Introduction
Allergic rhinitis (AR) is one of the most common
diseases affecting quality of life and work and school attendance
(1). AR is characterized by the
infiltration and activation of immune cells in the nasal mucosa,
which then produce T helper type 2 (Th2) cytokines and
proinflammatory molecules (2,3). It is
well-known that CD4+ Th2 cells serve an essential role
in allergic airway disease pathogenesis through producing the Th2
type cytokines interleukin (IL)-4, IL-5, and IL-13 (4). Following the identification of type 2
innate lymphoid cells (ILC2s) in 2010, this traditional
understanding of AR has been challenged (5).
Innate immunity comprises the first line of response
against invading pathogens and environmental insults. ILC2s are a
newly identified group of innate immune cells. ILC2s have
lymphocyte characteristics, but do not express T cell, B cell,
natural killer cell or other granulocyte-monocyte lineage markers
(6). They produce IL-5 and IL-13 in
response to the epithelium-derived cytokines IL-25, IL-33, and
thymic stromal lymphopoietin (7). It
remains unclear whether ILC2s aggregate in the peripheral blood in
AR. Bartemes et al (8)
suggested that ILC2s levels were increased in asthma, but did not
change in AR. Doherty et al (9) demonstrated that cat-sensitized AR
adults challenged locally by cat allergens exhibited a
significantly increased percentage of ILC2s in the peripheral blood
at 4 h after challenge. Subsequently, Fan et al (10) suggested that patients with AR
sensitized to house dust mite (HDM) or mugwort allergens have
different levels of ILC2 expression, which represent a critical
source of type 2 cytokines in HDM-AR (10). The present study determined whether
ILC2 levels were increased in systemic peripheral blood and their
association with clinical manifestations in pediatric patients with
AR.
Patients and methods
Clinical specimens
Patients with HDM-AR (n=12), non-HDM-AR (n=18) and
healthy controls (HCs) (n=12) were recruited from the Children's
Hospital of Chongqing Medical University from November in 2017 to
February in 2018. AR was diagnosed according to the criteria of the
Initiative on Allergic Rhinitis and its Impact on Asthma (11). Patients with AR presented with a
characteristic history of watery nasal discharge, nasal
obstruction, sneezing, itching in the nose, were positive for IgE
specific to antigens such as HDM, weeds (mugwort and ragweed),
animal danders (cat and dog) and Blattella germanica
(Pharmacia CAP System, Pharmacia Diagnostics). The severities of
clinical symptoms were further assessed using the Total 5 Symptom
Score (T5SS) (12). According to the
clinical symptoms of itching in the nose, nasal obstruction,
rhinorrhea, sneezing and itching in the eyes, the severity of
symptoms was assessed by a 0–3 point system [0, no symptoms; 1,
mild (symptoms exist, but not annoying); 2, moderate (symptoms
annoying, but easy to tolerate); 3, serious (symptoms annoying and
intolerable)].
Prior to inclusion in the present study, all
medications such as corticosteroids, antihistamines or leukotriene
receptor antagonists were prohibited for at least 4 weeks. Patients
with infectious, vasomotor, hormonal, drug-induced and occupational
rhinitis, or any complications, were excluded. HCs had to satisfy
the conditions of no history of allergic diseases, and a negative
skin prick test and absence of IgE specific to antigens (total IgE
levels <100 kU/l). Patient characteristics are summarized in
Table I. The present study was
approved by and performed in accordance with the local regulations
of the Ethical Committee of Chongqing Medical University (ethics
approval no. 038/2014) and the Declaration of Helsinki. Informed
consent was acquired from all subjects' legal guardians prior to
enrolment in the study. All participants showed no adverse
reactions.
 | Table I.Demographics and characteristics of
participants. |
Table I.
Demographics and characteristics of
participants.
|
| Patient groups |
|---|
|
|
|
|---|
|
Characteristics | Healthy
controls | HDM-AR | non-HDM-AR |
|---|
| Patients (n) | 12 | 12 | 18 |
| Age, years | 6.9±1.1 | 7.2±1.0 | 7.0±0.9 |
| Sex
(male/female) | 7/5 | 4/8 | 9/9 |
| Specific IgE,
kU/l |
|
HDM | <0.35 | 47±3.8 | 0 |
|
Weeds | <0.35 | 0 | 1.8±0.2 |
| Animal
danders | <0.35 | 0 | 3.4±0.4 |
|
Cockroach | <0.35 | 0 | 5.9±0.8 |
| T5SS | 0 | 9.5±1.69 | 7.56±1.92 |
| Allergen
positive/subjects tested | 0/12 | 12/12 | 18/18 |
Separation of peripheral blood
mononuclear cells (PBMCs)
A total of 5 ml blood was collected from each
subject into a heparin anticoagulant tube. Peripheral blood was
managed by Ficoll-Hypaque density gradient centrifugation at 800 ×
g for 30 min at 4°C. The PBMCs were collected from the cell layer
between the lymphocyte separation medium and the plasma.
