Introduction
Since the first ABO-incompatible (ABOi) kidney
transplantation (KT), was carried out in Japan in 1989 and
excellent long-term outcomes were obtained in subsequence surgeries
(1–3), the number of cases of ABOi-KT have
gradually increased worldwide. To avoid graft rejection in ABOi-KT,
immunosuppression is usually used to maintain the titre of ABO
blood group antibodies at a low level and to inhibit B-cell
activation (4–6). However, antibody-mediated graft
rejection is still one of the major causes of poor outcome in the
clinic.
Notably, in various ABOi-KT cases, the ABO blood
group antibody returned to baseline levels without
antibody-mediated graft rejection. For instance, Makroo et
al found that after ABOi-KT the graft survived normally, even
though the ABO blood group antibody titre returned to the
preoperative level (7). Snell et
al noted that the kidney graft survived normally for three
years while the recipient did not receive any B-cell-targeted
therapy, such as rituximab (8). In
other words, immunological accommodation occurred in those
cases.
Many researchers have found that the process of
immunological accommodation is associated with changes in graft
antigen and B-cell activation. After ABOi-KT, ABO blood group
antigens on the endothelium of the kidney graft were found to be
significantly decreased (9), and
soluble ABO blood antigen produced by the transplanted kidney are
able to bind to antibodies to reduce antibody-mediated immune
reactions (10), thus the graft
antigen may be one of the mechanisms underlying immunological
accommodation. On the other hand, in ABOi-KT, B cells could be
activated to produce enhancing antibodies (also named blocking
antibodies) that bind competitively with ABO blood group antigens
thus preventing an immune response (11). Consequently, ABO blood group
antibodies play an important role in the process of immunological
accommodation, but the mechanism of B-cell activation to produce
antibodies with different functions is still unclear.
Previous research has identified that haptoglobin is
associated with the activation of cytokine-induced killer cells
(CIKs) (12), and B cells in
peripheral blood mononuclear cells (PBMCs) from blood group A could
be activated by HK2 cells carrying blood group B antigen (13). In the present study, the role of HP
in B-cell activation was analyzed by flow cytometry, dot-ELISA and
bioinformatics. These results will provide novel insight for
further research on immunological accommodation in ABOi-KT
conditions.
Materials and methods
Peripheral blood was donated from blood group A
volunteers after informed consent. The study was approved by the
Animal Welfare and Research Ethics Committee of the Institute of
University of South China (Hengyang, Hunan, China).
Cell culture
Peripheral blood mononuclear cells (PBMCs) from
donors with blood group A were separated by the Ficoll density
gradient method as reported by Huang et al (14). The HK2 cells (human, non-tumor
cells), which carry human blood group B antigen, and PBMCs were
divided into three groups: The HK2 group contained HK2 cells, the
PBMC group contained PBMCs, and the HK2+PBMC group contained equal
quantities of PBMCs and HK2 cells. All groups were cultured with
RPMI-1640 medium (Thermo Fisher Scientific, Inc.) and 15% fetal
calf serum (FCS; Thermo Fisher Scientific, Inc.) at 37°C in 5%
CO2. In addition, previous research identified that
B-cell activation is significantly upregulated to the highest level
at day 4 (13). Thus, all of the
cells were collected at day 4 for further detection.
Flow cytometric assay
Day 0 PBMCs were collected and divided into two
groups: The isotype control group, which was mixed with 5 µl mouse
IgG2α FITC-conjugated antibody (BD Biosciences; cat no. 2137834)
and 5 µl mouse IgG1 APC-conjugated antibody (BD Biosciences; cat
no. 39573); and the target group, which was mixed with 5 µl mouse
anti-human CD3 FITC-conjugated antibody (BD Biosciences; cat. no.
4043995) and 5 µl mouse anti-human CD19 APC-conjugated antibody (BD
Biosciences; cat. no. 555415). Both groups were incubated at room
temperature for 15 min, and then treated with 200 µl Red Blood Cell
Lysis Buffer (Solarbio) at room temperature for 10 min, and
centrifuged at 150 × g for 10 min. Following resuspension with 1 ml
physiological saline and centrifugation at 150 × g for 10 min, the
cells were further treated following the instructions of the
Cytofix/Cytoperm™ Fixation/Permeabilization Kit (BD Biosciences).
In the process, 1 µl rabbit IgG monoclonal antibody (Abcam) was
added to the isotype control group, and 2 µl rabbit anti-human HP
(BosterBio) was added to the target group, and then the cells were
respectively incubated with 1 µl PE-conjugated goat anti-rabbit IgG
(4A Biotech Co., Ltd.). Finally, the cells were analyzed by BD FACS
Aria II, and at least 100,000 cells were collected per sample.
Extraction of cell proteins and
collection of supernatants
In the coculture system, HK2 cells were adhered on
the bottom of a culture dish, while lymphocytes exhibited suspended
growth. Thus, in the detection time, the lymphocytes were collected
with the culture medium after being shaken lightly, and HK2 cells
were collected using trypsinization (Solarbio). The supernatants of
all groups were collected and centrifuged at 13,000 × g for 10 min
and then transferred into clean EP tubes for further experiments.
HK2 cells of the HK2 group, PBMCs of the PBMC group and the
HK2+PBMC group were collected and then centrifuged at 900 × g for
10 min. The supernatants of all groups were collected and stored at
−20°C and the cells were resuspended in 2 ml physiological saline.
