Introduction
Cryptogenic organizing pneumonia (COP), also known
as bronchiolitis obliterans with organizing pneumonia (BOOP),
occurs without obvious pathogens or other concomitant diseases. It
presents as lesions involving mainly the alveolar spaces, alveolar
ducts, small airways and interstitial lung. Histopathological
findings demonstrate the proliferation of granulation tissue in the
alveolar spaces and bronchioles. Clinical manifestations include
interstitial lung disease with restrictive ventilatory dysfunction
(1,2). In 1983, Davision et al (3) referred to as-yet undescribed BOOP as
COP. In 2013, the American Thoracic Society and the European
Respiratory Society reclassified idiopathic interstitial pneumonia
(IIP) into three categories, i.e., main, rare and unclassifiable;
COP is categorised as main IIP (4).
The incidence of COP is low. It is sensitive to glucocorticoids and
has a good prognosis. However, COP presents with no specific
clinical manifestations and imaging features, lacks specific
biomarkers and is particularly difficult to distinguish from
community-acquired pneumonia.
Genetic, environmental, immune and other pathogenic
factors, including viral infections, interact to maintain a high
incidence of thyroid disease (5).
Among these, autoimmune thyroid diseases account for ~90% of
thyroid diseases (6). Hashimoto's
thyroiditis (HT), also known as chronic lymphocytic thyroiditis, is
a classic type of autoimmune thyroiditis. It usually manifests as a
local inflammatory response and is closely linked to autoimmunity.
The disease was first reported by the Japanese scholar Hakaru
Hashimoto in 1912 (7), and is thus
also known as Hashimoto's disease. Its pathogenesis is mediated by
a large number of lymphocytes that infiltrate the thyroid
parenchyma, repeatedly destroying the follicular structure of the
gland and causing hyperplasia of the interstitial fibres. This
results in diffuse or uneven nodularity. HT is accompanied by
thyroid tumours (8–11) and hypothyroidism but rarely causes
COP. It has been reported that the prevalence of HT with papillary
thyroid carcinoma is 0.5–38% (10).
Studies have also indicated that HT is an important risk factor for
primary non-Hodgkin's lymphoma (12), and it has even been suggested that HT
should be considered a pre-cancerous precursor of thyroid
cancer.
In the available literature, HT combined with COP
has been reported infrequently (13). One case series reporting on 18 cases
of organizing pneumonia indicated that it frequently occurred
concomitantly with infections and autoimmune disease. One of the 18
patients had organizing pneumonia that was considered to have been
caused by Hashimoto disease (13).
HT as well as COP are associated with viral infection and
autoimmunity, and there are certain similar risk factors.
Therefore, patients with Hashimoto's thyroiditis complicated by
concurrent COP may be more seriously ill and require longer
hospital stays. Whether long-term follow-up is able to detect a
higher incidence of thyroid tumours is currently elusive and
warrants further investigation.
Case report
Case presentation
A 49-year-old female developed chest tightness and
shortness of breath without apparent cause and presented at a local
hospital. Chest radiographs indicated increased thickening of the
lung texture and increased multiple patchy densities in the lower
lobes of the bilateral lungs, and a slightly increased thyroid was
seen on examination. The patient underwent electrocardiogram (ECG)
and echocardiography to exclude cardiogenic factors, was diagnosed
with ‘pulmonary infection, goitre’ and given antibiotic treatment
with levofloxacin and ribavirin for 9 days. The patient's symptoms
were not significantly relieved and the shortness of breath
gradually worsened, with associated fever, cough and a small amount
of white sticky sputum. Chest CT imaging indicated ‘pulmonary
infection’ and the antibiotics were broadened to meropenem,
vancomycin and fluconazole. After this treatment, the patient's
shortness of breath worsened progressively and she remained febrile
with a maximum temperature of 38.7°C. After 5 days of treatment,
chest CT indicated that the lung exudates were significantly
increased. The patient was transferred from the local hospital to
the critical care unit at The First Affiliated Hospital of Xi'an
Jiaotong University. The patient was conscious on admission, with
low body weight (49 kg). The patient's vital signs included a body
temperature of 37.2°C, heart rate of 97 beats/min, respiratory rate
of 37 breaths/min and blood pressure of 112/69 mmHg. On
examination, thyroid enlargement (grade 2) was noted. The thyroid
was firm and vascular murmur was absent. The lungs exhibited
dullness with percussion, and moist rales were heard at the
bilateral lung bases.
The patient was admitted to the hospital and
subsequently, her respiratory status further deteriorated. Arterial
blood gas analysis demonstrated the following: pH 7.42; oxygen
partial pressure, 54.0 mmHg; carbon dioxide partial pressure, 35.7
mmHg. These findings were consistent with Type 1 respiratory
failure and the patient was put on non-invasive mechanical
ventilation. Chest CT indicated ground-glass changes in the
bilateral lungs and multiple large high-density lesions. The
C-reactive protein level was 65.6 mg/l and a diagnosis of severe
pneumonia was considered. The patient received a combination
broad-spectrum anti-microbial treatment with imipenem,
moxifloxacin, ganciclovir and voriconazole. After one week of
treatment, the patient still had difficulty breathing and was not
able to be weaned from the non-invasive ventilator; the patient was
still experiencing intermittent fever with a body temperature of up
to 38.6°C, without any significant decrease in C-reactive protein
levels. Bronchoscopy with alveolar lavage and lung biopsy was
performed in order to exclude any infectious or malignant
aetiologies for the patient's respiratory symptoms.
Assessments
The initial and subsequent complete blood cell
counts revealed low and fluctuating haemoglobin, a normal to low
white blood cell count, elevated neutrophil percentage, as well as
normal platelets and eosinophils. Liver function indicators were
within normal ranges except for serum sodium, which was low. Serum
inflammatory markers (C-reactive protein and erythrocyte
sedimentation rate) were elevated. The patient's coagulation
profile indicated elevated activated partial thromboplastin time,
fibrinogen content and D-dimer. All values are provided in Table I.
 | Table I.Summary of diagnostic indicators for
the patient. |
Table I.
