Introduction
Acute respiratory distress syndrome (ARDS) is a
life-threatening disease with high mortality rates of 40–50%
(1), and can lead to hypoxemic
respiratory failure requiring mechanical ventilation (2,3). There
are many causes for this syndrome, including shock, aspiration
pneumonia, infection, sepsis and a combination of other reasons
(1). This condition can give rise to
excessive and persistent inflammation by increasing vascular
permeability, extravasation of plasma and leucocyte infiltration
(2), resulting in a series of
complications, such as sepsis, acute pancreatitis, pulmonary
fibrosis and disseminated intravascular coagulation. These diseases
can lead to secondary systemic inflammatory reactions that result
in multiple organ failure, such as respiratory, circulatory and
renal failure (4). If there were no
effective and timely treatments, this disease would exacerbate
quickly and even become life-threatening within just a short period
of time.
Glucocorticoids have been reported as important
substances in maintaining endothelial integrity and vascular
permeability (5–8). Glucocorticoids also modulate many
pro-inflammatory and anti-inflammatory cytokines, and play a key
role in immune homeostasis (9).
Consequently, corticosteroids are considered to represent an
effective therapy for patients with ARDS. Previous randomized
trials and some meta-analyses (10–13) have
demonstrated the efficacy of corticosteroids in reducing the
mortality of ARDS.
However, several important issues remain unresolved
and are under dispute. First and foremost, it is not clear as to
what dose and time-course of corticosteroids can reduce mortality
in patients with ARDS. Previously published meta-analyses were
limited because they illustrated the problem from just one or a few
perspectives (12–14), such as the dose of glucocorticoid.
Secondly, glucocorticoids have many side effects, including an
increase in infectious and neuromyopathic complications. Additional
potential risks include hyperglycemia, poor wound healing,
psychosis, pancreatitis and prolonged muscle weakness with impaired
functional status (15,16).
Furthermore, it is difficult to control the side
effects of glucocorticoid while achieving an effective therapeutic
effect. Previous meta-analyses have not fully addressed the issues
relating to complications (17,18).
Previous trials showed that methylprednisolone could improve the
ratio of arterial oxygen partial pressure to fractional inspired
oxygen (PaO2/FIO2 ratio) and reduce periods
of mechanical ventilation in ARDS (10,12).
Furthermore, the impact of dose or treatment duration on the
efficacy of corticosteroids remains unclear. The present study
conducted a systematic review and quantitative synthesis to address
these issues.
Materials and methods
Search strategy
A systematic search into the PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), Embase
(EMBASE.com) and Cochrane Library databases (www.update-software.com/clibng/cliblogon.htm) was
conducted, from inception to May 2017, restricting the publication
language to English. The following key words were used as search
terms: ‘ARDS’; ‘adult respiratory distress syndrome’; ‘acute
respiratory distress syndrome’; ‘respiratory insufficiency’; ‘shock
lung’; ‘respiratory failure’; ‘lung injury’ AND ‘steroids’;
‘corticosteroids’; ‘glucocorticoids’; ‘hydrocortisone’;
‘prednisolone’ AND ‘randomized controlled clinical trials’;
‘controlled trials’; and ‘randomized trials’ and ‘random control
test’.
Inclusion and exclusion criteria
The present study included all randomized controlled
trial (RCT) designs that reported mortality outcomes, users of
different doses of corticosteroid along with non-users for
comparison, and the users of corticosteroid in different phases of
ARDS.
The diagnosis criterion of ARDS was in accordance
with the current Berlin Definition of Acute Respiratory Distress
Syndrome (19). In order to increase
the reliability of data and experiments, all patients with ARDS
were included, depending upon the standard of the relative trial
period.
In order to assess the efficiency of corticosteroids
on ARDS, data relating to the length of mechanical ventilation, the
length of intensive care unit stay and the
PaO2/FIO2 ratio were included. All patients
were required to have a diagnosis of ARDS/acute lung injury and be
18 years of age, or older.
Previous studies were excluded if they were
duplicated studies, did not use a control group, involved animal
experiments, in vitro studies or multiple subjects, or if
the identified study was a meeting abstract.
Quality assessment and data
extraction
Data were extracted independently by two reviewers
and any differences were resolved through discussion. Quality
assessment of the previous studies was performed using The Cochrane
Collaboration's Risk of Bias tool (20), reporting of randomization method,
allocation concealment, blinding of outcome assessment,
completeness of follow-up and bias of selective reporting. Review
Manager 5.2 software (version 5.2; The Nordic Cochrane Centre, The
Cochrane Collaboration; ims.cochrane.org/revman) was also used to create
funnel plots with which to evaluate publication bias.
Statistical analysis
All data were assimilated using Review Manager 5.2
software provided by The Cochrane Collaboration Group. For
dichotomous data, the Mantel-Haenszel (M-H) method was used to
estimate the odds ratio (OR) with a 95% confidence interval (CI)
(21). The mortality rate by the
usage of low-dose and high-dose of corticosteroids, and mortality
outcomes in patients with ARDS who were administered with steroids
at different course using the M-H method were compared. The mean
difference (MD) was calculated between treatment and control groups
for continuous outcomes, such as the duration of mechanical
ventilation, the length of intensive care unit stay and
PaO2/FIO2 ratios. The number of patients
required for treatment was calculated as the inverse of the
absolute risk reduction, based upon the pooled risk ratio and the
baseline risk (20). P<0.05 was
considered to indicate a statistically significant difference. The
I2 test was used to evaluate the pooled variation
between all eligible trials and Cochran's Q statistic to assess
heterogeneity. In addition, bias risk was also assessed, based upon
standards reported in the Cochrane Handbook (22).