Following centrifugation at 900 × g for 10 min, the cell pellet was
collected and total protein was extracted using Native lysis Buffer
(Solarbio). The total protein concentration was determined by a BCA
Protein Assay Kit (Tiangen). HP concentration was detected by a
Haptoglobin Kit (Dialab). All cell protein extracts were stored at
−20°C with 0.01 M phenylmethylsulfonyl fluoride (PMSF).
Dot-ELISA analysis
A polyvinylidene fluoride (PVDF) membrane was
activated with methyl alcohol for 15 sec and then soaked in TBS
(Solarbio) for 10 min. For maintaining the comparability among
different samples, the total protein concentration of culture
supernatant was detected. After the PVDF membrane had dried at room
temperature, 2 µl (0.5 µg/µl) protein of each sample was
respectively applied to the PVDF membrane. Then, the membrane was
blocked with blocking buffer (TBS containing 0.05% Tween-20 and 5%
skim milk) at room temperature for 1 h. Following 3 washes with TBS
for 5 min each, the membrane was incubated with rabbit anti-human
HP (dilution 1:200; BosterBio; cat. no. BA3744) or mouse anti-human
blood group B antibody (dilution 1:1,000; Abcam; cat. no. 3968) for
1 h at room temperature and rinsed 3 times with TBS for 5 min each
time. The PVDF membrane was incubated with goat anti-rabbit IgG-HRP
(dilution 1:5,000; Cell Signaling Technology; cat. no. 7074P2) or
goat anti-mouse IgG-HRP (dilution 1:3,000; Thermo Fisher
Scientific, Inc.; cat. no. 31431) for 40 min at room temperature.
Finally, the membrane was rinsed 2 times with TBS for 15 min each.
For the expression analysis of IgM and IgG, PVDF membranes were
incubated with goat anti-human IgM-HRP (1:3,000; Thermo Fisher
Scientific, Inc.; cat. no. 31415) or goat anti-human IgG-HRP
(dilution 1:3,000; Thermo Fisher Scientific, Inc.; cat. no. 31410)
for 1 h at room temperature and rinsed 2 times with TBS for 15 min
each. All samples were dyed with ECL Plus (Solarbio) and analyzed
by ChemiDoc XRS+ (Bio-Rad).
In addition, the interaction of HP and Smad3 was
also analyzed using this method. In this experiment, rabbit
anti-human Smad3 (dilution 1:200; BosterBio; cat. no. BA4559) and
mouse anti-human Smad4 (dilution 1:200; BosterBio; cat. no. BM1601)
were used as the primary antibodies, and goat anti-rabbit IgG-HRP
(dilution 1:5,000; Cell Signaling Technology; cat. no. 7074S) and
goat anti-mouse IgG-HRP (dilution 1:5,000; Sigma-Aldrich; Merck
KGaA; cat. no. AP308P) were used as the secondary antibodies. After
1 µl mouse anti-human HP (dilution 1:1,000; Abcam; cat. no.
ab13429), rabbit anti-human HP (dilution 1:200), or mouse
anti-human Smad4 (dilution 1:200; BosterBio) was applied to PVDF
membranes, the membranes were blocked with blocking buffer at room
temperature for 1 h. Following 3 washes with TBS for 5 min each,
the cell protein extraction of the HK2+PBMC group (dilution 1:50)
was added to the membranes and incubated at room temperature for 1
h and then rinsed 3 times with TBS for 5 min each. Then, the
membranes were incubated with primary antibodies at room
temperature for 1 h. After rinsing 3 times with TBS for 5 min each,
the membranes were incubated with secondary antibodies at room
temperature for 40 min. Finally, the membranes were rinsed 2 times
with TBS for 15 min each, dyed with ECL Plus (Solarbio) and
analyzed by ChemiDoc XRS+ (Bio-Rad).
RNA exaction and cDNA synthesis
After stimulation with HK2 cells for 4 days, cells
from the PBMC and HK2+PBMC groups were collected, and the total RNA
was extracted using an RNAsimple Total RNA kit (TianGen Biotech
(Beijing) Co., Ltd.). cDNA was synthesized using a RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.).
PCR and sequence analysis
The primers used in this study are shown in Table I. The 40 µl premix for RT-qPCR was
composed of 20 µl 2X PCR Master Mix (Thermo Fisher Scientific), 2
µl forward primer, 2 µl reverse primer, 2 µl cDNA template and 14
µl ddH2O. The reaction programme was as follows: 94°C
for 2 min; 32 cycles at 94°C for 30 sec, 55°C for 30 sec, and 72°C
for 30 sec; and 72°C for 10 min. A 15 µl PCR product was used to
analyze the change in HP expression by agarose gel electrophoresis
(1% agarose, 80 V for 1 h), and 25 µl PCR product of HP was used
for sequencing.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer | Sequence |
|---|
| HP-F |
CAGCCAGAAACATAACCC |
| HP-R |
TCTACACCCTAACTACTCCC |
| β-actin-F |
ATCGTGCGTGACATTAAGGAG |
| β-actin-R |
TAGGTGCTTTGATGGAAGTTGAG |
Quantitative real-time PCR
analysis
The primers are listed in Table I. The 20 µl premix for RT-qPCR was
composed of 10 µl 2X SYBR Green PCR Mastermix (Beijing Solarbio
Science & Technology Co., Ltd., China), 1 µl forward primer, 1
µl reverse primer, 1 µl cDNA template and 7 µl ddH2O.
