Introduction
The immune mechanism of the digestive system is very
complex and consists of many synergistic interacting elements. The
basis of this mechanism is glandular cells and inflammatory cells.
Under normal conditions, inflammatory cells located in the
glandular ducts are responsible for the balance in the immune
system of the large intestine. Intestinal bacteria stimulate
GALT-associated lymphoid tissue. The system consists of lymphocytes
located in intestinal lymph nodes (Peyer's tufts), endothelial
cells, and in the lamina propria (1).
Cancer cells stimulate hypoxia and local
inflammatory mediators that activate numerous inflammatory cells,
including a diverse population of lymphoid cells (2). Tumor Associated Antigens (TAA) are
recognized and presented by the so-called antigen presenting cells
(APCs) which MHC class II molecules on their surface. They include
tumor-infiltrating lymphocytes (TILs) with phenotypes B and Th
(3). In addition, tumor antigens can
be bound by CD8+ T lymphocytes with MHC class I
molecules (4). The heterogeneous
density of tumor infiltrating immune cells was observed according
to tumor location (5). Kwak et
al (5) showed that
CD3+ positive lymphocytes was the highest in invasive
margin compare to those in the tumor center and distant metastasis.
Moreover, authors confirmed a low density of CD8+ TILs
in center of tumor in advanced colorectal cancer.
The tumor cells exhibit high histological and
clinical heterogeneity and thus a high variability within the TAA.
Decay or change in TAA is conditioned by the selection of clones
that consist in submitting tumor cells to the action of an immune
response. As a result of elimination, the cells with the highest
survival and proliferation are selected. Next, the selected clones
of altered antigenicity proliferate in an uncontrolled manner and
condition further invasion of tumors to nearby tissues and distant
organs (6). In colorectal cancer,
metastatic spread of cancer was located mainly in the liver and
lungs. Firstly, disseminating CRC cells come into portal
circulation, then they situated in the liver parenchyma due to
vessel fenestration. On the other hand, CRC cells can get into
general circulation and lead to development of pulmonary metastases
(7). Free antigens or fragments of
their cell membranes inhibit co-stimulatory molecules on APC cells
and block cytotoxic T lymphocyte activity by CLTA-4 (8). In addition, tumor cells may have
numerous Fas (FasL) ligands on their surface that bind to its
receptor on the surface of cytotoxic T lymphocytes. This leads to
the death of these inflammatory cells and the elimination of an
important element of the immune response (9). Therefore, immunotheraphies that aim to
stimulate immune response have been successfully used in many
malignant tumors such prostate cancer and malignant melanoma
(7).
Therefore, it seems reasonable to assess the
activity of the immune system in tissue material, in the form of
TILs located in the invasive primary tumor front as well as those
surrounding tumor cell deposits and in distal metastases to the
liver in patients with colorectal cancer correlated with
anatomoclinical parameters.
Materials and methods
The study was performed in conformity with the
Declaration of Helsinki for Human Experimentation and the protocol
was approved by the Bioethics Committee of the Medical University
of Bialystok (no. R-I-002/352/2016). Written informed consent was
obtained from all participants.
Patients
The study group consisted of 163 patients diagnosed
with colorectal carcinoma (female-56, male-88) and operated in the
Department of Oncological Surgery, in Comprehensive Cancer Center
of Bialystok, in years 2014–2016. Patients were divided into 3
groups: i) 123 cases of primary tumor colorectal cancer without
distant metastasis, ii) 25 cases with deposits of colorectal cancer
cells and iii) 15 cases of colorectal cancer liver metastasis.
During routine diagnostics all patients underwent basic diagnostic
laboratory tests, ECG, spirometry, arterial blood gasometric test
as well X-ray and computerized tomography of the chest. The
clinical efficiency was performed with a 5-point scale of Zubroda
(WHO) (10). The clinical staging of
CRC was evaluated according to TNM classification (11).
Patients diagnosed with neoplasms in rectum received
preoperative therapy (N=60): Chemotherapy (N=9), radiotherapy
(N=39) or radio-chemotherapy (N=12). They were administered a dose
of 25 Gy in fractions of 5 Gy during one week in the pelvic area.
Preoperatively, patients with tumors localized in a different site
received neither inflammatory nor immunosuppressive therapy.
Postoperatively, 64 cases received additional therapy: Chemotherapy
(N=57), radiotherapy (N=3) or radio-chemotherapy (N=5). The type of
pre/postoperative therapy was chosen on the basis of current
recommendation for colorectal cancer treatment. The response to
preoperative therapy was estimated according to the RECIST
(Response Evaluation Criteria in Solid Tumors) criteria (12).
