Introduction
Pioglitazone is a type of thiazolidinedione
anti-diabetic drug and a highly selective peroxisome
proliferator-activated receptor (PPAR) agonist. PPAR, a nuclear
hormone receptor, combines with the retinoic acid X receptor (RXR)
to form a heterodimer, which then binds to the peroxisome
proliferator response element of the target gene to activate the
PPAR signaling pathway (1). It is
able to significantly improve glycemic control in type 2 diabetes
mellitus and was approved by the US Food and Drug Administration in
1999 (2,3). A prospective clinical trial has
indicated that pioglitazone affects the incidence and mortality of
macrovascular disease in type 2 diabetes mellitus (4). It has been suggested that pioglitazone
not only improves insulin resistance and blood glucose, but also
displays anti-oxidant activity. It exerts a protective effect on
the body by affecting the formation of arteriosclerosis and
instability of plaques. Its mechanism of action may include: i)
Inhibition of the function of monocytes and/or macrophages, as well
as inhibition of the production of inflammatory cytokines. ii)
Inhibition of the expression of endothelial-cell adhesion molecules
and reduction of the interaction between leukocytes and endothelial
cells. iii) Direct action on vascular smooth muscle cells,
inhibition of the growth and calcium intake of vascular smooth
muscle cells and dilation of blood vessels. iv) Promotion of
reverse transportation of cholesterol, increase of plaque stability
and delay of the process of arteriosclerosis. v) Reduction of
apoptosis of cardiomyocytes and improvement of myocardial function
(5,6). Other studies have indicated that
pioglitazone improves insulin resistance and hyperandrogenism in
patients with polycystic ovary syndrome (PCOS), and this effect may
also enhance the responsiveness of PCOS patients to clomiphene
(7). In recent years, the
correlation between pioglitazone and tumor incidence has been
increasingly investigated. Certain observational and retrospective
studies have indicated that pioglitazone increases the risk of
bladder cancer in a dose-dependent manner, compared with that in a
control group (8,9). The present bioinformatics study set out
to identify the direct or indirect targets of pioglitazone, as well
as to aim to calculate or predict the most significant or
comprehensive pathways or mechanisms of action using data from
published databases.
Digital gene expression profiling (DGE) is a
high-throughput sequencing technique used for detecting gene
expression in specific tissues of a species. Through a
bioinformatics search, analysis and comparison of gene expression
profiles, specific information may be obtained, including that on
gene regulation, gene transcription, signal transduction pathways
and protein function (10). At
present, the technology for gene expression profiling is mainly
based on gene chip and high-throughput sequencing. Compared with
traditional gene expression profiling chips, DGE technology has
unique advantages: i) High-throughput-testing of almost all
expressed genes in cells; ii) digitalization-the digital signals
obtained by sequencing are direct and their resolution may reach 1
base difference between discriminable sequences; iii) good
real-time performance-the sequence to be tested does not require to
be designed beforehand; iv) ability to detect novel transcripts due
to being independent on known genes. Therefore, it has been widely
applied in the fields of life science and medicine (11–13).
Mining these rich databases may become an effective novel method
for studying the links and mechanisms between drugs and diseases
(14,15). DrugBank is the only bioinformatics
and chemical informatics database that combines detailed drug data
with comprehensive drug target information (16,17). The
DrugBank database contains information in numerous fields,
including drug type, drug profile, chemical structure, drug
ingredients, clinical trials, drug targets, enzymes, transporters,
carriers, drug images, approval status, approved prescription
drugs, foreign market names, drug interactions, manufacturers and
packers. DrugBank has been widely used for computer-assisted
retrieval of drug-associated data, drug recovery, retrieval of drug
structural data, drug-target docking or screening, drug metabolism
prediction and drug target prediction.
The purpose of the present study was to perform a
functional network analysis of gene-phenotype connectivity based on
pioglitazone. Direct and indirect target proteins of pioglitazone
were identified. Enrichment analysis was then performed to identify
the connective mechanism between pioglitazone and bladder
cancer.
Materials and methods
Identification of drug target
genes
DrugBank is the only bioinformatics and chemical
informatics database that combines detailed drug data with
comprehensive drug target information (17). Network interaction associations may
be classified into four types: Drug-drug, drug-target, drug-gene
and target-gene-protein associations. In the present study,
drug-target associations were selected to build a
pioglitazone-targeted network and the information collected is
provided in Table I.
 | Table I.Characterization of direct targets of
pioglitazone using DrugBank. |
Table I.
Characterization of direct targets of
pioglitazone using DrugBank.
| Accession no. | Name | Target symbol | Uniprot ID | Uniprot name |
|---|
| DB01132 | Pioglitazone | PPARG | P37231 | Peroxisome
proliferator-activated receptor gamma |
| DB01132 | Pioglitazone | PPARD | Q03181 | Peroxisome
proliferator-activated receptor delta |
| DB01132 | Pioglitazone | PPARA | Q07869 | Peroxisome
proliferator-activated receptor alpha |
| DB01132 | Pioglitazone | MAOB | P27338 | Monoamine oxidase
B |
Network visualization and Kyoto
Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
The Search Tool for the Retrieval of Interacting
Genes and proteins (STRING; version 10.5) is a database that
searches for interactions between proteins (18). It includes not only the direct
physical interactions between proteins, but also the indirect
functional correlations between proteins. In addition to
experimental data, it also includes results mined from PubMed
abstracts and data from other databases. It also uses
bioinformatics methods to predict results, including interacting
proteins. In the present study, STRING was used to construct the
first- and second-level protein networks that are either directly
or indirectly associated with pioglitazone. All protein data were
imported into Cytoscape software (version 2.8.0) (19) in order to construct the protein
network, while the Cluego plug-in was used for KEGG enrichment
analysis in order to predict associated pathways and functions. The
top 15 pathways with a significant P-value of <0.05 were
selected for further analysis.
cBio cancer genomics portal cancer
gene data visualization tool
The cBio Cancer Genomics Portal may aid in the
analysis of molecular data obtained from cancer tissues and
cytology, in order to recognize and understand heredity,
epigenetics, gene expression and proteomics (20). The cBio portal provides graphical
results that make complex cancer genomics data more understandable
and acceptable, without the requirement of any special
bioinformatics knowledge. Biological pathways, survival rates and
numerous other pathways may also be retrieved through this
portal.
The present study aimed to link
pioglitazone-associated genes with all data on bladder cancer
available in the cBio Portal in order to explore the association
between target genes of pioglitazone and cancer. All
pioglitazone-associated genes in all bladder cancer samples were
explored and classified, while an integrative analysis of complex
cancer genomics and clinical profiles was also performed (21).