Results
Literature search results and
population characteristics
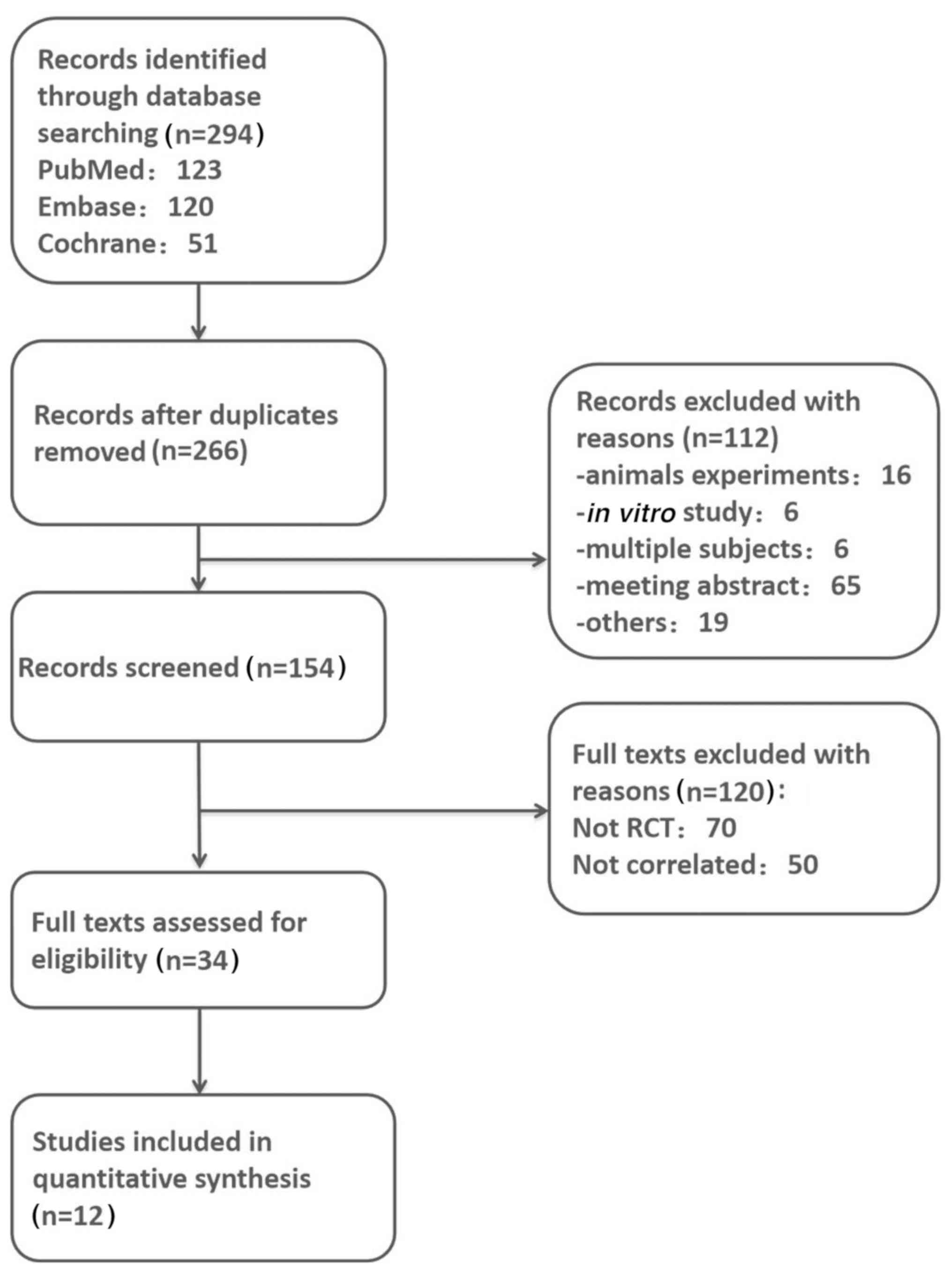
Using the aforementioned methods, 294 published
articles were identified, which were relevant to the topic of ARDS
and glucocorticoids. A total of 28 duplicates and 112 other
articles (animal experiments, in vitro studies, multiple
subjects and wrong abstracts) were excluded, leaving 154 articles.
Next, 120 more citations were excluded after careful review of the
titles and abstracts. According to the specific inclusion and
exclusion criteria of the present study, a total of 12 articles
were eligible for the final meta-analysis. A flowchart of the
present meta-analysis is presented in Fig. 1.
A total of 1,505 patients with ARDS were included,
with 780 cases in the treatment group and 725 cases in the control
group. The treatment group was divided into two groups depending on
the dose and time-course.
In total, 8 trials used low-dose therapy and 4
trials used high-dose therapy (Table
I). To keep consistency, the same cutoff value was used, and a
dose of corticosteroid (≤2.0 mg/kg/day or equivalent) of
methylprednisolone or equivalent) was defined as low dose, while a
dose of corticosteroids (>2.0 mg/kg/day methylprednisolone or
equivalent) was defined as a high dose, according to the guidelines
for clinical usage of glucocorticoids (23).
 | Table I.Detailed features of the included
studies. |
Table I.
Detailed features of the included
studies.
|
| Participants,
n |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Author, year | Treatment | Control | Clinical
trials | Phases of clinical
trials | Treatment
phase | Regimen | (Refs.) |
|---|
| Annane et
al, 2006 | 85 | 92 | Yes | IV | Early phase | Hydrocortisone, 200
mg/day for 7 days, low dose | (10) |
| Confalonieri et
al, 2005 | 23 | 23 | Yes | IV | Early phase | Hydrocortisone, 240
mg/day for 7 days, low dose | (11) |
| Lee et al,
2005 | 12 | 8 | No |
| Early phase | Methylprednisolone
2 mg/kg/day for 25 days, low dose | (24) |
| Liu et al,
2012 | 12 | 14 | No |
| Early phase | Hydrocortisone, 300
mg/day for 7 days, low dose | (25) |
| Meduri et
al, 2007 | 63 | 28 | No |
| Early and late
phase | Methylprednisolone,
1 mg/kg/day for 28 days, low dose | (12) |
| Seam et al,
2012 | 55 | 24 | No |
| Early phase | Methylprednisolone,
low dose not available | (26) |
| Steinberg et
al, 2006 | 89 | 91 | Yes | IV | Early and late
phase | Methylprednisolone,
2 mg/kg/day for 25 days, low dose | (27) |
| Wan et al,
2011 | 38 | 43 | Yes | IV | Early phase | Dexamethasone, 1
mg/kg/day for 3 days, low dose | (28) |
| Foster, 2010 | 39 | 42 | No |
| Not reported | Methylprednisolone,
3 mg/kg/day for 3 days, high dose | (13) |
| Meduri and Eltorky
2015 | 67 | 49 | No |
| Not reported | Glucocorticoid, 3
mg/kg/day for 3 days, high dose | (3) |
| Weigelt et
al, 1985 | 39 | 42 | No |
| Early phase | Methylprednisolone,
120 mg/kg/day for 2 days, high dose | (17) |
| Bernard et
al, 1987 | 50 | 49 | No |
| Early phase | Methylprednisolone,
120 mg/kg/day for 1 day, high dose | (29) |
The treatment group was divided into 2 groups
depending on the specific phase of ARDS (Table I); there were 8 trials involving
glucocorticoid intervention during the early phase of ARDS onset
(course of disease ≤7 days) and 2 trials involving the treatment of
late ARDS with glucocorticoid (course of disease >7 days); other
trials were excluded because of a lack of reporting. Standard care,
mechanical ventilation and other supportive care were applied to
patients in both groups.