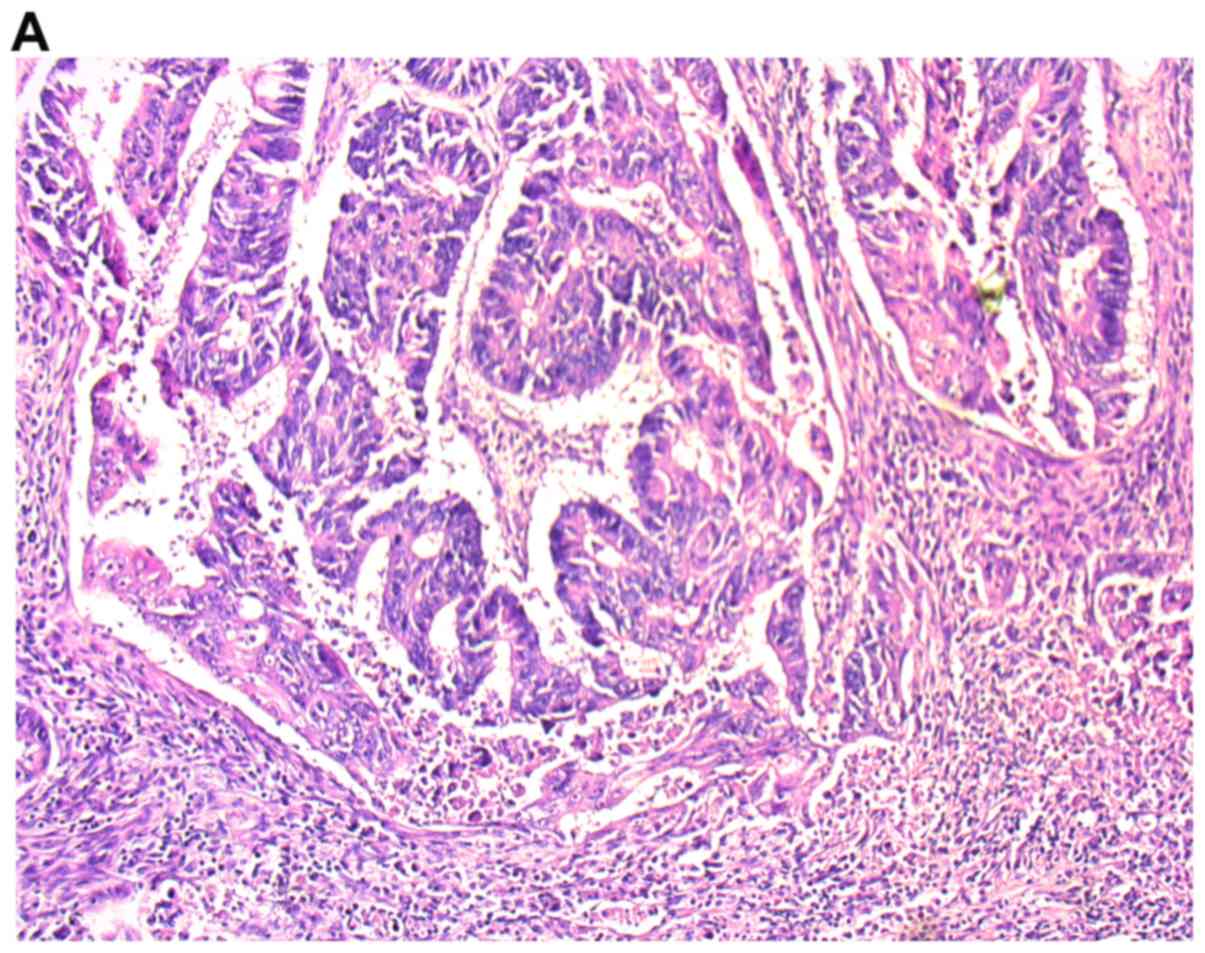
Histopathological examination
Sections, 4 µm-thick, were cut from paraffin blocks
and stained with hematoxylin and eosin (H&E) (no. cat.
468802128; POCH S.A.). The routine histopathological assessment of
the sections referred to type of tumor growth, tumor size,
histological type and percentage of the mucinous component, grade
of malignancy, pTNM and Duke stages. We also analyzed venous,
lymphatic and perineural invasions, characteristic features of
lymph node invasion such as number of resected and invaded lymph
nodes, presence of micro- and macrometastases, invasion of the
pouch lymph node; presence of distant metastases and their size in
millimeters. We also assessed the presence of deposits, their
number and size in millimeters (13). Tumor deposits (TDs) are defined as
focal aggregates of adenocarcinoma located in the pericolic or
perirectal fat disassociated with the primary tumor (14). The inflammation infiltrate in both
invasive front of the tumor and the center of the main mass was
classified according the Klintrup-Makinen criteria (15). A detailed demographic characteristic
of patients is shown in Table I.
 | Table I.Demographic details of the study
groups. |
Table I.
Demographic details of the study
groups.
| Variables | Primary tumor (N=123)
(%) | Deposits of cancer
cells (N=25) (%) | Liver metastases
(N=15) (%) |
|---|
| Age (years) |
|
|
|
|
<60 | 29 (23.7) | 6 (24) | 1 (6.6) |
|
>60 | 94 (76.3) | 19 (76) | 14 (93.4) |
| Sex |
|
|
|
|
Female | 48 (39.0) | 10 (40) | 5 (33.3) |
| Male | 75 (61.0) | 15 (60) | 10 (66.7) |
| Localization |
|
|
|
|
Right-side | 13 (10.5) | 3 (12) | 2 (13.3) |
|
Transverse | 5 (4.0) | 1 (4) | 1 (6.6) |
|
Left-side | 4 (3.2) | 1 (4) | 2 (13.3) |
|
Sigmoid | 21 (17.3) | 5 (20) | 1 (6.6) |
|
Rectum | 80 (65.0) | 15 (60) | 9 (60.2) |
| Tumor growth |
|
|
|
|
Expanding | 103 (83.7) | 20 (80) | 13 (98.7) |
|
Infiltrate | 20 (16.3) | 5 (20) | 2 (1.3) |
| Tumor size |
|
|
|
| <2.5
cm | 20 (16.3) | 3 (12) | 2 (1.3) |
| 2.5–5.0
cm | 81 (65.8) | 20 (80) | 13 (98.7) |
| >5.0
cm | 22 (17.9) | 2 (8) | 0 (0) |
| TNM stage |
|
|
|
| 1 | 17 (13.8) | 0 (0) | 0 (0) |
| 2 | 43 (34.9) | 0 (0) | 0 (0) |
| 3 | 58 (47.1) | 21 (84) | 0 (0) |
| 4 | 2 (4.2) | 4 (16) | 15 (100) |
| Lymph node
metastasis |
|
|
|
|
Absent | 65 (52.8) | 1 (4) | 6 (39.8) |
|
Present | 58 (47.2) | 24 (96) | 9 (60.2) |
| Distant
metastasis |
|
|
|
|
Absent | 123 (100) | 21 (84) | 0 (0) |
|
Present | 0 (0) | 4 (16) | 15 (100) |
| No. of
metastases |
|
|
|
|
Single | 0 (0) | 3 (75) | 8 (53.4) |
|
Multiple | 0 (0) | 1 (25) | 7 (46.6) |
| Distant metastasis
size (mm) |
|
|
|
|
<5 | 0 (0) | 1 (33.3) | 7 (46.6) |
|
>5 | 0 (0) | 2 (66.7) | 8 (53.4) |
| Tumour
deposits |
|
|
|
|
Absent | 123 (100) | 0 (0) | 11 (63.7) |
|
Present | 0 (0) | 25 (100) | 4 (36.3) |
| Preoperative
treatment |
|
|
|
|
Yes | 40 (32.6) | 7 (28) | 3 (2) |
| No | 83 (67.4) | 18 (72) | 12 (80) |
| Type of
preoperative treatment |
|
|
|
|
CHT | 5 (14) | 2 (28.5) | 0 (0) |
|
RHT | 29 (70) | 1 (14.2) | 3 (100) |
|
RHT+CHT | 6 (16) | 4 (57.3) | 0 (0) |
| Preoperative
treatment response |
|
|
|
| SD | 13 (26) | 3 (60) | 2 (66.7) |
| PR | 17 (24) | 2 (40) | 1 (33.3) |
| Postoperative
treatment |
|
|
|
|
Yes | 46 (37.3) | 5 (20) | 8 (53.4) |
| No | 77 (62.7) | 20 (80) | 7 (46.6) |
| Type of
postoperative treatment |