Results
Pioglitazone-linked target gene search
using Drugbank and STRING/visualization of the pioglitazone-target
network using Cytoscape
The core of Cytoscape is the identification of
networks. A simple network graph includes nodes and edges, with
each node being a gene, microRNA or other entity. The edge between
nodes represents the interaction between these nodes, including
protein-protein interaction and DNA-protein interaction. In the
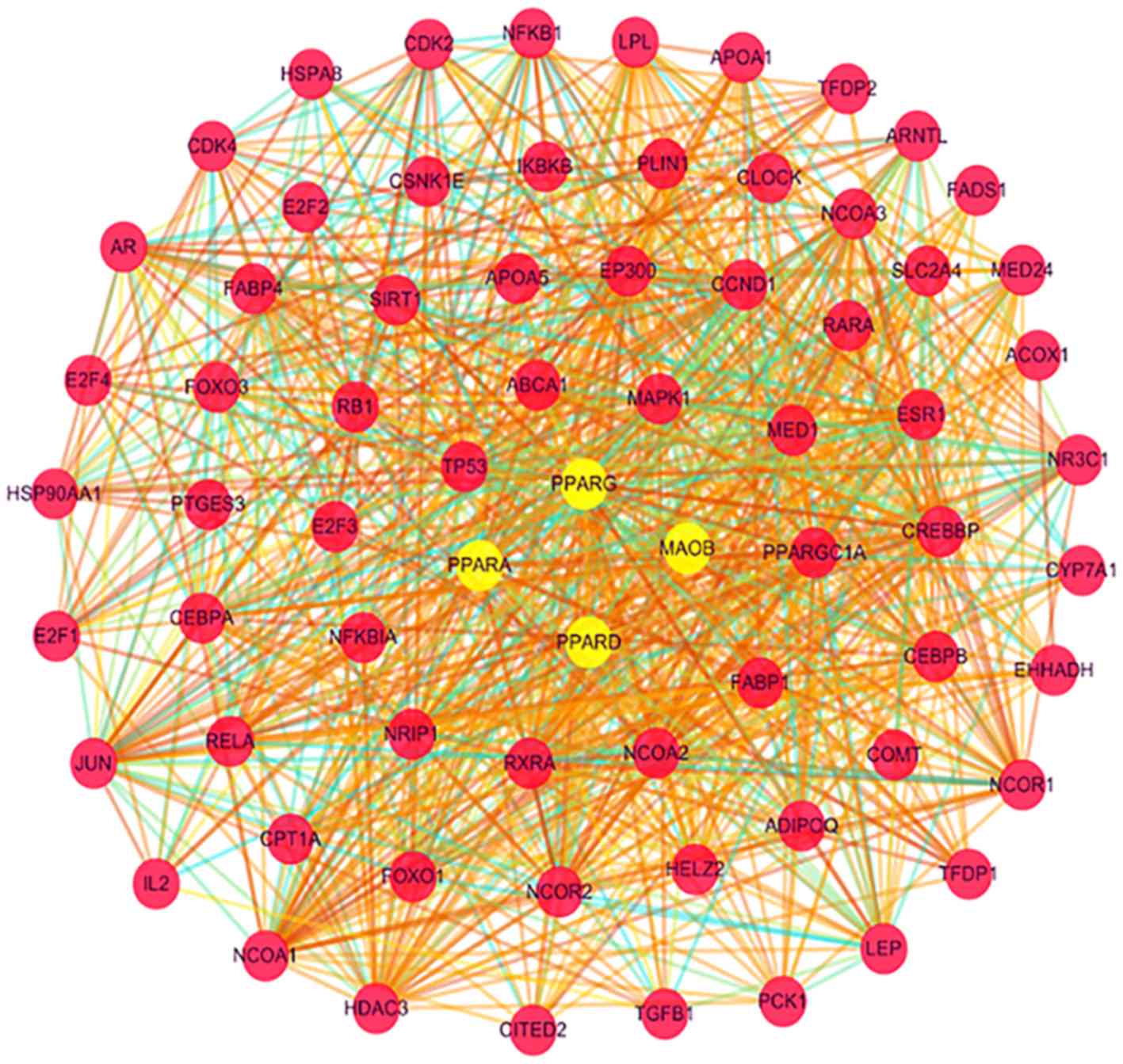
present analysis, 4 directly associated target proteins [PPAR gamma
(PPARG), PPAR delta (PPARD), PPAR alpha (PPARA) and monoamine
oxidase B (MAOB) were identified using DrugBank and are listed in
Table I. STRING software was then
used to predict the interacting proteins of the 4 direct target
proteins. The confidence value was set to 0.5 and the number of
interacting proteins was set to 50. All proteins identified were
either directly or indirectly associated with pioglitazone, and
Cytoscape was used to create the relevant network presented in
Fig. 1. All of these procedures were
performed to obtain the pioglitazone network and visualize
pioglitazone-associated target protein interactions.