The reaction programme was as follows: 95°C for 2 min and 40 cycles
at 95°C for 5 sec and 60°C for 30 sec. The experiment was detected
by LightCycler 96 (Roche).
Bioinformatics analysis
Proteins interacting with HP were predicted by
Cytoscape software 3.7.1 (https://cytoscape.org). Information on the HP
interaction proteins was obtained from the National Center for
Biotechnology Information (NCBI) website (https://www.ncbi.nlm.nih.gov/gene/?term=).
Statistical analysis
All experiments were repeated at least 3 times. The
data were analyzed with SigmaPlot 12.0 software, and the results
are shown as the mean ± SEM. Student's t-test was used to assess
the statistical significance, and P<0.05 and 0.01 were
considered to indicate significant and very significant
differences, respectively.
Results
Analysis of lymphocytes expressing
HP
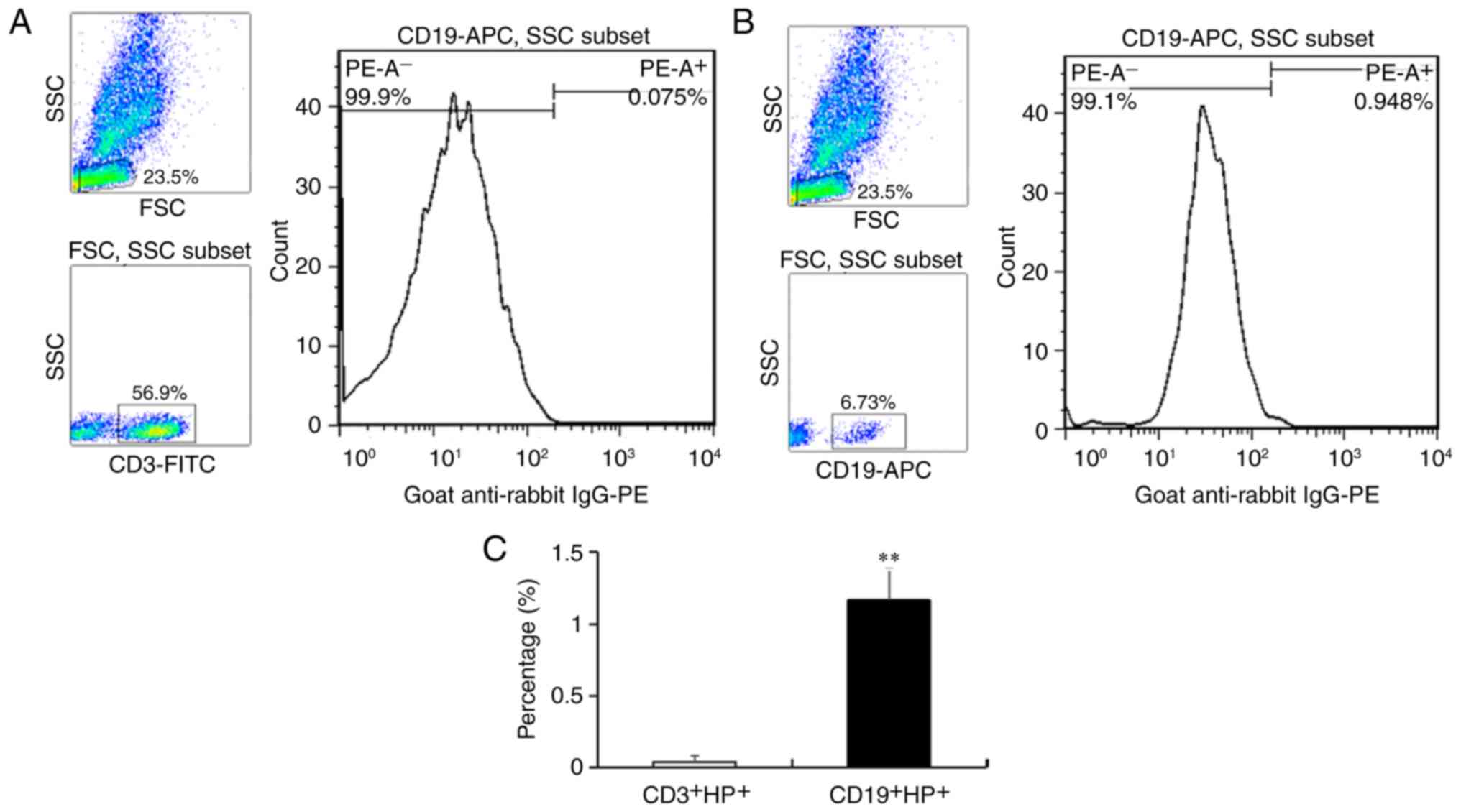
To identify the type of lymphocytes that express HP,
PBMCs were analyzed by flow cytometry (Fig. 1). To avoid the influence from the
sample, the starting samples of Fig.
1B was the same as those of Fig.
1A in this experiment. The results showed that the percentage
of CD3+ T cells was 56.90% without an obvious
HP+ cell population (Fig.
