrointestinal hemorrhage. Esophagogastric
varices rupture hemorrhage is one of the serious clinical
complications of cirrhosis due to large amount of hemorrhage,
dangerous onset and high mortality rate (5–7).
Clinically, TIPS technology is widely used to treat
esophageal and gastric variceal bleeding caused by portal
hypertension. TIPS is a minimally invasive method to establish
stents to artificially distribute blood, which reduces portal
pressure to prevent esophageal and gastric variceal bleeding
(8–10). However, the restenosis rate of blood
shunts and the incidence rate of hepatic encephalopathy are high
after TIPS is used, and the mid- and long-term curative effects of
patients after treatment are poor, which are defects of TIPS
technology. With the appearance of covered stent, evidence-based
medicine shows that the stenosis rate of blood shunt after covered
stent is significantly improved, but there are still some adverse
prognosis problems (11,12). Subsequently, FID of the United States
recommended using Viatorr stent for TIPS (13), but the stent price is higher and the
usage rate is lower. Therefore, clinical workers simulated Viatorr
stent and applied double stents in TIPS (14–16). As
the clinical efficacy of double stent simulated Viatorr stent in
TIPS, is not very clear we studied and compared the clinical
efficacy of single covered stent and double stents in TIPS for the
treatment of cirrhotic esophageal and gastric variceal bleeding,
providing certain reference for clinical treatment of cirrhotic
esophageal and gastric variceal bleeding under pressure.
Patients and methods
Collection of specimens
Altogether 124 patients with liver cirrhosis and
esophageal and gastric varices bleeding admitted to the First
People's Hospital of Neijiang (Neijiang, China) from February 2012
to April 2016 were selected as research objects and divided into
groups A and B. Among them, 65 patients with simple covered stent
were selected as group A, including 47 males and 18 females, with
an average age of 43.5±2.3 years. Another 59 patients treated with
double stent technique simulating Viatorr method were taken as
group B, including 38 males and 21 females, with an average age of
42.8±2.8 years.
This study was approved by the Medical Ethics
Committee of the First People's Hospital of Neijiang. Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients or the
guardians.
Inclusion and exclusion criteria
Inclusion criteria: Patients were diagnosed as
cirrhosis and with complete clinical data; with poor therapeutic
effect through drugs and endoscopy and high risk of surgical
operation; without cerebrovascular diseases and malignant tumor
diseases; who can be followed up and with Budd-Chiari syndrome.
Exclusion criteria: Patients with coagulation
dysfunction, severe infectious diseases, combined with other liver
and kidney diseases, hepatic vein occlusion, or polycystic liver
disease.
Treatment methods
Firstly, according to the patient's imaging data,
the positional relationship between hepatic vein and portal vein
was determined before operation to ensure the safety of the
puncture route. The patient was required to take the supine
position, the operation site was disinfected and anesthetized. The
position relationship between hepatic vein and portal vein was
determined again after preoperative preparation to ensure the
accuracy of the puncture. The puncture position was the right
internal jugular vein of the patient, and the portal vein pressure
was measured after the puncture was confirmed to be successful and
the position was appropriate. The varicose vein was embolized by
springs according to the direct portal angiography result. After
the puncture was completed, a balloon catheter was introduced to
expand the intrahepatic puncture under fluoroscopy, and a stent was
selected according to the incision of the balloon. The covered
stent was required not to block the ipsilateral portal vein blood
flow in human liver. Double stents were first implanted with bare
stents to connect the portal vein, and then implanted with film or
bare stents. The stents were released in a precise location. After
completion, the walking and expansion of stents in the shunt were
observed again through direct portal venography. After
confirmation, the catheter could be removed, and then the puncture
opening was bandaged.
Postoperative treatment and
follow-up
After the operation, the puncture point of the
patient was pressed to stop bleeding, blood routine and liver
function of the patient were tested, and anticoagulants were
administered orally every day. All patients were followed up by
telephone and outpatient review for two years after surgery, every
three months in the first year of follow-up and every six months in
the second year of follow-up. The follow-up ended in April
2018.
Observation indicators
Main observation indicators: The clinical efficacy
of two groups of patients after different stent operations was
observed (Table I) as well as the
immune function of patients after operation.
 | Table I.Evaluation criteria for efficacy. |
Table I.