Summary of diagnostic indicators for
the patient.
| A, Blood cell
count |
|---|
|
|---|
| Variable | Patient value | Normal
rangea | Characterization of
patient level compared with normal level |
|---|
| Haemoglobin
(g/l) | 85 | 110–150 | Low |
| White cell count
(×109/l) | 5.93 | 3.5–9.5 | Normal to low |
| Neutrophils (%) | 79.8 | 40–75 | Elevated |
| Platelets
(×109/l) | 280 | 125–350 | Normal |
| Eosinophils
(×109/l) | 0.11 | 0.02–0.52 | Normal |
|
| B, Liver
function |
|
| Variable | Patient
value | Normal
range | Characterization
of patient level compared with normal level |
|
| Ca (mmol/l) | 2.06 | 2.11–2.52 | Low |
| Mg (mmol/l) | 0.94 | 0.75–1.02 | Normal |
| Urea (mmol/l) | 2.46 | 2.6–7.5 | Low |
| Creatinine
(µmol/l) | 46 | 41–73 | Normal |
| Serum Na
(mmol/l) | 131 | 137–147 | Low |
|
| C, Inflammatory
markers |
|
| Variable | Patient
value | Normal
range | Characterization
of patient level compared with normal level |
|
| C-reactive protein
(mg/l) | 65.6 | 0.0–10.0 | Elevated |
| Erythrocyte
sedimentation rate (mm/h) | 91 | 0.0–20 | Elevated |
|
| D, Coagulation
profile |
|
| Variable | Patient
value | Normal
range | Characterization
of patient level compared with normal level |
|
| Activated partial