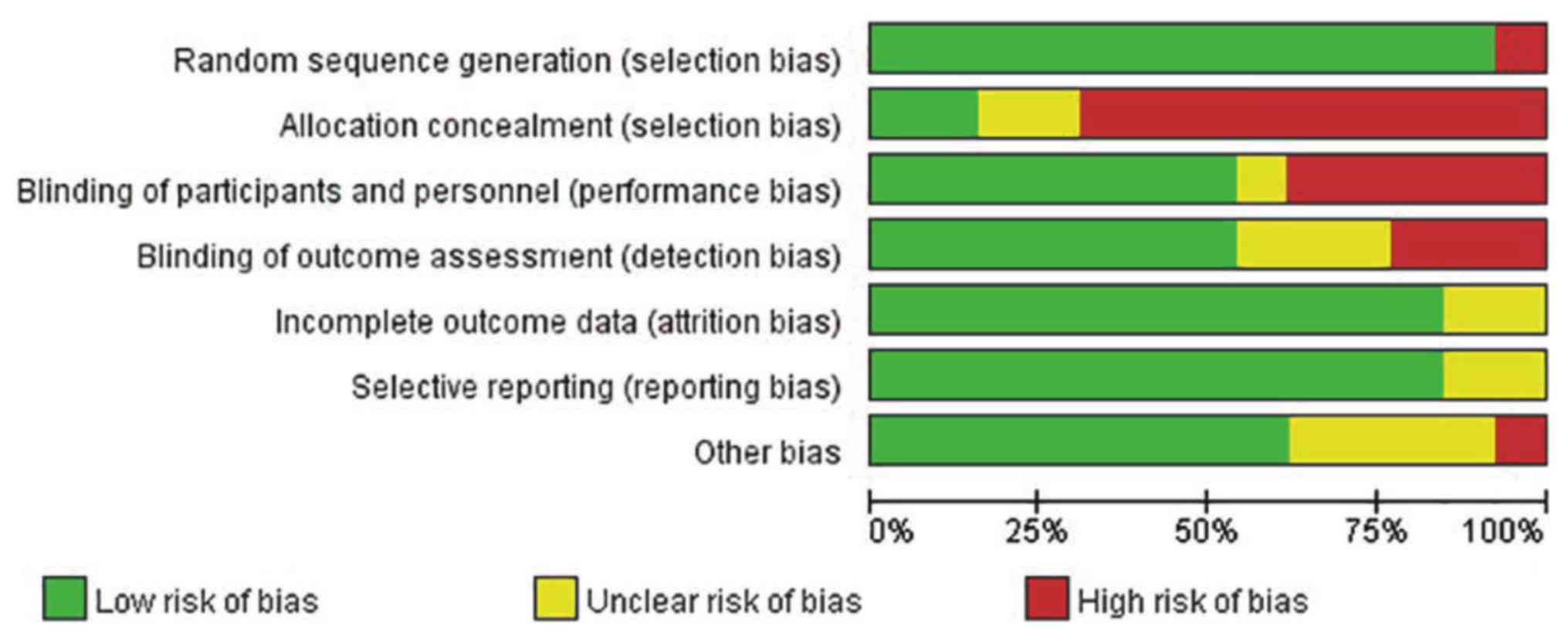
Quality assessment
As presented in Fig.
2, the quality of the studies included in the present
investigation was assessed using the Cochrane Risk of Bias Tool.
Some studies failed to provide a clear method of blinding
(including the blinding of participants, and personnel and outcome
assessment), while a few studies revealed limitations in sample
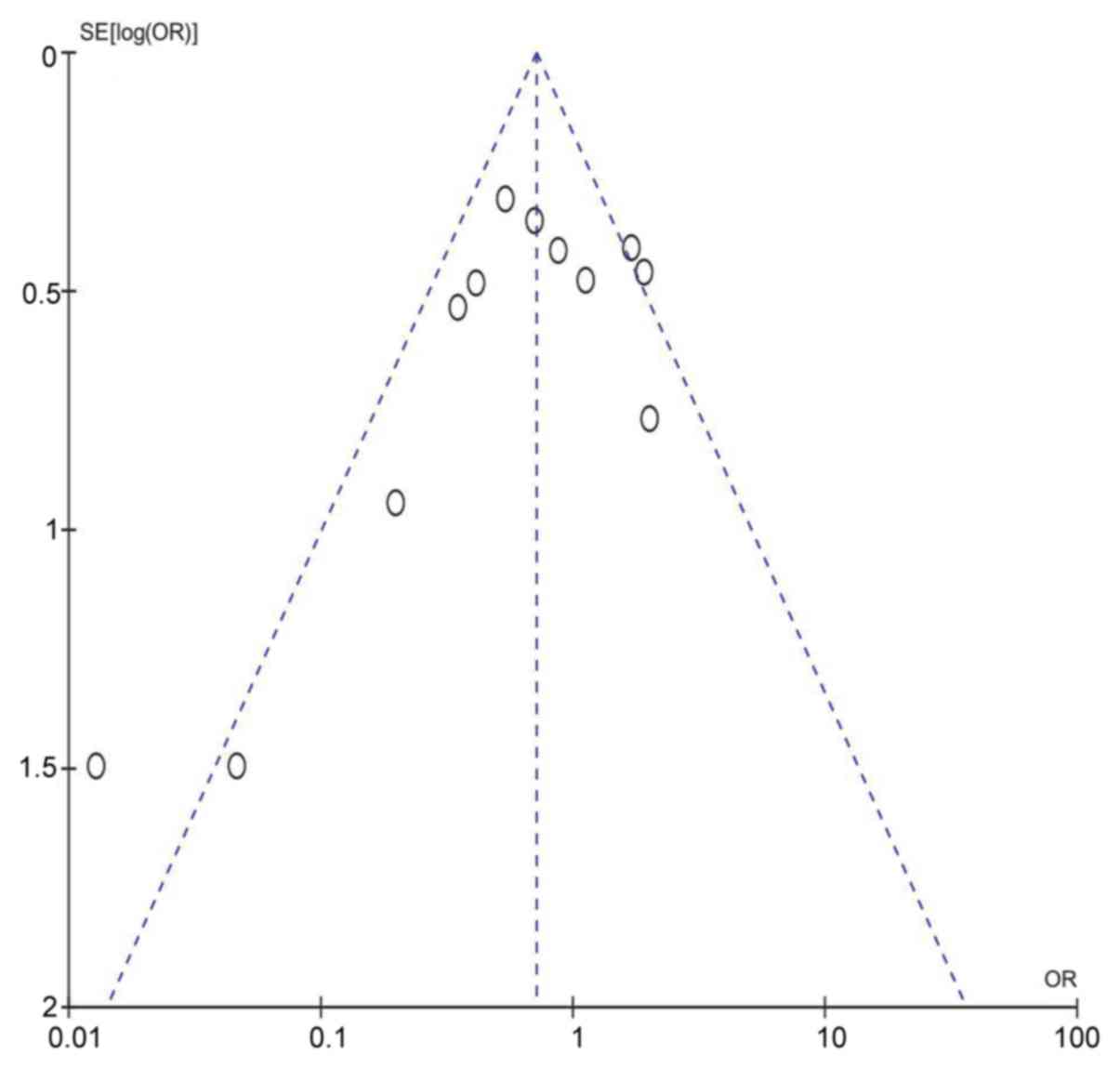
size. A funnel plot of publication bias is presented in Fig. 3; the plot is symmetrical and
distributed at the top of the scale, thus indicating that there is
either no publication bias or only minor publication bias.
Mortality outcomes in patients with
ARDS who were treated with different doses of steroids
In total, 12 trials (10–13,17,24–30) were
deemed to be suitable to assess whether glucocorticoid treatment