1A), and the percentage of CD19+ B cells was 6.73%
and that of HP+ cells was 0.95% (Fig. 1B). After statistical analysis, the
average percentage of CD19+HP+ cells was
calculated to be 1.16%. These results indicated that
CD19+ B cells were the lymphocytes that expressed
HP.
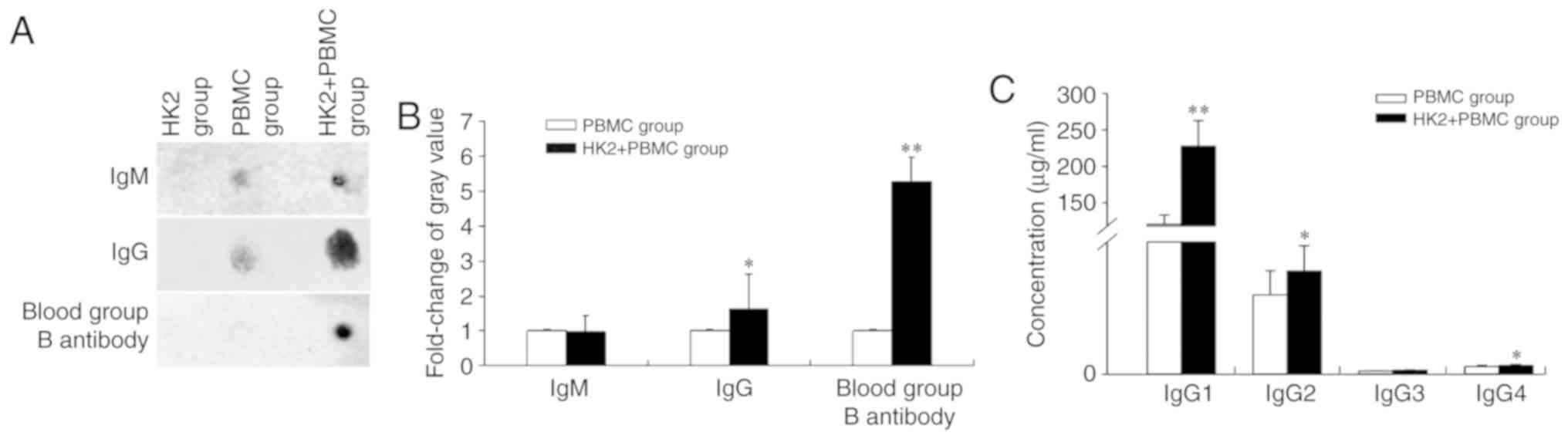
Expression of blood group B antibody,
IgM, total IgG and IgG subclasses
Human blood group antibodies are divide into two
major groups: IgM and IgG (15).
Thus, in this study, the expression of IgM, IgG, blood group B
antibody and IgG subclasses in cell culture supernatants were
detected after co-culture with HK2 cells. Dot-ELISA analysis showed
that IgG and blood group B antibody levels were significantly
increased 1.63- and 5.28-fold, respectively, and IgM was not
significantly altered (Fig. 2A and
B). Moreover, an ELISA assay showed that IgG1, IgG2 and IgG4
were increased 1.89-, 1.30- and 1.16-fold, respectively, and IgG3
was increased 1.21-fold, which was not statistically significant
(Fig. 2C, Table II). These results indicated that the
B cells were activated to produce antibodies with different
biological activities.
 | Table II.ELISA was used to detect the
concentration of IgG subclasses in PBMC and HK2+PBMC groups. |
Table II.
ELISA was used to detect the
concentration of IgG subclasses in PBMC and HK2+PBMC groups.
| IgG subclasses | PBMC group | HK2+PBMC group |
|---|
| IgG1 (µg/ml) | 120.45±12.71 | 227.85±34.94 |
| IgG2 (µg/ml) | 2.39±0.73 | 3.12±0.77 |
| IgG3 (µg/ml) | 0.1±0.01 | 0.12±0.02 |
| IgG4 (µg/ml) | 0.23±0.05 | 0.27±0.03 |
Expression of HP at the mRNA and
protein levels
As shown in Fig. 3,
the expressed sequence tags (EST) of HP were highly similar to the
sequence of human HP (Fig. 3A and
B). After B cells were activated by HK2 cells, HP mRNA was
significantly downregulated 14.38-fold (Fig. 3C and D). Moreover, dot-ELISA assay
revealed that HP was detected in the cell protein extract of the
PBMC and HK2+PBMC groups but not in the cell protein extract of the
HK2 cells or in the culture supernatant of any of the groups
(Fig. 3E). In addition, the
concentration of HP in the HK2+PBMC group (0.069 mg/ml) was
significantly increased 1.34-fold compared with that in the PBMC
group (0.052 mg/ml; Fig. 3F). These
results indicated that HP was associated with the activation of B
cells.