|
|
|
|
CHT | 40 (86.9) | 5 (100) | 6 (75) |
|
RHT | 2 (4.5) | 0 (0) | 1 (12.5) |
|
RHT+CHT | 4 (8.6) | 0 (0) | 1 (12.5) |
| Disease-free
survival |
|
|
|
| <6
months | 38 (30.8) | 9 (36) | 6 (40) |
| 6–12
months | 27 (22.9) | 5 (20) | 3 (20) |
| >12
months | 57 (46.3) | 11 (44) | 6 (40) |
Analysis of Tumor-Infiltrating
Lymphocytes
Tumor-Infiltrating Lymphocytes were assessed in
tissue material stained with hematoxylin-eosin and by light
microscopy (Leica DM6 B; KAWA.SKA) and evaluated by two independent
pathologists blinded to the clinical information. The analyses of
TILs were performed in i) the invasive front of primary tumor mass,
ii) the areas surrounding deposits of cancer cells and iii) the
invasive front of colorectal cancer liver metastasis. TILs were
determined as a percentage of mononuclear inflammatory cells over
total intratumoral stromal area and counted in 5 HPF (total
magnification, ×200–400) in the invasive front or areas surrounding
the deposits, except for tumor areas with crush artifacts, necrosis
or regressive hyalinization (16,17). We
defined a lymphocyte as a small, rounded cell a large,
dark-staining nucleus with little eosinophilic cytoplasm in
diameter no more than 7 µm. There is the smallest nucleated cell
which can be found in colorectal cancer tissue. For statistical
analysis, three levels of infiltration in the stroma TILs were
determined: 1 weak (0–10% of stromal TILs), 2-moderate (20–40% of
stromal TILs) and 3-strong (50–90% of stromal TILs). Also, in order
to conduct statistical analysis, we divided study group into: Group
1-(1 level of stromal TILs) and group 2-(2 and 3 levels of stromal
TILs).
Statistical analysis
Statistical analysis was conducted using the
STATISTICA 10.0 program (Statsoft). χ2 test was used to
compare the groups. Correlations between the parameters were
calculated by the Spearman's correlation coefficient and
χ2 tests. A P-value of <0.05 was considered
statistically significant.
Results
Tumor-Infiltrating Lymphocytes in
primary tumor, around the deposits and in the invasive front of
distant metastases to liver
TILs in the invasive front of primary tumor were
weak in 72 cases, moderate in 30 cases and strong in 21 cases.
Infiltration of TILs around the deposits of cancer cells was weak
in 18 cases, moderate in 4 cases and strong in 3 cases. Patients
with distant metastases to liver showed weak infiltration of TILs
in 11 cases, moderate in 3 cases and strong in 1 case. The
differences between all groups were statistically significant
(P=0.003). The rults are shown in Table
II and Fig. 1.
 | Table II.Distribution of TILs. |
Table II.
Distribution of TILs.
| TIL location | N | Weak levels of
TILs | Moderate levels of
TILs | Strong levels of
TILs | P-value |
|---|
| Invasive front of
primary tumor | 123 | 72 | 30 | 21 | 0.003 |
| Deposits of cancer
cells | 25 | 18 | 4 | 3 |
|
| Invasive front of
distant metastasis to liver | 15 | 11 | 3 | 1 |
|
Correlations between TILs in the
invasive front of primary tumor mass and anatomoclinical
features
TILs in the invasive front of primary tumor mass
were negatively correlated with venous, lymphatic and perineutral
invasions (R=−0.321; P=0.009; R=−0.434, P=0.004; R-0.197, P=0.013),
lymph node metastases, their number and invasion of pouch
(R=−0.197, P=0.019; R=−0.358, P=0.004; R=−0.419, P=0.030).