thromboplastin time (sec) | 45 | 28–43.5 | Elevated |
| Fibrinogen content
(g/l) | 5.69 | 2.0–4.0 | Elevated |
| D-dimer (mg/l) | 1.14 | 0–1 | Elevated |
|
| E,
Rheumatology |
|
| Variable | Patient
value | Normal
range | Characterization
of patient level compared with normal level |
|
| Anti-nuclear
antibodies (ratio) | 1:80 | ≤1:80 | Normal |
|
CD3+CD8+ Killer
T-cells (%) | 31.93 | 18.1–29.6 | Elevated |
| CD4/CD8 | 0.58 | 0.8–2.1 | Low |
| Procalcitonin
(ng/ml) | 0.043 | <0.5 | Normal |
| 1-3-b-D glucans
(pg/ml) | <10 | <60 | Normal |
| Galactomannan
(µg/l) | 0.96 | <0.5 | Elevated |
|
| F, Thyroid
function |
|
|
Variable | Patient
value | Normal
range | Characterization
of patient level compared with normal level |
|
| TPOAb (U/ml) | >3,000 | <15 | Greatly
elevated |
| T3
(ng/ml) | 0.660 | 0.78–2.20 | Low |
| FT4
(pmol/l) | 7.96 | 9.05–25.5 | Low |
| TSH (uIU/ml) | 9.61 | 0.25–5 | Elevated |
| TgAb (%) | 22.1 | <30 | Normal |
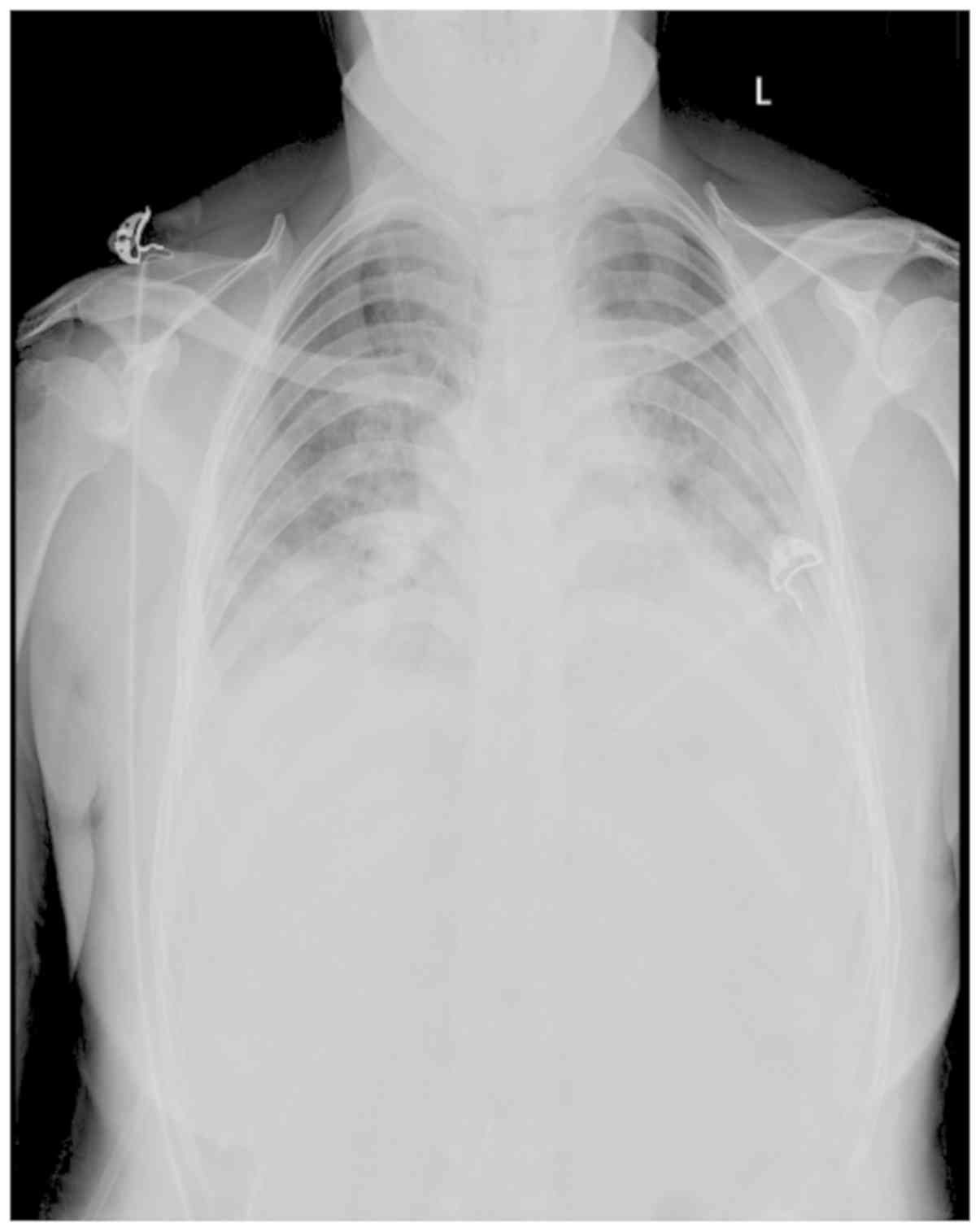
Initial chest radiographs indicated increased
thickening of the lung texture, increased multiple patchy densities
in the lower lobes of the bilateral lungs and a small bilateral
pleural effusion (Fig. 1). After the
patient was admitted to the intensive care unit of our hospital,