was beneficial to patients with ARDS in terms of reducing
mortality. Sub-group analyses of the mortality data, according to
different doses and duration of glucocorticoid therapy, were also
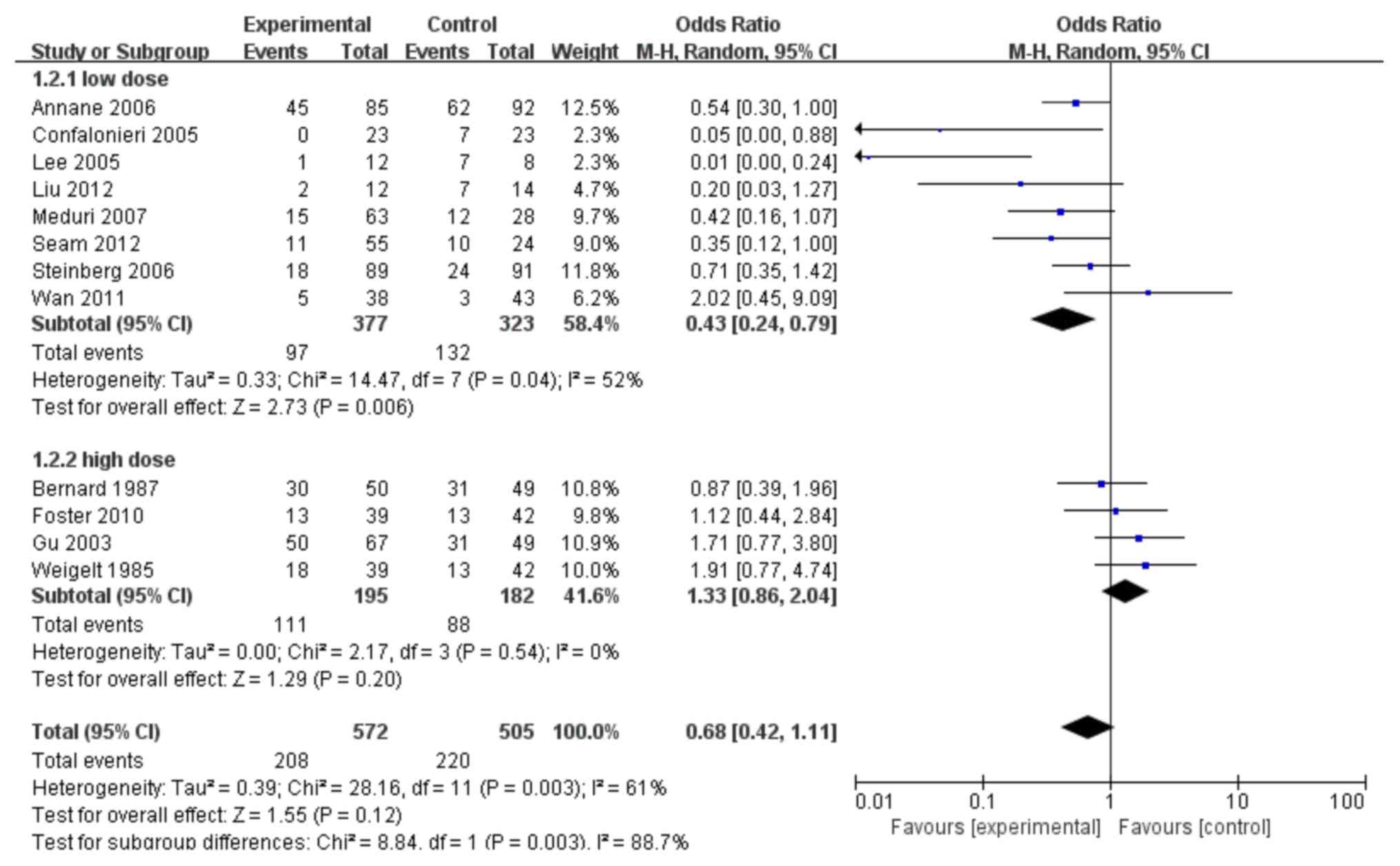
performed. As shown in Fig. 4, 8
trials (10–12,24–28) were
identified comparing the impact of low-dose glucocorticoid
treatment on the mortality rates of patients with ARDS with
appropriate controls. A significantly lower mortality was
identified in the low-dose group compared with the controls
(combined OR: 0.43; 95% CI: 0.24–0.79; P<0.05). Another
sub-group with only 4 trials identified (13,17,29,30)
studied the effect of high-dose glucocorticoid treatment on the
mortality rates of ARDS. As shown in Fig. 4, the group of patients with ARDS
receiving high-dose glucocorticoid treatment failed to show any
benefit (combined OR: 1.33; 95% CI: 0.86–2.04; P>0.05).