Sorting ILC2s with flow cytometry
To examine the role of ILC2 in AR, ILC2s were sorted
from PBMCs using the lineage cocktail method. Firstly, PBMC cells
were stained with fluorescein isothiocyanate (FITC)-conjugated
antibodies against lineage markers [cluster of differentiation
(CD)3, B-lymphocyte antigen CD19, hematopoietic progenitor cell
antigen CD34, natural killer cells antigen CD94, IL-3 receptor, T
cell receptor (TCR)αβ, C-type lectin domain family 4 member C and
high-affinity IgE receptor) and phycoerythrin (PE)-conjugated
lineage markers (T-cell surface glycoprotein CD1a, integrin
alpha-X, monocyte differentiation antigen CD14, TCRγδ and neural
cell adhesion molecule), which aimed to exclude T cells, monocytes,
macrophages, B cells, mast cells, basophils, dendritic cells and
hematopoietic progenitor cells. Lineage-negative (Lin−)
cells were collected from PBMCs by depleting lineage-positive
(Lin+) cells, which express the aforementioned markers.
Secondly, lineage-negative (Lin−) cells were further
sorted following staining with Alexa Fluor 647-conjugated
prostaglandin D2 receptor (CRTH2/CD294) and
phycoerythrin-Cy7-conjugated interleukin-7 receptor subunit α
(CD127; both from BD Biosciences). ILC2s were defined by their
Lin−CRTH2+CD127+ expression
(Fig. 1). In each analysis, at least
20,000 events were collected in order to conduct an analysis of the
data. Flow cytometry was conducted on a FACScan flow cytometer (BD
Biosciences). The number of ILC2s was expressed as a percentage of
PBMCs. Data were analyzed with CellQuest software (CellQuest Pro
5.2.1; BD Biosciences).
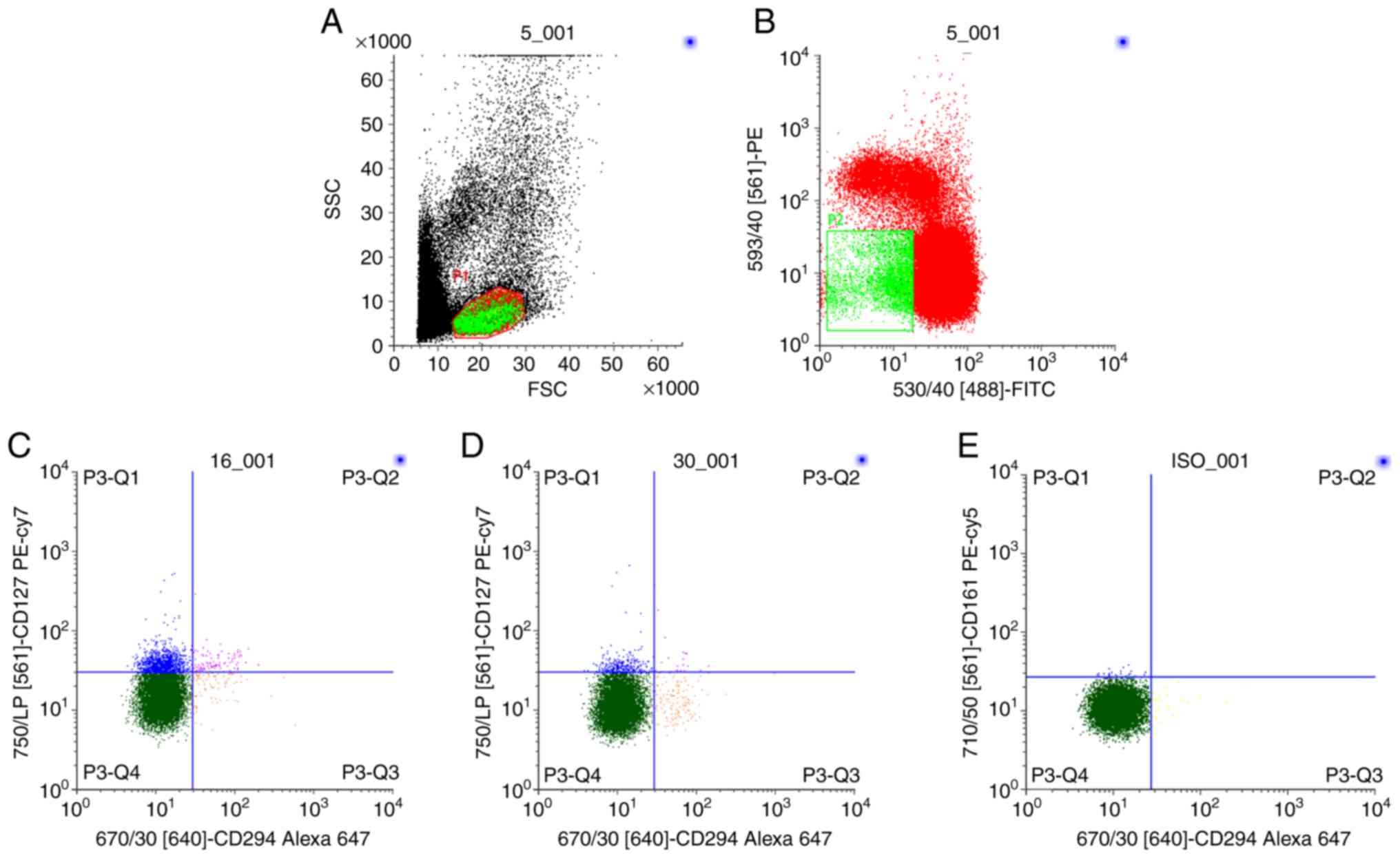 | Figure 1.Sequential gating to determine ILC2s
frequencies in lymphocyte fractions of HCs (n=12), patients with