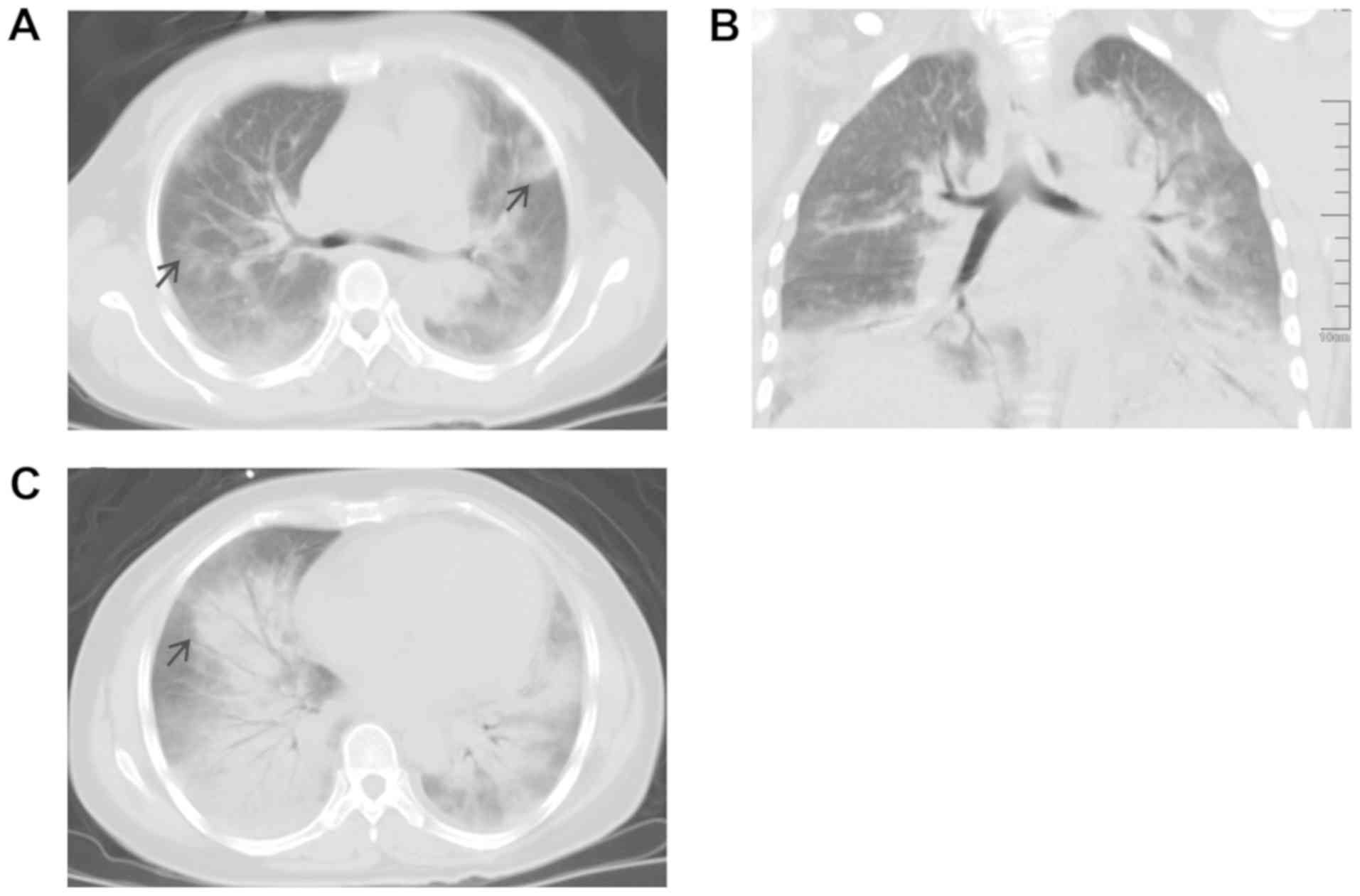
non-invasive mechanical ventilation was provided. Chest CT revealed
decreased translucency and increased ground-glass changes in the
bilateral lung fields, with multiple large high-density lesions
obscuring the borders of the lobar fissures. Air bronchograms were
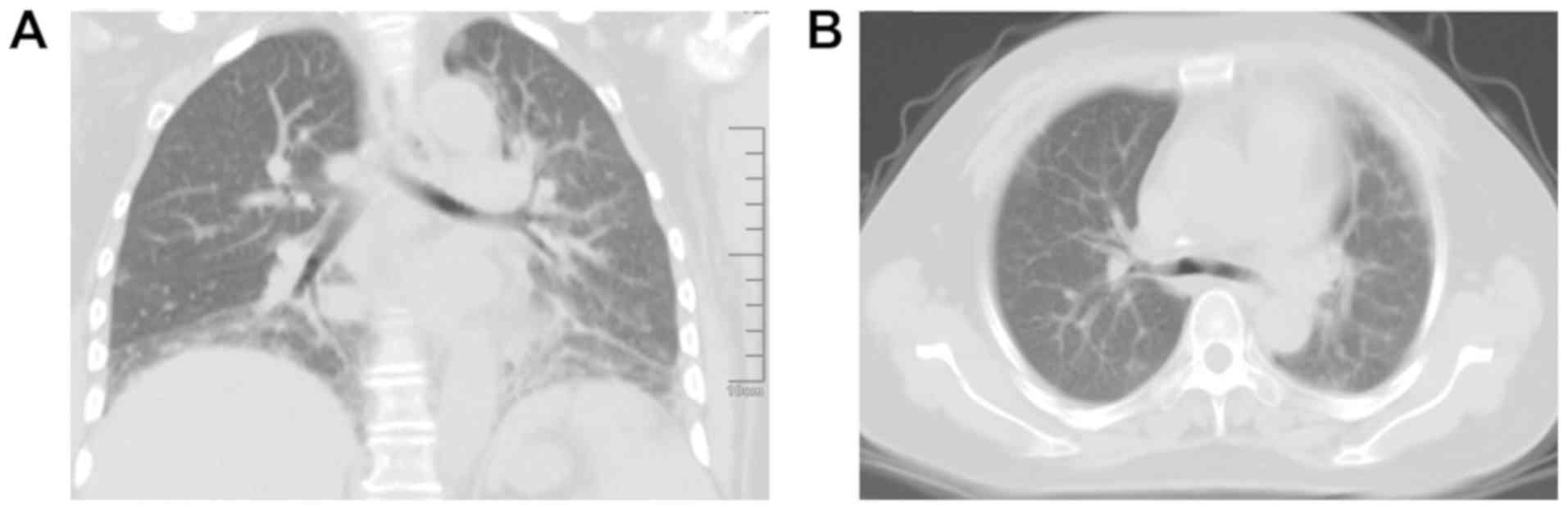
observed within a large high-density lesion (Fig. 2A-C).
Bronchoscopy revealed mild bilateral bronchial
oedema but no masses, haemorrhage or stenosis that may have
suggested inflammation of the bronchial mucosa. Examination of the
bronchoalveolar lavage fluid indicated negativity on tuberculosis
smears, tuberculosis DNA test, bacterial and fungal smears, and
bacterial and fungal cultures. Blood and lavage fluids were
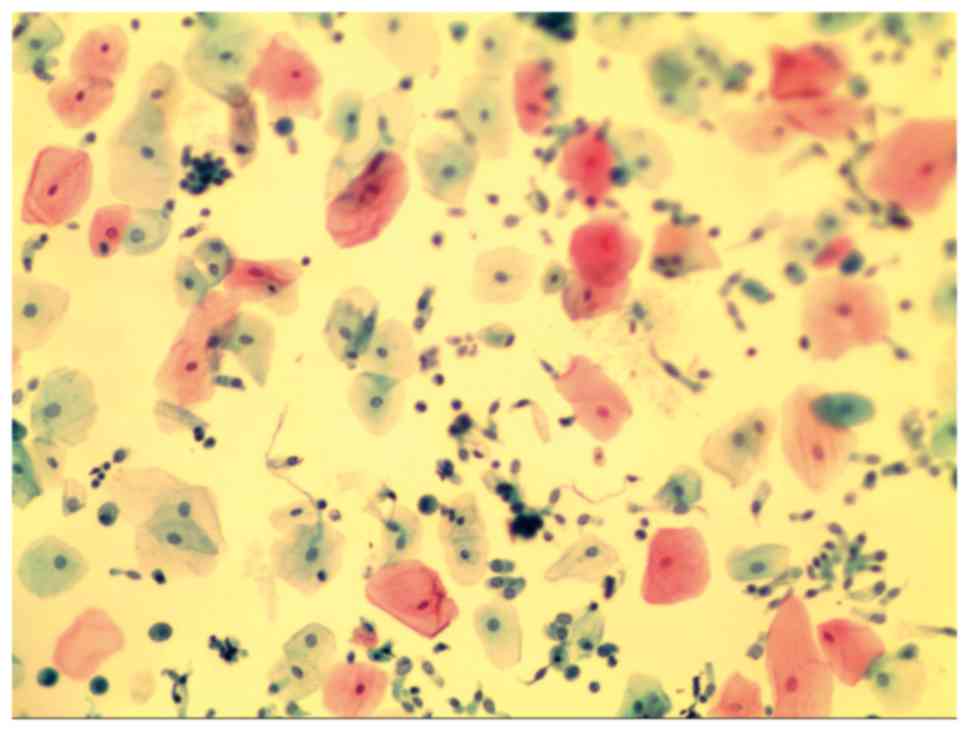
negative for tumour-associated markers. Cytological examination of
lavage fluid revealed no malignant cells, but columnar cells,
squamous cells, alveolar macrophages, lymphocytes and neutrophils
were observed (Fig. 3). The
diagnoses of systemic lupus erythematosus and sarcoidosis were not
supported, and all of the above results did not support a diagnosis
of bacterial, fungal, viral or atypical pathogenic infections,
including pneumocystis.
