Introduction
Nanoparticles (NPs) and other nanomaterials have
entered virtually all areas of everyday life which has raised
concerns over their toxicity and other potential effects on the
body. The biological effects of NPs are determined by various
factors including particle size, shape and ability to interact with
the surrounding tissue (1).
Metal nanoparticles have widespread applications. Of
these, iron oxide NPs (Fe2O3NPs) and silver
NPs (AgNPs) are the most prominent. Fe2O3NPs
are found in the environment as particulate matter originating from
air pollution and volcanic eruptions.
Fe2O3NPs particles can also be generated by
traffic, industry and power station emissions. In addition, they
are purposely chemically synthesised for a wide variety of
applications (2). Similarly, AgNPs
are widespread in the environment due to various different
industries. Specifically, AgNPs are of interest due to their unique
properties (e.g. the size and shape affecting its optical,
electrical, and magnetic properties), meaning that they have roles
in antimicrobial applications, biosensor materials, composite
fibers, cryogenic superconducting materials, cosmetic products and
electronic components (3). AgNPs
have been predominately utilized for the development of medicines,
drug delivery systems, and medical device coatings. An increase in
AgNP usage has led to greater concentrations of NPs in the
atmosphere and general environment. The most concerning element is
their ability to infiltrate groundwater and soil, which are the
greatest avenues of exposure. Ingested
Fe2O3NPs and AgNPs can be translocated into
the bloodstream and distributed throughout vital organs such as the
heart, liver, kidney, brain and lungs (4), raising concerns about their acute and
chronic toxic effects.
There is a compelling body of evidence that
addresses the toxicological effects of
Fe2O3NPs and AgNPs on animal cells and
tissues (5,6). NPs may cause inflammation, cytokine
production, cytoskeletal changes, altered vesicular trafficking,
oxidative stress, apoptosis and changes in gene expression and cell
signalling (1). Toxicity is further
heightened by the metallic nature of Fe2O3NPs
and AgNPs. It has been reported that silver ions (Ag+)
are released from AgNPs in aqueous environments (7) and a study also demonstrated that the
cytotoxicity of AgNPs was eliminated after Ag+ was
chelated by a thiol ligand (8).
Furthermore, Fe2O3NPs release free iron,
which induces reactive oxygen species (ROS) production through the
Fenton reaction process.
The toxicities of Fe2O3NPs and
AgNPs are well documented; however, the effects of simultaneous
co-exposure to both NPs remain uninvestigated, therefore the
present study aimed to address this issue. Rats were subchronically
exposed to Fe2O3NPs and AgNPs, as well as to
a combination of both, for 79 days and the effects on different
organs were assessed. The present study focused on the toxic
effects with regards to the lungs and heart, as the role of NP
exposure in the development of cardiovascular diseases is of
particular concern in nanotoxicology. The aims were to determine
the cardiotoxicity and lung toxicity of long-term
Fe2O3NPs and AgNPs exposure, alone and in
combination, and also to investigate the different mechanisms that
may be involved, including oxidative stress, oxidative DNA damage,
and dysregulated cytokine production.
Materials and methods
Tested compounds and doses
Fe2O3NPs (spherical; 50 nm
particle size; 50–245 m2/g surface area) and AgNPs
(spherical; 50 nm particle size; 5.0 m2/g surface area)
were purchased from Sigma-Aldrich (Merck KGaA).
Fe2O3NPs and AgNPs were dispersed in
distilled water by sonication for 30 sec to form suspensions before
use at doses of 5 mg/ml and 50 mg/ml, respectively. The
hydrodynamic size distribution of each NP in suspension was
determined by dynamic light scattering using a Zetasizer Nano ZS
(Malvern Instruments, Ltd.). Szalay et al (9) and Sharma et al (10) were consulted to determine the
appropriate dosage of Fe2O3NPs (5 mg/kg/day)
and AgNPs (50 mg/kg/day).
In vivo study and experimental
groups
Forty adult male Wistar rats weighing 160–170 g at
5–6 months of age were used in the present study. Animals were
provided by the Faculty of Medicine of Alexandria University. Rats
had free access to tap water and a basal diet, which were provided
ad libitum. Following two weeks of acclimation, animals were
divided into 4 equal groups (n=10): Group 1 served as the control,
group 2 was administered with Fe2O3NPs (5
mg/kg) by oral gavage, group 3 was administered with AgNPs (50
mg/kg) by oral gavage, and group 4 was administered with a
combination of Fe2O3NPs (5 mg/kg) and AgNPs
(50 mg/kg) by oral gavage. The rats were dosed with
Fe2O3NPs and AgNPs every day for 79 days with
this time period selected to cover two reproductive cycles of
spermatogonia to study the reproductive toxicity (unpublished
data). No signs of stress were observed in rats from any of the
experimental groups during the study period.
All experimental procedures, animal handling,
sampling, and scarification were performed in accordance with the
Guide for the Care and Use of Laboratory Animals, 8th edition
(National Research Council 2011) and were approved by the Research
Ethical Committee of the Medical Research Institute of Alexandria
University. All efforts were made to minimise the rats' suffering
during the experimental period.
Blood sample collection and tissue
preparation
On day 79 of the experimental period, all animals
were sacrificed by cervical dislocation under anaesthesia (ketamine
100 mg/kg and xylazine 10 mg/kg intraperitoneally) in accordance
with the literature (11). The final
body weight was <200 g. There were no signs of toxicity at the
stated doses of anaesthetic agents. Blood samples were collected in
test tubes containing heparin as an anticoagulant and placed
immediately on ice. The blood samples were centrifuged at 860 × g
for 20 min to separate the plasma. The plasma was kept at −80°C
until the experimental parameters were measured and analysed. The
hearts and lungs were immediately removed and washed with chilled
saline solution (0.9%) followed by the removal of the adhering fat
and connective tissues. Then each tissue was divided for the
following experiments: DNA isolation for
8-hydroxy-2−deoxyguanosine (8-OHdG) assessment, DNA
fragmentation, RNA isolation for gene expression analysis,
histological analysis and finally for ELISA.
Tissue homogenization
Heart and lung tissue was minced and homogenised
(10%, w/v), separately, in ice-cold sucrose buffer (0.25 M) in a
Potter-Elvehjem type homogeniser. The homogenates were then
centrifuged at 10,000 × g for 20 min at 4°C, to pellet the cell
debris. The supernatant was collected and stored at −80°C for
analysis.
Index of oxidative DNA damage measured
by 8-OHdG assay
A widely accepted sensitive marker of oxidative DNA
damage and oxidative stress is 8-OHdG. The determination of DNA
damage using 8-OHdG began with the isolation of genomic DNA using a
DNeasy kit (Qiagen Inc.) following the manufacturer's instructions.
The concentration was determined using Nanodrop. DNA was briefly
digested then 5 µg/µl (total DNA 200 µg) was incubated with 100
units of DNase I (Bio Basic, Inc) in 40 µl Tris/hydrochloric acid
10 mM and 10 µl of 0.5 M MgCl2 (final concentration of
20 mM) at 37°C for 1 h. The pH of the reaction mixture was then
lowered with the addition of 15 µl of 0.5 M sodium acetate (pH
5.1), 10 µl of nuclease P1 (Bio Basic, Inc.) and 30 µl of 10 mM
ZnSO4, and the mixture was incubated for 1 h. After
readjusting the pH with 100 µl of 0.4 M Tris/HCl (pH 7.8) and 20 µl
alkaline phosphatase (Bio Basic, Inc.), the samples were incubated
at 37°C for 30 min then boiled for 10 min. The resultant DNA
hydrolysate was used for experimentation using the 8-OH-dG ELISA
kit (cat. no. ab201734; Abcam) according to the manufacturer's
protocol.
p53 and cytokine assessment using
ELISA
The homogenates of heart and lung tissues were used
for the determination of p53 (cat. no. ELR-p53-1; RayBiotech,
Inc.), tumor necrosis factor-alpha (TNF-α; cat. no. ab100785) and
interleukin-6 (IL-6; cat. no. ab100772) by using respective ELISA
kits (Abcam) according to the manufacturer instructions.