HDM-AR (n=12) and non-HDM-AR (n=18). (A) Lymphocytes were isolated
from whole peripheral blood mononuclear cells, and (B)
Lin− cells were gated and further assessed for
co-expression of CD127 and CD294 (CRTH2). ILC2s were identified as
Lin−CRTH2+CD127+ lymphocytes. (C)
ILC2s frequencies in the HDM-AR and non-HDM-AR groups. (D)
Expression of ILC2s frequencies in the HC group. (E) Isotype
control staining. The numbers in the figures indicate the
percentages of ILC2s in Lin- cells. ILC2s, type 2 innate lymphoid
cells; HCs, healthy controls; HDM, house dust mite; AR, allergic
rhinitis; Lin−, lineage-negative; SSC, side scatter;
FSC, forward scatter; CD127, interleukin-7 receptor subunit α;
CD294, prostaglandin D2 receptor; PE, phycoerythrin; FITC,
fluorescein isothiocyanate. |
Statistical analysis
All data are presented as the mean ± standard
deviation. Analysis of variance followed by Tukey's post hoc test
was used to compare ILC2 data among the HDM-AR, non-HDM-AR and HCs
groups. The association between ILC2 levels and T5SS scores was
examined using Spearman's correlation analysis. SPSS v.19.0
statistical software (IBM Corp.) was used for statistical analysis,
and GraphPad Prism software (v.5.00; GraphPad Software, Inc.) was
used to generate the figures. P<0.05 was considered to indicate
a statistically significant difference.
Results
Clinical characteristics of the
patients
In the HDM-AR group (n=12), the patients were AR
were only allergic to HDM. In the non-HDM-AR group (n=18), 6
patients were monosensitized to weeds, 6 patients were
monosensitized to animal danders, and 6 patients were
monosensitized to cockroach. In the HCs group (n=12), all subjects
had no symptoms of AR or other allergic disease. Their T5SS scores