Mortality outcomes in patients with
ARDS who were administered steroids at different times
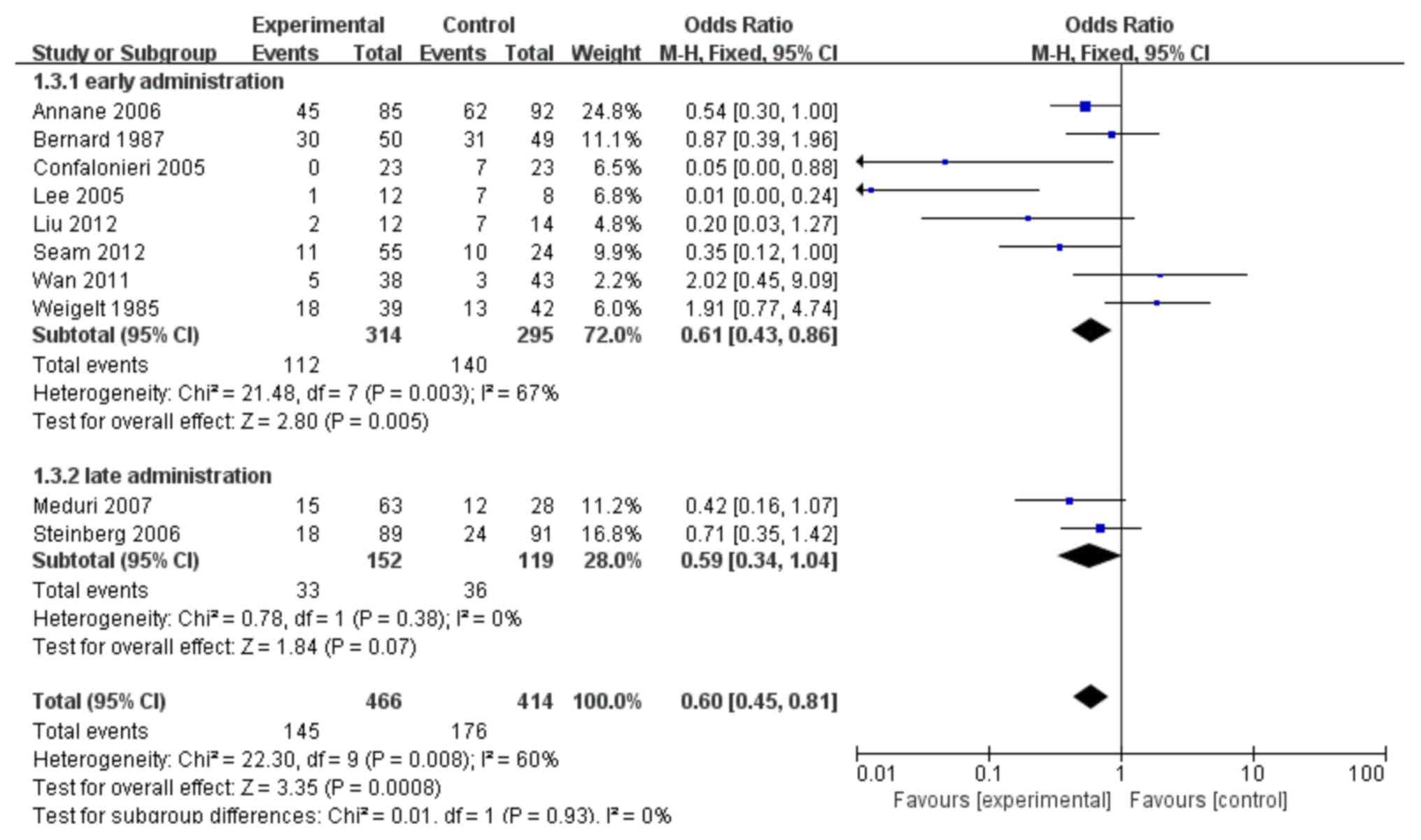
As shown in Fig. 5, 8
trials (10,11,17,24–26,28,29) were
identified which compared the impact of early glucocorticoid
administration on the mortality rates of patients with ARDS with
the controls. Patients who were given corticosteroids early,
compared with no corticosteroids, showed lower levels of mortality
(OR: 0.61; 95% CI: 0.43–0.86; P=0.005). This analysis failed to
show any benefit of late administration of corticosteroids compared
with controls (no corticosteroids), in terms of mortality in
patients with late ARDS (P>0.05).