Lipid peroxidation assay
The process of lipid peroxidation results in the end
product of malondialdehyde (MDA), therefore its quantification is
generally used as a marker for lipid peroxidation activity. MDA
levels were determined using thiobarbituric acid-reactive
substances assay (12). In brief,
the heart and lung tissues homogenates were heated with
thiobarbituric acid at a low pH to produce a pink chromogen, which
was analysed at a wavelength of 532 nm.
Nitric oxide (NOx) end products
The Griess reaction was used to determine the
concentration of NOx end products, nitrite and nitrate, in the
deproteinised heart and lung supernatants (13). The Griess reaction was supplemented
with the reduction of nitrate to nitrite by nicotinamide-adenine
dinucleotide phophate-dependent nitrate reductase. In brief, the
first step required the diazotisation of sulphanilic acid with
nitrite ions followed by the coupling of this product with diamine,
resulting in a measurable pink metabolite, which was analysed at a
wavelength of 540 nm.
Antioxidant determination
The total antioxidant capacity (TAC) and the
activities of superoxide dismutase (SOD), glutathione peroxidase
(GPX), glutathione S-transferase (GST) and catalase (CAT) in the
tissue homogenates were measured using colorimetric kits
(Bio-Diagnostic, Ltd.). Reduced glutathione (GSH) content was
assessed after protein precipitation using a metaphosphoric acid
reagent. The assay was based on the oxidation of GSH by
5,5′−dithiobis-(2-nitrobenzoic acid) (DTNB) to yield
glutathione disulphide (GSSG) and 5-thio-2-nitrobenzoic acid (TNB).
The rate of TNB formation was assessed at 412 nm and was
proportional to the GSH amount present in the sample (14). The rate of formation of TNB was
monitored by recording the change in the absorbance at 412 nm. The
total GSH content in the samples was determined from a GSH standard
curve. The results were subsequently expressed as nmol/g tissue by
dividing the concentration of GSH in the sample by the total weight
(g) of tissue used to prepare the sample.
Determination of paraoxonase (PON1)
and creatine kinase-muscle/brain (CK-MB) activities in the
heart
The PON1 enzyme activity in the heart was measured
by applying the method of Mueller et al (15). The CK-MB was assayed using an ELISA
kit (cat. no. K777; BioVision, Inc.).
Lipid profile assessment
Stored plasma samples were analysed for total lipids
(cat. no. 8.05.36.0.0250; Atlas Medical UK), cholesterol (cat. no.
11805), triacylglycerol (TAG; cat. no. 11828) and high-density
lipoprotein cholesterol (HDL-c; cat. no. 11557) using commercial
kits (BioSystems S.A.). Very low-density lipoprotein-cholesterol
(vLDL-c) was calculated by dividing the values of TAG by a factor
of 5. Low-density lipoprotein-cholesterol (LDL-c) was determined by
the following calculation: LDL-c=cholesterol-(HDL-c + vLDL-c). Rat
Apolipoprotein B (ApoB) ELISA kit (cat. no. KT-7394; Kamiya
Biomedical Company) was used for assessment of plasma level of
ApoB. All assays were carried out in accordance with manufacturers'
instructions.
Histological analysis of heart and
lung
Heart and lung specimens were obtained from rats,
and immediately fixed in 10% formalin for 18 h at room temperature
before being treated with a conventional grade of alcohol and
xylol, embedded in paraffin and sectioned at 4–6 mm thickness. The
sections were stained with haematoxylin and eosin (H&E) in
order to study the histopathological changes using a light
microscope at a magnification of ×400 as previously described
(16).
Statistical analysis
Results are expressed as mean ± standard error. All
statistical analysis was carried out using the general linear model
(SAS Institute, Inc.). Multiple comparisons were performed using
one-way analysis of variance followed by Duncan's new multiple
range test. To test for interactions between the individual
treatments when given in combination, a factorial design test was
used. P<0.05 was considered to indicate statistical
significance.
Results
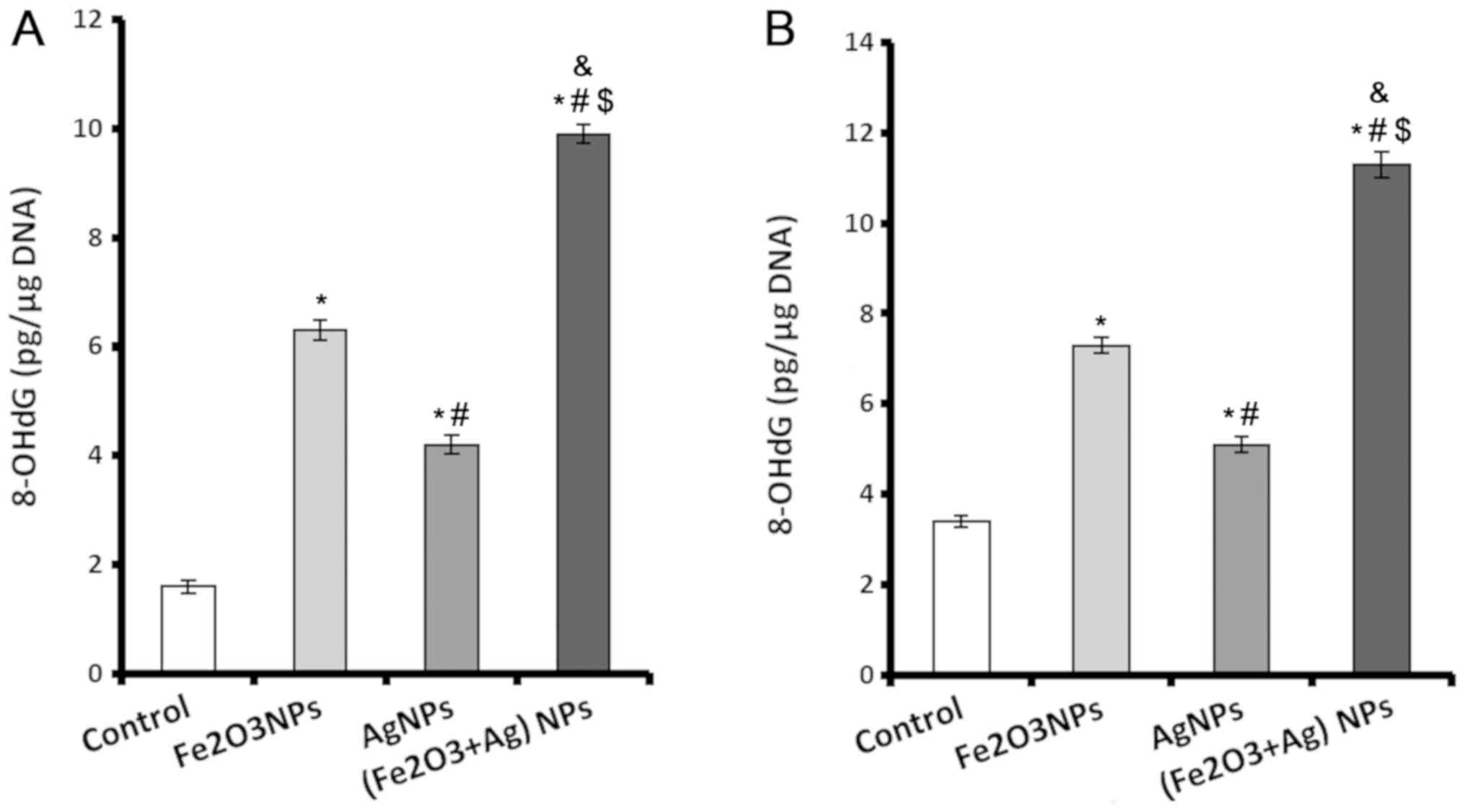
Levels of 8-OHdG increase in cardiac
and lung tissue following exposure to NPs
A sensitive marker of oxidative DNA damage is
8-OHdG. Rats exposed to Fe2O3NPs and AgNPs
alone or in combination displayed significantly higher levels of
8-OHdG in their cardiac and lung tissue compared with rats in the
control group (Fig. 1). The rats
exposed to Fe2O3NPs had higher cardiac and
lung 8-OHdG contents compared with rats exposed to AgNPs (Fig. 1). The rats coexposed to both NPs
exhibited markedly higher 8-OHdG contents in both cardiac (Fig. 1A) and lung (Fig. 1B) tissues compared with other
groups.