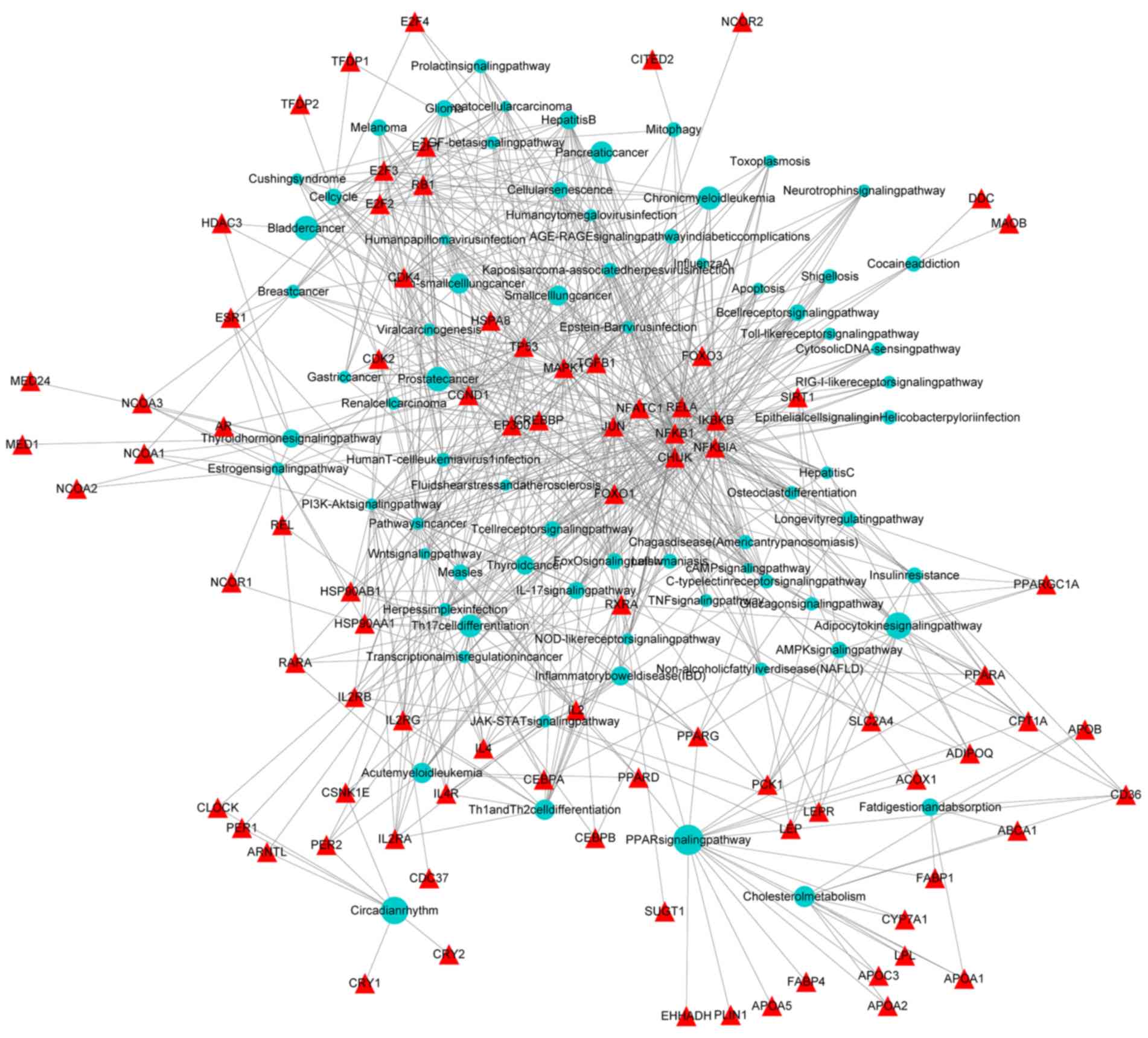
KEGG enrichment analysis of
pioglitazone-associated genes using Cluego
KEGG is a database for the systematic analysis of
gene function and genome information. It helps researchers to study
genes and their expression information as a whole network. Pathway
databases store information on gene functions and graphically
represent biological processes within cells, including metabolism,
membrane transport, signal transduction and cell growth cycle.
Therefore, a KEGG pathway analysis of pioglitazone-associated genes
was performed using Cluego. The top 15 pathways were selected,
which included the PPAR signaling pathway (19 genes), Wnt signaling
pathway (8 genes), thyroid hormone signaling pathway (15 genes),
glucagon signaling pathway (8 genes), non-alcoholic fatty liver
disease (11 genes), AGE/RAGE signaling pathway in diabetic
complications (9 genes), fat digestion and absorption (5 genes),
cholesterol metabolism (8 genes), cocaine addiction (5 genes),
mitophagy (6 genes), JAK/STAT signaling pathway (11 genes),
pathways in cancer (36 genes), transcriptional misregulation in
cancer (13 genes) and adenosine monophosphate kinase signaling
pathway (13 genes). The results are provided in Table II and Fig. 2. All of the pathways obtained through
the enrichment analysis may be linked to the mechanism of action of
pioglitazone. Of these pioglitazone-associated genes, certain
candidates may also be involved in the pathogenesis of tumors,
which warrants further research. PPARG is a member of the nuclear
hormone receptor family and has been indicated to be expressed in
numerous tumor types (22). At
present, it is thought that it mainly induces cell differentiation,
and that cell terminal transformation may stop cell growth, but it
may also induce apoptosis of cancer cells (23,24). The
Wnt signaling pathway is a conserved signaling pathway that has an
important role in regulating normal embryonic development, cell
proliferation and differentiation. Abnormal activation or
imbalanced regulation of this gene may lead to tumor formation
(25).
 | Table II.List of KEGG pathways enriched by
pioglitazone-associated genes determined using Cluego. |
Table II.
List of KEGG pathways enriched by
pioglitazone-associated genes determined using Cluego.
| KEGG ID | Associated Pathway
name | Genes genes(%) | number | Associated
genes |
|---|
| KEGG:03320 | PPAR signaling
pathway | 25.68 | 19 | ACOX1, ADIPOQ,
APOA1, APOA2, APOA5, APOC3, CD36, CPT1A, CYP7A1, EHHADH, FABP1,
FABP4, LPL, PCK1, PLIN1, PPARA, PPARD, PPARG, RXRA |
| KEGG:04310 | Wnt signaling
pathway | 5.48 | 8 | CCND1, CREBBP,
CSNK1E, EP300, JUN, NFATC1, PPARD, TP53 |
| KEGG:04919 | Thyroid hormone
signaling pathway | 12.93 | 15 | CCND1, CREBBP,
EP300, ESR1, FOXO1, HDAC3, MAPK1, MED1, MED24, NCOA1, NCOA2, NCOA3,
NCOR1, RXRA, TP53 |
| KEGG:04922 | Glucagon signaling
pathway | 7.77 | 8 | CPT1A, CREBBP,
EP300, FOXO1, PCK1, PPARA, PPARGC1A, SIRT1 |
| KEGG:04932 | Non-alcoholic fatty
liver disease | 7.38 | 11 | ADIPOQ, CEBPA,
IKBKB, JUN, LEP, LEPR, NFKB1, PPARA, RELA, RXRA, TGFB1 |
| KEGG:04933 | AGE/RAGE signaling