Evaluation criteria for efficacy.
| Grade | Criteria |
|---|
| Markedly
effective | Hemostasis within 8
h, no black stool and haematemesis within 2 weeks. |
| Effective | Hemostasis within 24
h, no black stool and haematemesis within 2 weeks. |
| Ineffective | Active bleeding was
still present within 24 h and recurrence occurred within 2
weeks. |
Secondary observation indicators: The changes of
portal vein pressure before and after operation in the two groups,
and the changes of esophageal and gastric varices in the two groups
were observed (Table II). The
survival conditions of the groups two years after operation were
recorded.
 | Table II.Grading criteria of esophagogastric
varices. |
Table II.
Grading criteria of esophagogastric
varices.
| Grade | Form of esophageal
varicosis (F) | Red color (RC) |
|---|
| Mild (G1) | Straight or slightly
circuitous (F1) | No |
| Moderate | Straight or slightly
circuitous (F1) | Yes |
| (G2) | Snake roundabout
uplift (F2) | No |
| Severe | Snake roundabout
uplift (F2) | Yes |
| (G3) | Beads, nodules,
tumors (F3) | No or yes |
Statistical analysis
SPSS 19.0 software (SPSS Inc.) was used in this
study for statistical analysis. GraphPad Prism 7 software was used
to visualize the data in this study. The enumeration data was
expressed by rate (%) and detected by Chi-square test, while the
measurement data was expressed by mean ± standard deviation (mean ±
SD). Independent sample t-test was used to compare the two groups,
and K-M survival curve was used to analyze the recurrence of
patients within two years. Statistical significance is indicated by
P<0.05.
Results
General clinical data of groups A and
B
There was no difference in age, sex, BMI
(kg/m2), liver function Child-Pugh classification,
etiology of liver cirrhosis, preoperative portal vein width,
residence, smoking, alcoholism and other general clinical baseline
data between groups A and B (P>0.05) (Table III).
 | Table III.General clinical data of groups A and
B [n (%)]. |
Table III.
General clinical data of groups A and
B [n (%)].
| Factor | A (n=65) | B (n=59) | t/χ2
value | P-value |
|---|
| Sex |
|
| 0.896 | 0.344 |
| Male | 47 (72.31) | 38 (64.41) |
|
|
|
Female | 18 (27.69) | 21 (35.59) |
|
|
| Age (years) | 43.5±2.3 | 42.8±2.8 | 1.527 | 0.129 |
| BMI
(kg/m2) | 24.35±1.72 | 23.87±1.69 | 1.565 | 0.120 |
| Liver function
Child-Pugh grading |
|
| 0.505 | 0.477 |
| Grade
A | 30 (46.15) | 31 (52.54) |
|
|
| Grade
B | 35 (53.85) | 28 (47.46) |
|
|
| Etiology of liver
Cirrhosis |
|
| 1.110 | 0.775 |
|
Posthepatitic B Cirrhosis | 32 (49.23) | 26 (44.07) |
|
|
|
Posthepatitic C Cirrhosis | 5 (7.69) | 3 (5.08) |
|
|
| Alcoholic
cirrhosis | 7 (10.77) | 6 (10.17) |
|
|
|
Others | 21 (32.31) | 24 (40.68) |
|
|
| Preoperative portal
vein width (cm) | 1.27±0.14 | 1.34±0.21 | 2.202 | 0.030 |
| Residence |
|
| 1.343 | 0.247 |
|
Urban | 44 (67.69) | 34 (57.63) |
|
|
|
Rural | 21 (32.31) | 25 (42.37) |
|
|
| Smoking
history |
|
| 0.016 | 0.898 |
|
Yes | 29 (44.62) | 27 (45.76) |
|
|
| No | 36 (55.38) | 32 (54.24) |
|
|
| Drinking
history |
|
| 0.482 | 0.488 |
|
Yes | 36 (55.38) | 29 (49.15) |
|
|
| No | 29 (44.62) | 30 (50.85) |
|
|
Evaluation of curative effect of two
groups of patients
Comparing the curative effect evaluation of the two
groups of patients, it was found that the total effective rate of
group A was 83.08%, and of B was 93.22%. The total effective rate
of patients in group B was higher than that in group A (P>0.05)
(Table IV).
 | Table IV.Efficacy evaluation of two groups of
patients [n(%)]. |
Table IV.
