Introduction
Triple-negative breast cancer (TNBC) is a subtype of
breast cancer that is characterized by the lack of estrogen
receptor, progesterone receptor and human epidermal growth factor
receptor 2 expression (1). In total,
TNBC accounts for ~10–25% of all breast cancer cases (1), and is associated with unique clinical
and pathophysiological phenotypes, including strong invasiveness,
low overall survival rate and poor prognosis (2). Compared with other types of breast
cancer, TNBC carries an increased probability of metastasis and
recurrence (3) due to metastasis to
other organs, including the lungs, liver, and brain via the
systemic circulation. Targeted therapy cannot be administered to
patients with TNBC due to the lack of hormone receptor expression.
Although previous studies have reported that TNBC is more sensitive
to chemotherapy compared with other types of breast cancer, TNBC
remains to exhibit the worst prognosis compared with other subtypes
(4). Therefore, the development of
novel anti-metastatic therapeutic strategies is required for the
effective treatment of TNBC.
Brucea javanica is the fruit of the Brucea
javanica (L.) Merr (Simaroubaceae). It has been used to treat a
number of diseases, including cancer, dysentery, malaria and
stomach ulcers (5). Bruceine D (BD;
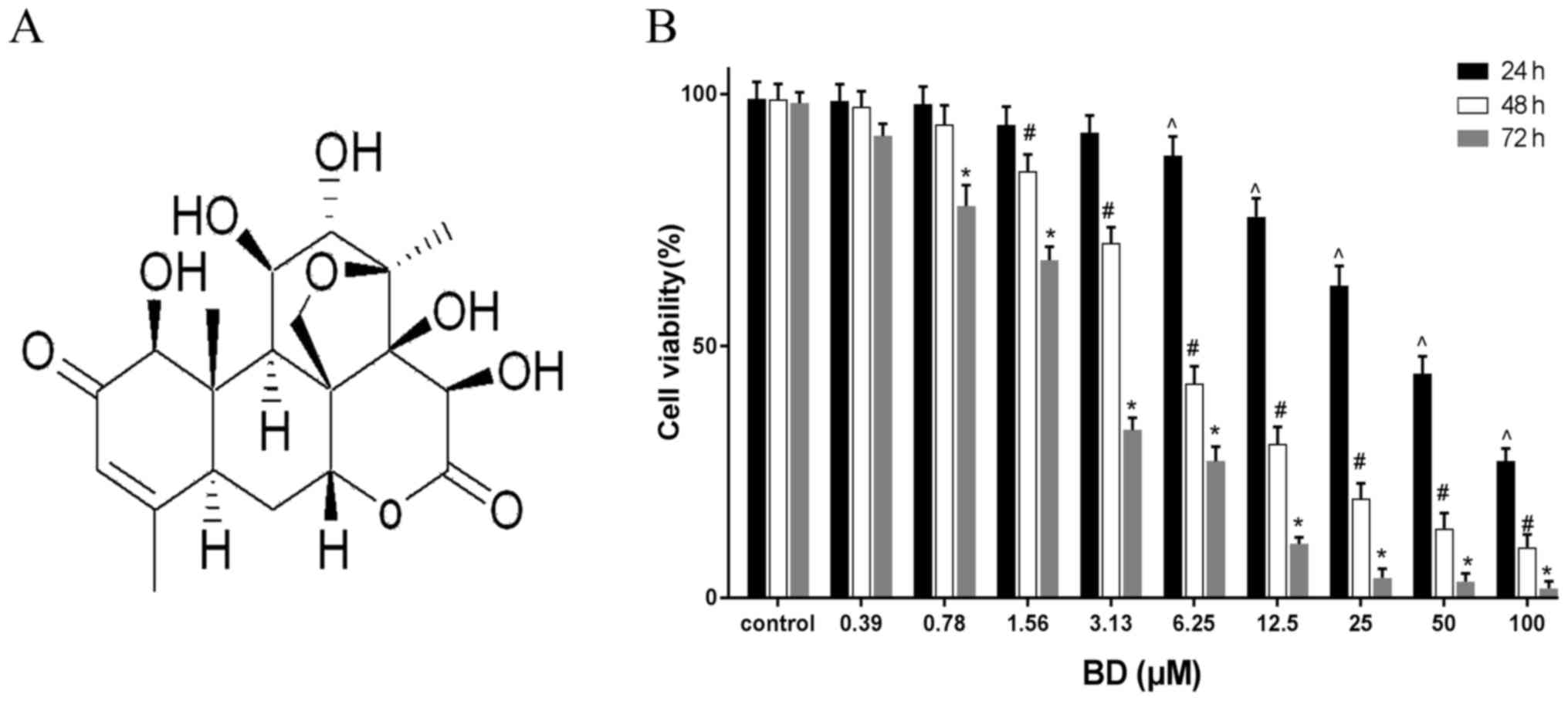
Fig. 1A) is a bioactive component
that can be isolated from Brucea javanica (6), which has previously been reported to
induce apoptosis in pancreatic cancer cell lines PANC1, SW1990 and
Capan1 and inhibit hepatoma cell proliferation (7–10).