pathway in diabetic complications | 9.09 | 9 | CCND1, CDK4, FOXO1,
JUN, MAPK1, NFATC1, NFKB1, RELA, TGFB1 |
| KEGG:04975 | Fat digestion and
absorption | 12.2 | 5 | ABCA1, APOA1, APOB,
CD36, FABP1 |
| KEGG:04979 | Cholesterol
metabolism | 16 | 8 | ABCA1, APOA1,
APOA2, APOB, APOC3, CD36, CYP7A1, LPL |
| KEGG:05030 | Cocaine
addiction | 10.2 | 5 | DDC, JUN, MAOB,
NFKB1, RELA |
| KEGG:04137 | Mitophagy | 9.23 | 6 | CITED2, E2F1,
FOXO3, JUN, RELA, TP53 |
| KEGG:04630 | JAK/STAT signaling
pathway | 6.79 | 11 | CCND1, CREBBP,
EP300, IL2, IL2RA, IL2RB, IL2RG, IL4, IL4R, LEP, LEPR |
| KEGG:05200 | Pathways in
cancer | 6.84 | 36 | AR, CCND1, CDK2,
CDK4, CEBPA, CHUK, CREBBP, E2F1, E2F2, E2F3, EP300, ESR1, FOXO1,
HSP90AA1, HSP90AB1, IKBKB, IL2, IL2RA, IL2RB, IL2RG, IL4, IL4R,
JUN, MAPK1, NCOA1, NCOA3, NFKB1, NFKBIA, PPARD, PPARG, RARA, RB1,
RELA, RXRA, TGFB1, TP53 |
| KEGG:05202 | Transcriptional
misregulation in cancer | 6.99 | 13 | CEBPA, CEBPB,
FOXO1, IL2RB, NCOR1, NFKB1, PER2, PPARG, RARA, REL, RELA, RXRA,
TP53 |
| KEGG:04152 | AMPK signaling
pathway | 10.83 | 13 | ADIPOQ, CCND1,
CD36, CPT1A, FOXO1, FOXO3, LEP, LEPR, PCK1, PPARG, PPARGC1A, SIRT1,
SLC2A4 |
| KEGG:05216 | Thyroid cancer | 13.51 | 5 | CCND1, MAPK1,
PPARG, RXRA, TP53 |
Mining of genes (PPARD, PPARG and
RXRA) associated with pioglitazone-associated genes in bladder
cancer using the cBio portal
KEGG enrichment analysis was performed on
pioglitazone-associated genes and the relevant pathways obtained
are presented in Table II. In order
to further explore the association between pioglitazone-associated
genes and tumor pathways, a web-based data mining database (cBio
portal) was used to search for genes associated with bladder
cancer. Since the PPAR signaling pathway is the major pathway among
the pioglitazone-associated genes and the aim of the present study
was to identify a common link with gene expression in bladder
cancer, the genes in the PPAR signaling pathway and pathways in
cancer were intersected, which led to the discovery of three
overlapping genes (PPARD, PPARG and RXRA). These three overlapping
genes were selected and queried for bladder cancer using the cBio
portal. All of the 11 bladder cancer studies published prior to
2018 (26–35) were analyzed; however, certain studies
are not listed on PubMed. Alterations, including gene mutation and
amplification, ranged from 0–27%, and a total of 2,887 samples were
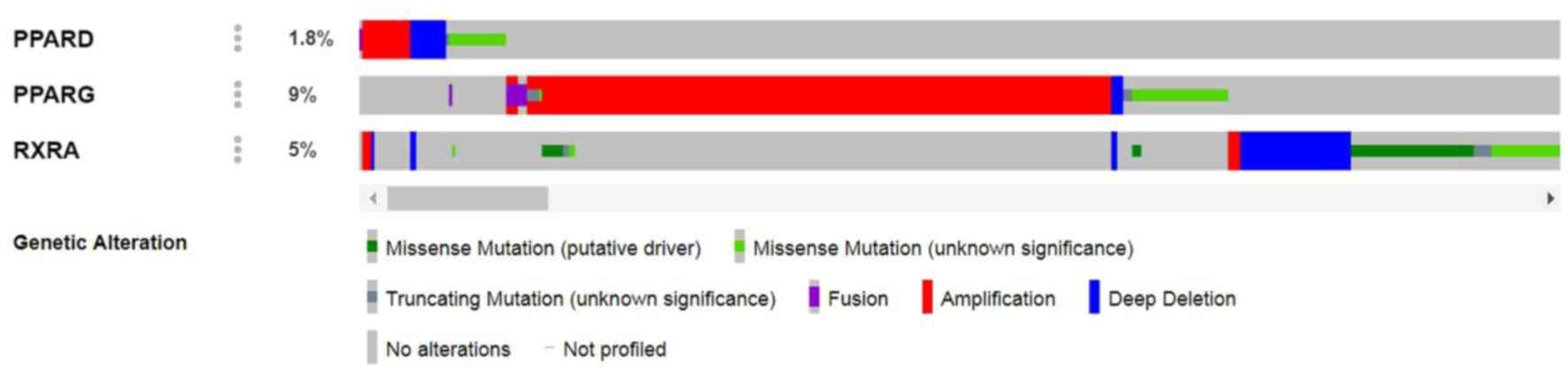
queried (Fig. 3). For PPARG, most
alterations were amplifications, but they also included deep
deletions, fusions and missense mutations (Fig. 4). Alterations of the three selected
genes in bladder cancer are provided in Fig. 4. Analysis of The Cancer Genome Atlas
data published in 2014 (31)
indicated that the most obvious alteration among all of the studies
was in the one by Oncoprint (27).
The query contained 1 gene pair (PPARD and PPARG) with mutually
exclusive alterations (none significant) and 2 gene pairs with
co-occurrent alterations (1 significant). Co-occurrence of PPARD
and RXRA across samples was indicated to be significant (P=0.020),
as presented in Table III.