Efficacy evaluation of two groups of
patients [n(%)].
|
| A (n=65) | B (n=59) | χ2
value | P-value |
|---|
| Markedly
effective | 25 (38.46) | 32 (54.24) | 3.099 | 0.078 |
| Effective | 29 (44.62) | 23 (38.98) | 0.403 | 0.536 |
| Ineffective | 11 (16.92) | 4 (6.78) | 2.993 | 0.084 |
| Total
effective | 54 (83.08) | 55 (93.22) | 2.993 | 0.084 |
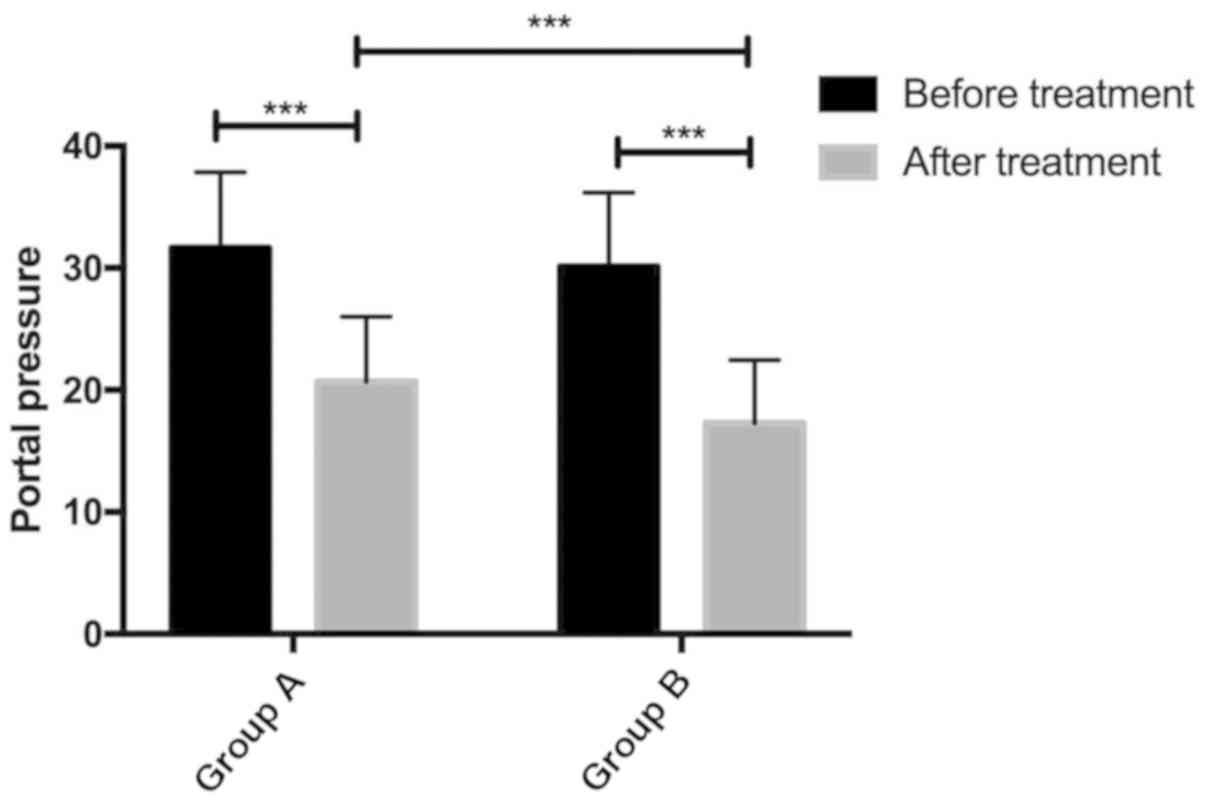
The changes of portal vein pressure before and after
operation in the two groups. Preoperative portal pressure in group
A was 31.64±6.21 and postoperative portal pressure was 20.67±5.34.
Preoperative portal pressure in group B was 30.12±6.07 and
postoperative portal pressure was 17.29±5.16. There was no
difference in portal vein pressure between the two groups
(P>0.05). The portal vein pressure in group B was significantly
lower than that in group A (P<0.05) (Fig. 1).
Comparison of changes of
esophagogastric varices between two groups
The degree of esophageal and gastric varices after
operation in both groups was significantly improved compared with
that before operation. The disappearance rate and overall effective
rate of varicose veins in group B were better than those in group A
(P>0.05) (Table V).
 | Table V.Degree of esophagogastric varices in
two groups of patients after treatment. |
Table V.