Metastasis is an important feature of malignant
tumors that impede the clinical treatment of cancer (11). Epithelial-mesenchymal transition
(EMT) is an important stage in the metastasis of cancer cells.
During this process, epithelial cell polarity is lost, and is
coupled with concomitant enhancements in migratory and invasive
abilities (12). As a result, the
epithelial phenotype disappears, whereas the mesenchymal phenotype
gradually develops (12). The
PI3K/AKT signaling pathway has been previously indicated to be
associated with cancer cell proliferation, differentiation,
migration and invasion (13). AKT
activation in epithelial cells reduces cell polarity and
intercellular adhesion and promotes EMT in cancer cells by altering
the expression and distribution of epithelial and mesenchymal
markers (14).
In the present study, the TNBC cell line MDA-MB-231
was used to investigate the potential inhibitory effects of BD on
cell viability, migration and invasion. In addition, the effect of
BD on the EMT process and the PI3K/AKT signaling pathway were
evaluated in this cell type.
Materials and methods
Materials
The sample of Bruceine D (≥98% purity) used in the
current study was provided by the Institute of Traditional Chinese
Medicine and Natural Products, Jinan University (Guangzhou, China).
RPMI-1640 medium and FBS were purchased from Gibco; Thermo Fisher
Scientific, Inc. The MTT cell proliferation and cytotoxicity assay
kits and penicillin/streptomycin solution (PS) were purchased from
Nanjing KeyGen Biotech Co., Ltd. The antibody targeting PI3K (cat.
no. AF5112) was obtained from Affinity Biosciences. Antibodies
against AKT (cat. no. 60203-2-Ig), phosphorylated (p)-AKT (cat. no.
66444-1-Ig), E-cadherin (cat. no. 20874-1-AP) and β-catenin (cat.
no. 51067-2-AP) were purchased from Proteintech Group, Inc.
Vimentin antibody (cat. no. BS1491) was purchased from Bioworld
Technology, Inc. Antibodies against GAPDH (cat. no. ab181602) and
horseradish peroxidase (HRP)-conjugated goat anti-rabbit (cat. no.
ab6721) immunoglobulin (Ig) G were purchased from Abcam.
Cell culture
Human triple-negative breast cancer MDA-MB-231 cells
were donated by Nanjing Pharmaceutical Co., Ltd. Cells were
cultured in RPMI 1640 medium supplemented with 10% FBS and 1% PS
solution in a humidified atmosphere with 5% CO2 at 37°C.
BD was dissolved with DMSO and diluted in complete RPMI-1640 medium
to required concentrations (1, 2 and 4 µM). The final DMSO
concentration in the culture medium was ≤0.1% and control cells
were treated with 0.1% DMSO at 37°C.
Cell viability assay
MTT assay was performed to measure cell viability.
Cells were seeded into 96-well plates (5×103
cells/well), cultured overnight and subsequently treated at 37°C
with ascending concentrations of BD (100, 50, 25, 12.5, 6.25, 3.13,
1.56, 0.78 and 0.39 µM) for 24, 48 and 72 h. Each well then
received 20 µl MTT (5 mg/ml) and the cells were cultured for an
additional 4 h at 37°C. Subsequently, cells were rinsed using PBS
and each well received 150 µl DMSO. Optical density was then
measured at the wavelength of 490 nm using a microplate reader
(Mutiskan™ MK3; Thermo Fisher Scientific, Inc.). Data were
presented as the percentage of survival rate relative to that of
control.
Wound-healing assay
A wound-healing assay was performed to evaluate the
migratory ability of MDA-MB-231 cells. Cells in the logarithmic
growth phase were inoculated into six-well plates (5×103
cells/well). The following day, when ~100% of the surface was
occupied a straight cell-free wound was introduced by scratching
the bottom of the plate using a sterile pipette tip. Subsequently,
the detached cells were washed twice with PBS and then re-incubated
with BD (1, 2 and 4 µM) or 0.1% DMSO dissolved in serum-free RPMI
1640 medium for 24 h at 37°C. The wound images were obtained using
a fluorescence inverted microscope (magnification, ×100) at 0 and
24 h, respectively, where the wound distance was measured using the
following formula: Migration distance=scratch distance at 0
h-scratch distance at 24 h.
Transwell assay
The invasive capabilities of MDA-MB-231 cells were
evaluated using a Transwell® assay (24 wells; Matrigel
gel; Corning, Inc.). The cells were first cultured in serum-free
RPMI medium for 24 h at 37°C. Matrigel was incubated at 4°C
overnight, and melted Matrigel was diluted twice with incomplete
medium. A total of 30 µl diluted Matrigel was added to the upper
chamber of each Transwell insert, which was then incubated at 37°C
for 120 min. The upper chamber of the Transwell contained 100 µl
cells (1×104 cells/chamber) suspended in serum-free RPMI
medium containing different concentrations of BD (1, 2 and 4 µM) or
0.1% DMSO, and the lower chamber was supplemented with 500 µl
RPMI-1640 medium containing 20% FBS. Following incubation for 24 h
at 37°C, cells on the upper surface of the membrane were removed
using a cotton swab, whereas cells on the bottom surface of insert
membrane were fixed with 95% alcohol for 10 min at room temperature
and subsequently stained with 0.1% crystal violet for 30 min at
room temperature. Invasive cells were photographed and counted in
five random fields of view under a fluorescence inverted microscope
(magnification, ×200).