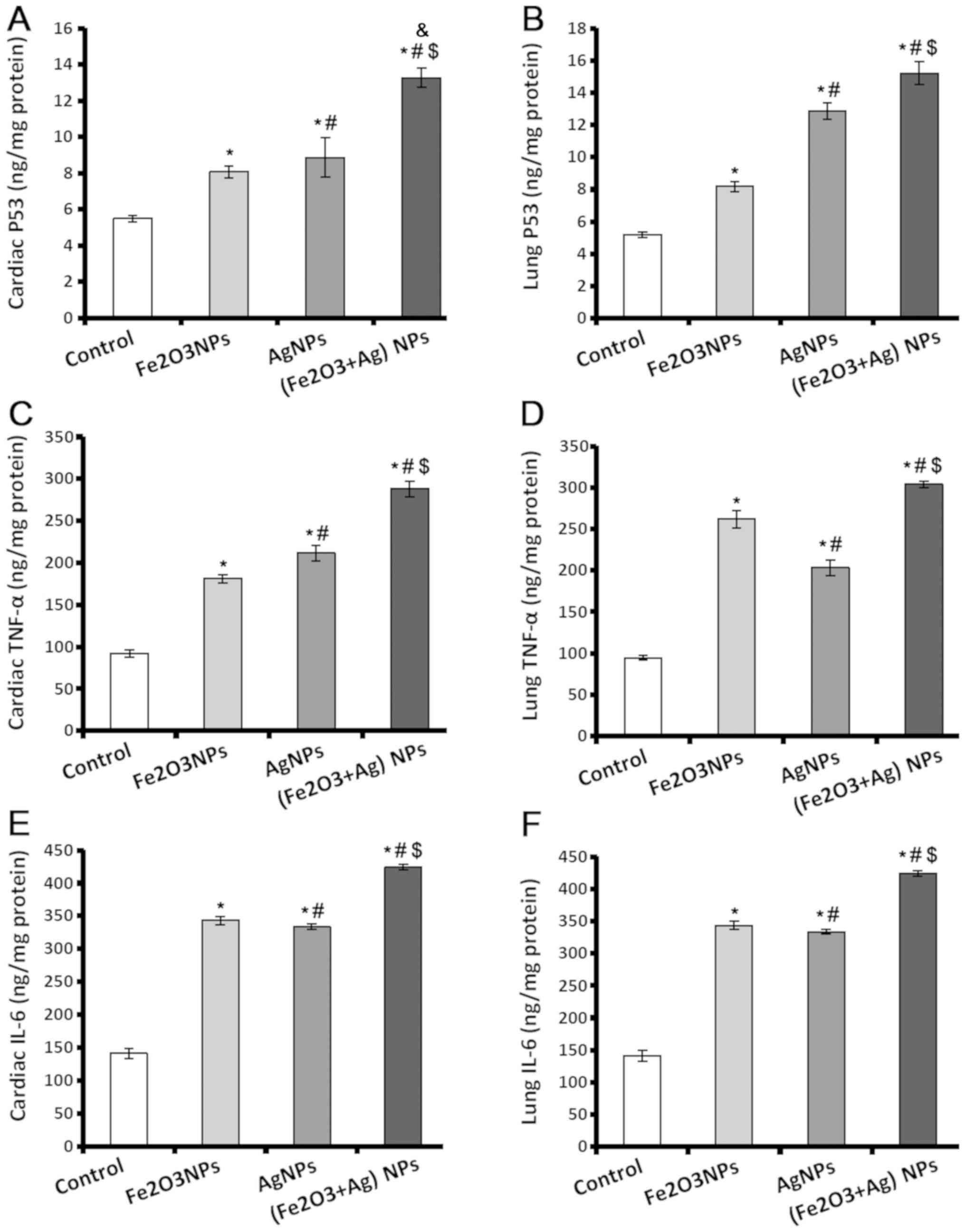
Levels of p53, TNF-α, and IL-6
increase in cardiac and lung tissue following exposure to NPs
p53 controls the cell cycle to suppress the
production of tumours, and acts as genome guard and as an inducer
of apoptosis. TNF-α and IL-6 have important roles in tissue injury
and oxidative stress. The results indicated that p53, TNF-α and
IL-6 levels were significantly higher in heart and lung tissues of
rats exposed to Fe2O3NPs and AgNPs (Fig. 2). Exposure to AgNPs caused the
production of a significantly higher level of p53 in cardiac and
lung tissues (Fig. 2A and B), and
TNF-α in cardiac tissues (Fig. 2C)
compared to the rats exposed to Fe2O3NPs. The
amount of TNF-α present in lung tissues was lower in the rats
exposed to AgNPs compared to the rats exposed to
Fe2O3NPs. Regarding IL-6 levels (Fig. 2E and F), there was no significant
difference in the tissues of rats exposed to
Fe2O3NPs and AgNPs. The rats coexposed to
both NPs demonstrated significantly higher p53, TNF-α, and IL-6
levels compared with the control rats or rats exposed to individual
NPs (Fig. 2).
Lipid peroxidation and nitric oxide
end products increase following exposure to NPs
NOx is a pleotropic molecule that serves an
important role in cell signalling; however, at high concentrations
it can act as a pro-oxidant leading to catastrophic cell damage. It
has a short half-life, so upon analysis, it is typically identified
as total nitrite and nitrate as these are both NOx end products.
The male rats exposed to Fe2O3NPs and AgNPs
demonstrated significantly higher tissues levels of thiobarbituric
acid-reactive substances (TBARS) and NOx compared with the control
rats (Table I). The rats exposed to
AgNPs demonstrated significantly higher values of both TBARS and
NOx in lung tissue and NOx in heart tissue compared with the rats
exposed to Fe2O3NPs. The rats coexposed to
both NPs exhibited a significant elevation in TBARS and NOx content
in cardiac and lung tissues compared with all other groups
(Table I).
 | Table I.Heart and lung tissues levels of
TBARS, NOx, GSH, SOD, CAT, GST, GPx and TAC of male rats exposed to
Fe2O3NPs and AgNPs. |
Table I.