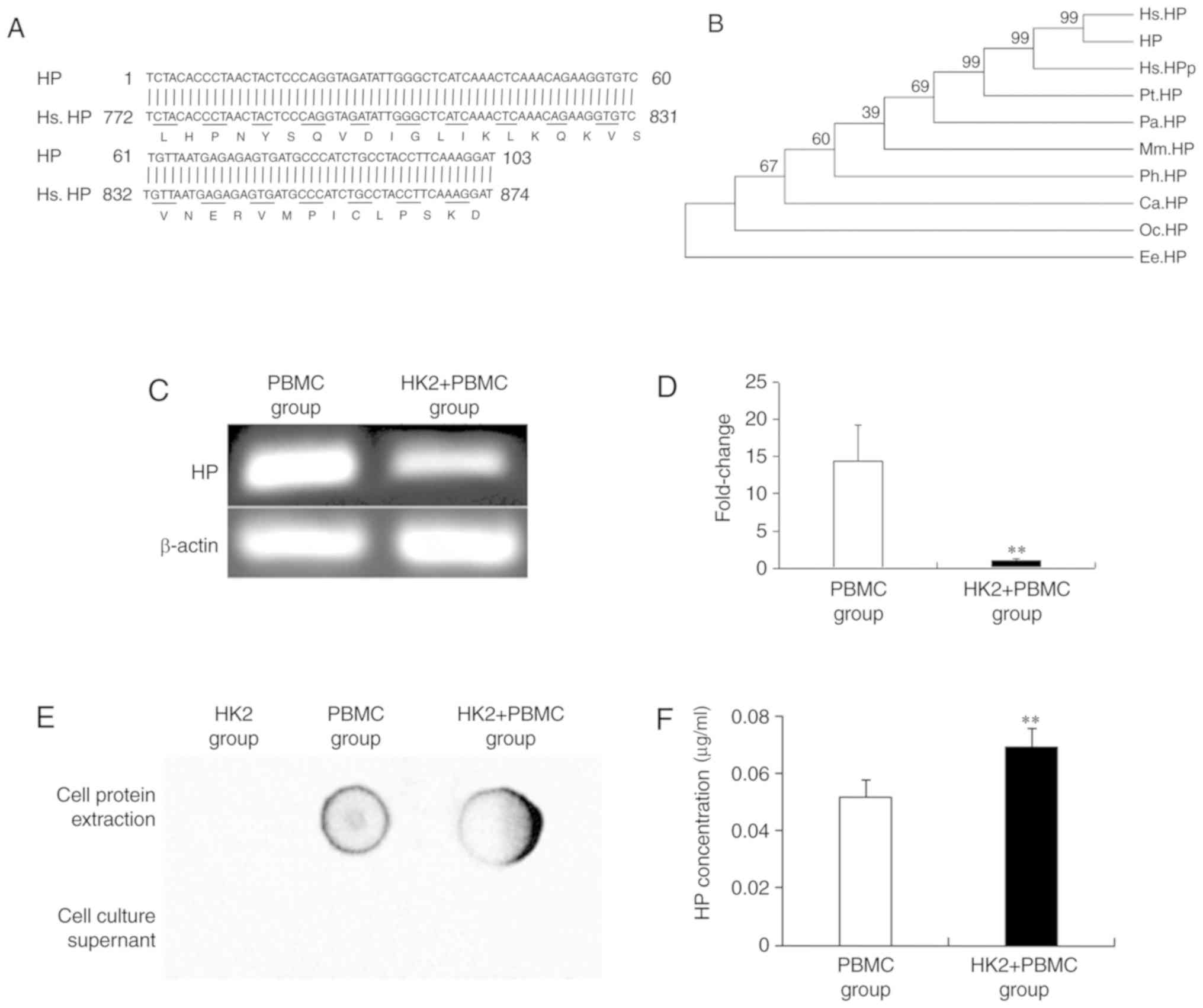 | Figure 3.Change in HP expression in B cells of
the PBMC and HK2+PBMC groups. (A) Sequence analysis of the HP EST.
(B) Phylogenetic tree analysis of HP. Hs, Homo sapiens; Pt,
Pan troglodytes; Ph, Papio hamadryas; Ca,
Cercocebus atys; Pa, Pongo abelii; Mm, Microcebus
murinus; Oc, Oryctolagus cuniculus; Ee, Elephantulus
edwardii; HP, haptoglobin; HPP, haptoglobin isoform 3
preproprotein. (C) Agarose gel electrophoresis analysis of the
expression of HP mRNA. (D) RT-qPCR analysis of the expression of HP
mRNA. (E) Dot-ELISA analysis of the expression of HP. Rabbit
anti-human HP (dilution 1:200) and goat anti-rabbit IgG-HRP
(dilution 1:5,000) antibodies were used as the primary and
secondary antibodies, respectively. (F) Immunoturbidimetric
analysis of the concentration of HP. Bars represent the mean ± SD
(n=3). **P<0.01, significant differences between the PBMC and
HK2+PBMC groups. PBMC, peripheral blood mononuclear cell; HP,
haptoglobin. |
Analysis of the interaction protein of
HP
To reveal the signaling pathway of HP in B cells,
bioinformatics analysis and dot-ELISA were performed. The results
showed that of the 24 potential proteins that interacted with HP,
Smad3 (4088), which possessed higher indegree and edge betweenness,
was selected as the main candidate (Fig.
4A). The protein-protein interaction network indicated that
Smad3 could interact with other SMAD family proteins such as Smad4
(4089, Fig. 4B). Furthermore,
dot-ELISA verified that the HP-Smad3 and Smad4-Smad3 complexes were
detected in the cell protein extraction of PBMCs (Fig. 4C and E) but not the HP-Smad4 complex
(Fig. 4D). These results indicated
that Smad3 was the target protein that interacted with HP.