Western blot analysis
Cells were first treated with BD (1–4 µM) or 0.1%
DMSO for 24 h at 37°C before being washed with cold PBS, lysed
using RIPA buffer (Gibco; Thermo Fisher Scientific, Inc.) and
centrifuged at 13,000 × g for 15 min at 4°C. The supernatant was
collected and stored at −20°C for further use. A BCA protein assay
kit (Nanjing KeyGen Biotech Co., Ltd.) was used to quantify the
total protein concentration for each sample. Protein samples (30
µg) were subsequently separated by 12% SDS-PAGE (90 min, 100 V) and
transferred onto nitrocellulose membranes (90 min; 300 mA). The
membranes were then blocked with 5% non-fat dry milk for 1 h at
room temperature and washed three times with TBS supplemented with
Tween-20 (TBS-T) following which the membranes were incubated with
primary antibodies against vimentin (1:1,000), E-cadherin
(1:5,000), β-catenin (1:5,000), PI3K (1:1,000), AKT (1:5,000),
p-AKT (1:5,000) or GAPDH (1:10,000) overnight at 4°C. The membranes
with the primary antibodies were then incubated on a shaker at room
temperature for 30 min. After further rinsing with TBS-T three
times, the membranes were incubated with HRP-conjugated goat
anti-rabbit (1:2,000) IgG secondary antibodies at room temperature
for 1 h. The protein bands were then visualized with enhanced
chemiluminescent substrates (cat. no. 32106; Thermo Fisher
Scientific, Inc.) using a Syngene G:BOX Chemi XR5 imaging system
(Syngene International). Gel-Pro Analyzer software (version 4.0;
Media Cybernetics, Inc.) was used to perform densitometric analysis
on each membrane. GAPDH was used as a loading control.
Statistical analysis
All experimental data were presented as the mean ±
standard deviation. All experiments were repeated in triplicate.
The data were analyzed using a one-way ANOVA, followed by Tukey's
test using GraphPad 7 (GraphPad Software, Inc). P<0.05 was
considered to indicate a statistically significant difference.
Results
BD reduces MDA-MB-231 cell
viability
MTT assay was performed to evaluate the effect of BD
on the viability of MDA-MB-231 cells. The cells were treated with
varying concentrations of BD (0–100 µM) for 24, 48 and 72 h at
37°C. BD treatment markedly reduced cell viability in a time- and