Heart and lung tissues levels of
TBARS, NOx, GSH, SOD, CAT, GST, GPx and TAC of male rats exposed to
Fe2O3NPs and AgNPs.
| A, Heart
tissues |
|---|
|
|---|
|
| Experimental
groups |
|---|
|
|
|
|---|
| Parameter | Control |
Fe2O3NPs | AgNPs |
Fe2O3NPs+AgNPs |
|---|
| TBARS (nmol/g
tissue) | 59.9±1.2 |
78.8±1.4a |
87.5±3.4a,b |
116.4±3.6a–d |
| NO (µmol/g
tissue) | 28.24±0.74 |
33.35±0.30a |
40.78±2.5a,b |
47.80±0.27a–c,e |
| GSH (nmol/g
tissue) | 5.0±0.20 |
3.7±0.27a |
4.0±0.06a |
3.3±0.14a,c |
| SOD (mU/mg
protein) | 38.3±2.2 |
24.3±1.6a |
25.4±1.7a |
16.2±0.8a,b,c,c |
| CAT (U/mg
protein) | 55.0±2.94 |
40.0±1.11a |
38.0±1.88a |
18.0±0.89a–d |
| GST (U/mg
protein) | 0.46±0.03 |
0.36±0.01a |
0.32±0.02a |
0.29±0.01a,b |
| GPX (mU/mg
protein) | 26.6±1.81 |
18.4±1.18a |
17.1±1.29a |
13.4±0.63a–c,e |
| TAC (µmol/g
tissue) | 21.50±0.10 |
17.27±0.31a |
17.08±0.16a |
14.14±0.12a–d |
|
| B, Lung
tissues |
|
|
| Experimental
groups |
|
|
|
|
Parameter | Control |
Fe2O3NPs | AgNPs |
Fe2O3NPs+AgNPs |
|
| TBARS (nmol/g
tissue) | 41.0±1.5 |
75.2±2.1a | 70.6 ±
0.9a |
89.5±2.7a–c,e |
| NO (µmol/g
tissue) | 36.6±1.73 |
51.8±0.81a |
69.7±0.73a,b |
75.6±0.57a–c |
| GSH (nmol/g
tissue) | 5.7±0.13 |
4.7±0.21a |
4.4±0.24a |
3.5±0.18a–c |
| SOD (mU/mg
protein) | 9.0±0.2 |
6.7±0.2a |
7.2±0.1a |
4.4±0.2a–d |
| CAT (U/mg
protein) | 42.9±0.85 |
28.5±1.16a |
27.1±1.66a |
15.5±0.87a–d |
| GST (U/mg
protein) | 0.57±0.02 |
0.44±0.01a |
0.41±0.01a |
0.35±0.02a–c |
| GPX (mU/mg
protein) | 33.8±0.844 |
25.1±0.976a |
26.1±0.901a |
18.6±0.879a–d |
| TAC (µmol/g
tissue) | 22.8±0.02 |
21.1±0.13a |
18.7±0.07a,b |
16.3±0.07a–c |
Antioxidant levels decrease following
exposure to NPs
The antioxidant parameters detected in the present
study included GSH, which represents 90% of the reducing power of
the cell, and antioxidant enzymes SOD, CAT, GPX, and GST, and TAC.
Exposure to Fe2O3NPs or AgNPs caused a
significant decline in the heart and lung antioxidant parameters
compared with the levels found in control rats. (Table I) When comparing rats exposed to
Fe2O3NPs and the group exposed to AgNPs the
only significant difference was a significantly lower level of TAC
in the lung tissues of rats exposed to AgNPs. Of note, rats
coexposed to both NPs had significantly lower levels of all
antioxidant parameters compared with control rats and also rats
exposed to single NPs only (Table
I).
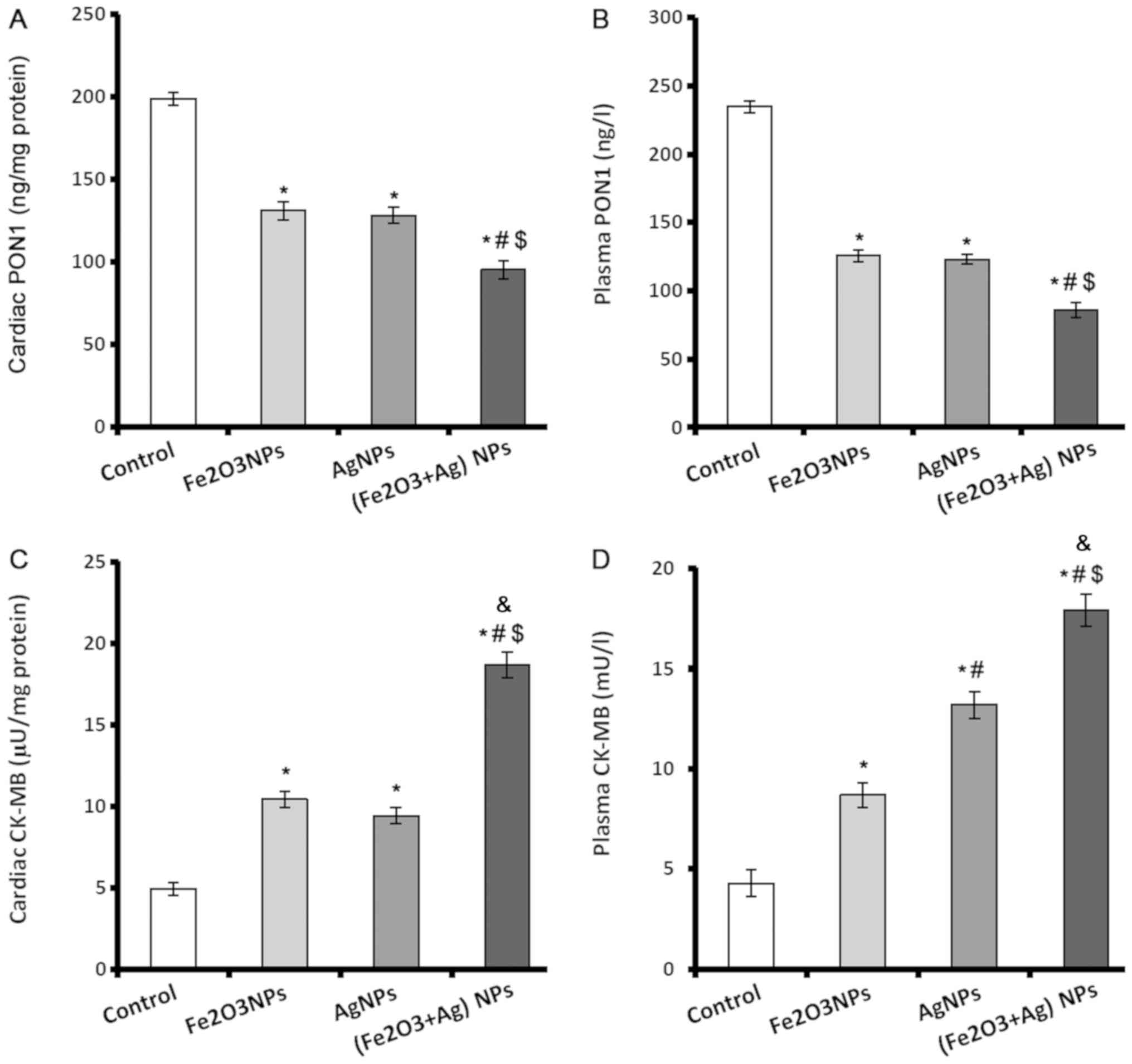
PON1 and creatine kinase-MB (CK-MB)
levels in cardiac and lung tissue decrease following exposure to
NPs
PON1 enzyme is a major antiatherosclerotic component
of HDL and has an important role in reducing atherosclerosis. PON1
levels in plasma and heart tissues were significantly lower in rats
exposed to Fe2O3NPs or AgNPs compared with
control rats (Fig. 3A and B).
However, there was no significant difference in PON1 levels between
the rats exposed to Fe2O3NPs or AgNPs.
Coexposure to both NPs caused a significant decline in the plasma
and cardiac levels of PON1 compared with the control group and rats
exposed to single NPs only (Fig. 3A and
B).
CK-MB is an important cardiac marker used to assist
diagnoses of myocardial infarction. The activity of CK-MB was
significantly elevated in the cardiac tissues and plasma of rats
exposed to Fe2O3NPs or AgNPs (Fig. 3C and D). AgNPs exposure caused
significantly higher plasma activity of CK-MB than exposure to
Fe2O3NPs. Coexposure to both NPs
significantly elevated the activity of CK-MB in plasma and cardiac
tissues by >3-fold (Fig. 3C and
D).