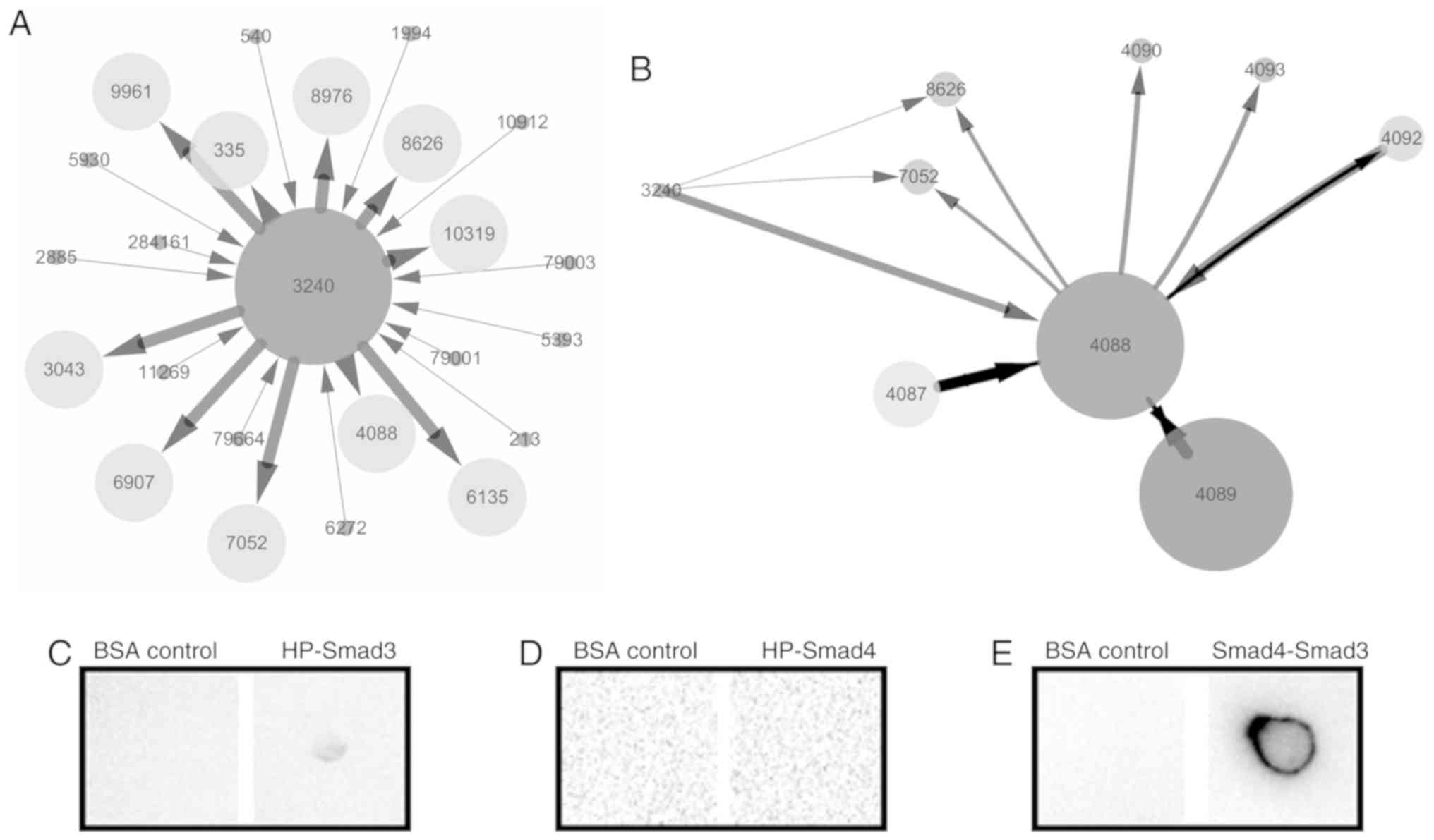 | Figure 4.Analysis of the interaction protein of
HP. (A) Bioinformatics analysis of protein interactions with HP
(3240). The numbers in the figure are the Gene IDs obtained from
the National Center for Biotechnology Information (NCBI) database
(https://www.ncbi.nlm.nih.gov/gene/?term=). The
indegree is indicated by the size of the nodes. The edge
betweenness and shared interaction are indicated with the width and
length of the lines, respectively. The direction of interaction is
indicated with an arrow. (B) The protein-protein interaction
network of HP and Smad3 (4088). (C) Interaction analysis of HP and
Smad3. PVDF membranes were blotted with mouse anti-human HP
(dilution 1:1,000), followed by incubation with cell protein
extract of the HK2+PBMC group, rabbit anti-human Smad3 (dilution
1:200) and goat anti-rabbit IgG-HRP (dilution 1:5,000). (D)
Interaction analysis of HP and Smad4. PVDF membranes were blotted
with rabbit anti-human HP (dilution 1:200), followed by incubation
with cell protein extract of the HK2+PBMC group, mouse anti-human
Smad4 (dilution 1:200) and goat anti-mouse IgG-HRP (dilution
1:5,000). (E) Interaction analysis of Smad3 and Smad4. PVDF
membranes were blotted with rabbit anti-human Smad3 (dilution
1:200), followed by incubation with cell protein extract of the
HK2+PBMC group, mouse anti-human Smad4 (dilution 1:200) and goat
anti-mouse IgG-HRP (dilution 1:5,000). The experiments were
repeated at least 3 times. PBMC, peripheral blood mononuclear cell;
HP, haptoglobin. |
Discussion
The present study found that HP was mainly expressed
by B cells and not by T cells (Fig.