dose-dependent manner (Fig. 1B). The
half maximal inhibitory concentrations (IC50) of BD were
calculated to be 40.805, 5.84 and 2.364 µM at 24, 48 and 72 h,
respectively.
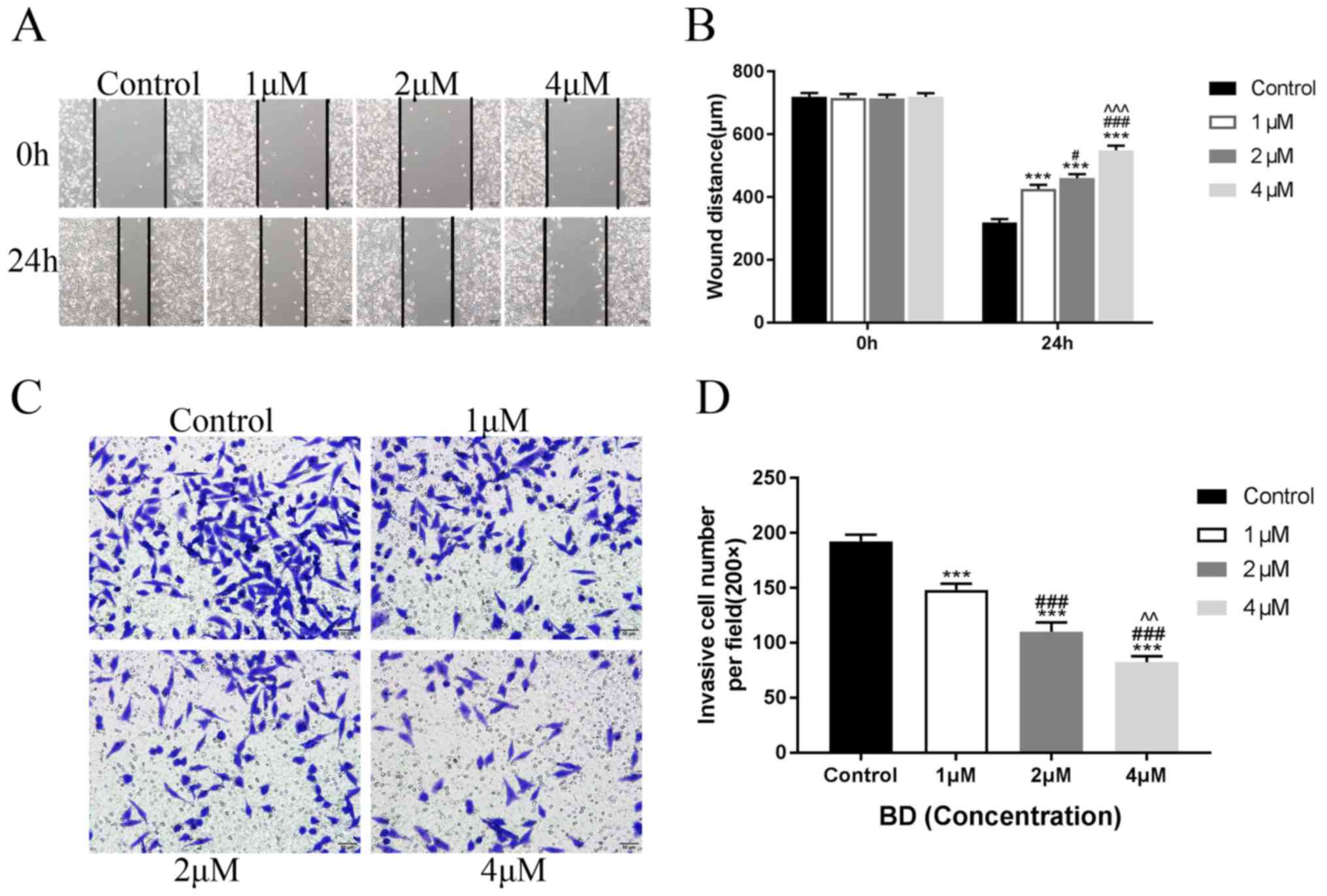
BD suppresses the migratory and
invasive capabilities of MDA-MB-231 cells
Wound healing and Transwell assays were performed to
explore the effects of BD on cell migration and invasion,
respectively. In this assay, a low dose of BD (1, 2 and 4 µM),
which exerted little to no effects on cell viability, was used. BD
reduced the wound healing ability of MDA-MB-231 cells in a
dose-dependent manner (Fig. 2A). The
width of the wounds observed following treatment with BD at 1, 2
and 4 µM were 715.79±0.8, 714.62±1.01 and 718.71±1.01 µm at 0 h,
respectively, which were not significantly different compared with
the control group (719.01±1.34 µm; Fig.
2A and B). Following treatment with different concentrations of
BD for 24 h, the width of the wounds were 425.44±13.82,
460.53±12.16 and 549.12±14.15 µm in the 1, 2 and 4 µM treatment
groups, respectively, all of which were significantly higher
compared with that in the control group (318.13±11.68 µm; Fig. 2A and B). In addition, BD reduced the
invasive capabilities of MDA-MB-231 cells in a dose-dependent
manner. The numbers of invasive cells were 148.33±5.53, 110±9.21
and 82.33±5.55 in the 1, 2 and 4 µM treatment groups, respectively
(Fig. 2C and D), all of which were
significantly lower compared with the control group (192.33±6.08).
Altogether, these results indicated that BD suppressed the
migratory and invasive capabilities of MDA-MB-231 cells in a
dose-dependent manner.
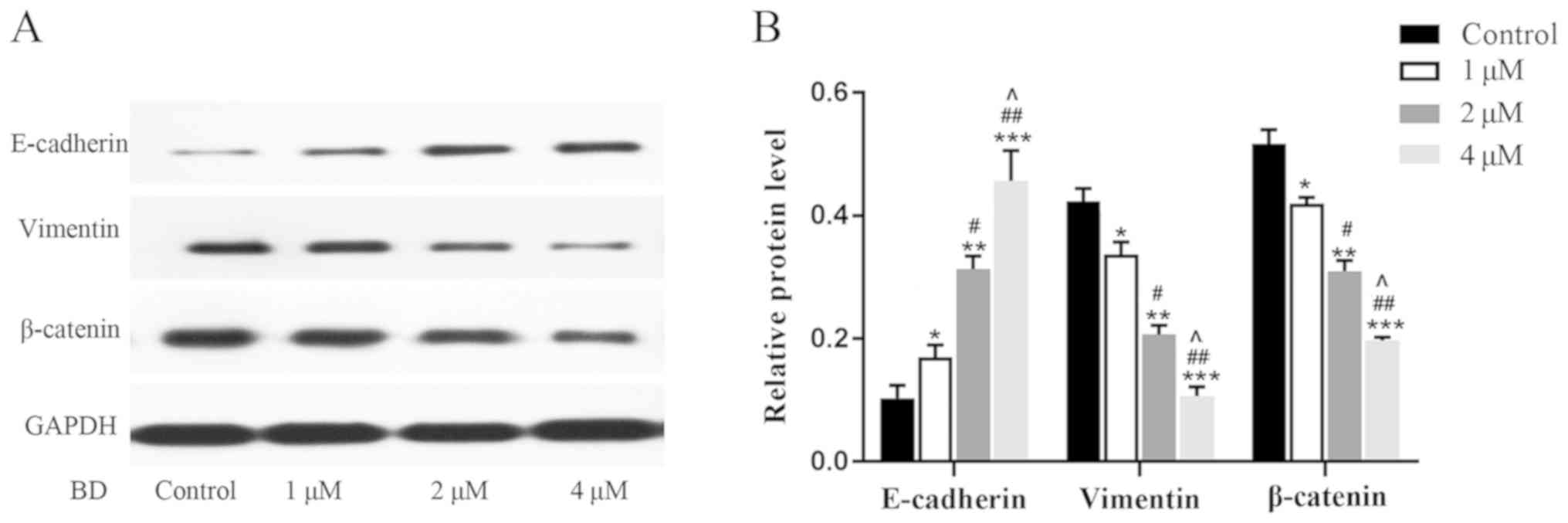
BD reverses the EMT process of
MDA-MB-231 cells
EMT is an important physiological process in the
migration and invasion of malignant tumor cells (12). Following treatment with 1–4 µM BD or
0.1% DMSO for 24 h, western blot analysis was performed to evaluate
the expression of E-cadherin, vimentin and β-catenin, which are
proteins associated with EMT. E-cadherin expression in cells
treated with BD were found to be significantly higher compared with
the control group (Fig. 3A and B).