Lipid profile parameters
The plasma levels of total lipid, triglycerides,
cholesterol, LDL-c, vLDL-c and ApoB of rats exposed to
Fe2O3NPs or AgNPs were significantly higher
compared with control rats. The total lipids, LDL-c and ApoB were
significantly higher in the rats exposed to AgNPs compared with
those exposed to Fe2O3NPs, whilst the other
lipid profile parameters demonstrated no significant difference
between the two groups (Table II).
There were significantly lower levels of HDL-c in the rats exposed
to either Fe2O3NPs or AgNPs compared with the
control group. The rats coexposed to both NPs displayed
significantly higher levels of all lipid profile parameters, with
the exception of HDL-c which displayed significantly lower levels,
compared with the control rats and rats exposed to single NPs only
(Table II).
 | Table II.Plasma total lipid, triglyceride,
cholesterol, LDL-c, vLDL-c, HDL-C, and ApoB of male rats exposed to
Fe2O3NPs and AgNPs. |
Table II.
Plasma total lipid, triglyceride,
cholesterol, LDL-c, vLDL-c, HDL-C, and ApoB of male rats exposed to
Fe2O3NPs and AgNPs.
|
| Experimental
groups |
|---|
|
|
|
|---|
| Parameter
(mg/dl) | Control |
Fe2O3NPs | AgNPs |
Fe2O3NPs+AgNPs |
|---|
| Total lipid | 505±9.0 |
610±14.8a |
654±18.9a,b | 706±
21.0a–d |
| Triglycerides | 112.9±2.49 |
130.5±1.06a |
134.8±1.17a |
155.1±2.38a–c,e |
| Cholesterol | 141.6±5.38 |
169.1±3.29a |
182.6±3.10a |
221.6±5.10a–d |
| LDL-c | 50±6.9 | 88±6.9a |
107±4.3a,b |
157±7.1a–c,e |
| VLDL-c | 23±0.5 | 26±0.5a | 27±0.2a | 31±0.5a–c |
| HDL-c | 69±1.8 | 55±1.8a | 48±1.9a | 33±2.0a–d |
| ApoB | 51±4.5 | 79±
4.5a | 91±2.8a,b |
127±4.6a–d |
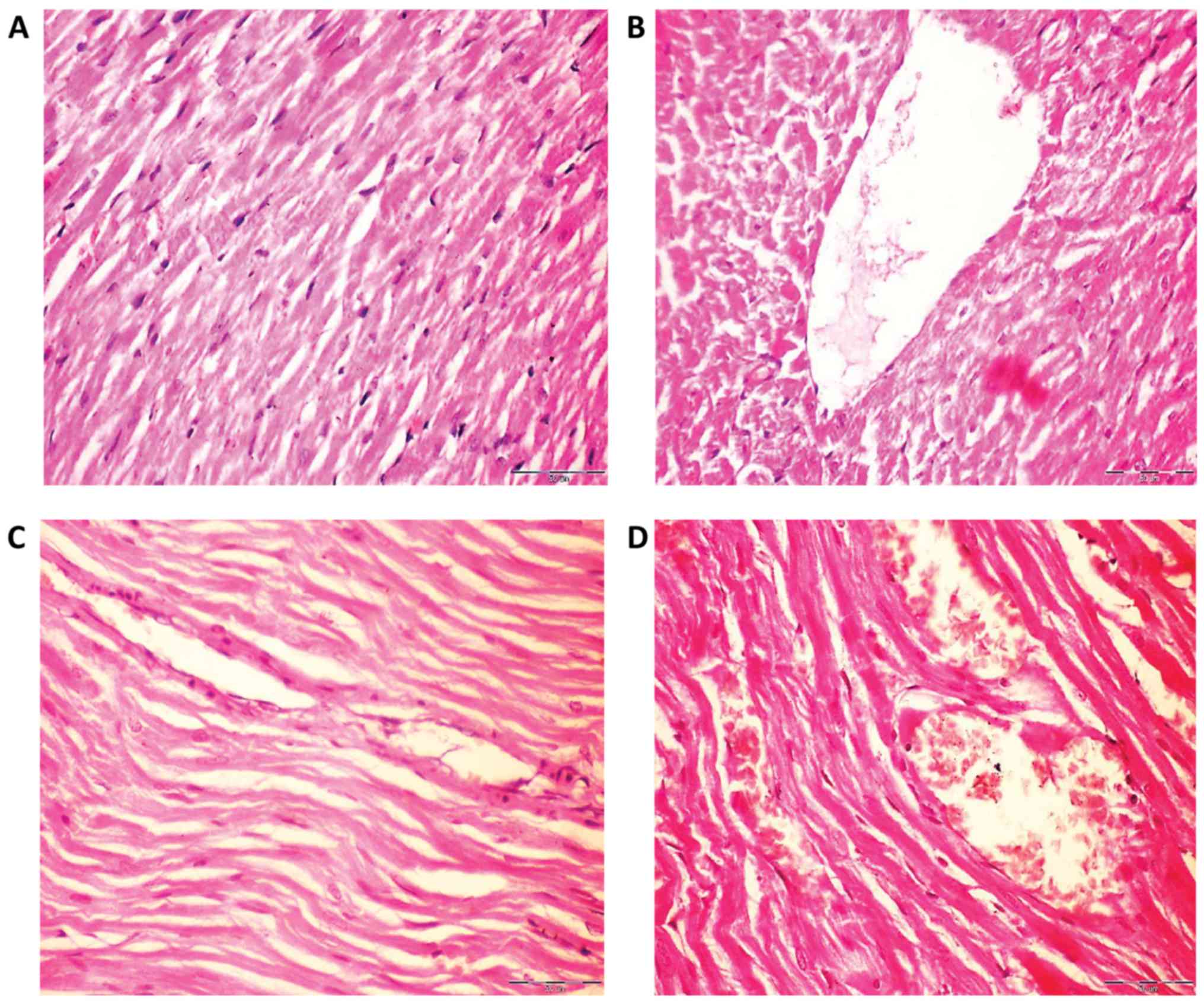
Histological changes in rat lung and
heart following exposure to NPs
The hearts of rats in the control group had normal
myofiber cells with unaffected architectures (Fig. 4A), whilst rats exposed to
Fe2O3NPs demonstrated effects of myocardial
degeneration (Fig. 4B). Similarly,
analysis of the rat heart tissue exposed to AgNPs (Fig. 4C) revealed fragmentation of
sarcoplasm and degeneration changes in myocardial fibers. The heart
tissues of the coexposed rats revealed loss of cross striation and
also myocardial degeneration changes (Fig. 4D).