Degree of esophagogastric varices in
two groups of patients after treatment.
| Degree of
varicose | A (n=65) | B (n=59) | χ2
value | P-value |
|---|
| G1 | 26 (40.00) | 25 (42.37) | 0.072 | 0.789 |
| G2 | 16 (24.62) | 14 (23.73) | 0.013 | 0.908 |
| G3 | 12 (18.46) | 5 (8.47) | 2.607 | 0.106 |
| Disappearance | 11 (16.92) | 15 (25.42) | 1.349 | 0.246 |
Changes of immune function in two
groups of patients
The changes of CD3+, CD4+ and
CD4+/CD8+ before treatment, 3 and 7 days
after treatment in the two groups were observed. Compared with the
changes of CD3+, CD4+ and
CD4+/CD8+ 3 and 7 days after treatment in
group A, there was no statistical difference (P>0.05). After
treatment for 3 days in group B, CD3+, CD4+
and CD4+/CD8+ were higher than those in group
A. After 7 days of treatment, the decrease rate of CD3+,
CD4+ and CD4+/CD8+ in group B was
higher than that in group A (P>0.05) (Table VI).
 | Table VI.Changes of CD3+
CD4+ and CD4+/CD8+ before, after 3
and 7 days of treatment in the two groups of patients. |
Table VI.
Changes of CD3+
CD4+ and CD4+/CD8+ before, after 3
and 7 days of treatment in the two groups of patients.
|
|
CD3+ |
CD4+ |
CD4+/CD8+ |
|---|
|
|
|
|
|
|---|
| Groups | Before
treatment | Three days after
treatment | Seven days after
treatment | Before
treatment | Three days after
treatment | Seven days after
treatment | Before
treatment | Three days after
treatment | Seven days after
treatment |
|---|
| A (n=65) | 50.35±6.23 | 53.47±4.75 | 52.32±4.43 | 43.12±5.07 | 46.87±5.11 | 43.47±4.06 | 0.83±0.34 | 1.25±0.39 | 0.92±0.36 |
| B (n=59) | 50.87±6.45 | 55.23±5.47 | 51.07±3.23 | 42.69±4.58 | 48.32±4.72 | 42.13±3.89 | 0.76±0.28 | 1.36±0.42 | 0.82±0.31 |
| t value | 0.649 | 1.917 | 1.780 | 0.494 | 1.636 | 1.872 | 1.244 | 1.512 | 1.649 |
| P-value | 0.456 | 0.057 | 0.078 | 0.622 | 0.104 | 0.064 | 0.216 | 0.133 | 0.102 |
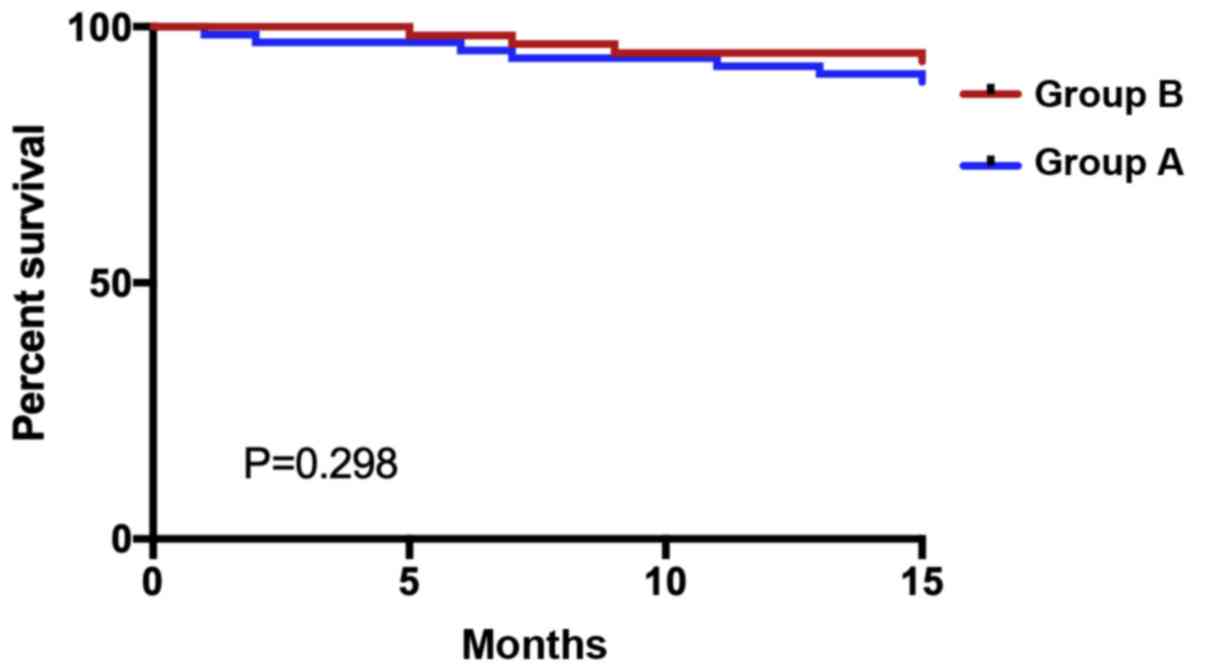
Two-year survival of two groups of
patients
Statistics on the survival of the two groups of
patients were made for two years. Altogether 124 patients or their
families were followed up, with 0 patients lost to follow-up.