Effect of steroid treatment on the
duration of mechanical ventilation
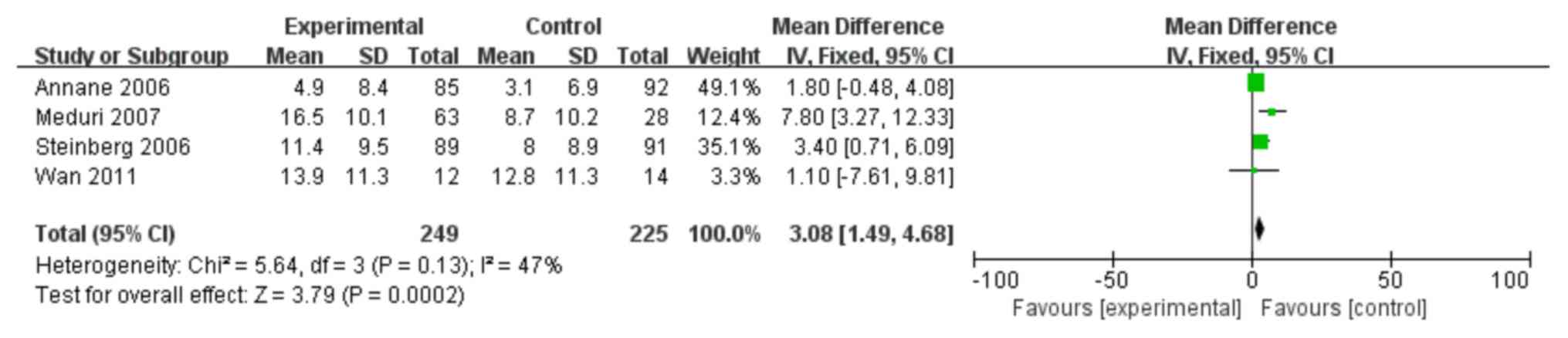
A total of 4 trials (10,12,27,28) were
identified which investigated whether steroids could reduce the
number of days on which patients with ARDS were mechanically
ventilated. As shown in Fig. 6, the
number of mechanical ventilation-free days was significantly higher
in the treatment group compared with the control group (MD: 3.08;
95% CI: 1.49–4.68; P<0.05).
Effect of steroid treatment on the
PaO2/FiO2 ratio
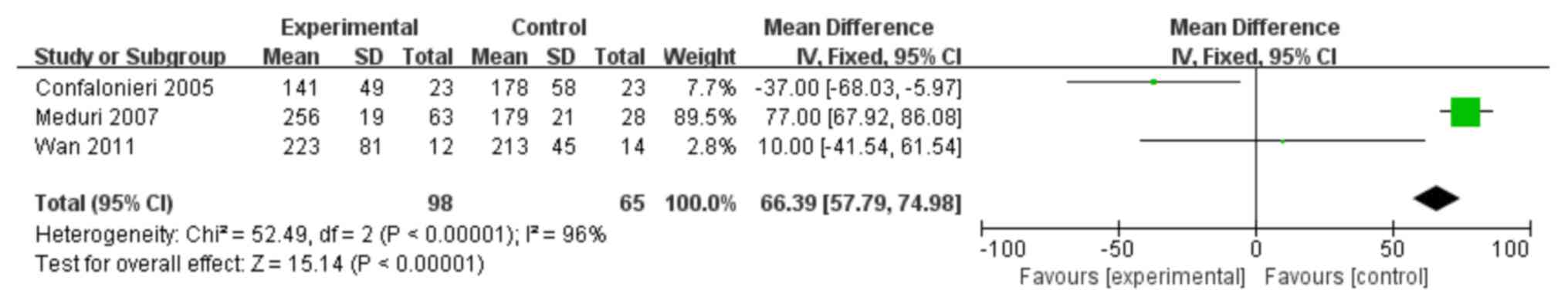
A total of 3 trials (11,12,28) were
identified as having investigated whether corticosteroids could
augment the PaO2/FiO2 ratio in patients with
ARDS. As shown in Fig. 7, the
PaO2/FiO2 ratio was significantly increased
in the treatment group when compared with the controls (MD: 66.39;
95% CI: 57.79–74.98; P<0.05).
Meta-analysis of the risk of new
infection in patients with ARDS treated with steroids
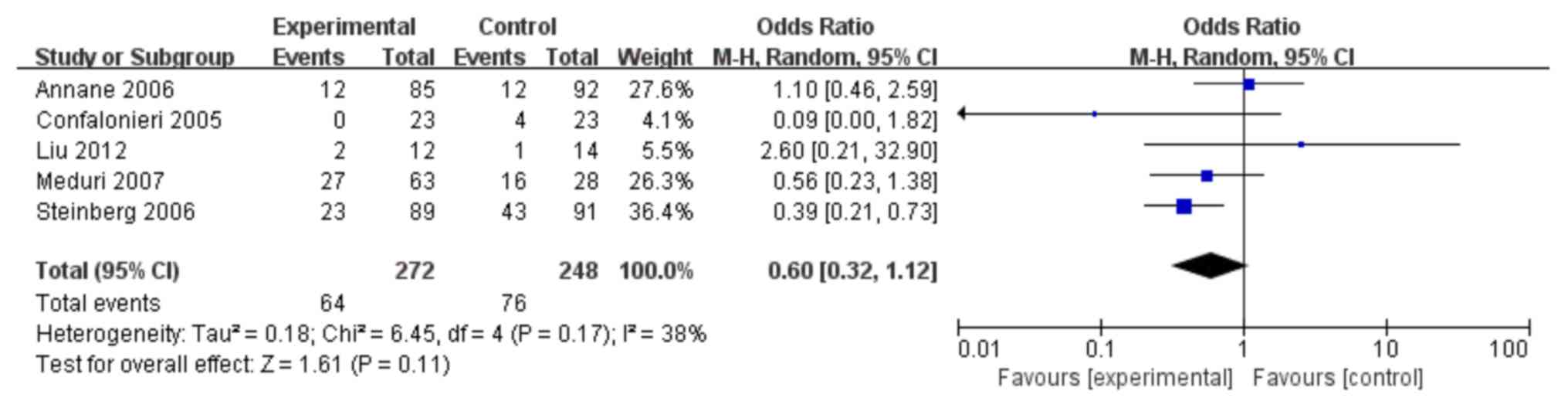
In total, 5 trials (10–12,25,27) were
identified which investigated whether corticosteroids could
increase the risk of a new infection (side effects). As shown in
Fig. 8, the use of corticosteroids
did not increase the risk of a new infection when compared with
controls (OR: 0.60; 95% CI: 0.32–1.12; P>0.05).