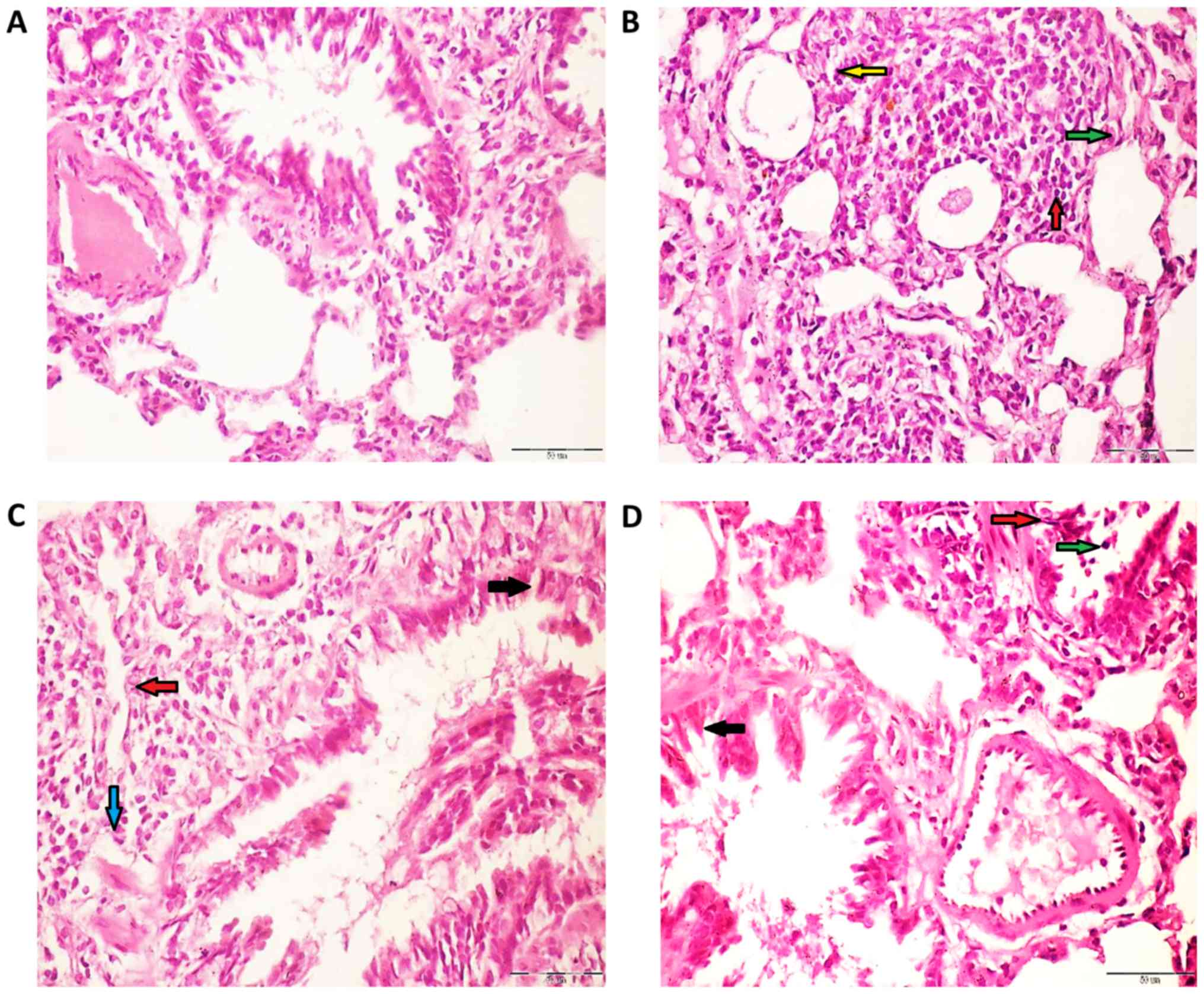
The lung tissue of control rats demonstrated normal
alveoli and bronchi lined mucous secreting columnar respiratory
epithelium (Fig. 5A). However, the
lung tissues belonging to rats exposed to
Fe2O3NPs (Fig.
5B) demonstrated a moderate influx of polymorph, lymphocytes,
and macrophages into perivascular tissue. Similarly, the lung
tissues of rats administered with AgNPs (Fig. 5C) displayed an influx of polymorph,
lymphocytes, and macrophages into the surrounding lung alveoli and
perivascular cuff. The lung tissue from coexposed rats (Fig. 5D) demonstrate a moderate influx of
polymorph, lymphocytes, and histiocytes into the surrounding
perivascular tissue and surrounding bronchi. Semi-quantitative
analysis was carried out on the heart and lung histology images of
the different rat groups (Table
III).
 | Table III.Semi-quantitative analysis of heart
and lung histology of male rats exposed to
Fe2O3NPs and AgNPs. |
Table III.
Semi-quantitative analysis of heart
and lung histology of male rats exposed to
Fe2O3NPs and AgNPs.
| A, Heart
tissue |
|---|
|
|---|
|
| Experimental
groups |
|---|
|
|
|
|---|
| Parameter
(mg/dl) | Control |
Fe2O3NPs | AgNPs |
Fe2O3NPs+AgNPs |
|---|
| Loss of cross
striation | − | ++ | + | +++ |
| Fragmentation of
sarcoplasm | − | ++ | + | +++ |
| Cytoplasmic
vacuolization in cardiac muscles | − | ++ | + | +++ |
| Degenerative
changes in myocardial fibers | − | ++ | ++ | +++ |
|
| B, Lung
tissue |
|
|
| Experimental
groups |
|
|
|
| Parameter
(mg/dl) | Control |
Fe2O3NPs | AgNPs |
Fe2O3NPs+AgNPs |
|
| Extravasations of
red blood cells into long parenchyma | − | ++ | +++ | ++++ |
| Interstitial
inflammation | − | ++ | ++ | ++++ |
| Alveolar wall
thickening | − | ++ | +++ | ++++ |
| Collapse of
terminal bronchioles | − | ++ | +++ | ++++ |
| Fibrosis | − | + | ++ | +++ |
| Alveolar
macrophages | − | ++ | +++ | ++++ |
| Polymorphs
infiltration | − | ++ | +++ | ++++ |
Discussion
Widespread medical and industrial NP applications
increase the risk of human exposure to various NPs that may cause
synergistic toxicities on internal organs. The cardiovascular and
respiratory systems are of particular concern as they face the most
interaction with NPs in medical processes and through general
environmental exposure. The present study confirmed the
cardiotoxicity and lung toxicity of both
Fe2O3NPs and AgNPs, and also demonstrated
their synergistic and additive effects in inducing these
toxicities.
Damage to DNA is a fundamental example of cellular
toxicity, and it is critical to identify damage caused by NPs as
DNA damage is highly correlated with an increased risk of cancer.
In the present study, 8-OHdG was employed as a sensitive indicator
for oxidative DNA damage. Analysis demonstrated a significant
8-OHdG elevation in heart and lung tissues as a result of the
exposure to Fe2O3NPs and AgNPs, with a even
more prominent increase when rats were subjected to the combined
NPs. Furthermore, NOx and TBARS were used as markers of lipid
peroxidation with increased levels detected following exposure to
NPs. The accumulation of these forms of oxidative damage results in
DNA mutations and genotoxicity that may cause cell death (apoptotic
or necrotic) or malignant transformation.
The ability of NPs to induce cell death is well
documented (17). A previous study
reported that AgNPs induces genotoxicity and cytotoxicity in cancer
and normal cell lines, alters cell morphology, reduces cell
viability, and causes oxidative stress in lung fibroblast and
glioblastoma cells (18).
AgNP-induced cytotoxicity may be partially caused by the direct
action of Ag+ ions being released from AgNPs (19) and the subsequent enhanced generation
of intracellular ROS and reactive nitrogen species (RNS), which are
responsible for inducing oxidative and nitrosative damage. In turn,
this results in lipid peroxidation of biological membranes, and
elicits oxidative DNA and structural protein damage (20,21).
Previous nanotoxicity studies conducted with
Fe2O3NPs have conclusively determined that
the production of ROS is a major causal factor of cell death
(22). Rate of uptake of
Fe2O3NPs is mediated by the mononuclear
phagocytic system via endocytosis and then degraded in the
lysosomes. This releases the free iron from
Fe2O3NPs, which affects the iron homeostasis
(23). Free iron is then stored in
the form of proteins, such as ferritin and haemosiderin, for
further use in the body. However, when the iron storage capacity of
these proteins is exceeded, iron overload occurs which triggers the
production of ROS via the Fenton reaction. In accordance with the
present results, many studies have documented the toxic effects of
Fe2O3NPs resulting in cellular and DNA damage
(9,22–25).
Fe2O3NPs causes cell death, mitochondrial
damage, and DNA damage in A549 cells (24). The genotoxic effects of
Fe2O3NPs are mainly caused by the direct
interaction with leached iron ions or various indirect factors,
such as excessive ROS. The direct and indirect contact of
Fe2O3NPs with DNA can affect the structure of
DNA causing strand breaks, cross-linking, and oxidation of
nucleotides as well as affecting DNA transcription and replication.