Within two years, 12 patients died, 112 survived, with a survival
rate of 90.32%. Eight patients died in group A, 57 survived, and
the survival rate was 87.69%. Four patients died in group B, 55
survived, and the survival rate was 93.22%. The two-year survival
rate of the patients in group B was higher than that in group A,
with no statistical significance (P=0.298) (Fig. 2).
Discussion
Esophagogastric varices are common complications of
liver cirrhosis. Liver cirrhosis is pathologically defined as
liver, which leads to metabolic liver failure and portal
hypertension (17,18). Portal hypertension is the most common
complication of liver cirrhosis, and esophageal varices are portal
mesangium of patients with liver cirrhosis with portal hypertension
(19). Every year, a certain
proportion of cirrhotic patients develop esophageal varices, which
is the main cause of death for cirrhotic patients (20). TIPS is the main treatment method for
esophageal varices rupture and hemorrhage. In recent years, the
appearance of covered stent and double-stent simulated Viatorr
methods have further optimized TIPS treatment (21). Therefore, this study investigated the
efficacy of the two stent methods in TIPS.
We compared the clinical efficacy of covered stent
and double stent in TIPS treatment of liver cirrhosis patients with
esophageal and gastric varices bleeding. The results showed that
the therapeutic efficacy of patients with double stent was higher
than that of patients with single covered stent. In the study of
Sommer et al (22), the
hemodynamics, patency rate and complications of Viatorr stent in
TIPS treatment were higher than those of bare metal stent, and the
clinical success rate was better than those of bare metal stent,
which was similar to our results. Furthermore, we compared the
therapeutic effects of single covered stent and double stents (i.e.
simulated Viatorr stent method) in TIPS. The single covered stent
was improved on the basis of bare metal stents and was more
effective than bare metal stents in preventing variceal rebleeding.
This view has also been confirmed in the study conducted by Bucsics
et al (23). However, the
appearance of Viator stents in the United States provides a new
concept in TIPS therapy. In our study, we also show that the
simulated Viatorr double stent method has better curative effect,
which further supports our research results. In addition, we also
observed that the portal vein pressure of patients applying the two
stent methods is lower than that of patients applying single
covered stent. In the study of the Zhao et al (24), the portal vein pressure of patients
after Viatorr stent treatment was reduced, which was similar to our
results. We speculate that the double stent can effectively improve
the patency of the shunt and reduce the pressure of the shunt to
prevent varicose veins. Double stents are used to simulate Viatorr
stents with the same effect for TIPS therapy, further supporting
our research results. Moreover, we observed that the varicose
degree of patients after both stent operations was improved, and
the varicose disappearance rate in patients using double stents was
higher. Then, we observed the immune function of patients after
surgery. Compared with patients with single covered stent, the
immune function of patients with double covered stent increased
three days after surgery, and the rate of recovery was higher than
that of patients with single covered stent. According to the
recovery of patients' immune function, we consider that the double
covered stent method can effectively reduce bleeding symptoms and
complications. In the study of Ferral et al (15), Viatorr stent, which is the double
covered stent we simulated, has excellent shunt unblocking rate and
better therapeutic effect. After two years of follow-up
investigation, the results showed that the survival rate of
patients with double stents is higher than that of patients with
single covered stents, which suggests that the application of
double stents in TIPS may be beneficial to the survival of
patients.