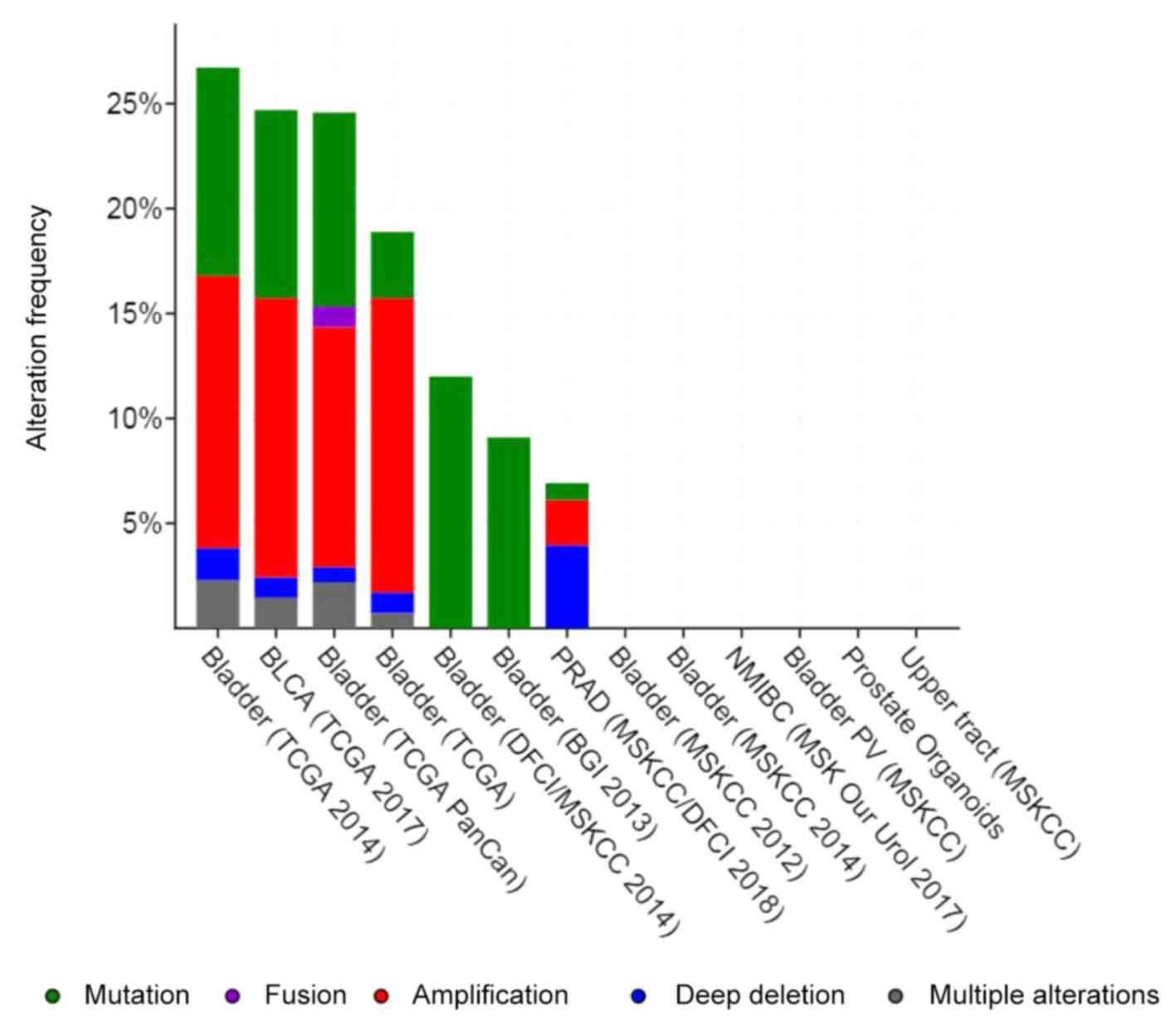 | Figure 3.Summary of alterations for
pioglitazone-associated target genes (PPARD, PPARG, RXRA) in
bladder cancer by cBioportal. TCGA, The Cancer Genome Atlas; PPARD,
peroxisome proliferator-activated receptor delta; RXRA, retinoid X
receptor alpha; BLCA, bladder cancer; DFCI, dana-farber cancer
institute; MSKCC, memorial sloan kettering cancer center; BGI,
beijing genomics institution; NMIBC, nonmuscle invasive bladder
cancer; PV, provisional. |
 | Table III.Mutual exclusivity of gene sets. |
Table III.
Mutual exclusivity of gene sets.
| Gene A | Gene B | Neither | A not B | B not A | Both | Log odds ratio | Adjusted
P-value | Characteristic |
|---|
| PPARD | RXRA | 2711 | 42 | 127 | 7 | 1.269 | 0.020 | Co-occurrence |
| PPARD | PPARG | 2597 | 48 | 241 | 1 | −1.494 | 0.220 | Mutual
exclusivity |
| PPARG | RXRA | 2527 | 226 | 118 | 16 | 0.416 | 0.273 | Co-occurrence |
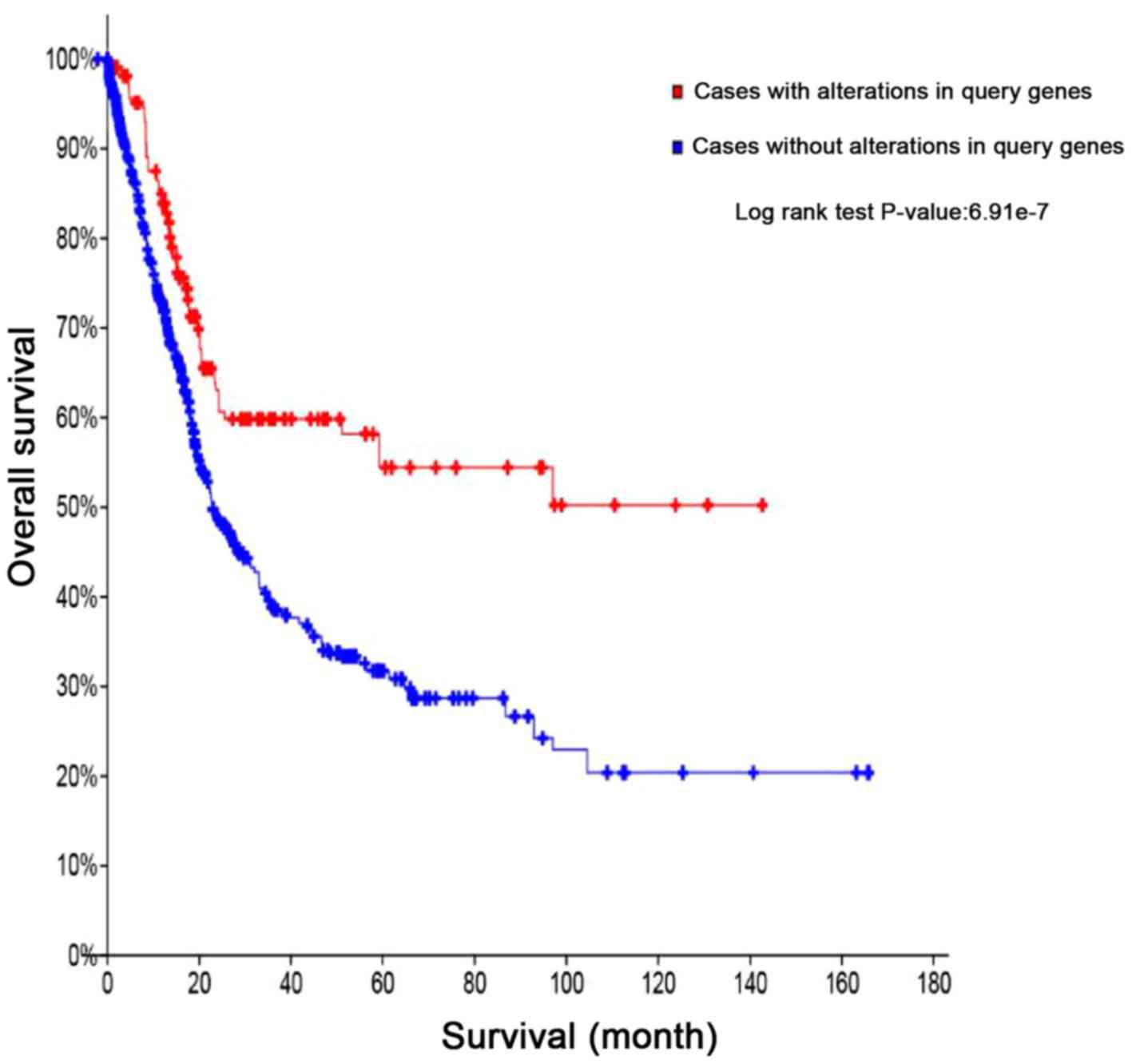
Creating a survival curve for bladder
cancer using the cBio portal
Kaplan-Meier curves provide prognostic maps
regarding overall or disease-free survival. They are plots of the
percentage of surviving patients in different groups against the
time, with stratification by the presence or absence of least one
variant in the tumor sample, and the P-value regarding the
difference between the curves may be determined to assess the
significance of the feature in question as a prognostic factor.