were not detectable. All the subjects with AR expressed high
specific IgE in the plasma and had a high T5SS. As indicated in
Table I, there were no significant
differences in age or the sex ratio among the three groups.
However, significant differences appeared in the specific IgE
levels and T5SS between the HDM-AR, non-HDM-AR and HCs groups.
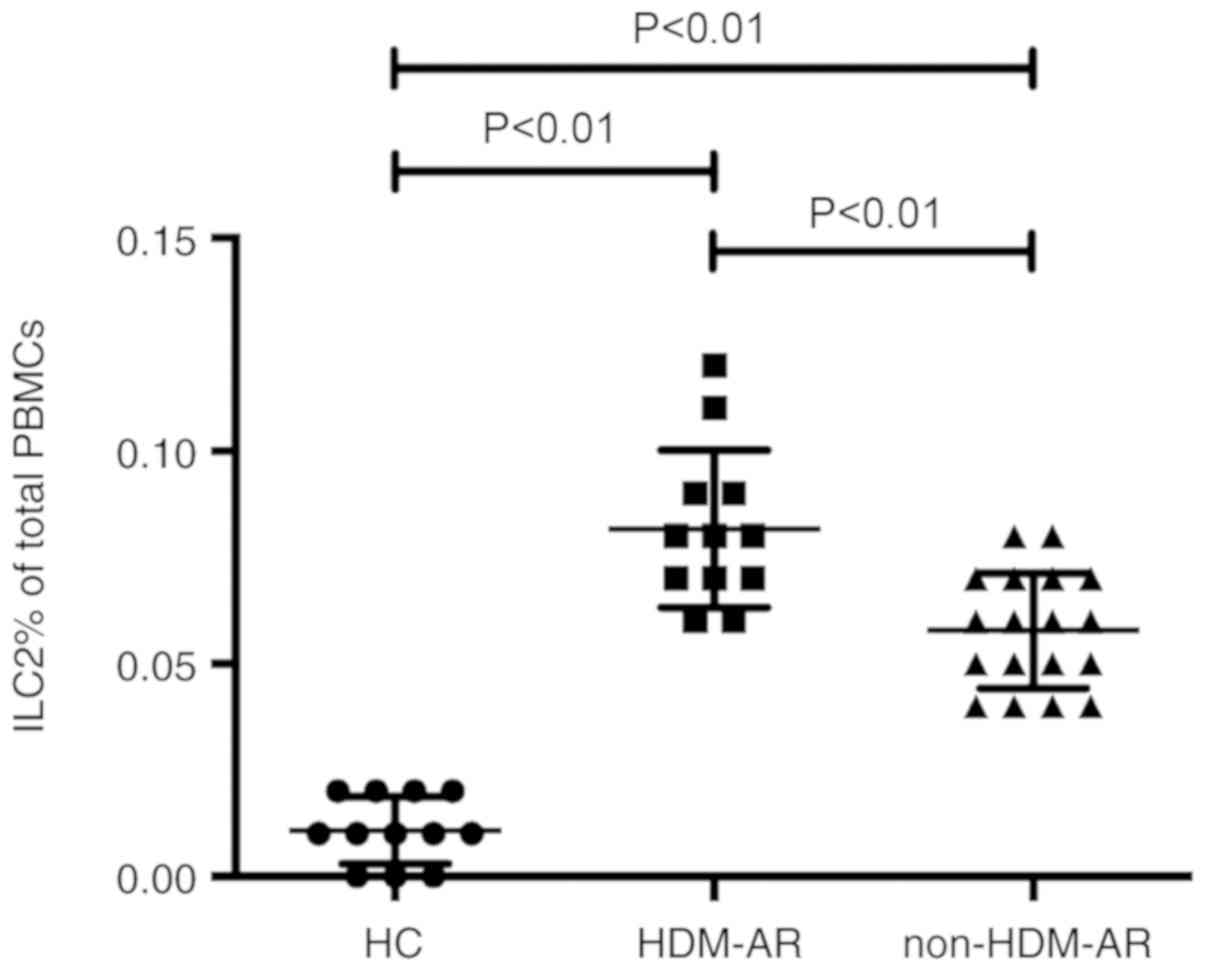
Differences in ILC2s frequencies
between HDM-AR, non-HDM-AR and HC subjects
PBMCs were resuspended into single cell suspension
for flow cytometry. Lin− cells that co-expressed CRTH2
and CD127 were defined as ILC2s (Fig.
1). HCs exhibited rare ILC2s expression on PBMCs (0.01±0.008%;
Fig. 2). However, in the HDM-AR
(0.08±0.018%; Fig. 2) and non-HDM-AR
groups (0.06±0.013%; Fig. 2), ILC2s
percentages were significantly increased when compared to the HC
group (P<0.01). Furthermore, subgroup analysis revealed that
subjects with HDM-AR exhibited a higher level of ILC2s compared
with those with non-HDM-AR (Fig. 2;
P<0.01). In addition, there were no significant differences
among the three non-HDM-AR patient subgroups (P>0.05, data not
shown).
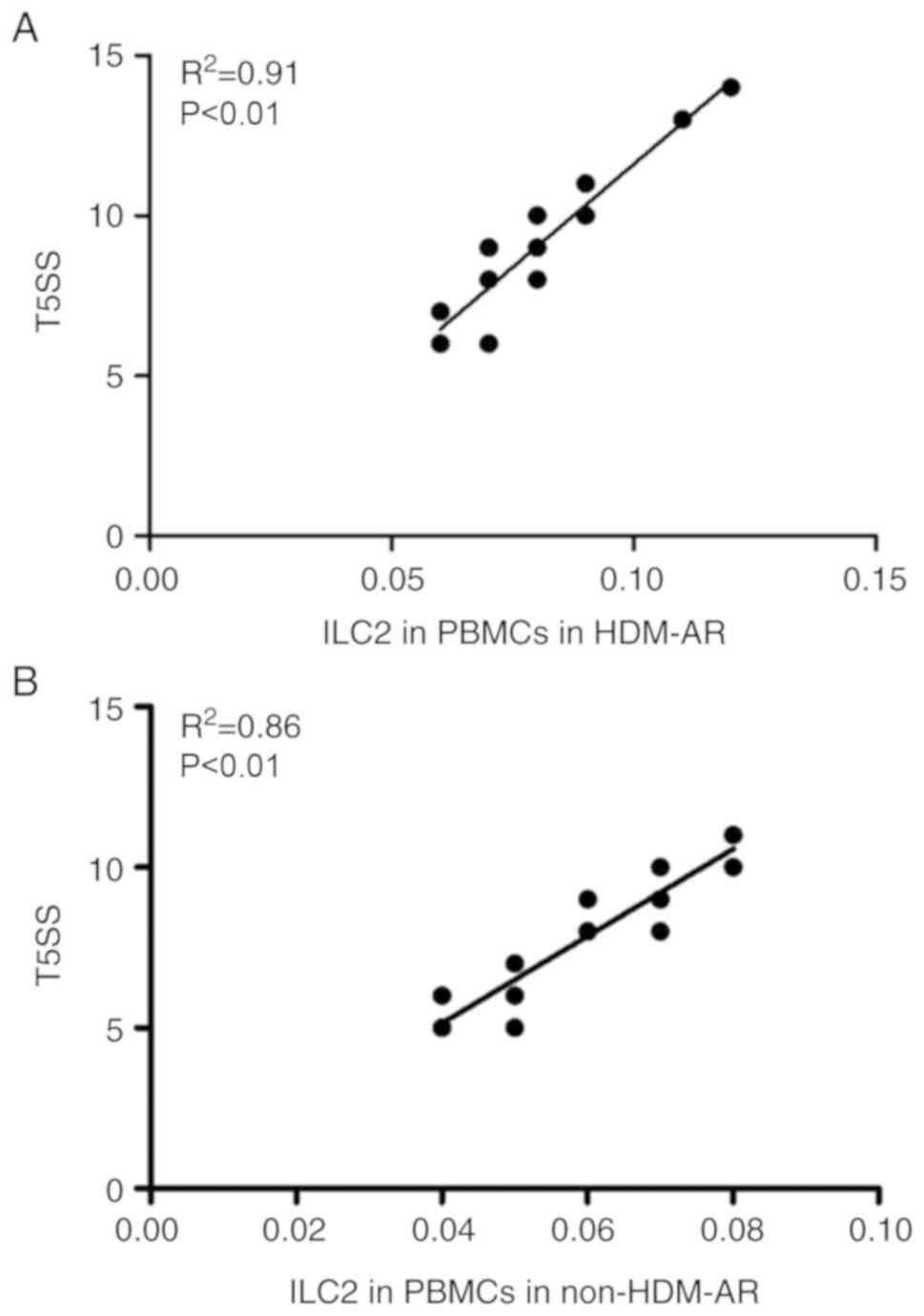
Circulating ILC2 level is positively
correlated with clinical parameters
The correlation between ILC2 levels and clinical
parameters was further analyzed. There was a positive linear
correlation between the proportion of ILC2s in PBMCs in the HDM-AR
group (R2=0.91; P<0.01) and the T5SS score (Fig. 3A). Similarly, a positive correlation
between the percentages of circulating ILC2s and the T5SS score was
observed in patients with non-HDM-AR (Fig. 3B; R2=0.86; P<0.01).
Discussion
In the present study, it was demonstrated that blood
ILC2s levels were significantly increased in pediatric patients