Correlation between TILs localized in
areas around the deposits of cancer cells and in the invasive front
of distant metastases to liver and anatomoclinical variables
TILs distributed around the deposits of cancer cells
were associated with postoperative treatment (R=0.452; P=0.017) and
stronger inflammatory cell infiltrate in the invasive front of
tumor (R=0.439, P=0.040). TILs localized in the invasive front of
liver metastases were positively correlated with preoperative
treatment (R=0.559, P=0.030), pT stage (R=0.554, P=0.003) and
inflammatory cell infiltrate in the invasive front of primary tumor
(R=0.664, P=0.017). Moreover, TILs of liver metastases were
negatively associated with lymph node metastases (R=−0.686,
P=0.004) and presence of tumor deposits (R=−0.543, P=0.036)
(Tables III and IV).
 | Table III.Correlation between TILs in invasive
primary tumor front, tumor cell deposits, and distant metastasis to
liver and anatomoclinicaal parameters in patients with colorectal
cancer. |
Table III.
Correlation between TILs in invasive
primary tumor front, tumor cell deposits, and distant metastasis to
liver and anatomoclinicaal parameters in patients with colorectal
cancer.
|
| TILs associated
with invasive front of primary tumor (N=123) | TILs associated
with deposits of cancer cells (N=25) | TILs associated
with invasive front of distant metastastic cells in liver
(N=15) |
|---|
|
|
|
|
|
|---|
| Variable | Low group (%) | High group (%) | P-value | Low group (%) | High group (%) | P-value | Low group (%) | High group (%) | P-value |
|---|
| Age |
|
<60 | 20 (16.2) | 9 (7.3) | NS | 3 (12.0) | 3 (12.0) | NS | 1 (6.6) | 0 (0) | NS |
|
>60 | 42 (38.2) | 42 (38.2) |
| 15 (60) | 4 (16) |
| 10 (66.6) | 4 (26.6) |
|
| Sex |
|
Female | 24 (19.5) | 24 (19.5) | NS | 8 (40) | 2 (8) |
| 2 (13.3) | 3 (20.0) | NS |
|
Male | 48 (39.1) | 27 (21.9) |
| 10 (60) | 5 (22) | NS | 9 (60.1) | 1(6.66) |
|
| Localization |
|
Right-side | 10 (8.3) | 3 (2.4) | NS | 1 (6.6) | 2 (8) | NS | 1 (6.66) | 0 (0) |
|
|
Transverse | 3 (2.4) | 2 (1.6) |
| 1 (6.6) | 0 (0) |
| 6 (40.0) | 2 (13.3) |
|
|
Left-side | 4 (3.2) | 0 (0) |
| 1 (6.6) | 0 (0) |
| 1 (6.66) | 1 (6.66) |
|
|
Sigmoid | 10 (8.3) | 11 (8.9) |
| 14 (56) | 4 (16) |
| 1 (6.66) | 1 (6.66) |
|
|
Rectum | 44 (35.7) | 36 (29.2) |
| 5 (20) | 1 (6.6) |
| 2 (13.3) | 0 (0) |
|
| Tumor growth |
|
Expanding | 60 (49) | 44 (35.7) | NS | 15 (80) | 5 (22) | NS | 10 (66.6) | 3 (20.0) | NS |
|
Infiltrate | 12 (9.7) | 7 (5.6) |
| 3 (12.0) | 2 (8) |
| 1 (6.66) | 1 (6.66) |
|
| Tumor size |
| <2.5
cm | 18 (14.6) | 6 (4.8) | NS | 2 (8) | 1 (6.6) | NS | 1 (13.3) | 1 (6.66) | NS |
| 2.5–5.0
cm | 41 (33.6) | 34 (27.6) |
| 14 (80) | 6 (21.4) |
| 10 (86.7) | 3 (20.0) |
|
| >5.0
cm | 13 (10.5) | 11 (8.9) |
| 2 (8) | 0 (0) |
|
|
|
|
| TNM stage |
| 1 | 23 (18.6) | 20 (16.2) | NS | 0 (0) | 0 (0) | NS | 0 (0) | 0 (0) | NS |
| 2 | 20 (16.2) | 11 (8.9) |
| 0 (0) | 0 (0) |
| 0 (0) | 0 (0) |
|
| 3 | 7 (5.6) | 5 (4) |
| 2 (8) | 3 (12.0) |
| 0 (0) | 0 (0) |
|
| 4 | 2 (1.6) | 15 (12.1) |
| 17 (80) | 4 (16) |
| 11 (73.4) | 4 |
|
| Duke stage |
| A | 39 (31.7) | 25 (20.3) | NS | 0 (0) | 0 (0) | NS | 0 (0) | 0 (0) | NS |
| B | 34 (27.6) | 8 (6.5) |
| 5 (28) | 2 (8) |
| 0 (0) | 0 (0) |
|
| C | 11 (8.9) | 5 (4) |
| 12 (60) | 3 (12.0) |
| 1 (6.66) | 0 (0) |
|
| D | 39 (31.7) | 13 (10.5) |
| 1 (12) | 2 (8) |
| 10 (93.33) | 3 (20.0) |
|
| Adenocarcinoma
type |
| Partim
mucnous | 55 (44.7) | 48 (39.1) |
| 6 (21.4) | 2 (8) | NS | 2 (13.3) | 0 (0) | NS |
|
Nonmucinous | 17 (13.8) | 3 (2.4) | NS | 12 (48) | 5 (22.6) |
| 9 (60.1) | 4 (26.6) |
|
| Grade of
malignancies |
| 2 | 68 (53.8) | 49 (39.8) | NS | 16 (64) | 7 (28) | NS | 11 (73.4) | 4 (26.6) | NS |
| 3 | 4 (4.8) | 2 (1.6) |
| 2 (8) | 0 (0) |
| 0 (0) | 0 |
|
| Preoperative
treatment |
|
Yes | 14 (11.3) | 12 (9.7) | NS | 2 (8) | 1 (6.6) | NS | 0 (0) | 0 (0) | 0.037 |
| No | 67 (57.8) | 30 (21.2) |
| 16 (64) | 6 (21.4) |
| 11 (73.4) | 4 (26.6) |
|
| Postoperative
treatment |
|
Yes | 21 (19.5) | 15 (12.1) | NS | 8 (48) | 4 (16) | 0.018 | 4 (26.6) | 2 (13.3) | NS |
| No | 51 (41.6) | 3 6 (29.2) |
| 10 (52) | 3 (12.0) |
| 7 (46.8) | 2 (13.3) |
|
 | Table IV.Correlation between TILs in invasive
primary tumor front, tumor cell deposits, and distant metastasis to
liver and parameters of disease progression in patients with
colorectal cancer. |
Table IV.