Using the cBio portal, the survival curves for mutation and
non-mutation of three target genes in the bladder cancer samples
were obtained, as provided in Fig.
5. Mutations in either of the three target genes (PPARD, PPARG
and RXRA) resulted in significant differences in survival rates,
compared with those of bladder cancer patients without the mutation
(P<0.05). This suggests that pioglitazone may affect the
survival rate of bladder cancer patients by targeting these three
genes and associated pathways.
Discussion
At present, the association between pioglitazone and
bladder cancer remains controversial. Meta-analysis also proves
that high doses of pioglitazone exposure may be linked to a higher
risk of developing bladder cancer (36,37).
However, the relevant mechanisms have remained to be elucidated.
Pioglitazone may produce a hypoglycemic effect by activating PPARG
(2), and it also acts on bladder
tissue (38). It was also reported
that activation of PPARG may affect the proliferation of tumor
cells (39). Bishop-Bailey and
Warner (40) first proposed the role
of the epidermal growth factor receptor signaling pathway in
bladder epithelial cell differentiation. PPARγ has been proven to
affect cell differentiation by regulating epithelial
differentiation-associated antigens and keratin. However, this
study did not determine when PPARγ has a role in the cell cycle.
Sato et al (41) indicated
that pioglitazone induces solid crystallization in urine of male
rats, while an acidic diet inhibits pioglitazone-induced bladder
tumors in male rats. Microcrystallization of advanced proliferative
lesions, particularly calcium crystallization, may induce
cytotoxicity and stimulate the proliferation of epithelial cells,
which may be the mechanism of development of pioglitazone-induced
bladder cancer (42).
New methods and technologies are required to explore
intermolecular interactions and mechanisms in order to develop
precise medical treatment methods. Bioinformatics is a discipline
that studies the collection, processing, storage, dissemination,
analysis and interpretation of biological information. In the
present study, a functional network analysis of gene-phenotype
connectivity based on pioglitazone was created with the aim to
explore the molecular mechanisms of pioglitazone and its
association with bladder cancer using web-based tools and software
(DrugBank, Cytoscape and the cBio portal). First, searches with
DrugBank and STRING were performed to identify direct and indirect
target proteins of pioglitazone. The alterations of the target
genes were then verified in bladder cancer samples using the cBio
portal. Using this process, the pioglitazone-associated genes were
linked with bladder cancer in particular common pathways. This is a
novel type of bioinformatics application.
The 4 direct target proteins of pioglitazone
identified are PPARA, PPARD, PPARG and MAOB. Genes associated with
those target genes were also predicted in order to then perform
pathway analysis. Analysis of all associated genes yielded 15
significant pathways. The PPAR signaling pathway and Wnt signaling
pathway are the first two most significant pathways that were found
to be enriched by the pioglitazone-associated genes. It has been
reported that activation of PPARγ may affect the proliferation of
tumor cells (39). Therefore, it is
indicated that the PPAR signaling pathway is associated with
cancer. The Wnt signaling pathway is a conserved signaling pathway
that has an important role in regulating normal embryonic
development, cell proliferation and differentiation. Abnormal
activation or imbalanced regulation of genes may lead to tumor
formation (25). It is an
interesting phenomenon that most enriched pathways shared certain
mechanisms with cancer. The PPARD, PPARG and RXRA genes were
indicated to participate in the PPAR signaling pathway and also in
pathways in cancer. PPARG and PPARG are involved in regulating the
cell cycle, which may have an effect on bladder cancer. Mutations
of the RXRA gene are also associated with bladder cancer, as a
result of activating peroxisome proliferator-activated receptors,
and this mechanism has been previously observed in urothelial cell
proliferation (43). Mutation of
RXRA has been reported to be accountable for 20–25% of bladder
cancers. The results of the current study suggested that mutations
of PPARD include amplification, missense mutations and deep
deletions, with the major change being amplifications. Mutations of
PPARG include amplifications, fusions, missense mutations and deep
deletions, while amplifications were indicated to be the most
significant change. Mutations in RXRA comprise deep deletions,
missense mutations, amplifications and truncating mutations. This
indicates that multidirectional mixed mutations may be involved in
the mechanisms of the development of bladder cancer.
Biological processes or pathways in cancer are
frequently regulated by a variety of genes or mechanisms. Mutual
exclusivity may be discovered using the cBio portal in order to
reveal previously unknown mechanisms of cancer, which may have an
important role in tumorigenesis and cancer progression. According
to this concept, genes associated with a particular tumor type tend
to be mutually exclusive. Genetic exclusion is when a specific
tumor only involves one specific gene. On the contrary, if there
are multiple genes involved in tumors, these genes may coexist and
have a role in the occurrence and development of tumors, while
these tumors may not be the result of a single gene problem. The
present results demonstrate that the PPARD and RXRA genes have a
co-occurrence association, which indicates multiple gene
alterations in bladder cancer. The results of the survival curve
analysis reveal that alterations of the three genes selected
(PPARD, PPARG and RXRA) lead to significant differences in overall
survival (P<0.01). The use of pioglitazone may induce bladder
cancer through causing variations in PPARD, PPARG and RXRA. These
alterations result from amplifications, fusions, missense mutations
or deep deletions.
Bioinformatics has become an important part and a
frontier of biomedical research. Bioinformatics aims to clarify and
interpret the biological significance from massive quantities of
biomedical data, reveal and understand the complexity of genomic
information structure and the fundamental laws of genetic language,
and it features a combination of genomes, information structure and
complexity. In fact, along with the rapid development of
high-throughput sequencing technology, the large amount of
biomedical data available has changed the focus of bioinformatics
from data generation to data analysis. Methods of searching for and
identifying trends using the data available in order to further
interpret mechanisms associated with human health and diseases has
become one of the major problems that require to be urgently solved
at present. Functional analysis of drugs using relevant databases,
including DrugBank and cBio portal, may help us create a more
in-depth understanding of the mechanisms of action of drugs without
experimental error that is included in traditional research
(44,45). Disease-specific gene expression
analysis may be used as a novel method of bioinformatics (46). Similarly, functional network analysis
may serve as a strategy for further basic research in the
traditional sense, as a method that may be more conducive for the
discovery of mechanisms of disease development and drug action, and
to also further develop biomedicine.