In addition, Fe2O3NPs upregulate genes that
are associated with endothelial layer integrity and lysosomal
function (25).
A further effect of exposure to NPs is an elevated
inflammatory response at the cellular level, as demonstrated by
various studies (1,2,5,6,9,26). A large variety of soluble factors
including ILs, TNF-α, ROS and RNS are inflammatory response factors
that mediate inflammation. These inflammatory factors promote DNA
damage such as chromosomal fragmentation, DNA point mutations,
inhibition of DNA repair and formation of methylation patterns,
that may lead to altered gene expression profiles and the formation
of DNA adducts (26).
The present study confirmed the proinflammatory
effects of Fe2O3NPs and AgNPs particularly
when combined. For example, significantly higher levels of TNF-α
and IL-6 in cardiac and lung tissues were detected. In accordance
with these results, Zhu et al (6) reported that intravascular
Fe2O3NPs induces endothelial system
inflammation and dysfunction via either direct interaction with the
endothelial monolayer or indirectly by releasing free iron, thus
impacting the endothelial cells and provoking oxidative stress
responses. Fe2O3NPs have evident
intratracheal toxic effects as they cause specific
pathomorphological damage in rat lungs and irreversible injury to
the membranes of alveolar cells (9).
NPs are known to upregulate the transcription of
various proinflammatory genes, including TNF-α, IL-1, IL-6, and
IL-8, by activating NF-κB signaling. These sequential molecular and
cellular events are known to cause oxidative stress, followed by
severe cellular genotoxicity and then programmed cell death through
activation of the JNK, p53 and NF-κB pathways.
Fe2O3NP- and AgNP-induced inflammatory
responses in murine tissues, such as the heart and lung have been
well documented (27,28). It has also been documented that
particle deposition in the lung causes recruitment of inflammatory
cells that generate ROS, clastogenic factors, and cytokines either
harming or stimulating resident lung cells (29).
Indices of oxidative damage on lipids and DNA
molecules indicate that heart and lung tissues suffer oxidative
stress damage not only as a result of increased ROS generation but
also due to the impairment of the antioxidant mechanism caused by
NP exposure. The present study demonstrated that
Fe2O3NPs and AgNPs significantly hampered the
main antioxidant processes in heart and lung tissues including
levels of antioxidant enzymes SOD, GST, GPx, and CAT, TAC, and the
GSH system. Therefore, a growing body of evidence, including the
present findings, confirm the pro-oxidant effects of
Fe2O3NPs and AgNPs (30,31).
Besides their general pro-oxidant and
proinflammatory effects, Fe2O3NPs and AgNPs
also induced specific metabolic alterations that may have important
roles as atherogenic factors. PON1 is a hydrolytic enzyme with a
wide range of substrates that is able to protect against lipid
oxidation. The atheroprotective effects of PON1 are achieved
through inhibition of LDL oxidation, reducing the risk of
cardiovascular disease (32). The
present results demonstrated that exposure to
Fe2O3NPs and AgNPs alone or in combination
caused depletion of PON1 in the plasma and heart tissues. The PON1
decline, together with the documented dyslipidemia including HDL-c
decrease and elevation of triglycerides, cholesterol, LDL-c and
total lipids, are strong atherogenic factors that predisposed the
rats towards developing cardiovascular diseases. This risk is
evidenced by the significant elevation of CK-MB plasma and cardiac
activity as well as the histological abnormalities of cardiac
tissues including myocardial congestion and degeneration. The
increased activity of CK-MB in cardiac tissues due to
Fe2O3NPs and AgNPs exposure may lead to
disruption of the specific role of CK in the excitation-contraction
mechanism. In addition, the high plasma activity of CK-MB is an
indication of myocardial injury. In agreement with the present
results, Nemmar et al (33)
reported that Fe2O3NPs increases CK-MB levels
in the heart and plasma tissues. Similarly, Shen et al
(34) reported that
Fe2O3NPs attack the myocardium muscles
inducing myocardial iron overload, resulting in myocardial injury
and deterioration of cardiac function via oxidative stress-mediated
iron toxicity. Histology findings revealed myocyte apoptosis,
inflammation, and fibrosis. Furthermore, Rathore et al
(35) concluded that the heart
muscle fibers treated with AgNPs demonstrated mild edema and
separation of myofibrils. In addition, Adeyemi and Faniyan
(36) documented that AgNPs cause
inflammation, loss of cross striation with myocardial degeneration
and cellular alteration in the cardiac tissue of male Wistar rats.
Warheit et al (37) reported
that in rat lungs exposed to Fe2O3NPs, the
interstitium widened and was infiltrated with lymphocytes,
macrophages, and plasma cells and fibrosis developed.
In summary, the present study determined the adverse
effects of long-term exposure to Fe2O3NPs and
AgNPs on the heart and lungs. Cardiotoxicity and lung toxicity were
induced via different pathways including oxidative DNA
modification, induction of inflammation, free radical generation,
and inhibition of antioxidant mechanisms. In addition, NPs caused
alterations in heart and lung histology and lipid profiles.
Furthermore, the results evidently demonstrated that simultaneous
co-exposure to Fe2O3NPs and AgNPs resulted in
greater ramifications to the heart and lungs compared to the effect
of individual NPs. The possible adverse effects on other rat organs
coexposed to Fe2O3NPs and AgNPs will be
further investigated with focus on the male reproductive system,
liver and brain. In conclusion, the present findings raise concerns
about the synergistic effects of different NPs in inducing chronic
toxicity on the heart and lungs, and the impact this may have on
the subsequent predisposition to cardiovascular and pulmonary
diseases.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
MIY suggested the research topic, participated in
the experimental design and contributed to the writing of the
paper. AAA performed most experiments, carried out the statistical
analysis of data and helped in the writing of the paper. MANK
designed the experiments, performed the molecular experiments, and
contributed to the writing and revising of the paper. All authors
have read and approved the final paper.
Ethics approval and consent to
participate
All experimental procedures, animal handling,
sampling, and scarification were conducted in accordance with the
Guide for the Care and Use of Laboratory Animals, 8th edition
(National Research Council 2011) and were approved by the Research
Ethical Committee of the Medical Research Institute, Alexandria
University. All efforts were made to minimise the rats' suffering
during the experimental period.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
8-OHdG
|
8-hydroxy-2′-deoxyguanosine
|
|
TBARS
|
thiobarbituric acid-reactive
substances
|
|
GSH
|
reduced glutathione
|
|
GST
|
glutathione S-transferase
|
|
SOD
|
superoxide dismutase
|
|
CAT
|
catalase
|
|
ROS
|
reactive oxygen species
|
|
GPX
|
glutathione peroxidase
|
|
LPO
|
lipid peroxidation
|
|
PON1
|
paraoxinase 1
|
|
vLDL
|
very low-density lipoprotein
|
|
ApoB
|
apolipoprotein B
|
|
CK
|
creatine kinase
|
|
HDL-c
|
high-density
lipoprotein-cholesterol
|
|
NOx
|
nitric oxide
|
|
LDL
|
low-density lipoprotein
|
|
TL
|
total lipids
|
|
RNS
|
reactive nitrogen species
|
|
TAC
|
total antioxidant capacity
|
References
|
1
|
Oberdörster G, Stone V and Donaldson K:
Toxicology of nanoparticles: A historical perspective.
Nanotoxicology. 1:2–25. 2007. View Article : Google Scholar
|
|
2
|
Karlsson HL, Gustafsson J, Cronholm P and
Möller L: Size-dependent toxicity of metal oxide particles-a
comparison between nano-and micrometer size. Toxicol Lett.
188:112–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Senapati S, Ahmad A, Khan MI, Sastry M and
Kumar R: Extracellular biosynthesis of bimetallic AuAg alloy
nanoparticles. Small. 1:517–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan I, Saeed K and Khan I: Nanoparticles:
Properties, applications and toxicities. Arab J Chem. MAy
18–2017.(Epub ahead of print). doi: 10.1016/j.arabjc.2017.05.011.