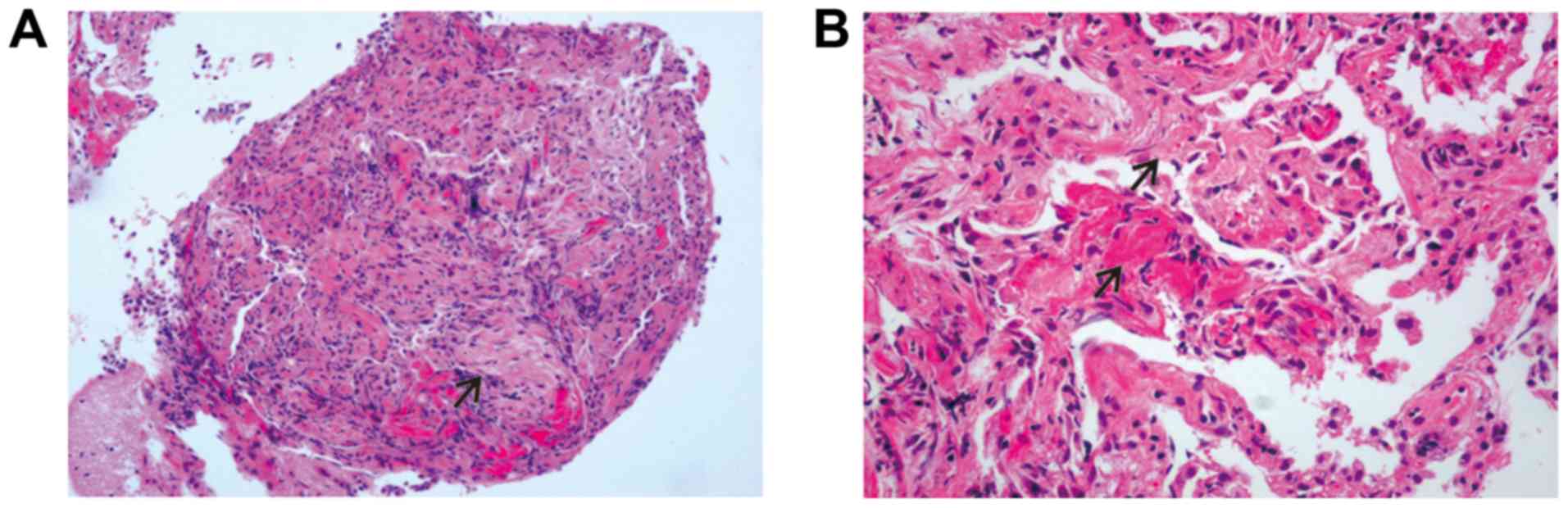
The lung biopsy revealed characteristic changes of
COP, with loose fibroblast plugs in the alveolar space and
extensive proliferation of fibroblasts (Fig. 4). Eosinophils were not observed in
the lung biopsy and Churg-Strauss syndrome was ruled out.
Blood and urine cultures and urine smears were
negative. Procalcitonin and 1,3-β-d-glucan were normal and
galactomannan was elevated (Table
I). Tests for Epstein-Barr virus, cytomegalovirus, human
immunodeficiency virus, hepatitis B virus DNA, hepatitis and
respiratory syncytial virus antibodies were all negative, as were
the antigen tests for Streptococcus pneumoniae, Mycoplasma
pneumoniae, Toxoplasma gondii, Legionella pneumophila and
Treponema pallidum.
The rheumatology work-up was negative for
perinuclear anti-neutrophil cytoplasmic antibodies, cytoplasmic
anti-neutrophil antibodies and double-stranded DNA antibodies.
Anti-nuclear antibodies (1:80) were weakly positive, a non-specific
finding. The percentage of killer T cells
(CD3+CD8+) was slightly elevated and the
CD4/CD8 ratio was low (Table I).
Thyroid ultrasound revealed a diffusely enlarged
symmetric thyroid gland with enlargement of the isthmus, a smooth
and well-demarcated capsule, hypoechoic nodules visible in the
bilateral lobes and regular morphology. Colour Doppler imaging
indicated slightly increased vascularity of the gland. Thyroid
function tests revealed markedly increased thyroid peroxidase
auto-antibody (TPOAb), low triiodothyronine, normal free thyroxine,
elevated thyroid stimulatory hormone and normal thyroglobulin
antibody (TgAb) (Table I).
Differential diagnosis
The early onset of the patient's respiratory
symptoms and early tests led us to consider bacterial pneumonia,
fungal pneumonia or rare pathogens. However, subsequent testing for
these infections and extensive tests mentioned above were all
negative. The patient received antibiotic treatment directly after
admission to the critical care unit, mainly due to her clinical
symptoms and associated imaging.
It is noteworthy that anti-microbial therapy in
patients with COP has not led to any improvement in pulmonary
status. The cause of COP itself remains elusive. Conditions
including aspneumonia, drug toxicity, lung cancer and non-specific
interstitial pneumonia may cause COP-like pathological pulmonary
symptoms.
It has been reported that the presence of multiple
migratory patchy opacities seen on CT in the bilateral lungs is a
characteristic radiographic manifestation of COP, and the area of
lung bearing tho lesions may reflect disease severity to a certain
extent (14). However, recent
studies have indicated that conditions including hypersensitivity
pneumonitis, eosinophilic pneumonia and fungal infections may
present with similar radiographic patterns. It is difficult to
diagnose COP from the clinical manifestations and imaging alone,
particularly in the early stages of COP, given the wide variety of
differential diagnoses encompassing numerous mimicking conditions
(15).
Rheumatological causes, including systemic lupus
erythematosus, sarcoidosis, polymyositis and Churg-Strauss
syndrome, were also considered, but the blood work and lung biopsy
were not suggestive of these aetiologies. Malignancy, including
lymphoma or leukaemia, was also considered, and a bone marrow
biopsy was performed to exclude these diagnoses.
HT may be distinguished from subacute thyroiditis
due to its association with autoimmunity. HT frequently presents
with clinical hypothyroidism and elevated TPOAb and TgAb. Subacute
thyroiditis is associated with viral infections and is
self-limited, usually without any long-term sequelae. HT is easy to
confuse with certain types of thyroid tumour (8–11),
requiring careful diagnostic attention.