1). The results were similar to studies that demonstrated that
HP is an important activator of immune cells (16–18).
Based on these results, we conjectured that HP plays important
roles in B-cell activation.
To analyze the activation of B cells, IgM, IgG and
blood group B antibody were detected using Dot-ELISA. The results
showed that the antibodies were not detected in the sample from HK2
cells. Meanwhile, B-cell activation was detected by dot-ELISA and
ELISA methods. Dot-ELISA showed that IgG and blood group B
antibodies were all significantly increased while IgM was not
significantly altered (Fig. 2A and
B), and ELISA results showed that IgG1, IgG2 and IgG4 were all
significantly upregulated (Fig. 2C).
Ig is the main effector molecule of humoral immunity, and its
concentration is related to the number of B cells (19). That is, B cells in the ABOi kidney
cell model were activated to produce different functional
antibodies.
Following these results, the expression of HP was
analyzed at the mRNA and protein levels. After the EST sequence of
HP was verified to be that of human HP (Fig. 3A and B), PCR and RT-qPCR analyses
showed that HP mRNA was significantly downregulated (Fig. 3C and D). In contrast to the change in
HP mRNA levels, HP protein was only detected in PBMCs by dot-ELISA
(Fig. 3E), and the concentration was
significantly upregulated after HK2 stimulation (Fig. 3F). As Liu et al reported
(20), the changes in gene
expression at the mRNA and protein levels are not always in line.
Therefore, the results suggested that HP was closely related to
B-cell activation.
In addition, further research was carried out to
explore the possible mechanism by which HP is involved in B-cell
activation. Bioinformatics analysis found that HP had 24
interaction proteins (Fig. 4A).
Among them, Smad3 (4088) was selected as the most likely
interaction protein because of its higher indegree and edge
betweenness. Smad3 is associated with the regulation,
differentiation and survival of cells because it is a key signal
transducer in multiple signalling pathways (21–23). One
example is the classic TGF-β signaling pathway, in which Smad3 is a
major downstream mediator of TGF-β and is phosphorylated by the
activated TGF-β receptor (24,25).
After phosphorylation, Smad3 is directly involved in Ig class
switch recombination in B cells, which allows the production of
antibodies of different subtypes and subclasses (26,27).
Most important for this study, the HP-Smad3 complex was detected in
the cell protein extract by the Dot-ELISA method (Fig. 4C-E). To ensure the reliability of the
results of Dot-ELISA, multiple negative and positive controls were
selected in this study. For example, Smad3 and Smad4 were
identified with interaction (28),
thus they were selected as positive controls; Bio-informatic
analysis showed that there is no interaction between HP and Smad4,
thus they were selected as a negative control. In addition, the BSA
group was the negative control for the three groups. After
comparison of those control groups, we confirmed that the result of
HP combined with Smad3 was reliable.
In conclusion, the results of this study indicate
that HP is involved in B-cell activation via interaction with
Smad3. This research provides novel insight on immunological
accommodation in ABOi-KT, and further research will explore whether
the HP-Smad3 complex affects B-cell activation via the TGF-β
signaling pathway.
Acknowledgements
Not applicable.
Funding
This study was supported by the Zhengxiang Scholar
Program of The University of South China (Hengyang, Hunan,
China).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Laboratory experiments, data analysis and manuscript
writing were accomplished by JC and CC. Manuscript revision and
data analysis were accomplished by LL, YZ and HZ. The guidance of
the experimental design was performed by JX and YW. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The study was approved by the Animal Welfare and
Research Ethics Committee of the Institute of University of South
China. Informed consent was received from the volunteers.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Koo TY and Yang J: Current progress in
ABO-incompatible kidney transplantation. Kidney Res Clin Pract.
34:170–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takahashi K and Saito K: ABO-incompatible
kidney transplantation. Transplant Rev (Orlando). 27:1–8. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takahashi K, Tanabe K, Ooba S, Yagisawa T,
Nakazawa H, Teraoka S, Hayasaka Y, Kawaguchi H, Ito K, Toma H, et
al: Prophylactic use of a new immunosuppressive agent,
deoxyspergualin, in patients with kidney transplantation from
ABO-incompatible or preformed antibody-positive donors. Transplant
Proc. 23:1078–1082. 1991.PubMed/NCBI
|
|
4
|
Muramatsu M, Gonzalez HD, Cacciola R,
Aikawa A, Yaqoob MM and Puliatti C: ABO incompatible renal
transplants: Good or bad? World J Transplant. 4:18–29. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morimoto H, Ide K, Tanaka Y, Ishiyama K,
Ohira M, Tahara H, Akita T, Tanaka J and Ohdan H: Different
sensitivity of rituximab-treatment to B-cells between
ABO-incompatible kidney and liver transplantation. Hum Immunol.
77:456–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee KW, Park JB, Oh DK, Na BG, Choi JY,
Cho WT, Lee SH, Park HJ, Cho D, Huh WS and Kim SJ: Short-term
outcomes of ABO-incompatible living donor kidney transplantation
with uniform protocol: Significance of baseline anti-ABO titer.