with AR compared with those in the HCs. Furthermore, there was a
positive correlation between ILC2 levels and T5SS. These data
suggest that ILC2s may serve a significant role in AR.
Allergic rhinitis is caused by IgE-mediated and type
2 inflammatory responses to inhaled allergens, which results in
nasal symptoms that include watery nasal discharge, nasal
obstruction, sneezing and itching in the nose (13,14). The
pathogenesis of AR is not fully clear. ILC2s are a newly recognized
subset of the innate lymphoid cell family and have been gaining
attention in AR research. To the best of our knowledge, the first
study to verify the association between ILC2s and AR indicated that
the percentage of ILC2s in the peripheral blood of patients with
cat-sensitized AR increased significantly after nasal cat allergen
challenge, relative to the control challenge (9). Subsequently, Lao et al (15) identified that ILC2s levels were
elevated in patients with grass pollen-sensitized AR during the
pollen season compared with the control group, and that the ILC2
levels were decreased following subcutaneous immunotherapy. A
recent study identified that ILC2s levels were significantly
increased in patients with AR who were monosensitized to HDM
compared with the HCs (16). In
addition, elevated ILC2s levels were detected in patients with
other allergic airway diseases, including asthma, chronic
rhinosinusitis and aspirin exacerbated respiratory disease
(17–23). Consistent with these results, the
present study identified that blood ILC2s levels were significantly
increased in pediatric patients with AR compared with the HCs.
ILC2s respond to IL-25, IL-33 and leukotrienes to promote features
of allergic airway diseases via the production of Th2 type
cytokines IL-4, IL-5 and IL-13 (24–28).