Correlation between TILs in invasive
primary tumor front, tumor cell deposits, and distant metastasis to
liver and parameters of disease progression in patients with
colorectal cancer.
|
| TILs associated
with the invasive front of primary tumor (N=123) | TILs associated
with the deposits of cancer cells (N=25) | TILs associated
with the invasive front of distant metastasis to liver (N=15) |
|---|
|
|
|
|
|
|---|
| Variables | Low (%) | High (%) | P-value | Low (%) | High (%) | P-value | Low (%) | High (%) | P-value |
|---|
| pT stage |
| 1 | 2 (1.6) | 1 (0.8) | NS | 0 (0) | 0 (0) | NS | 0 (0) | 0 (0) | 0.004 |
| 2 | 31 (25.2) | 23 (18.6) |
| 6 (24) | 3 (12.0) |
| 1 (6.6) | 0 (0) |
|
| 3 | 38 (31.1) | 27 (21.9) |
| 12 (45,4) | 3 (12.0) |
| 9 (60) | 3 (20.0) |
|
| 4 | 1 (0.8) | 0 (0) |
| 1 (6.6) | 0 (0) |
| 1 (6.6) | 1 (6.6) |
|
| Venous
invasion |
|
Absent | 51 (41.6) | 39 (31.7) | 0.012 | 10 (40) | 4 (16) | NS | 4 (60) | 2 (13.3) | NS |
|
Present | 21 (17) | 12 (9.7) |
| 8 (32) | 3 (12.0) |
| 7 (40) | 2 (13.3) |
|
| Lymphatic
invasion |
|
Absent | 58 (60.3) | 39 (31.7) | 0.009 | 13 (38) | 2 (8) | NS | 6 (66.6) | 4 (26.6) | NS |
|
Present | 26 (21.2) | 12 (9.7) |
| 5 (22) | 5 (22) |
| 5 (33.6) | 0
(0) |
|
| Perineural
invasion |
|
Absent | 65 (52.9) | 48 (39.1) | 0.017 | 15 (57.4) | 6 (24) | NS | 10 (93.3) | 4 (26.6) | NS |
|
Present | 7 (5.6) | 3 (2.4) |
| 3 (12.0) | 1
(6.6) |
| 1 (6.6) | 0 (0) |
|
| Number of removed
lymph nodes |
|
<5 | 8 (6.5) | 2 (1.6) | NS | 2 (8) | 1 (6.6) | NS | 1 (6.6) | 0 (0) | NS |
|
5–10 | 13 (10.5) | 12 (9.7) |
| 2 (8) | 1 (6.6) |
| 2 (13.3) | 0 (0) |
|
|
>10 | 51
(41.6) | 37 (30.1) |
| 14 (48.8) | 5 (22) |
| 8 (53.4) | 4 (26.6) |
|
| Lymph node
metastasis |
|
Absent | 42 (38.2) | 34 (27.6) | 0.022 | 7 (28) | 3 (12.0) | NS | 6 (40) | 2 (13.3) | 0.006 |
|
Present | 30 (21.2) | 16 (13) |
| 11
(44) | 4 (16) |
| 5 (33.4) | 2 (13.3) |
|
| Number of
metastatic lymph nodes |
|
<5 | 28 (85.4) | 8 (6.5) | 0.007 | 8 (53.3) | 2 (13.3) | NS | 3 (20.0) | 1 (6.6) | NS |
|
>5 | 8 (6.5) | 2 (1.6) |
| 4
(20.1) | 2 (13.3) |
| 2 (13.3) | 1 (6.6) |
|
| Lymph node pouch
invasion |
|
Absent | 10 (8.3) | 2(1.6) | NS | 2 (13.3) | 1 (6.6) | NS | 7 (66.7) | 3 (20.0) | NS |
|
Present | 30
(21.2) | 4 (4.8) |
| 6 (40) | 6 (40) |
| 4 (26.6) | 1 (6.6) |
|
| Distant
metastasis |
|
Absent | – | – | – | 15 (62) | 5 (22) | NS | 0 (0) | 0 (0) | NS |
|
Present |
|
|
| 2 (8) | 2 (8) |
| 11 (100) | 4 (26.6) |
|
| Distant metastasis
size (mm) |
|
<10 | – | – | – | 2 (8) | 0 (0) | NS | 9 (60) | 3 (20.0) | NS |
|
>10 |
|
|
| 2 (8) | 0 (0) |
| 3 (26.7) | 1 (6.6) |
|
| Tumor deposits |
|
Absent | – | – | – | 0 (0) | 0 (0) | NS | 9 (60) | 2 (13.3) | 0.052 |
|
Present |
|
|
| 18 (72) | 7 (28) |
| 2 (13.3) | 2 (13.3) |
|
| Inflammatory cell
infiltrate in the invasive front of tumor |
|
Absent | 8 (6.5) | 7 (5.6) | 0.002 | 2 (8) | 1 (6.6) | 0.042 | 2 (13.3) | 1 (6.6) | NS |
|
Weak | 31
(25.2) | 23 (18.6) |
| 6 (24) | 4 (16) |
| 3 (20.0) | 2 (13.3) |
|
|
Moderate | 17 (14.2) | 15
(12.1) |
| 7 (28) | 2 (8) |
| 5 (33.6) | 1 (6.6) |
|
|
Strong | 16 (13) | 6 (4.8) |
| 3 (12.0) | 0 (0) |
| 1 (6.6) | 0 (0) |
|
| Inflammatory cell
infiltrate in the center of tumor mass |
|
Absent | 6 (4.8) | 3 (2.4) | 0.003 | 1 (6.6) | 0 (0) | NS | 1 (6.6) | 0 (0) | NS |
|
Weak | 21 (19.5) | 33 (24.5) |
| 11 (34.2) | 5 (22) |
| 9 (60) | 3 (20.0) |
|
|
Moderate | 32 (26) | 12 (9.7) |
| 3 (12.0) | 1 (6.6) |
| 0 (0) | 1 (6.6) |
|
|
Strong | 10 (8.3) | 6 (4.8) |
| 3 (12.0) | 1 (6.6) |
| 1 (6.6) | 0 (0) |
|
Discussion
Lymphocytic infiltration is a major immunological
defense against tumor cells in solid tumors and is a potential
predictor of colorectal cancer (18–20). In
our studies, we found a weak infiltration in 72 cases, moderate in
30 cases and strong in 21 cases. TILs may be stimulated by
APC-lymphocytes, while the degree and direction of activation and
selectivity of stromal TILs are dependent on the type of antigen on
the tumor cell (21). TILs located