Transplant Proc. 48:820–826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Makroo RN, Nayak S, Chowdhry M, Jasuja S,
Sagar G, Rosamma NL and Thakur UK: Role of therapeutic plasma
exchange in reducing ABO titers in patients undergoing
ABO-incompatible renal transplant. Apollo Medicine. 13:31–36. 2016.
View Article : Google Scholar
|
|
8
|
Snell GI, Davis AK, Menahem S, Kotecha S,
Whitford HM, Levvey BJ, Paraskeva M, Webb A, Westall GW and Walker
RG: ABO incompatible renal transplantation following lung
transplantation. Transpl Immunol. 39:30–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanabe T, Ishida H, Horita S, Yamaguchi Y,
Toma H and Tanabe K: Decrease of blood type antigenicity over the
long-term after ABO-incompatible kidney transplantation. Transpl
Immunol. 25:1–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim J, Kim S, Hwang IS, Choi JR, Lee JG,
Kim YS, Kim MS and Kim HO: Effects of neutralization by soluble ABH
antigens produced by transplanted kidneys from ABO-incompatible
secretor donors. Ann Lab Med. 37:254–260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen C and Guo H: Transplantation
pathology. People's Medical Publishing House (edition 1); Beijing,
China: 2009
|
|
12
|
Cao J, Chen C, Gao Y, Hu L, Liang Y and
Xiao J: Identification of a protein associated with the activity of
cytokine-induced killer cells. Oncol Lett. 14:6937–6942.
2017.PubMed/NCBI
|
|
13
|
Cao J, Liu L, Zhang Y, Xiao J and Wang Y:
The influence of HK2 blood group antigen on human B cell activation
for ABOi-KT conditions. BMC Immunol. 18:492017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang J, Kan Q, La n, Zhao X, Zhang Z,
Yang S, Li H, Wang L, Xu L, Cheng Z and Zhang Y: Chemotherapy in
combination with cytokine-induced killer cell transfusion: An
effective therapeutic option for patients with extensive stage
small cell lung cancer. Int Immunopharmacol. 46:170–177. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khalili I, Koch M, Thaiss F, Plaetke R,
Bruegger J and Peine S: Systematic comparison of IgM and IgG ABO
antibody titers by using tube and gel card techniques and its
relevance for ABO-incompatible kidney transplantation. Clin Lab.
63:1393–1401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tan W, Wang F, Guo D, Ke Y, Shen Y, Lv C
and Zhang M: High serum level of haptoglobin is associated with the
response of 12 weeks methotrexate therapy in recent-onset
rheumatoid arthritis patients. Int J Rheum Dis. 19:482–489. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Delanghe JR, Langlois MR and De Buyzere
ML: Haptoglobin polymorphism: A key factor in the proatherogenic
role of B cells? Atherosclerosis. 217:80–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huntoon KM, Russell L, Tracy E, Barbour
KW, Li Q, Shrikant PA, Berger FG, Garrett-Sinha LA and Baumann H: A
unique form of haptoglobin produced by murine hematopoietic cells
supports B-cell survival, differentiation and immune response. Mol
Immunol. 55:345–354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang J, Doria-Rose NA, Longo NS, Laub L,
Lin CL, Turk E, Kang BH, Migueles SA, Bailer RT, Mascola JR and
Connors M: Isolation of human monoclonal antibodies from peripheral
blood B cells. Nat Protoc. 8:1907–1915. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Beyer A and Aebersold R: On the
dependency of cellular protein levels on mRNA abundance. Cell.
165:535–550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hao Y, Yang X, Zhang D, Luo J and Chen R:
Long noncoding RNA LINC01186, regulated by TGF-β/SMAD3, inhibits
migration and invasion through Epithelial-Mesenchymal-Transition in
lung cancer. Gene. 608:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Letterio JJ: Murine models define the role
of TGF-beta as a master regulator of immune cell function. Cytokine
Growth Factor Rev. 11:81–87. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
DiRenzo DM, Chaudhary MA, Shi X, Franco
SR, Zent J, Wang K, Guo LW and Kent KC: A crosstalk between
TGF-β/Smad3 and Wnt/β-catenin pathways promotes vascular smooth
muscle cell proliferation. Cell Signal. 28:498–505. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kashiwagi I, Morita R, Schichita T, Komai
K, Saeki K, Matsumoto M, Takeda K, Nomura M, Hayashi A, Kanai T and
Yoshimura A: Smad2 and Smad3 inversely regulate TGF-β autoinduction
in Clostridium butyricum-activated dendritic cells. Immunity.
43:65–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lan HY: Smads as therapeutic targets for
chronic kidney disease. Kidney Res Clin Pract. 31:4–11. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McKarns SC, Letterio JJ and Kaminski NE:
Concentration-dependent bifunctional effect of TGF-beta 1 on
immunoglobulin production: A role for Smad3 in IgA production in
vitro. Int Immunopharmacol. 3:1761–1774. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y and Derynck R: Transcriptional
regulation of the transforming growth factor-beta-inducible mouse
germ line Ig alpha constant region gene by functional cooperation
of Smad, CREB, and AML family members. J Biol Chem.
275:16979–16985. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chacko BM, Qin BY, Tiwari A, Shi G, Lam S,
Hayward LJ, De Caestecker M and Lin K: Structural basis of
heteromeric smad protein assembly in TGF-beta signaling. Mol Cell.
15:813–823. 2004. View Article : Google Scholar : PubMed/NCBI
|