In contrast, the expression of vimentin and β-catenin were
significantly lower compared with the control group (Fig. 3A and B). Additionally, the
upregulation of E-cadherin expression and the downregulation of
vimentin and β-catenin expression appeared to be dependent on the
dose of BD applied. These observations suggest that BD reversed the
EMT process in MDA-MB-231 cells in a dose-dependent manner.
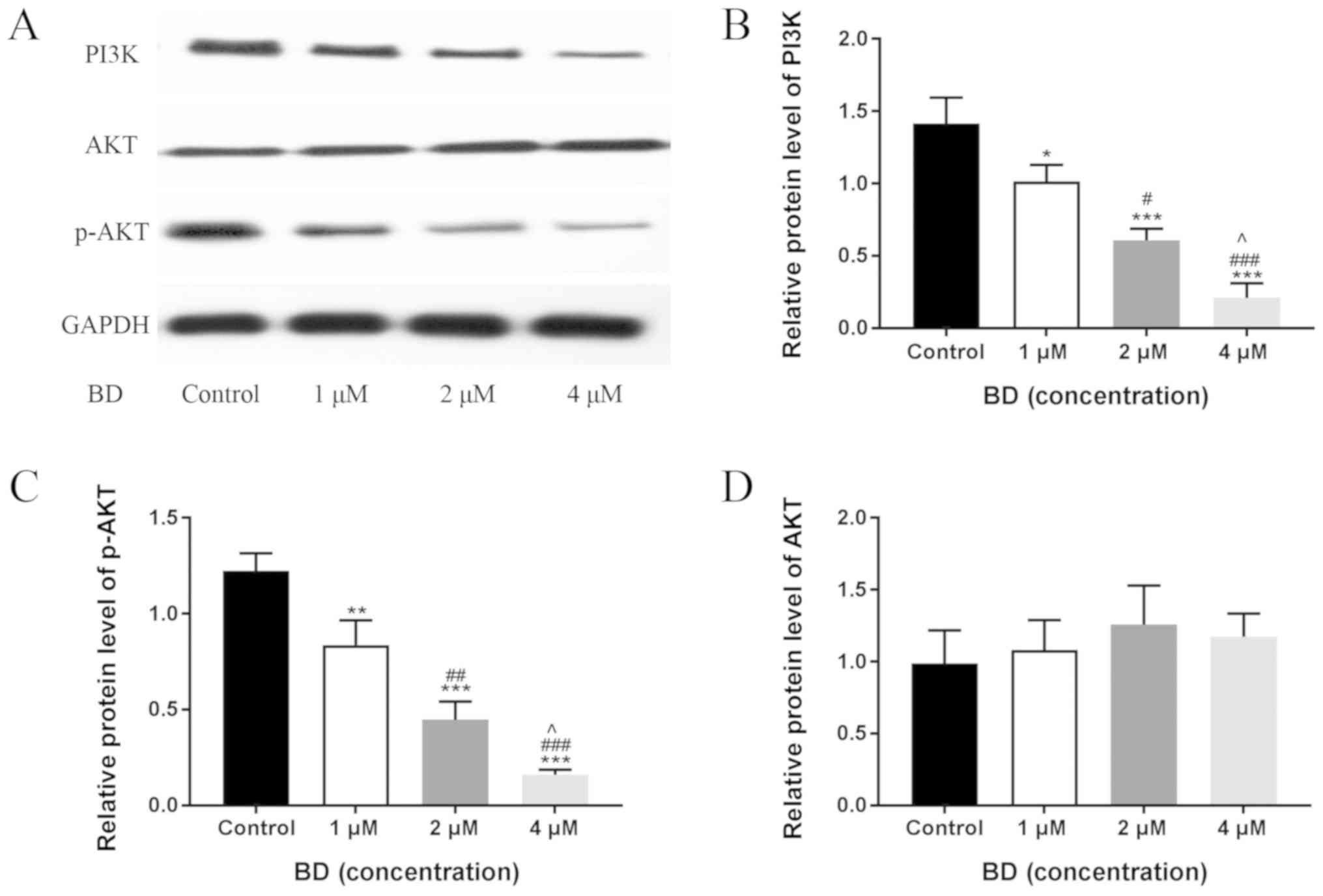
BD inhibits the activation of the
PI3K/AKT pathway in MDA-MB-231 cells
The PI3K/AKT signaling pathway has been previously
reported to serve a role in cancer cell migration and invasion
(13). Therefore, western blot
analysis was performed to determine the expression of PI3K, AKT,
and p-AKT, which are key components in the PI3K/AKT signaling
pathway. BD significantly reduced PI3K expression and AKT
phosphorylation in a dose-dependent manner after 24 h, whilst no
significant changes were observed in total AKT levels (Fig. 4). These results indicated that BD
inhibited the PI3K/AKT signaling pathway in MDA-MB-231 cells in a
dose-dependent manner.