in the invasive front are one of the first elements of host defense
against invasive tumor cells that further stimulate or inhibit
cellular response (22). In brief,
there are correlations in which we observed invasions of tumor
cells into blood vessels and lymph vessels, as well as perineutral
space with a decrease in the amount of TILs. In addition, in
patients with a low incidence of TILs in the invasive primary tumor
front, we found metastases to the local lymph nodes and tumor
invasion beyond the nodule to the surrounding tissues. The results
of our study are consistent with the observations of Perez et
al (18), Huh et al
(23) and Pagès et al
(24,25). Also, Mlecnik et al (26) confirmed that the decrease in
intratumoral immune T-cell densities correlated with the growth of
the primary tumor and the metastatic spread of colorectal cancer
cells. In our studies, we reported a significantly higher TILs
infiltration in the primary tumor compared to their presence in the
deposits and metastatic carcinoma of the intestinal carcinoma. The
failure of TILs in subsequent stages of tumor invasion can be
determined by several factors. It has been reported that TILs in
colorectal cancer can undergo apoptosis (19). The phenotype of the lymphocytes which
commonly contains Treg lymphocytes exerts a significant influence
on the organization of TILs and its effectiveness (27). The status of microsatellite
instability is not trivial. It has been shown that TILs of MSI-H
colorectal cancers may be more immunoactive and cytotoxic than in
MSS tumors (28,29).
Cancer cells of primary tumors acquire an invasive
phenotype and show features of metastasis (30). In the first place, the involvement of
blood vessels and lymph nodes, regional lymph nodes and tumor cells
crossing the border of the nodule are demonstrated. In some cases,
patients with colorectal cancer show deposits of cancer cells (TDs)
that are defined and classified as a feature N1C in the 7th edition
of the AJCC/TNM staging system (31). There are tumor cells in the
pericolorectal or adjacent mesocolic fats far away from the leading
edge of the tumor. There should be no exclusion of residual lymph
node tissue. Moreover, tumor deposits should be within the lymph
drainage area of the primary carcinoma (31). It has been shown that the presence of
colorectal cancer deposits may be an unfavorable predictor
(31). To our knowledge, this was
the first study to investigate the TILs surrounded by tumor
deposits. We have mainly reported weak TILs in most cases. The
infiltrate of TILs around cancer deposits positively correlated
with postoperative treatment in the form of chemo or radiotherapy.
Previous studies confirmed that the presence of deposits in
patients with rectal cancer who underwent preoperative
chemoradiotherapy was associated with a trend of higher local
recurrence rate and significantly decreased survival (32,33). The
analysis of patients with rectal cancer who underwent preoperative
radiotherapy treatment showed the relevance of TDs with several
aggressive tumor features, including more intensive regional lymph
nodes metastasis and more perineural invasion (34). Perhaps the preoperative treatment
modifies the immune response around the deposits, probably into a
poor TIL response, which results in the fact that they are an
exponent of disease progression. There is also a possibility that
it is the selected clones of tumor cells that build deposits
escaping from the control of local inflammatory response and
directly inhibit its organization, which is also expressed by the
presence of a few cells. Therefore, perhaps the increase in the
number of TILs around the deposit improves the effect of
postoperative treatment. A limitation of our analysis in this case
is the fact that the group of patients is not great and the study
included neither a detailed breakdown of the deposits of patients
with preoperative therapy used nor assessment of its effectiveness.
In the future, we will expand our TILs analysis based on these
criteria.