Pioglitazone, as a commonly used hypoglycemic agent,
may act through PPARG, but at the same time, its association with
side effects requires to be considered (47). A previous functional network analysis
has indicated that pioglitazone may increase the risk of prostate
and pancreatic cancer (48). The
effect of PPARG agonists on bladder cancer remains controversial.
Yang et al (49) indicated
that pioglitazone did not promote malignant alterations of normal
urothelial transitional epithelium cells or stimulate the
proliferation of bladder cancer cells. Lv et al (50) demonstrated that PPARγ activation by
pioglitazone markedly induced cell cycle arrest in G2 phase and
apoptosis in bladder cancer cells, which resulted in suppression of
tumor growth. Survival analysis was used in the present study to
propose that pioglitazone could alter the survival rate of bladder
cancer through associated the presence of target genes. Therefore,
it should be used with caution.
Acknowledgements
Not applicable.
Funding
Sponsorship for the present study was provided and
the article-processing charges were covered by the National Natural
Science Foundation of China (grant nos. 81670763 and 81471050).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LG and QP made substantial contributions to
conception and design of the study and revised it critically for
important intellectual content. WW and LZ made substantial
contributions to acquisition of data, analysis and interpretation
of data. XW and DL were involved in data analysis and drafting the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DTP
|
direct target protein
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
PPAR
|
peroxisome proliferator-activated
receptor
|
|
RXR
|
retinoic acid X receptor
|
|
PCOS
|
polycystic ovary syndrome
|
|
DGE
|
digital gene expression profiling
|
References
|
1
|
Mylona E, Giannopoulou I, Diamantopoulou
K, Bakarakos P, Nomikos A, Zervas A and Nakopoulou L: Peroxisome
proliferator-activated receptor gamma expression in urothelial
carcinomas of the bladder: Association with differentiation,
proliferation and clinical outcome. Eur J Surg Oncol. 35:197–201.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kemnitz JW, Elson DF, Roecker EB, Baum ST,
Bergman RN and Meglasson MD: Pioglitazone increases insulin
sensitivity, reduces blood glucose, insulin, and lipid levels, and
lowers blood pressure, in obese, insulin-resistant rhesus monkeys.
Diabetes. 43:204–211. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller JL: FDA approves pioglitazone for
diabetes. Am J Health Syst Pharm. 56:16981999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dormandy JA, Charbonnel B, Eckland DJ,
Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre
PJ, Murray GD, et al: Secondary prevention of macrovascular events
in patients with type 2 diabetes in the PROactive Study
(PROspective pioglitAzone Clinical Trial in MacroVascular Events):
A randomised controlled trial. Lancet. 366:1279–1289. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marx N, Duez H, Fruchart JC and Staels B:
Peroxisome proliferators-activated receptors and atherogenesis:
Regulators of gene expression in vascular cells. Circ Res.
94:1168–1178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Wijk JP and Rabelink TJ: Impact of
thiazolidinedione therapy on atherogenesis. Curr Atheroscler Rep.
7:369–374. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hernández-Valencia M, Hernández-Rosas M
and Zárate A: Care of insulin resistance in polycystic ovary
syndrome. Ginecol Obstet Mex. 78:612–616. 2010.(In Spanish).
PubMed/NCBI
|
|
8
|
Lewis JD, Ferrara A, Peng T, Hedderson M,
Bilker WB, Quesenberry CP Jr, Vaughn DJ, Nessel L, Selby J and
Strom BL: Risk of bladder cancer among diabetic patients treated
with pioglitazone: Interim report of a longitudinal cohort study.
Diabetes Care. 34:916–922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neumann A, Weill A, Ricordeau P, Fagot JP,
Alla F and Allemand H: Pioglitazone and risk of bladder cancer
among diabetic patients in France: A population-based cohort study.
Diabetologia. 55:1953–1962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ness RW, Siol M and Barrett SC: De novo
sequence assembly and characterization of the floral transcriptome
in cross- and self-fertilizing plants. BMC Genomics. 12:2982011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alagna F, D'Agostino N, Torchia L, Servili
M, Rao R, Pietrella M, Giuliano G, Chiusano ML, Baldoni L and
Perrotta G: Comparative 454 pyrosequencing of transcripts from two
olive genotypes during fruit development. BMC Genomics. 10:3992009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Severin AJ, Woody JL, Bolon YT, Joseph B,
Diers BW, Farmer AD, Muehlbauer GJ, Nelson RT, Grant D, Specht JE,
et al: RNA-Seq Atlas of Glycine Max: A guide to the soybean
transcriptome. BMC Plant Biol. 10:1602010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morrissy AS, Morin RD, Delaney A, Zeng T,
McDonald H, Jones S, Zhao Y, Hirst M and Marra MA: Next-generation
tag sequencing for cancer gene expression profiling. Genome Res.
19:1825–1835. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barabási AL and Oltvai ZN: Network
biology: Understanding the cell's functional organization. Nat Rev
Genet. 5:101–113. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barabási AL, Gulbahce N and Loscalzo J:
Network medicine: A network-based approach to human disease. Nat
Rev Genet. 12:56–68. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun J, Wu Y, Xu H and Zhao Z: DTome: A
web-based tool for drug-target interactome construction. BMC
Bioinformatics. 13 (Suppl 9):S72012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Law V, Knox C, Djoumbou Y, Jewison T, Guo
AC, Liu Y, Maciejewski A, Arndt D, Wilson M, Neveu V, et al:
DrugBank 4.0: Shedding new light on drug metabolism. Nucleic Acids
Res. 42:D1091–D1097. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: New features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jackson L, Wahli W, Michalik L, Watson SA,
Morris T, Anderton K, Bell DR, Smith JA, Hawkey CJ and Bennett AJ:
Potential role for peroxisome proliferator activated receptor
(PPAR) in preventing colon cancer. Gut. 52:1317–1322. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawa S, Nikaido T, Unno H, Usuda N,
Nakayama K and Kiyosawa K: Growth inhibition and differentiation of
pancreatic cancer cell lines by PPAR gamma ligand troglitazone.