Diagnostic focus was initially centred on the
imaging features in the lungs and various pulmonary infections were
carefully considered as the causative agent. The final diagnosis of
COP was achieved with lung biopsies and histopathologic review. The
patient's condition improved with corticosteroid treatment.
Subacute thyroiditis requires regular review and follow-up for
recurrence, rather than a particular treatment.
Treatment
Blood gas analysis of the patient indicated type I
respiratory failure, which was treated with non-invasive mechanical
ventilation. According to the chest CT findings and the patient's
clinical presentation, severe pneumonia was considered to be the
most likely diagnosis, even though the specific causative pathogen
was not clear. The patient received broad-spectrum anti-microbial
treatment with imipenem, moxifloxacin, ganciclovir and voriconazole
for the treatment of the suspected pneumonia. The patient also
received human intravenous immunoglobulin 0.4 g/kg/day for 5
days.
During the course of treatment, there was no
significant improvement seen on chest CT examinations. Testing for
all associated pathogens was negative, and infection-associated
indices, including white blood cell count and plateletcrit, were
normal, yet the symptoms of the patient's clinical condition,
including chest tightness and shortness of breath, did not improve.
At one week after the start of anti-microbial therapy,
fibrobronchoscopy with lung biopsy indicated characteristic changes
of COP on histopathological analysis, thus establishing a diagnosis
of COP.
After the definitive diagnosis, antibiotics were
discontinued, and intravenous methylprednisolone (80 mg daily) was
initiated. After 5 days of treatment with methylprednisolone, the
dose was reduced to 40 mg daily. At this point, the patient's body
temperature returned to normal, her shortness of breath gradually
improved and the non-invasive ventilator was intermittently
disengaged. After a week of treatment, chest CT indicated that the
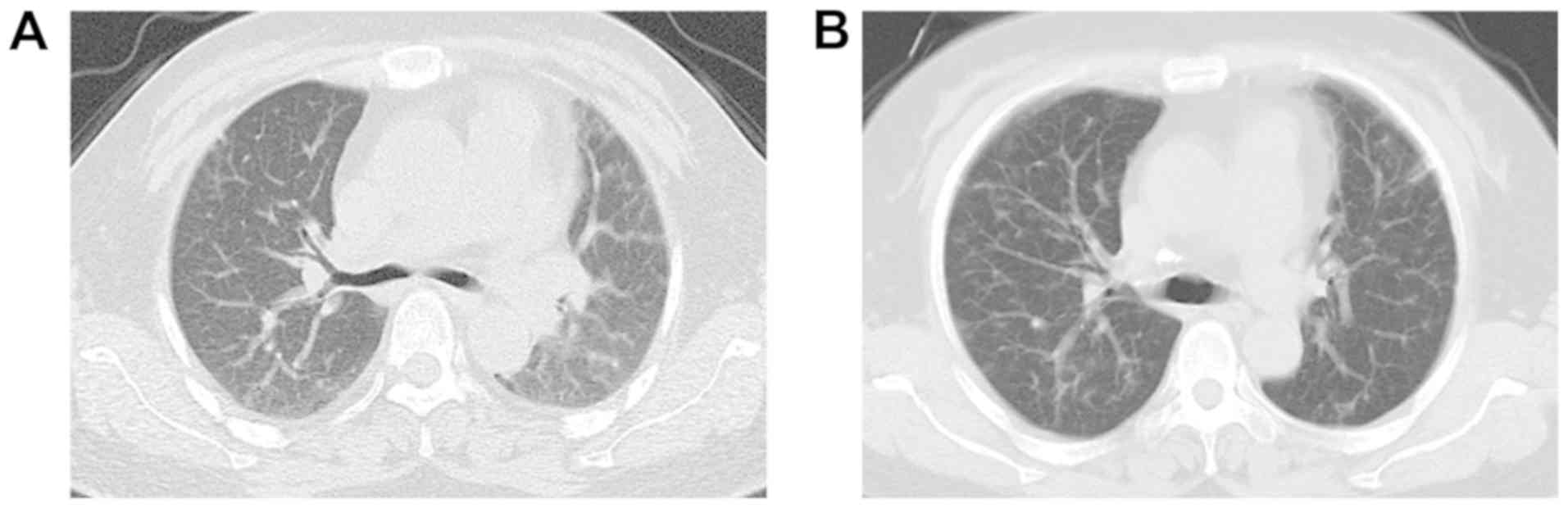
lung consolidations were decreased (Fig.
5), the shortness of breath and other symptoms resolved and the
non-invasive ventilator was completely disengaged. The patient was
discharged and continued treatment with oral prednisone (0.75
mg/kg/day), with a gradual reduction in symptoms noted on regular
follow-up that was completed at 6 months. As HT presents with no
specific symptoms, after excluding the possibility of thyroid
tumours, it was decided not to provide any treatment, and to
perform regular follow-up with thyroid ultrasound and thyroid
function tests.
Outcome and follow-up
After 1 week of methylprednisolone treatment, the
chest CT and radiograph indicated the gradual absorption of lung
exudates and the symptoms of the patient were markedly improved,
with no shortness of breath or fever. The serum white blood cell
count and C-reactive protein were normal. The patient's lung
function was normal at the 6- and 12-month follow-up visits and the
chest CT indicated complete resolution of the previously seen
opacities (Fig. 6). The patient
demonstrated no further symptoms, including shortness of breath,
and her activities of daily life were not affected. The size of the
thyroid gland was essentially normal and no obvious abnormalities
were observed on ultrasound or laboratory assessment of thyroid
function.
Discussion
COP occurs most frequently in 40–60-year-old
individuals and occasionally in patients aged <40 years. Its
onset is usually subacute or acute. Symptoms typically comprise
respiratory complaints, including cough and dyspnoea (particularly
with exertion), and are frequently accompanied by fever, fatigue,
weight loss and other systemic symptoms (16). Physical examination may at times
reveal wet rales on auscultation of the lungs. Common laboratory
abnormalities include elevated white blood cell count, erythrocyte
sedimentation rate and C-reactive protein levels (17,18).