With the progression of cancer disease, tumor cells
of the subsequent tissue structures, including the distal organs,
can be observed. In our studies, we have shown a weak infiltrate of
TILs in the invasive front of liver metastatic sites in
approximately 73% of cases. In turn, Kwak et al (5) and Lee et al (35) have observed that higher densities of
CD3+, CD8+ and CD45RO (+) TILs in distant
metastases of colorectal cancer were significantly correlated with
better prognosis. In contrast to the above studies, Schweiger et
al (36) demonstrated that
patients with pulmonary metastases from colorectal cancer have a
high density of FOXP3 + TILs at invasive front and low density of
CD8+ cells in TILs may have worse prognosis. In our studies, we
have found correlations between TILs in the invasive frontal
metastases of liver cancer and the use of preoperative treatment.
Halama et al (37), showed
that TIL densities at the invasive margin of liver metastases
allowed the prediction of response to chemotherapy with a high
sensitivity and specificity. Also Morris et al (38) TILs in patients treated with
5-fluorouracil-based chemotherapy were associated with
significantly improved survival. There are indications that the
presence of TILs in distant metastases may be conditioned and
modified by both pre- and postoperative treatment.
In conclusion, the results of our study confirmed
that the histopathological evaluation of TILs infiltration in both
the primary tumor of colon cancer and its metastases in the form of
deposits and cancer focus of liver may help to determine the extent
of immune defense activation and provide a potential pathological
outcome.
Acknowledgements
Not applicable.
Funding
The author(s) received funding support from Medical
University of Bialystok for this work (grant no.
N/ST/ZB/18/002/1194).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KJ prepared the conception and designed the study,
collected the data, made the analysis and wrote the paper. MK
performed the data analysis and provided suggestions for important
content. WF and WK performed the microscopic examination. LKK
interpreted the data. WF and WK reviewed the manuscript. All the
authors approved the final version of manuscript.
Ethics approval and consent to
participate
The study was performed in conformity with the
Declaration of Helsinki for Human Experimentation and the protocol
was approved by the Bioethics Committee of the Medical University
of Bialystok (approval no. R-I-002/352/2016).
Patient consent for publication
Written informed consent was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jakóbisiak M and Lasek W: Immunologia
nowotworówImmunologia. Gołąb J, Jakóbisiak M, Lasek W and Stokłosa
T: 5th. Wydawnictwo Naukowe PWN; Warszawa: pp. 478–484. 2007
|
|
2
|
Kuss I, Hathaway B, Ferris RL, Gooding W
and Whiteside TL: Decreased absolute counts of T lymphocyte subsets
and their relation to disease in squamous cell carcinoma of the
head and neck. Clin Cancer Res. 10:3755–3762. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edge SB, Byrd D, Compton CC, Fritz AG,
Greene F and Trotti A: American Joint Committee On CancerAJCC
Cancer Staging Manual. 7th. Springer; New York, NY: 2010
|
|
4
|
Kumar S, Skeen MJ, Adiri Y, Yoon H, Vezys
VD, Lukacher AE, Evavold BD, Ziegler HK and Boss JM: A cytokine
promoter/yellow fluorescent protein reporter transgene serves as an
early activation marker of lymphocyte subsets. Cell Immunol.
237:131–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwak Y, Koh J, Kim DW, Kang SB, Kim WH and
Lee HS: Immunoscore encompassing CD3+ and
CD8+ T cell densities in distant metastasis is a robust
prognostic marker for advanced colorectal cancer. Oncotarget.
7:81778–81790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quatromoni JG, Wang Y, Vo DD, Morris LF,
Jazirehi AR, McBride W, Chatila T, Koya RC and Economou JS: T cell
receptor (TCR)-transgenic CD8 lymphocytes rendered insensitive to
transforming growth factor beta (TGFβ) signaling mediate superior
tumor regression in an animal model of adoptive cell therapy. J
Transl Med. 10:1272012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tauriello DV, Calon A, Lonardo E and
Batlle E: Determinants of metastatic competency in colorectal
cancer. Mol Oncol. 11:97–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramnath N, Tan D, Li Q, Hylander BL,
Bogner P, Ryes L and Ferrone S: Is downregulation of MHC class I
antigen expression in human non-small cell lung cancer associated
with prolonged survival? Cancer Immunol Immunother. 55:891–899.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salvi S, Fontana V, Boccardo S, Merlo DF,
Margallo E, Laurent S, Morabito A, Rijavec E, Dal Bello MG, Mora M,
et al: Evaluation of CTLA-4 expression and relevance as a novel
prognostic factor in patients with non-small cell lung cancer.
Cancer Immunol Immunother. 61:1463–1472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamilton SR and Aaltonen LA: Tumours of
the colon and rectum In: World health organization classification
of tumoursPathology and genetics of tumours of the digestive
system. IARC Press; Lyon: pp. 103–104. 2000
|
|
12
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, national cancer
institute of the united states, national cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin Q, Wei Y, Ren L, Zhong Y, Qin C, Zheng
P, Xu P, Zhu D, Ji M and Xu J: Tumor deposit is a poor prognostic
indicator in patients who underwent simultaneous resection for
synchronous colorectal liver metastases. Onco Targets Ther.
8:233–240. 2015.PubMed/NCBI
|
|
14
|
Song YX, Gao P, Wang ZN, Liang JW, Sun Z,
Wang MX, Dong YX, Wang XF and Xu HM: Can the tumor deposits be
counted as metastatic lymph nodes in the UICC TNM staging system
for colorectal cancer? PLoS One. 7:e340872012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klintrup K, Mäkinen JM, Kauppila S, Väre
PO, Melkko J, Tuominen H, Tuppurainen K, Mäkelä J, Karttunen TJ and
Mäkinen MJ: Inflammation and prognosis in colorectal cancer. Eur J
Cancer. 41:2645–2654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salgado R, Denkert C, Demaria S, Sirtaine
N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL,
Penault-Llorca F, et al: The evaluation of tumor-infiltrating
lymphocytes (TILs) in breast cancer: Recommendations by an
international TILs working group 2014. Ann Oncol. 26:259–271. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iseki Y, Shibutani M, Maeda K, Nagahara H,
Fukuoka T, Matsutani S, Kashiwagi S, Tanaka H, Hirakawa K and Ohira
M: A new method for evaluating tumor-infiltrating lymphocytes
(TILs) in colorectal cancer using hematoxylin and eosin
(H-E)-stained tumor sections. PLoS One. 13:e01927442018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perez RO, Habr-Gama A, dos Santos RM,
Proscurshim I, Campos FG, Rawet V, Kiss D and Cecconello I:
Peritumoral inflammatory infiltrate is not a prognostic factor in
distal rectal cancer following neoadjuvant chemoradiation therapy.