View Article : Google Scholar
|
|
5
|
Akter M, Sikder T, Rahmana M, Ullah A,
Hossain KFB, Banik S, Hosokawa T, Saito T and Kurasaki M: A
systematic review on silver nanoparticles-induced cytotoxicity:
Physicochemical properties and perspectives. J Adv Res. 9:1–16.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu MT, Wang B, Wang Y, Yuan L, Wang HJ,
Wang M, Ouyang H, Chai ZF, Feng WY and Zhao YL: Endothelial
dysfunction and inflammation induced by iron oxide nanoparticle
exposure: Risk factors for early atherosclerosis. Toxicol Lett.
203:162–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J and Hurt RH: Ion release kinetics
and particle persistence in aqueous nano-silver colloids. Environ
Sci Technol. 44:2169–2175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bar-Ilan O, Albrecht RM, Fako VE and
Furgeson DY: Toxicity assessments of multisized gold and silver
nanoparticles in zebrafish embryos. Small. 5:1897–1910. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szalay B, Tátrai E, Nyírő G, Vezér T and
Dura G: Potential toxic effects of iron oxide nanoparticles in vivo
and in vitro experiments. J Appl Toxicol. 32:446–453. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharma VK, Yngard RA and Lin Y: Silver
nanoparticles: Green synthesis and their antimicrobial activities.
Adv Colloid Interface Sci. 145:83–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
The University of Iowa. Office of animal
resources, . Institutional animal care and use committee. IACUC
Guidelines: Anesthesia. https://animal.research.uiowa.edu/iacuc-guidelines-anesthesiaDecember
26–2018
|
|
12
|
Draper HH and Hadley M: Malondialdehyde
determination as index of lipid peroxidation. Methods Enzymol.
186:421–431. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guevara I, Iwanejko J, Dembińska-Kieć A,
Pankiewicz J, Wanat A, Anna P, Gołabek I, Bartuś S,
Malczewska-Malec M and Szczudlik A: Determination of
nitrite/nitrate in human biological material by the simple Griess
reaction. Clin Chim Acta. 274:177–188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Griffith OW: Determination of glutathione
and glutathione disulfide using glutathione reductase and
2-vinylpyridine. Anal Biochem. 106:207–212. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mueller RF, Hornung S, Furlong CE,
Anderson J, Giblett ER and Motulsky AG: Plasma paraoxonase
polymorphism: A new enzyme assay, population, family, biochemical,
and linkage studies. Am J Hum Genet. 35:393–408. 1983.PubMed/NCBI
|
|
16
|
Drury RA and Wallington EA: Carleton's
Histological Techniques6th. Oxford University Press; New York, NY,
Toronto: 1980
|
|
17
|
Fumagalli M, Rossiello F, Clerici M,
Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V,
Beausejour CM, et al: Telomeric DNA damage is irreparable and
causes persistent DNA-damage response activation. Nat Cell Biol.
14:355–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asharani PV, Hande MP and Valiyaveettil S:
Anti-proliferative activity of silver nanoparticles. BMC Cell Biol.
10:652009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh RP and Ramarao P: Cellular uptake,
intracellular trafficking and cytotoxicity of silver nanoparticles.
Toxicol Lett. 213:249–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Foldbjerg R, Olesen P, Hougaard M, Dang
DA, Hoffmann HJ and Autrup H: PVP-coated silver nanoparticles and
silver ions induce reactive oxygen species, apoptosis and necrosis
in THP-1 monocytes. Toxicol Lett. 190:156–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim SH, Ko JW, Koh SK, Lee IC, Son JM,
Moon C, Kim SH, Shin DH and Kim JC: Silver nanoparticles induce
apoptotic cell death in cultured cerebral cortical neurons. Mol
Cell Toxicol. 10:173–179. 2014. View Article : Google Scholar
|
|
22
|
Patil US, Adireddy S, Jaiswal A, Mandava
S, Lee BR and Chrisey DB: In vitro/in vivo toxicity evaluation and
quantification of iron oxide nanoparticles. Int J Mol Sci.
16:24417–24450. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Voinov MA, Sosa Pagán JO, Morrison E,
Smirnova TI and Smirnov AI: Surface-mediated production of hydroxyl
radicals as a mechanism of iron oxide nanoparticle biotoxicity. J
Am Chem Soc. 133:35–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JH, Ju JE, Kim BI, Pak PJ, Choi EK,
Lee HS and Chung N: Rod-shaped iron oxide nanoparticles are more
toxic than sphere-shaped nanoparticles to murine macrophage cells.
Environ Toxicol Chem. 33:2759–2766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alarifi S, Ali D and Alkahtani S:
Nanoalumina induces apoptosis by impairing antioxidant enzyme
systems in human hepatocarcinoma cells. Int J Nanomedicine.
10:3751–3760. 2015.PubMed/NCBI
|
|
26
|
Park EJ and Park K: Oxidative stress and
pro-inflammatory responses induced by silica nanoparticles in vivo
and in vitro. Toxicol Lett. 184:18–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pujalté I, Passagne I, Brouillaud B,
Tréguer M, Durand E, Ohayon-Courtès C and L'Azou B: Cytotoxicity
and oxidative stress induced by different metallic nanoparticles on
human kidney cells. Part Fibre Toxicol. 8:102011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mukaetova-Ladinska EB, Garcia-Siera F,
Hurt J, Gertz HJ, Xuereb JH, Hills R, Brayne C, Huppert FA, Paykel
ES, McGee M, et al: Staging of cytoskeletal and beta-amyloid
changes in human isocortex reveals biphasic synaptic protein
response during progression of Alzheimer's disease. Am J Pathol.
157:623–636. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nel A, Xia T, Mädler L and Li N: Toxic
potential of materials at the nanolevel. Science. 311:622–627.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang A, Pu K, Dong B, Liu Y, Zhang L,
Zhang Z, Duan W and Zhu Y: Role of surface charge and oxidative
stress in cytotoxicity and genotoxicity of graphene oxide towards
human lung fibroblast cells. J Appl Toxicol. 33:1156–1164. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McShan D, Ray PC1 and Yu H: Molecular
toxicity mechanism of nanosilver. J Food Drug Anal. 22:116–127.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Litvinov D, Mahini H and Garelnabi M:
Antioxidant and anti-inflammatory role of paraoxonase 1:
Implication in arteriosclerosis diseases. N Am J Med Sci.
4:523–532. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nemmar A, Beegam S, Yuvaraju P, Yasin J,
Tariq S, Attoub S and Ali BH: Ultrasmall superparamagnetic iron
oxide nanoparticles acutely promote thrombosis and cardiac
oxidative stress and DNA damage in mice. Part Fibre Toxicol.
13:222016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen Y, Huang Z, Liu X, Qian J, Xu J, Yang
X, Sun A and Ge J: Iron-induced myocardial injury: An alarming side
effect of superparamagnetic iron oxide nanoparticles. J Cell Mol
Med. 19:2032–2035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rathore M, Mohanty IR, Maheswari U, Dayal
N, Suman R and Joshi DS: Comparative in vivo assessment of the
subacute toxicity of gold and silver nanoparticles. J Nanopart Res.
16:23382014. View Article : Google Scholar
|
|
36
|
Adeyemi OS and Faniyan TO: Antioxidant
status of rats administered silver nanoparticles orally. J Taibah
Univ Med Sci. 9:182–186. 2014.
|
|
37
|
Warheit DB, Sayes CM, Reed KL and Swain
KA: Health effects related to nanoparticle exposures:
Environmental, health and safety considerations for assessing
hazards and risks. Pharmacol Ther. 120:35–42. 2008. View Article : Google Scholar : PubMed/NCBI
|