Discussion
BD is a potent quassinoid extracted from the
Brucea javanica plant, which has been demonstrated to
exhibit anticancer effects in a number of previous studies
(15). Xiao et al (10) reported that BD induced apoptosis of
hepatoma cells by regulating microRNA-95 expression without
affecting the growth of normal hepatocytes. Cheng et al
(16) demonstrated that BD inhibited
liver cancer proliferation by synergizing with the protein kinase
inhibitor sorafenib to induce tumor necrosis and apoptosis.
However, since the anticancer effects of BD on TNBC cells remain
unclear, the present study examined the potential effects of BD on
the MDA-MB-231 cell line. Data from the present study demonstrated
that BD reduced the viability of MDA-MB-231 cells in a time- and
dose-dependent manner. Additionally, low concentrations of BD (1–4
µM) were used for wound healing and Transwell assays, which
indicated that BD significantly inhibited MDA-MB-231 cell migration
and invasion in a dose-dependent manner, suggesting that BD exerts
a potent anti-migratory effect on MDA-MB-231 cells.
Tumor metastasis is a process in which malignant
tumor cells migrate from the primary site to other organs via
lymphatic channels, blood vessels and body cavities (11). Previous studies have revealed that
the EMT serves a pivotal role in the primary invasion and secondary
metastasis of breast, colon and liver cancer (17), where the reduction or loss of
E-cadherin expression is a key landmark change during the EMT
process (18). E-cadherin is an
important protein that is associated with cell-cell adhesion and
attachment, and promotes adhesion between cells to maintain
structural integrity (19). A number
of studies have previously reported that E-cadherin is involved in
the metastasis of malignancies, including colorectal, breast and
cervical squamous cell carcinoma (20–23).
Sloan and Anderson (24) confirmed
that breast cancer patients with lower E-cadherin expression were
associated with higher rates of bone and lung metastasis. Indeed,
the intracellular cytoskeletal structure is altered during the EMT,
which is mainly characterized by upregulated vimentin expression
(25). During the EMT process in
breast cancer, the cytoskeleton profile changes from cytokeratin to
vimentin, significantly increasing cell viability (26). In contrast, silencing vimentin
expression reduces the invasiveness of breast cancer (27,28).
β-catenin is a cytoskeletal protein that binds to E-cadherin and
α-catenin to form the E-cadherin/catenin complex, which serves an
important role in cell adhesion and maintaining the structural
integrity of epithelial cells (29,30).
However, the downregulation of E-cadherin expression results in the
dissociation of this complex, releasing β-catenin into the nucleus
to activate the TCF/Lef transcription factor, inducing the
transcription of genes that regulate invasion and migration
(31). Cheng et al (16) indicated that BD inhibited the
expression of β-catenin in hepatoma cells. The results of the
present study demonstrated that BD upregulates E-cadherin
expression whilst downregulating β-catenin and vimentin expression
in a concentration-dependent manner, suggesting that BD effectively
reversed EMT in MDA-MB-231 cells.
The PI3K/AKT signaling pathway has been found to be
aberrantly activated in a large number of tumor cells (32). PI3K is a specific class of kinases
that catalyze the synthesis of phosphatidylinositol lipids
(33). Activated PI3K activates its
downstream target AKT via a second messenger, phosphatidylinositol
3,4,5-triphosphate [PI(3,4,5)P3] (34). Previous studies have demonstrated
that inhibition of PI3K inhibits AKT activation, subsequently
downregulating the expression of key regulatory factors of the
cytoskeleton by suppressing adhesion, thereby inhibiting EMT
(13,35,36).
Wang et al (37) reported
that inhibition of AKT activation inhibited ovarian cancer cell
proliferation and invasion. In another study, Nakanishi et
al (38) detected AKT
phosphorylation in serum-free cultured Li7 cells, which is a liver
cancer cell line, suggesting that AKT activation is associated with
intrahepatic hematogenous metastasis. Treatment with a PI3K/AKT
blocker LY294002 in the implanted model inhibited intrahepatic
metastasis, demonstrating that PI3K/AKT serves an important role in
metastasis (38). Lai et al
(39) previously confirmed that BD
inhibited the PI3K/AKT signaling pathway in pancreatic cancer
cells. Similarly, the present study revealed that BD treatment
reduced PI3K expression and AKT activation in a
concentration-dependent manner, suggesting that BD inhibits the
PI3K/AKT signaling pathway in MDA-MB-231 cells, which can serve as
the underlying mechanism behind the anti-invasion and
anti-migratory effects of BD.