J Gastrointest Surg. 11:1534–1540. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Liang L, Dai W, Cai G, Xu Y, Li X,
Li Q and Cai S: Prognostic impact of programed cell death-1 (PD-1)
and PD-ligand 1 (PD-L1) expression in cancer cells and tumor
infiltrating lymphocytes in colorectal cancer. Mol Cancer.
15:552016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Radzikowski C, Opolski A and Wietrzyk J:
Postęp w badaniach procesu wzrostu inwazyjnego i przerzutowania.
Nowotwory. 53:57–65. 2002.(In Polish).
|
|
21
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
LeBoit G, Burg G, Weedon D and Sarasin A:
Pathology and Genetics of Skin TumoursIARC Press; Lyon: 2005
|
|
23
|
Huh JW, Lee JH and Kim HR: Prognostic
significance of tumor-infiltrating lymphocytes for patients with
colorectal cancer. Arch Surg. 147:366–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pagès F, Galon J and Fridman WH: The
essential role of the in situ immune reaction in human colorectal
cancer. J Leukoc Biol. 84:981–987. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pagès F, Berger A, Camus M, Sanchez-Cabo
F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte
D, et al: Effector memory T cells, early metastasis, and survival
in colorectal cancer. N Engl J Med. 353:2654–2666. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mlecnik B, Tosolini M, Kirilovsky A,
Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman
WH, Pagès F and Galon J: Histopathologic-based prognostic factors
of colorectal cancers are associated with the state of the local
immune reaction. J Clin Oncol. 29:610–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buckowitz A, Knaebel HP, Benner A, Bläker
H, Gebert J, Kienle P, von Knebel Doeberitz M and Kloor M:
Microsatellite instability in colorectal cancer is associated with
local lymphocyte infiltration and low frequency of distant
metastases. Br J Cancer. 9:1746–1753. 2005. View Article : Google Scholar
|
|
28
|
Youssef MM, Paish EC, Murray JC, Farag NM,
Saleh K and Ellis IO: Tumor infiltrating T lymphocytes and
apoptosis in colorectal cancer. Egypt J Immunol. 22:19–28.
2015.PubMed/NCBI
|
|
29
|
Phillips SM, Banerjea A, Feakins R, Li SR,
Bustin SA and Dorudi S: Tumour-infiltrating lymphocytes in
colorectal cancer with microsatellite instability are activated and
cytotoxic. Br J Surg. 91:469–475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salama P, Phillips M, Grieu F, Morris M,
Zeps N, Joseph D, Platell C and Iacopetta BJ: Tumor-infiltrating
FOXP3+ T regulatory cells show strong prognostic
significance in colorectal cancer. Clin Oncol. 27:186–192. 2009.
View Article : Google Scholar
|
|
31
|
Ohtani H: Focus on TILs: Prognostic
significance of tumor infiltrating lymphocytes in human colorectal
cancer. Cancer Immun. 7:42007.PubMed/NCBI
|
|
32
|
Qi QH, Wang T, Mao Y and Hua D: Prognostic
significance of tumor deposits in patients with stage III colon
cancer. Zhonghua Zhong Liu Za Zhi. 38:784–789. 2016.(In Chinese).
PubMed/NCBI
|
|
33
|
Wei X, Qiu MZ, Zhou XY, He MM, Luo HY,
Wang FH, Zhang DS, Li YH and Xu RH: The clinicopathologic relevance
and prognostic value of tumor deposits and the applicability of N1c
category in rectal cancer with preoperative radiotherapy.
Oncotarget. 7:75094–75103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gopal P, Lu P, Ayers GD, Herline AJ and
Washington MK: Tumor deposits in rectal adenocarcinoma after
neoadjuvant chemoradiation are associated with poor prognosis. Mod
Pathol. 27:1281–1287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee WS, Kang M, Baek JH, Lee JI and Ha SY:
Clinical impact of tumor-infiltrating lymphocytes for survival in
curatively resected stage IV colon cancer with isolated liver or
lung metastasis. Ann Surg Oncol. 20:697–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schweiger T, Berghoff AS, Glogner C,
Glueck O, Rajky O, Traxler D, Birner P, Preusser M, Klepetko W and
Hoetzenecker K: Tumor-infiltrating lymphocyte subsets and tertiary
lymphoid structures in pulmonary metastases from colorectal cancer.
Clin Exp Metastasis. 33:727–739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Halama N, Michel S, Kloor M, Zoernig I,
Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G,
Luber B, et al: Localization and density of immune cells in the
invasive margin of human colorectal cancer liver metastases are
prognostic for response to chemotherapy. Cancer Res. 71:5670–5677.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morris M, Platell C and Iacopetta B:
Tumor-infiltrating lymphocytes and perforation in colon cancer
predict positive response to 5-fluorouracil chemotherapy. Clin
Cancer Res. 14:1413–1417. 2008. View Article : Google Scholar : PubMed/NCBI
|