Pancreas. 24:1–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang TH and Szabo E: Induction of
differentiation and apoptosis by ligands of peroxisome
proliferator-activated receptor gamma in non-small cell lung
cancer. Cancer Res. 60:1129–1138. 2000.PubMed/NCBI
|
|
25
|
Kikuchi A, Kishida S and Yamamoto H:
Regulation of Wnt signaling by protein-protein interaction and
post-translational modifications. Exp Mol Med. 38:1–10. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim PH, Cha EK, Sfakianos JP, Iyer G,
Zabor EC, Scott SN, Ostrovnaya I, Ramirez R, Sun A, Shah R, et al:
Genomic predictors of survival in patients with high-grade
urothelial carcinoma of the bladder. Eur Urol. 67:198–201. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iyer G, Al-Ahmadie H, Schultz N, Hanrahan
AJ, Ostrovnaya I, Balar AV, Kim PH, Lin O, Weinhold N, Sander C, et
al: Prevalence and co-occurrence of actionable genomic alterations
in high-grade bladder cancer. J Clin Oncol. 31:3133–3140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Robertson AG, Kim J, Al-Ahmadie H,
Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA,
Akbani R, et al: Comprehensive molecular characterization of
muscle-invasive bladder cancer. Cell. 171:540.e25–556.e25. 2017.
View Article : Google Scholar
|
|
29
|
Al-Ahmadie HA, Iyer G, Lee BH, Scott SN,
Mehra R, Bagrodia A, Jordan EJ, Gao SP, Ramirez R, Cha EK, et al:
Frequent somatic CDH1 loss-of-function mutations in plasmacytoid
variant bladder cancer. Nat Genet. 48:356–358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo G, Sun X, Chen C, Wu S, Huang P, Li Z,
Dean M, Huang Y, Jia W, Zhou Q, et al: Whole-genome and whole-exome
sequencing of bladder cancer identifies frequent alterations in
genes involved in sister chromatid cohesion and segregation. Nat
Genet. 45:1459–1463. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of urothelial bladder
carcinoma. Nature. 507:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pietzak EJ, Bagrodia A, Cha EK, Drill EN,
Iyer G, Isharwal S, Ostrovnaya I, Baez P, Li Q, Berger MF, et al:
Next-generation sequencing of nonmuscle invasive bladder cancer
reveals potential biomarkers and rational therapeutic targets. Eur
Urol. 72:952–959. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Van Allen EM, Mouw KW, Kim P, Iyer G,
Wagle N, Al-Ahmadie H, Zhu C, Ostrovnaya I, Kryukov GV, O'Connor
KW, et al: Somatic ERCC2 mutations correlate with cisplatin
sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov.
4:1140–1153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Faltas BM, Prandi D, Tagawa ST, Molina AM,
Nanus DM, Sternberg C, Rosenberg J, Mosquera JM, Robinson B,
Elemento O, et al: Clonal evolution of chemotherapy-resistant
urothelial carcinoma. Nat Genet. 48:1490–1499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hoadley KA, Yau C, Hinoue T, Wolf DM,
Lazar AJ, Drill E, Shen R, Taylor AM, Cherniack AD, Thorsson V, et
al: Cell-of-origin patterns dominate the molecular classification
of 10,000 tumors from 33 types of cancer. Cell. 173:291.e6–304.e6.
2018. View Article : Google Scholar
|
|
36
|
Tang H, Shi W, Fu S, Wang T, Zhai S, Song
Y and Han J: Pioglitazone and bladder cancer risk: A systematic
review and meta-analysis. Cancer Med. 7:1070–1080. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Isabelle N. Colmer, Samantha L. Bowker, et
al: Pioglitazone and bladder cancer risk: A systematic review and
meta-analysis. CMAJ. 184:E675–E683. 2012.PubMed/NCBI
|
|
38
|
Guan Y, Zhang Y, Davis L and Breyer MD:
Expression of peroxisome proliferator-activated receptors in
urinary tract of rabbits and humans. Am J Physiol. 273:F1013–F1022.
1997.PubMed/NCBI
|
|
39
|
Bojková B, Orendáš P, Kubatka P, Péč M,
Kassayová M, Kisková T and Kajo K: Positive and negative effects of
glitazones in carcinogenesis: Experimental models vs. clinical
practice. Pathol Res Pract. 210:465–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bishop-Bailey D and Warner TD: PPARgamma
ligands induce prostaglandin production in vascular smooth muscle
cells: Indomethacin acts as a peroxisome proliferator-activated
receptor-gamma antagonist. FASEB J. 17:1925–1927. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sato K, Awasaki Y, Kandori H, Tanakamaru
ZY, Nagai H, Baron D and Yamamoto M: Suppressive effects of
acid-forming diet against the tumorigenic potential of pioglitazone
hydrochloride in the urinary bladder of male rats. Toxicol Appl
Pharmacol. 251:234–244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suzuki S, Arnold LL, Pennington KL,
Kakiuchi-Kiyota S, Wei M, Wanibuchi H and Cohen SM: Effects of
pioglitazone, a peroxisome proliferator-activated receptor gamma
agonist, on the urine and urothelium of the rat. Toxicol Sci.
113:349–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Halstead AM, Kapadia CD, Bolzenius J, Chu
CE, Schriefer A, Wartman LD, Bowman GR and Arora VK:
Bladder-cancer-associated mutations in RXRA activate peroxisome
proliferator-activated receptors to drive urothelial proliferation.
Elife. 6:2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rhodes DR, Kalyana-Sundaram S, Mahavisno
V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ,
Kincead-Beal C, Kulkarni P, et al: Oncomine 3.0: Genes, pathways,
and networks in a collection of 18,000 cancer gene expression
profiles. Neoplasia. 9:166–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gottlieb A, Stein GY, Ruppin E and Sharan
R: PREDICT: A method for inferring novel drug indications with
application to personalized medicine. Mol Syst Biol. 7:4962011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Devchand PR, Liu T, Altman RB, FitzGerald
GA and Schadt EE: The pioglitazone trek via human PPAR Gamma: From
discovery to a medicine at the FDA and beyond. Front Pharmacol.
9:10932018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wen W, Wu P, Gong J, Zhao M, Zhang Z, Chen
R, Chen H and Sun J: Association of pioglitazone with increased
risk of prostate cancer and pancreatic cancer: A functional network
study. Diabetes Ther. 9:2229–2243. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang SL, Wang JJ, Chen M, Xu L, Li N, Luo
YL, Bu L, Zhang MN, Li H and Su BL: Pioglitazone use and risk of
bladder cancer: An in vitro study. Int J Med Sci. 15:228–237. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lv S, Wang W, Wang H, Zhu Y and Lei C:
PPARγ activation serves as therapeutic strategy against bladder
cancer via inhibiting PI3K-Akt signaling pathway. BMC Cancer.
19:2042019. View Article : Google Scholar : PubMed/NCBI
|