However, it should be noted that the present study
only explored the inhibitory effects of BD on the PI3K/AKT
signaling pathway. Whether this inhibition was mediated by BD
directly and if this leads to the suppression of EMT remains
unclear, since multiple signaling pathways are likely to be
involved in the regulation of EMT. In addition, whether BD directly
regulates the expression of genes downstream of AKT was not been
investigated in the present study. Since only one cell line was
used, whether BD exerts similar anti-tumor effects on other TNBC
cell lines remains to be determined. The present study was
conducted in vitro, in vivo studies are required to
investigate whether BD exerts similar anti-migratory and
anti-invasive effects on TNBC xenografts or human samples.
Treatment with 1–4 µM BD exerted anticancer effects in
vitro, but whether it can safely reach the amount used in
vivo will also need to be studied in the future. Therefore,
addressing these questions will be the subject for further
research.
In conclusion, the present study demonstrated that
BD inhibited MDA-MB-231 cell viability migration and invasion
whilst suppressing EMT and PI3K/AKT signaling activation. These
results highlighted the use of BD as a therapeutic agent for the
treatment of TNBC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81241102).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZJ and CL designed the study. CL was a major
contributor in writing the manuscript. CL and YW performed cell
culture and MTT assay experiments. CL and CW performed the wound
healing and Transwell assay. CL and YC performed western blotting.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BD
|
Bruceine D
|
|
EMT
|
epithelial-mesenchymal
transformation
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Nakhjavani M, Hardingham JE, Palethorpe
HM, Price TJ and Townsend AR: Druggable molecular targets for the
treatment of triple negative breast cancer. J Breast Cancer.
22:341–361. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malla RR, Kumari S, Gavara MM, Badana AK,
Gugalavath S, Kumar DK and Rokkam P: A perspective on the
diagnostics, prognostics, and therapeutics of microRNAs of
triple-negative breast cancer. Biophys Rev. 11:227–234. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He MY, Rancoule C, Rehailia-Blanchard A,
Espenel S, Trone JC, Bernichon E, Guillaume E, Vallard A and Magne
N: Radiotherapy in triple-negative breast cancer: Current situation
and upcoming strategies. Crit Rev Oncol Hematol. 131:96–101. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bianchini G, Balko JM, Mayer IA, Sanders
ME and Gianni L: Triple-negative breast cancer: Challenges and
opportunities of a heterogeneous disease. Nat Rev Clin Oncol.
13:674–690. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan Z, Guo GF and Zhang B: Research of
Brucea javanica against cancer. Chin J Integr Med.
23:153–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dou YX, Zhou JT, Wang TT, Huang YF, Chen
VP, Xie YL, Lin ZX, Gao JS, Su ZR and Zeng HF: Self-nanoemulsifying
drug delivery system of bruceine D: A new approach for
anti-ulcerative colitis. Int J Nanomedicine. 13:5887–5907. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lau ST, Lin ZX, Liao Y, Zhao M, Cheng CH
and Leung PS: Bruceine D induces apoptosis in pancreatic
adenocarcinoma cell line PANC-1 through the activation of
p38-mitogen activated protein kinase. Cancer Lett. 281:42–52. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lau ST, Lin ZX and Leung PS: Role of
reactive oxygen species in brucein D-mediated p38-mitogen-activated
protein kinase and nuclear factor-kappaB signalling pathways in
human pancreatic adenocarcinoma cells. Br J Cancer. 102:583–593.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu L, Lin ZX, Leung PS, Chen LH, Zhao M
and Liang J: Involvement of the mitochondrial pathway in bruceine
D-induced apoptosis in Capan-2 human pancreatic adenocarcinoma
cells. Int J Mol Med. 30:93–99. 2012.PubMed/NCBI
|
|
10
|
Xiao Z, Ching Chow S, Han Li C, Chun Tang
S, Tsui SK, Lin Z and Chen Y: Role of microRNA-95 in the anticancer
activity of Brucein D in hepatocellular carcinoma. Eur J Pharmacol.
728:141–150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Irani S: Emerging insights into the
biology of metastasis: A review article. Iran J Basic Med Sci.
22:833–847. 2019.PubMed/NCBI
|
|
12
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Duan L, Zou Z, Li H, Yuan S, Chen
X, Zhang Y, Li X, Sun H, Zha H, et al: Activation of the
PI3K/Akt/mTOR/p70S6K pathway is involved in S100A4-induced
viability and migration in colorectal cancer cells. Int J Med Sci.
11:841–849. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Xu X, Li Y, Zou K, Zhang Z, Xu X,
Liao Y, Zhao X, Jiang W, Yu W, et al: Synergistic antitumor effect
of BKM120 with prima-1met via inhibiting PI3K/AKT/mTOR and
CPSF4/hTERT signaling and reactivating mutant P53. Cell Physiol
Biochem. 45:1772–1786. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mao G, Tian Y, Sun Z, Ou J and Xu H:
Bruceine D isolated from Brucea javanica (L.) merr. As a
systemic feeding deterrent for three major lepidopteran pests. J
Agric Food Chem. 67:4232–4239. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng Z, Yuan X, Qu Y, Li X, Wu G, Li C,
Zu X, Yang N, Ke X, Zhou J, et al: Bruceine D inhibits
hepatocellular carcinoma growth by targeting β-catenin/jagged1
pathways. Cancer Lett. 403:195–205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yap AS, Crampton MS and Hardin J: Making
and breaking contacts: The cellular biology of cadherin regulation.