Pulmonary function testing may indicate mild to moderate
restrictive ventilatory dysfunction, with or without reduced
diffusion capacity, and blood gas analysis may suggest mild
hypoxaemia. Chest radiographs demonstrate a similar appearance to
pneumonia, with no clear characteristic changes of COP. CT may
indicate unilateral or bilateral opacities, mostly in the
subpleural and peribronchial regions, and may also demonstrate air
bronchograms, ground-glass attenuation or pulmonary nodules with
rare isolated nodules displaying as a halo (19). Histopathological characteristics
include granulation tissue located around the respiratory
bronchioles and within the alveoli, or polypoid tissues composed of
fibroblasts. Macroscopically, these may have a patchy, strip-like
distribution. Mucinous fibroblast nodules known as Masson bodies
may be observed in the bronchial cavities, alveolar ducts and
alveolar cavities (20). These
oval-or polygonal-shaped bodies rich in mucopolysaccharides are
known as a hallmark pathological sign of COP.
Biomarkers including Krebs von den Lungen-6 (KL-6),
tenascin C and galectin-9 in bronchoalveolar lavage fluid may aid
in the diagnosis of COP, but with certain limitations (21,22). At
present, a definitive diagnosis of COP requires either open or
thoracoscopic lung biopsy. The current clinical treatment of COP is
still based on corticosteroid therapy, and 65–80% of patients with
COP achieve complete clinical and pathological remission after
corticosteroid therapy. Although COP responds well to
corticosteroids, it may be prone to relapse following treatment
(23,24). COP may be induced by numerous
factors, including viral infections and autoimmune diseases, but
its pathogenesis remains incompletely understood and warrants
further study.
HT is an organ-specific autoimmune disease. In the
present case, the thyroid gland revealed diffuse enlargement with
nodular changes of the gland surface. On thyroid biopsy, a large
number of dendritic cells was observed, which is noteworthy, as in
the presence of HT lesions, dendritic cells may activate immune
cells and cause immune-mediated injury (8). Elevation of TPOAb and TgAb levels is
sensitive and specific for the diagnosis of HT. Diffuse goitre,
particularly with vertebral lobe enlargement of the isthmus, or
changes in thyroid function, may indicate HT. If serum TPOAb and
TgAb are significantly increased, the diagnosis may be confirmed.
Following a diagnosis of HT, one should pay attention to the
emergence of thyroid tumours and clinical hypothyroidism (8–11). At
present, the treatment of HT is based replacement of deficient
innate thyroid hormone production with levothyroxine, an exogenous
thyroid hormone.
In the patient of the present study, pneumonia was
considered as the likely diagnosis at the local hospital early in
the onset of the disease, but no significant improvement was
observed after antibiotic treatment. However, in the early stages
of the disease, the patient exhibited fever, chest imaging changes
and a slight elevation of infection indicators, including white
blood cell count, all consistent with a diagnosis of pneumonia.
Severe pneumonia was still considered the likely diagnosis upon
transfer to the intensive care unit, but no significant improvement
was achieved after broad-spectrum anti-microbial treatment, and the
abnormalities on chest imaging did not improve. No causative
pathogens were detected after broncheolar lavage.. Bronchoscopy and
lung biopsy combined with imaging finally led to the diagnosis of
COP. At the same time, the combination of thyroid function test and
thyroid ultrasonography confirmed COP present concomitantly with
HT, although the patient's thyroid function was normal and no
thyroid tumour was observed. Therefore, antibiotics were
discontinued and the patient was treated with methylprednisolone.
After treatment, the patient's shortness of breath resolved and the
non-invasive ventilator was completely disengaged. Chest imaging
revealed that the lung consolidations gradually resolved over a
period of 6 months after treatment, at which point it was indicated
that the condition was completely controlled. After 12 months of
follow-up, the lung imaging was completely normal, pulmonary
function was completely normal and no recurrence was observed. The
thyroid examination was normal, the patient was generally in good
health and her activities of daily life were not affected. The two
diseases discussed in the present study may be induced by
autoimmune factors. The patient's relevant laboratory examinations
indicated a weakly positive anti-nuclear antibody ratio (1:80) and
a low CD4/CD8 cell ratio, giving rise to the speculation that
autoimmune factors may have been causative of these two conditions.
HT may lead to or exacerbate COP and the two diseases may clearly
coexist, as demonstrated in this patient. Such coexistence of HT
and COP in the same patient has rarely been reported in the
literature (13). The diagnosis and
treatment of this case are reported in the present study and
hopefully, clinicians will take note.
In short, when patients with pulmonary exudation and
consolidation appear in clinical practice and prove unresponsive to
the conventional anti-infective treatment, a differential diagnosis
should be considered. Bronchoscopy or lung biopsy are recommended
to confirm the diagnosis. It is important to identify whether
associated diseases including HT accompany COP in order to reduce
misdiagnosis and improve the cure rate and quality of life of
patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LG designed the study, collected and analysed the
data, selected the patient for the study and wrote the manuscript.
BC collected and analysed the data and revised the manuscript. LZ
collected the data and helped select the patient for the study. YD
designed the study and collected the data. HL collected and
analysed the patient data and revised the manuscript. QS designed
the study, collected the data and wrote the manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki. Informed consent was obtained from the
patient of the present study.
Patient consent for publication
The patient consented to the publication of her
images and data in this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim AR, Yoo KH, Lee KY, Kim SJ, Kim HJ,
Kim JH and Rhyu YA: A case of cryptogenic organizing pneumonia
after transarterial chemoembolization for the treatment of
hepatocellular carcinoma. Tuberc Respir Dis (Seoul). 78:469–472.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Izykowski N, Kuehnel M, Hussein K,
Mitschke K, Gunn M, Janciauskiene S, Haverich A, Warnecke G,
Laenger F, Maus U and Jonigk D: Organizing pneumonia in mice and
men. J Transl Med. 14:169–180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davison AG, Heard BE, McAllister WA and
Turner-Warwick ME: Cryptogenic organizing pneumonitis. Q J Med.