Discussion
ARDS is an inflammatory disease process of the lungs
which occurs in response to a variety of reasons. ARDS is
characterized clinically by severe hypoxemia, reduced lung
compliance and bilateral radiographic infiltrates (1). Although the prevalence of ARDS is
expected to increase worldwide, there is no effective treatment for
this fatal disease. Corticosteroids have been the most widely
studied drugs for ARDS and are the only agents that have shown
promise as a potential treatment. In the present study, the
increased sample size allowed the detection of a significant
treatment effect in terms of mortality reduction. As a result, a
lower mortality rate was identified in the low-dose group compared
with the control group, with a combined OR of 0.43 and a 95% CI of
0.24–0.79. Furthermore, there was a significantly lower mortality
in patients who were administered with steroids early compared with
the controls (OR: 0.46; 95% CI: 0.21–1.01), but not when comparing
patients with late administrations of corticosteroids with the
controls. The beneficial effects of corticosteroid therapy observed
in the present analysis concurred with previous trials, which
showed improved PaO2/FiO2 ratios and reduced
periods of mechanical ventilation in ARDS in response to treatment
with low doses of methylprednisolone (10–12).
Furthermore, the pooled estimates, provided by the random effects
model, considered the heterogeneity evident across existing
studies.
Over recent decades, the dosage and timing of
corticosteroid therapy for patients with ARDS has changed. Prior to
1990, previous studies usually used a high daily dose (30 mg/kg)
over a short period of time (≤2 days) in order to prevent or treat
ARDS. Some investigators suggested different treatment doses for
early ARDS and persistent ARDS in which a duration of ARDS ≤3 days
was considered as early ARDS and ≥5 days as persistent or
non-resolving ARDS (31,32). In addition, sub-group analysis of the
ARDS net steroid study recommended against starting corticosteroid
therapy >14 days after the onset of ARDS (25). These findings suggested that a
particular sub-group of patients with ARDS might benefit the most
from corticosteroid therapy: Those with persistent ARDS and an
onset of ARDS <14 days.
The results in a number of previous studies
(10–12,24,25) have
demonstrated that treatments with low doses of corticosteroids have
been associated with a lower mortality rate for patients with ARDS.
In 2006, a previous study conducted by Annane et al
(10) demonstrated that a 7 day
treatment, involving low doses of corticosteroids, was associated
with better outcomes in septic shock-associated early ARDS
non-responders, but not in responders. In this previous study,
patients underwent a short corticotropin test with 250 mg
tetracosactrin (Synacthène Ciba), which was administered
intravenously before randomization. Patients were then graded as
responders when cortisol increased by <9 g/dl (250 nmol/l)
(10). The effect on mortality was
consistent in both randomized and non-randomized studies (cohort
studies), and highlighted the need for multiple-center,
placebo-controlled, randomized and double-blind trials to confirm
efficacy. In 2014, a previous study conducted by Ruan et al
(33) showed that the effects of
corticosteroids on the mortality of patients with ARDS differed by
way of the duration of outcome measures and by etiologies.
Corticosteroids did not improve longer-term outcomes and may have
caused harm in certain sub-groups. However, the number of RCTs and
sample size were relatively small. There were only two studies in
some sub-group analyses and the statistical power was insufficient
in some cases. In the present study, different sub-groups were
analyzed and only RCT studies were selected, resulting in a more
scientifically rigorous conclusion.
Due to the limitations of the present data, it was
only possible to conduct a pooled analysis of mortality in relation
to incident infection, and the number of days alive and off
mechanical ventilation. Therefore, the effects of glucocorticoids
on ARDS was not investigated thoroughly. Compared with other
meta-analyses, the effect of prolonged glucocorticoid treatment was
not assessed, as outcome data from pooled studies were used. The
present study was also limited by the inclusion of small to
moderately sized RCTs with a relatively small number of events and
the satisfied and RCT designs were included to solve the problem of
corticoids in patients with ARDS, resulting in the inclusion of two
old studies. Furthermore, it was not possible to completely rule
out publication bias.
The present meta-analysis suggested that
corticosteroid treatment can reduce the mortality rate of patients
with ARDS, particularly in the early stages. Glucocorticoid
treatment can alleviate systemic inflammation and accelerate the
resolution of ARDS. However, high-dose glucocorticoid treatment and
the late administration of glucocorticoids did not significantly
improve the outcome of patients with ARDS. This result suggested
that the duration of glucocorticoid therapy is just as important,
in terms of treatment effects, as the dose itself. The present
results also suggested that the number of days the patients
remained alive, and the number of days for on/off mechanical
ventilation, as well as the PaO2/FiO2 ratio,
improved following the administration of glucocorticoid. The use of
steroids was not associated with the risk of new infection in
patients with ARDS undergoing steroid treatment. Therefore, the
present analyses demonstrated that corticosteroid therapy is
associated with a trend towards reduced mortality rates.
Acknowledgements
Not applicable.
Funding
The present study was supported by Jiangsu
Provincial Key Research and Development Program (grant no.
BE2016721), Natural science Foundation of Jiangsu Province (grant
no. BK20191351) and Jiangsu Planned Projects for Postdoctoral
Research Funds (grant no. 2019k178).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW and DDL conceptualized and designed this study.
SSS provided the study materials. DDL, HZ and XWZ collected and
assembled the data. SSS and XWZ analyzed and interpreted the data.
All authors wrote the manuscript and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ARDS
|
acute respiratory distress
syndrome
|
|
RCT
|
randomized controlled trial
|
|
OR
|
odds ratio
|
|
CI
|
confidence interval
|
|
MD
|
mean difference
|
|
M-H
|
Mantel-Haenszel
|
References
|
1
|
Ware LB and Matthay MA: The acute
respiratory distress syndrome. N Engl J Med. 342:1334–1349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meduri GU, Annane D, Chrousos GP, Marik PE
and Sinclair SE: Activation and regulation of systemic inflammation
in ARDS: Rationale for prolonged glucocorticoid therapy. Chest.
136:1631–1643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meduri GU and Eltorky MA: Understanding
ARDS-associated fibroproliferation. Intensive Care Med. 41:517–520.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roch A, Guervilly C and Papazian L: Fluid
management in acute lung injury and ards. Ann Intensive Care.
1:162011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lamberts SW, Bruining HA and de Jong FH:
Corticosteroid therapy in severe illness. N Engl J Med.
337:1285–1292. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chirousos GP: The
hypothalamic-pituitary-adrenal axis and immune-mediated
inflammation. N Engl J Med. 332:1351–1362. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Udelsman R, Ramp J, Gallucci WT, Gordon A,
Lipford E, Norton JA, Loriaux DL and Chrousos GP: Adaptation during
surgical stress. A reevaluation of the role of glucocorticoids. J
Clin Invest. 77:1377–1381. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
MacLaren R and Jung R: Stress-dose
corticosteroid therapy for sepsis and acute lung injury or acute
respiratory distress syndrome in critically ill patients.