Curr Opin Cell Biol. 19:508–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wells A, Yates C and Shepard CR:
E-cadherin as an indicator of mesenchymal to epithelial reverting
transitions during the metastatic seeding of disseminated
carcinomas. Clin Exp Metastasis. 25:621–628. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Loh CY, Chai JY, Tang TF, Wong WF, Sethi
G, Shanmugam MK, Chong PP and Looi CY: The E-cadherin and
N-cadherin switch in epithelial-to-mesenchymal transition:
Signaling, therapeutic implications, and challenges. Cells.
8:E1182019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li B, Shi H, Wang F, Hong D, Lv W, Xie X
and Cheng X: Expression of E-, P- and N-cadherin and its clinical
significance in cervical squamous cell carcinoma and precancerous
lesions. PLoS One. 11:e01559102016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berx G and Van Roy F: The
E-cadherin/catenin complex: An important gatekeeper in breast
cancer tumorigenesis and malignant progression. Breast Cancer Res.
3:289–293. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Debies MT and Welch DR: Genetic basis of
human breast cancer metastasis. J Mammary Gland Biol Neoplasia.
6:441–451. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilmanns C, Grossmann J, Steinhauer S,
Manthey G, Weinhold B, Schmitt-Graff A and von Specht BU: Soluble
serum E-cadherin as a marker of tumour progression in colorectal
cancer patients. Clin Exp Metastasis. 21:75–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sloan EK and Anderson RL: Genes involved
in breast cancer metastasis to bone. Cell Mol Life Sci.
59:1491–1502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scanlon CS, Van Tubergen EA, Inglehart RC
and D'Silva NJ: Biomarkers of epithelial-mesenchymal transition in
squamous cell carcinoma. J Dent Res. 92:114–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tanaka K, Tokunaga E, Inoue Y, Yamashita
N, Saeki H, Okano S, Kitao H, Oki E, Oda Y and Maehara Y: Impact of
expression of vimentin and axl in breast cancer. Clin Breast
Cancer. 16:520–526.e2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu R, Zhou Z, Yu W, Xia Y and Zhi X: CPEB4
promotes cell migration and invasion via upregulating vimentin
expression in breast cancer. Biochem Biophys Res Commun.
489:135–141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zelenko Z, Gallagher EJ, Tobin-Hess A,
Belardi V, Rostoker R, Blank J, Dina Y and LeRoith D: Silencing
vimentin expression decreases pulmonary metastases in a
pre-diabetic mouse model of mammary tumor progression. Oncogene.
36:1394–1403. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Conacci-Sorrell M, Zhurinsky J and
Ben-Ze'ev A: The cadherin-catenin adhesion system in signaling and
cancer. J Clin Invest. 109:987–991. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ren L, Chen H, Song J, Chen X, Lin C,
Zhang X, Hou N, Pan J, Zhou Z, Wang L, et al: MiR-454-3p-mediated
Wnt/β-catenin signaling antagonists suppression promotes breast
cancer metastasis. Theranostics. 9:449–465. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan X, Lyu T, Jia N, Yu Y, Hua K and Feng
W: Huaier aqueous extract inhibits ovarian cancer cell motility via
the AKT/GSK3β/β-catenin pathway. PLoS One. 8:e637312013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Liu C, Tan T, Li S, Tang S and Chen
X: Sinomenine sensitizes human gastric cancer cells to cisplatin
through negative regulation of PI3K/AKT/Wnt signaling pathway.
Anticancer Drugs. 30:983–990. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elmenier FM, Lasheen DS and Abouzid KA:
Phosphatidylinositol 3 kinase (PI3K) inhibitors as new weapon to
combat cancer. Eur J Med Chem. 183:1117182019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: Rationale and
promise. Cancer Cell. 4:257–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ning J, Liu W, Zhang J, Lang Y and Xu S:
Ran GTPase induces EMT and enhances invasion in non-small cell lung
cancer cells through activation of PI3K-AKT pathway. Oncol Res.
21:67–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang J, Xiao L, Luo CH, Zhou H, Hu J, Tang
YX, Fang KN and Zhang Y: Overexpression of TRPM7 is associated with
poor prognosis in human ovarian carcinoma. Asian Pac J Cancer Prev.
15:3955–3958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakanishi K, Sakamoto M, Yasuda J,
Takamura M, Fujita N, Tsuruo T, Todo S and Hirohashi S: Critical
involvement of the phosphatidylinositol 3-kinase/Akt pathway in
anchorage-independent growth and hematogeneous intrahepatic
metastasis of liver cancer. Cancer Res. 62:2971–2975.
2002.PubMed/NCBI
|
|
39
|
Lai ZQ, Ip SP, Liao HJ, Lu Z, Xie JH, Su
ZR, Chen YL, Xian YF, Leung PS and Lin ZX: Brucein D, a naturally
occurring tetracyclic triterpene quassinoid, induces apoptosis in
pancreatic cancer through ROS-associated PI3K/Akt signaling
pathway. Front Pharmacol. 8:9362017. View Article : Google Scholar : PubMed/NCBI
|