This study initially proved that the clinical effect
of double stents in TIPS treatment of liver cirrhosis esophageal
and gastric fundus varices hemorrhage is better, but this study
still has certain limitations as we did not study the complications
of patients and did not make a good prognosis. The number of
samples and the research time need to be increased in the follow-up
studies.
In conclusion, double stents are more effective in
TIPS in the treatment of liver cirrhosis with esophageal and
gastric variceal bleeding, promoting the rapid recovery of immune
function, and are worthy of clinical application and promotion.
Acknowledgements
Not applicable.
Funding
The study was funded by the project of ‘Clinical
application of TIPS in the treatment of ePTFE covered stent in
cirrhotic portal hypertension’, Neijiang Bureau of Science,
Technology and Intellectual Property (no. 2016105).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BX wrote the manuscript. BX, JL and LL conceived and
designed the study, and drafted the manuscript. BX, JL, SL and XX
collected, analyzed and interpreted the experimental data. LL
revised the manuscript for important intellectual content. All the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Medical Ethics
Committee of the First People's Hospital of Neijiang (Neijiang,
China). Patients who participated in this research had complete
clinical data. Signed informed consents were obtained from the
patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krzyżanowska P, Drzymała-Czyż S,
Pogorzelski A, Duś-Żuchowska M, Skorupa W, Bober L, Sapiejka E,
Oralewska B, Rohovyk N, Moczko J, et al: Vitamin K status in cystic
fibrosis patients with liver cirrhosis. Dig Liver Dis. 49:672–675.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu KC, Huang HC, Chang T, Lee WS, Chuang
CL, Hsin IF, Hsu SJ, Lee FY, Chang CC and Lee SD: Effect of
sirolimus on liver cirrhosis and hepatic encephalopathy of common
bile duct-ligated rats. Eur J Pharmacol. 824:133–139. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pimenta JR, Ferreira AR, Fagundes ED,
Queiroz TC, Baptista RA, de Araújo Moreira EG, de Resende CB,
Bittencourt PF, Carvalho SD, Neto JA, et al: Factors associated
with bleeding secondary to rupture of esophageal varices in
children and adolescents with cirrhosis. J Pediatr Gastroenterol
Nutr. 64:e44–e48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saito H, Ohmori M, Iwamuro M, Tanaka T,
Wada N, Yasunaka T, Takaki A and Okada H: Hepatic and gastric
involvement in a case of systemic sarcoidosis presenting with
rupture of esophageal varices. Intern Med. 56:2583–2588. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim DJ, Darcy MD, Mani NB, Park AW,
Akinwande O, Ramaswamy RS and Kim SK: Modified balloon-occluded
retrograde transvenous obliteration (BRTO) techniques for the
treatment of gastric varices: Vascular plug-assisted retrograde
transvenous obliteration (PARTO)/coil-assisted retrograde
transvenous obliteration (CARTO)/balloon-occluded antegrade
transvenous obliteration (BATO). Cardiovasc Intervent Radiol.
41:835–847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fullwood D: Portal hypertension and
varices in patients with liver cirrhosis. Nurs Stand. 26:52–57,
quiz 58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El Ray A, Azab MM, El-Aziz IM, El-Aleem
AA, El-Talkawy MD, El-Badea MA, El Ansary M, Safeem AA and Diab TM:
Non-invasive predictors for the presence, grade and risk of
bleeding from esophageal varices in patients with post-hepatitic
cirrhosis. J Egypt Soc Parasitol. 45:421–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lv Y, Yang Z, Liu L, Li K, He C, Wang Z,
Bai W, Guo W, Yu T, Yuan X, et al AVB-TIPS Study Group, : Early
TIPS with covered stents versus standard treatment for acute
variceal bleeding in patients with advanced cirrhosis: A randomised
controlled trial. Lancet Gastroenterol Hepatol. 4:587–598. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maimone S, Saffioti F, Filomia R,
Alibrandi A, Isgrò G, Calvaruso V, Xirouchakis E, Guerrini GP,
Burroughs AK, Tsochatzis E, et al: Predictors of re-bleeding and
mortality among patients with refractory variceal bleeding
undergoing salvage transjugular intrahepatic portosystemic shunt
(TIPS). Dig Dis Sci. 64:1335–1345. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lv Y, Zuo L, Zhu X, Zhao J, Xue H, Jiang
Z, Zhuge Y, Zhang C, Sun J, Ding P, et al: Identifying optimal
candidates for early TIPS among patients with cirrhosis and acute
variceal bleeding: A multicentre observational study. Gut.