52:382–394. 1983.PubMed/NCBI
|
|
4
|
Travis WD, Costabel U, Hansell DM, King TE
Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU,
et al: An official American Thoracic Society/European respiratory
society statement: Update of the international multidisciplinary
classification of the idiopathic interstitial pneumonias. Am J
Respir Crit Care Med. 188:733–748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Aurizio F, Villalta D, Metus P, Doretto
P and Tozzoli R: Is vitamin D a player or not in the
pathophysiology of autoimmune thyroid diseases? Autoimmun Rev.
14:363–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banga JP and Schott M: Autoimmune thyroid
diseases. Int J Dermatol. 47:699–701. 2015.
|
|
7
|
Hashimoto H: Zur Kenntniss der
lymphomatosen Veranderung der Schilddruse (Struma lymphomatosa).
Arch Klin Chir. 97:219–248. 1912.
|
|
8
|
Silva de Morais N, Stuart J, Guan H, Wang
Z, Cibas ES, Frates MC, Benson CB, Cho NL, Nehs MA, Alexander CA,
et al: The impact of hashimoto thyroiditis on thyroid nodule
cytology and risk of thyroid cancer. J Endocr Soc. 3:791–800. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YK, Lin CL, Cheng FT, Sung FC and Kao
CH: Cancer risk in patients with Hashimoto's thyroiditis: A
nationwide cohort study. Br J Cancer. 109:2496–2501. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SH, Choi JY and Yun JS: Features of
papillary thyroid micro carcinoma in the presence and absence of
lymphocytic thyroiditis. Endocr Pathol. 21:149–153. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bedir R and Yılmaz R: Coexistence of
papillary thyroid cancer and Hashimoto's thyroiditis developing
within an ovarian mature cystic teratoma. J Midlife Health.
10:45–47. 2019.PubMed/NCBI
|
|
12
|
Hwang YC, Kim TY, Kim WB, Shong YK, Yi KH,
Shong M, Jo YS, Kim WS and Chung JH: Clinical characteristics of
primary thyroid lymphoma in Koreans. Endoer J. 56:399–405.
2009.
|
|
13
|
Radzikowska E, Wiatr E, Remiszewski P,
Bestry I, Grudny J, Langfort R, Szopiński J, Zych J, Płodziszewska
M, Rudziński P, et al: Organizing pneumonia-analysis of 18 own
cases. Pneumonol Alergol Pol. 72:99–104. 2004.(In Polish).
PubMed/NCBI
|
|
14
|
Kligerman SJ, Franks TJ and Galvin JR:
From the radiologic pathology archives: Organization and fibrosis
as a response to lung injury in diffuse alveolar damage, organizing
pneumonia, and acute fibrinous and organizing pneumonia.
RadioGraphics. 33:1951–1975. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pardo J, Panizo A, Sola I, Queipo F,
Martinez-Peñuela A and Carias R: Prognostic value of clinical,
morphologic, and immunohistochemical factors in patients with
bronchiolitis obliterans-organizing pneumonia. Hum Pathol.
44:718–724. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niksarlıoğlu EY, Özkan GZ, Bakan ND, Yurt
S, Kılıç L and Çamsarı G: Cryptogenic organizing pneumonia:
Clinical and radiological features, treatment outcomes of 17
patients, and review of the literature. Turk J Med Sci.
46:1712–1718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Taniguchi H and Kondoh Y: Acute and
subacute idiopathic interstitial pneumonias. Respirology.
21:810–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yılmaz S, Akıncı Özyürek B, Erdoğan Y,
Cirit Koçer B, Demirağ F, Dadalı Y and Büyükyaylacı Özden S:
Retrospective evaluation of patients with organizing pneumonia: Is
cryptogenic organizing pneumonia different from secondary
organizing pneumonia? Tuberk Toraks. 65:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung MP, Nam BD, Lee KS, Han J, Park JS,
Hwang JH, Cha MJ and Kim TJ: Serial chest CT in cryptogenic
organizing pneumonia: Evolutional changes and prognostic
determinants. Respirology. 23:325–330. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen L, Liu J, Huang L, Zhang Y, Xiao X
and Yu H: Cryptogenic organizing pneumonia presenting as a solitary
mass: clinical, imaging, and pathologic features. Med Sci Monit.
25:466–474. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Radzikowska E, Roży A, Jaguś P, Wiatr E,
Gawryluk D, Chorostowska-Wynimko J and Roszkowski-Śliż K:
Cryptogenic organizing pneumonia: IL-1β, IL-6, IL-8, and TGF-β1
serum concentrations and response to clarithromycin treatment. Adv
Exp Med Biol. 911:77–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Katoh S, Ikeda M, Shimizu H, Abe M, Ohue
Y, Mouri K, Kobashi Y and Oka M: Increased Galectin-9 concentration
and number of CD4+Foxp3high+cells in bronchoalveolar lavage fluid
of patients with cryptogenic organizing pneumonia. Lung.
193:683–689. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Y, Wang L, Huang M, Ding J, Jiang H,
Zhou K, Meng F, Xiao Y, Cai H and Dai J: A long-term retrospective
study of patients with biopsy-proven cryptogenic organizing
pneumonia. Chron Respir Dis. 16:14799731198538292019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lebargy F, Picard D, Hagenburg J, Toubas
O, Perotin JM, Sandu S, Deslee G and Dury S: Micronodular pattern
of organizing pneumonia: Case report and systematic literature
review. Medicine (Baltimore). 96:e57882017. View Article : Google Scholar : PubMed/NCBI
|