Pharmacotherapy. 22:1140–1156. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Newton R: Molecular mechanisms of
glucocorticoid action: What is important? Thorax. 55:603–613. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Annane D, Sébille V and Bellissant E;
Ger-Inf-05 Study Group, : Effect of low doses of corticosteroids in
septic shock patients with or without early acute respiratory
distress syndrome. Crit Care Med. 34:22–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Confalonieri M, Urbino R, Potena A,
Piattella M, Parigi P, Puccio G, Della Porta R, Giorgio C, Blasi F,
Umberger R and Meduri GU: Hydrocortisone infusion for severe
community-acquired pneumonia: A preliminary randomized study. Am J
Respir Crit Care Med. 171:242–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meduri GU, Golden E, Freire AX, Taylor E,
Zaman M, Carson SJ, Gibson M and Umberger R: Methylprednisolone
infusion in early severe ARDS: Results of a randomized controlled
trial. Chest. 131:954–963. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Foster JR; Steroids for early acute
respiratory distress syndrome: Critical appraisal of, ; Meduri GU,
Golden E, Freire AX, et al: Methylprednisolone infusion in early
severe ARDS: Results of a randomized controlled trial. Chest.
2007.131:954–963, Pediatr Crit Care Med 11: 404–407. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lamontagne F, Briel M, Guyatt GH, Cook DJ,
Bhatnagar N and Meade M: Corticosteroid therapy for acute lung
injury, acute respiratory distress syndrome and severe pneumonia: A
meta-analysis of randomized controlled trials. J Crit Care.
25:420–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoogwerf B and Danese RD: Drug selection
and the management of corticosteroid-related diabetes mellitus.
Rheum Dis Clin North Am. 25:489–505. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McDonough AK, Curtis JR and Saag KG: The
epidemiology of glucocorticoid-associated adverse events. Curr Opin
Rheumatol. 20:131–137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weigelt JA, Norcross JF, Borman KR and
Snyder WH III: Early steroid therapy for respiratory failure. Arch
Surg. 120:536–540. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Agarwal R, Nath A, Aggarwal A and Gupta D:
Do glucocorticoids decrease mortality in acute respiratory distress
syndrome? A meta-analysis. Respirology. 12:585–590. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ranieri VM, Rubenfeld GD, Thompson BT,
Ferguson ND, Caldwell E, Fan E, Camporota L and Slutsky AS: Acute
respiratory distress syndrome: The berlin definition. JAMA.
307:2526–2533. 2012.PubMed/NCBI
|
|
20
|
Savović J, Weeks L, Sterne JA, Turner L,
Altman DG, Moher D and Higgins JP: Evaluation of the Cochrane
Collaboration's tool for assessing the risk of bias in randomized
trials: Focus groups, online survey, proposed recommendations and
their implementation. Syst Rev. 3:372014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeiss EE and Hanley JA: Mantel-Haenszel
techniques and logistic regression: Always examine one's data first
and don't overlook the simpler techniques. Paediatr Perinat
Epidemiol. 6:311–315. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Higgins JP and Green S: Cochrane handbook
for systematic reviews of interventions. Version 5.2.0The Cochrane
Collaboration. 2017
|
|
23
|
Ning G, et al: The guidelines for clinical
usage of glucocorticoid. Chin J Endocrinol Metab. 28:2012.
|
|
24
|
Lee HS, Lee JM, Kim MS, Kim HY, Hwangbo B
and Zo JI: Low-dose steroid therapy at an early phase of
postoperative acute respiratory distress syndrome. Ann Thorac Surg.
79:405–410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Li J, Huang YZ, Liu SQ, Yang CS,
Guo FM, Qiu HB and Yang Y: The effect of stress dose glucocorticoid
on patients with acute respiratory distress syndrome combined with
critical illness-related corticosteroid insufficiency. Zhonghua Nei
Ke Za Zhi. 51:599–603. 2012.(In Chinese). PubMed/NCBI
|
|
26
|
Seam N, Meduri GU, Wang H, Nylen ES, Sun
J, Schultz MJ, Tropea M and Suffredini AF: Effects of
methylprednisolone infusion on markers of inflammation,
coagulation, and angiogenesis in early acute respiratory distress
syndrome. Crit Care Med. 40:495–501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Steinberg KP, Hudson LD, Goodman RB, Hough
CL, Lanken PN, Hyzy R, Thompson BT and Ancukiewicz M: National
heart, lung, and blood institute acute respiratory distress
syndrome(ARDS) clinical trials network: Efficacy and safety of
corticosteroids for persistent acute respiratory distress syndrome.
N Engl J Med. 354:1671–1684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wan MH, Li J, Gong HL, Xue P, Zhu L, Chen
GY, Xia Q and Wen-Fu T: Clinical observation on the effect of
dexamethasone and Chinese herbal decoction for purgation in severe
acute pancreatitis patients. Chin J Integr Med. 17:141–145. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bernard GR, Luce JM, Sprung CL, Rinaldo
JE, Tate RM, Sibbald WJ, Kariman K, Higgins S, Bradley R and Metz
CA: High-dose corticosteroids in patients with the adult
respiratory distress syndrome. N Engl J Med. 317:1565–1570. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thompson BT: Glucocorticoids and acute
lung injury. Crit Care Med. 31 (4 Suppl):S253–S257. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bone RC, Fisher CJ, Clemmer TP, Slotman
GJ, Metz CA and Balk RA: Sepsis syndrome: A valid clinical entity.
Methylprednisolone severe sepsis study group. Crit Care Med.
17:389–393. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luce JM, Montgomery AB, Marks JD, Turner
J, Metz CA and Murray JF: Ineffectiveness of high-dose
methylprednisolone in preventing parenchymal lung injury and
improving mortality in patients with septic shock. Am Rev Respir
Dis. 138:62–68. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ruan SY, Lin HH, Huang CT, Kuo PH, Wu HD
and Yu CJ: Exploring the heterogeneity of effects of
corticosteroids on acute respiratory distress syndrome: A
systematic review and meta-analysis. Crit Care. 18:R632014.
View Article : Google Scholar : PubMed/NCBI
|