However, a different previous study demonstrated
that there were neither enhanced type 2 responses nor increased
ILC2 levels in the peripheral blood in patients with AR outside of
the allergy season (8). Fan et
al (10) demonstrated that the
ILC2s level of patients with monosensitized mugwort-AR and HCs were
similar, while the percentage of ILC2s in patients with HDM-AR was
significantly increased compared with those in the other two groups
(10). The results of the present
study were inconsistent with these aforementioned data. The present
study identified that pediatric patients with AR may have
significantly increased levels of blood ILC2s compared with the
HCs, irrespective of the type of allergen. In addition, a subgroup
analysis of patients with AR indicated that the proportion of ILC2s
in HDM-AR was significantly increased compared with that in non-HDM
AR. Another previous study indicated that the frequencies of ILC2s
were elevated in seasonal Timothy grass (Phleum
pratense)-sensitized AR subjects, 66.7% of whom were
polysensitized to HDM allergen (14). Miao et al (29) has suggested that during and outside
mugwort pollen season, an increased level of circulating ILC2s was
detected in patients with asthma monosensitized to mugwort or HDM
compared with the HCs (29). These
data suggest that there is different immunogenicity between dust
mites and other allergens such as mugwort pollen. Mugwort is one of
the most common pollen allergens in China (30,31).
Allergic immune responses to the major mugwort pollen allergen Art,
which belongs to the pectate lyase allergen family, are
characterized by IgE binding and T-cell proliferation (32–34). By
contrast, house dust mites (HDM) are a perennial allergen present
globally. HDM and their fecal particles contain several
trypsin/chymotrypsin-like enzymes that may directly lead to tissue
damage and increase the passage of allergens through the epithelial
barrier. These effects may additionally enhance allergic reactions
through different pathways, including an increase in the release of
IL-33 by epithelial cells (35,36). It
has been confirmed that IL-33 has a strong stimulating effect on
the activation and migration of ILC2s in vitro, and promotes
the aggregation of ILC2s in the airway during the initiation phase
of Th2 immunity induced by HDM (37). Wang et al (38) has demonstrated that
Dermatophagoides farinae−31, a type of HDM, upregulated the
levels of IL-33 in epithelial cells via the activation of Toll-like
receptor 2, which induced eosinophil-like airway allergy and
increased the number of lung-resident ILC2s (38).
The present study also observed a correlation
between peripheral ILC2s levels and disease severity in patients
with AR. The results demonstrated that the high expression of
peripheral ILC2s was positively correlated with T5SS scores,
representing the severity of the clinical symptoms. These results
were consistent with previous studies: Compared with mild atopic
asthma and the HCs group, the number of activated ILC2s in
peripheral blood and sputum of patients with severe asthma was
increased significantly (39). In
addition, recent studies have demonstrated that peripheral ILC2s
are significantly increased in patients with AR sensitized to HDM,
and that there is a positive correlation between ILC2 levels and
VAS scores (16). These data further
demonstrate the close association between ILC2s and allergic
inflammation.
In summary, the present study demonstrated that
blood ILC2s levels were significantly increased in pediatric
patients with AR, irrespective of the type of allergen. In
addition, the proportion of ILC2s in HDM-AR was significantly
increased compared with in non-HDM AR. Furthermore, there was a
positive correlation between ILC2 levels and the severity of the
clinical symptoms. These data indicate that ILC2s may serve an
important role in the pathogenesis of AR, and therefore may
represent a potential therapeutic target for AR.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project
National Natural Science Foundation Project (grant no., 81500776)
and the Fundamental and Advanced Research Program of Chongqing
(grant no., cstc2015jcyjA10103).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RS and XT made substantial contributions to the
experimental design, analysis of experimental results and selection
of subjects. YY and ZG were responsible for the collection of
clinical specimens and separation of PBMCs. QH and PW were
responsible for the flow cytometry to determine the expression
levels of ILC2s. All the authors have read and approved the final
version of this manuscript.
Ethics approval and consent to
participate
The present study was approved by and performed in
accordance with the local regulations of the Ethical Committee of
Chongqing Medical University (ethics approval no. 038/2014) and the
Declaration of Helsinki. Informed consent was acquired from all
subjects' legal guardians prior to enrolment in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Seidman MD, Gurgel RK, Lin SY, Schwartz
SR, Baroody FM, Bonner JR, Dawson DE, Dykewicz MS, Hackell JM, Han
JK, et al: Guideline otolaryngology development group. AAO-HNSF.
Clinical practice guideline: Allergic rhinitis. Otolaryngol Head
Neck Surg. 152 (Suppl 1):S1–S43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bachert C, Wagenmann M and Holtappels G:
Cytokines and adhesion molecules in allergic rhinitis. Am J Rhinol.
12:3–8. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang DY and Clement P: Pathogenic
mechanisms underlying the clinical symptoms of allergic rhinitis.