68:1297–1310. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang L, Xiao Z, Yue Z, Zhao H, Fan Z, Zhao
M, He F, Dai S, Qiu B, Yao J, et al: Efficacy of covered and bare
stent in TIPS for cirrhotic portal hypertension: A single-center
randomized trial. Sci Rep. 6:210112016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bureau C, Garcia-Pagan JC, Otal P,
Pomier-Layrargues G, Chabbert V, Cortez C, Perreault P, Péron JM,
Abraldes JG, Bouchard L, et al: Improved clinical outcome using
polytetrafluoroethylene-coated stents for TIPS: Results of a
randomized study. Gastroenterology. 126:469–475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vignali C, Bargellini I, Grosso M,
Passalacqua G, Maglione F, Pedrazzini F, Filauri P, Niola R, Cioni
R and Petruzzi P: TIPS with expanded
polytetrafluoroethylene-covered stent: Results of an Italian
multicenter study. AJR Am J Roentgenol. 185:472–480. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geeratikun Y, Timmermans H, Uchida B,
Jahangiri Y, Horikawa M, Kaufman JA and Farsad K: VIDEO: Technique
to restrict the gore viatorr stent-graft for TIPS reduction. AJR Am
J Roentgenol. 210:W172. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferral H, Gomez-Reyes E and Fimmel CJ:
Post-transjugular intrahepatic portosystemic shunt follow-up and
management in the VIATORR era. Tech Vasc Interv Radiol. 19:82–88.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai W, Zhuge Y, Zhang J, Li Z, He Q, Zhang
M, Ni J, Li Y, Ma Q and Peng C: Safety and clinical efficacy of
TIPS with various stents for treatment of cirrhosis with esophageal
gastric varices bleeding. Zhonghua Gan Zang Bing Za Zhi.
23:258–264. 2015.(In Chinese). PubMed/NCBI
|
|
17
|
Nan YM: Current status and perspectives of
diagnosis and treatment of complications related to liver
cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 25:241–245. 2017.(In
Chinese). PubMed/NCBI
|
|
18
|
Hung TH, Liang CM, Hsu CN, Tai WC, Tsai
KL, Ku MK, Wang JW, Tseng KL, Yuan LT, Nguang SH, et al:
Association between complicated liver cirrhosis and the risk of
hepatocellular carcinoma in Taiwan. PLoS One. 12:e01818582017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gioia S, Nardelli S, Pasquale C,
Pentassuglio I, Nicoletti V, Aprile F, Merli M and Riggio O:
Natural history of patients with non cirrhotic portal hypertension:
Comparison with patients with compensated cirrhosis. Dig Liver Dis.
50:839–844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wasfy E, Elkassas G, Elnawasany S,
Elkasrawy K, Abd-Elsalam S, Soliman S and Badawi R: Predicting
esophageal varices in cirrhotic hepatitis C virus patients using
noninvasive measurement of insulin resistance variables. Endocr
Metab Immune Disord Drug Targets. 18:573–580. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi X, Tian Y, Zhang W, Yang Z and Guo X:
Covered versus bare stents for transjugular intrahepatic
portosystemic shunt: An updated meta-analysis of randomized
controlled trials. Therap Adv Gastroenterol. 10:32–41. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sommer CM, Gockner TL, Stampfl U,
Bellemann N, Sauer P, Ganten T, Weitz J, Kauczor HU and Radeleff
BA: Technical and clinical outcome of transjugular intrahepatic
portosystemic stent shunt: Bare metal stents (BMS) versus viatorr
stent-grafts (VSG). Eur J Radiol. 81:2273–2280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bucsics T, Schoder M, Diermayr M,
Feldner-Busztin M, Goeschl N, Bauer D, Schwabl P, Mandorfer M,
Angermayr B, Cejna M, et al: Transjugular intrahepatic
portosystemic shunts (TIPS) for the prevention of variceal
re-bleeding - A two decades experience. PLoS One. 13:e01894142018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao JB, Ye P, Zeng QL, Pang HJ, He XF and
Chen Y: Transjugular intrahepatic portosystemic shunt with Viatorr
stent grafting: Report of 3 cases. Nan Fang Yi Ke Da Xue Xue Bao.
36:294–296. 2016.(In Chinese). PubMed/NCBI
|