Am J Rhinol. 14:325–333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pawankar R, Mori S, Ozu C and Kimura S:
Overview on the pathomechanisms of allergic rhinitis. Asia Pac
Allergy. 1:157–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cavagnero K and Doherty TA: Cytokine and
lipid mediator regulation of group 2 innate lymphoid cells (ILC2s)
in human allergic airway disease. J Cytokine Biol. 2(pii):
1162017.PubMed/NCBI
|
|
6
|
Björkström NK, Kekäläinen E and Mjösberg
J: Tissue-specific effector functions of innate lymphoid cells.
Immunology. 139:416–427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spits H, Artis D, Colonna M, Diefenbach A,
Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius
RE, et al: Innate lymphoid cells-a proposal for uniform
nomenclature. Nat Rev Immunol. 13:145–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartemes KR, Kephart GM, Fox SJ and Kita
H: Enhanced innate type 2 immune response in peripheral blood from
patients with asthma. J Allergy Clin Immunol. 134:671–678.e4. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doherty TA, Scott D, Walford HH, Khorram
N, Lund S, Baum R, Chang J, Rosenthal P, Beppu A, Miller M and
Broide DH: Allergen challenge in allergic rhinitis rapidly induces
increased peripheral blood type 2 innate lymphoid cells that
express CD84. J Allergy Clin Immunol. 133:1203–1205. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan D, Wang X, Wang M, Wang Y, Zhang L, Li
Y, Fan E, Cao F, Van Crombruggen K, Zhang L, et al:
Allergen-dependent differences in ILC2s frequencies in patients
with allergic rhinitis. Allergy Asthma Immunol Res. 8:216–222.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bousquet J, Schünemann HJ, Samolinski B,
Demoly P, Baena-Cagnani CE, Bachert C, Bonini S, Boulet LP,
Bousquet PJ, Brozek JL, et al: Allergic rhinitis and its impact on
asthma (ARIA): Achievements in 10 years and future needs. J Allergy
Clin Immunol. 130:1049–1062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bousquet J, Bachert C, Canonica GW, Mullol
J, Van Cauwenberge P, Bindslev Jensen C, Fokkens WJ, Ring J, Keith
P, Lorber R, et al: Efficacy of desloratadine in intermittent
allergic rhinitis: A GA (2)LEN study. Allergy. 64:1516–1523. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pilette C, Jacobson MR, Ratajczak C, Detry
B, Banfield G, VanSnick J, Durham SR and Nouri-Aria KT: Aberrant
dendritic cell function conditions Th2-cell polarization in
allergic rhinitis. Allergy. 68:312–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Skrindo I, Ballke C, Gran E, Johansen FE,
Baekkevold ES and Jahnsen FL: IL-5 production by resident mucosal
allergen-specific T cells in an explant model of allergic rhinitis.
Clin Exp Allergy. 45:1296–1304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lao-Araya M, Steveling E, Scadding GW,
Durham SR and Shamji MH: Seasonal increases in peripheral innate
lymphoid type 2 cells are inhibited by subcutaneous grass pollen
immunotherapy. J Allergy Clin Immunol. 134:1193–1195.e4. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhong H, Fan XL, Yu QN, Qin ZL, Chen D, Xu
R, Chen DH, Lin ZB, Wen W and Fu QL: Increased innate type 2 immune
response in house dust mite-allergic patients with allergic
rhinitis. Clin Immunol. 183:293–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Christianson CA, Goplen NP, Zafar I, Irvin
C, Good JT Jr, Rollins DR, Gorentla B, Liu W, Gorska MM, Chu H, et
al: Persistence of asthma requires multiple feedback circuits
involving type 2 innate lymphoid cells and IL-33. J Allergy Clin
Immunol. 136:59–68.e14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagakumar P, Denney L, Fleming L, Bush A,
Lloyd CM and Saglani S: type 2 innate lymphoid cells in induced
sputum from children with severe asthma. J Allergy Clin Immunol.
137:624–626.e6. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mjösberg JM, Trifari S, Crellin NK, Peters
CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T and Spits H: Human
IL-25- and IL-33-responsive type 2 innate lymphoid cells are
defined by expression of CRTH2 and CD161. Nat Immunol.
12:1055–1062. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walford HH, Lund SJ, Baum RE, White AA,
Bergeron CM, Husseman J, Bethel KJ, Scott DR, Khorram N, Miller M,
et al: Increased ILC2s in the eosinophilic nasal polyp endotype are
associated with corticosteroid responsiveness. Clin Immunol.
155:126–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miljkovic D, Bassiouni A, Cooksley C, Ou
J, Hauben E, Wormald PJ and Vreugde S: Association between group 2
innate lymphoid cells enrichment, nasal polyps and allergy in
chronic rhinosinusitis. Allergy. 69:1154–1161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ho J, Bailey M, Zaunders J, Mrad N, Sacks
R, Sewell W and Harvey RJ: Group 2 innate lymphoid cells (ILC2s)
are increased in chronic rhinosinusitis with nasal polyps or
eosinophilia. Clin Exp Allergy. 45:394–403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eastman JJ, Cavagnero KJ, Deconde AS, Kim
AS, Karta MR, Broide DH, Zuraw BL, White AA, Christiansen SC,
Doherty TA, et al: Group 2 innate lymphoid cells are recruited to
the nasal mucosa in patients with aspirin-exacerbated respiratory
disease. J Allergy Clin Immunol. 140:101–108.e3. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Doherty TA: At the bench: Understanding
group 2 innate lymphoid cells in disease. J Leukoc Biol.
97:455–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Johansson K, Malmhäll C, Ramos-Ramírez P
and Rådinger M: Bone marrow type 2 innate lymphoid cells: A local
source of interleukin-5 in interleukin-33-driven eosinophilia.
Immunology. 153:268–278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue L, Salimi M, Panse I, Mjösberg JM,
McKenzie AN, Spits H, Klenerman P and Ogg G: Prostaglandin D2
activates group 2 innate lymphoid cells through chemoattractant
receptor-homologous molecule expressed on TH2 cells. J Allergy Clin
Immunol. 133:1184–1194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salimi M, Stöger L, Liu W, Go S, Pavord I,
Klenerman P, Ogg G and Xue L: Cysteinyl leukotriene E4
activates human group 2 innate lymphoid cells and enhances the
effect of prostaglandin D2 and epithelial cytokines. J
Allergy Clin Immunol. 140:1090–1100.e11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doherty TA, Khorram N, Lund S, Mehta AK,
Croft M and Broide DH: Lung type 2 innate lymphoid cells express
cysteinyl leukotriene receptor 1, which regulates TH2 cytokine
production. J Allergy Clin Immunol. 132:205–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miao Q, Wang Y, Liu YG, Ren YX, Guan H, Li
Z, Xu W and Xiang L: Seasonal variation in circulating group 2
innate lymphoid cells in mugwort-allergic asthmatics during and
outside pollen season. Allergy Asthma Clin Immunol. 14:62018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang XY, Ma TT, Wang XY, Zhuang Y, Wang
XD, Ning HY, Shi HY, Yu RL, Yan D, Huang HD, et al: Prevalence of
pollen-induced allergic rhinitis with high pollen exposure in
grasslands of northern China. Allergy. 73:1232–1243. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen K, Liao YF and Zhang JT: The major
aeroallergens in Guangxi, China. Clin Allergy. 18:589–596. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jahn-Schmid B, Kelemen P, Himly M, Bohle
B, Fischer G, Ferreira F and Ebner C: The T cell response to Art v
1, the major mugwort pollen allergen, is dominated by one epitope.
J Immunol. 169:6005–6011. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fu W, Gao Z, Gao L, Jin J, Liu M, Sun Y,
Wu S, Wu L, Ma H, Dong Y, et al: Identification of a 62-kDa major
allergen from Artemisia pollen as a putative galactose oxidase.
Allergy. 73:1041–1052. 2017. View Article : Google Scholar
|
|
34
|
Wopfner N, Gadermaier G, Egger M, Asero R,
Ebner C, Jahn-Schmid B and Ferreira F: The spectrum of allergens in
ragweed and mugwort pollen. Int Arch Allergy Immunol. 138:337–346.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu CK and Chen CL: Activation of mast
cells is essential for development of house dust mite
Dermatophagoides farinae-induced allergic airway inflammation in
mice. J Immunol. 171:3808–3815. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chu DK, Llop-Guevara A, Walker TD, Flader
K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S,
Coyle AJ, et al: IL-33, but not thymic stromal lymphopoietin or
IL-25, is central to mite and peanut allergic sensitization. J
Allega Clin Immunol. 131:187–200.e1-8. 2013. View Article : Google Scholar
|
|
37
|
Jia Y, Fang X, Zhu X, Bai C, Zhu L, Jin M,
Wang X, Hu M, Tang R and Chen Z: IL-13+ type 2 innate lymphoid
cells correlate with asthma control status and treatment response.
Am J Respir Cell Mol Biol. 55:675–683. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang H, Lin J, Zeng L, Ouyang C, Ran P,
Yang P and Liu Z: Der f 31, a novel allergen from Dermatophagoides
farinae, activates epithelial cells and enhances lung-resident
group 2 innate lymphoid cells. Sci Rep. 7:85192017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Smith SG, Chen R, Kjarsgaard M, Huang C,
Oliveria JP, O'Byrne PM, Gauvreau GM, Boulet LP, Lemiere C, Martin
J, et al: Increased numbers of activated group 2 innate lymphoid
cells in the airways of patients with severe asthma and persistent
airway eosinophilia. J Allergy Clin Immunol. 137:75–86.e8. 2016.
View Article : Google Scholar : PubMed/NCBI
|