Introduction
Type 2 diabetes mellitus (T2DM) presents a major and
growing health problem throughout the world. Microvascular and
macrovascular complications often occur in T2DM, and chronic
low-grade inflammation plays a central role in this process
(1–3). The inflammatory response is associated
with both insulin resistance and the development of vascular
complications in T2DM patients (4,5). T2DM
patients with higher body mass index (BMI) and a large amount of
adipose tissue are considered to be at greater risk of developing
more severe insulin resistance (6,7) and
cardiovascular disease (8,9).
Adipose tissue is regarded as an important endocrine
organ and is known to be involved in regulating inflammation
(10–12). Studies have shown that the NACHT
leucine rich repeat and pyd domains-containing 3 (NLRP3)
inflammasome plays a role in the initiation of inflammation in
adipose tissue (3,13). A wide range of pathogens and cellular
damage can activate the NLRP3 inflammasome, and its activation
leads to caspase-1 activation and the release of the inflammatory
cytokines interleukin (IL)-1 and IL-18 (14,15). It
has been shown that the expression of NLRP3 is significantly
elevated in adipose tissues of patients with obesity, dyslipidemia
and diabetes, and is positively correlated with the severity of
atherosclerosis (16). It is
hypothesized that a high glucose (HG) environment may induce
aberrant expression of the NLRP3 inflammasome in adipose tissue of
T2DM patients, and that this may contribute to the development of
atherosclerosis. However, the effects of HG on the expression of
NLRP3 in adipocytes and adipose tissues remain to be
elucidated.
Hydrogen sulfide (H2S), a gaseous
signaling transmitter, can be produced by a wide spectrum of
mammalian tissues (17). Previous
studies have indicated that H2S plays numerous
regulatory effects in the cardiovascular, gastrointestinal and
neurological systems (18–21). The anti-inflammatory properties of
H2S have been identified in a range of cell types
(22–24). In the cardiovascular system,
H2S is suggested to inhibit the development of
atherosclerosis through suppression of inflammation (25,26).
Cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS) are
enzymes responsible for the synthesis of H2S. It has
been reported that both CSE and CBS are expressed in adipose
tissues and adipocytes (27,28). Pan et al (29) demonstrated that HG inhibits
expression of CSE, and thus the production of H2S, in
adipocytes. This was confirmed by our previous study, which
additionally revealed that HG did not affect the expression level
of CBS (30). Several studies have
also suggested that HG significantly increases the secretion of
cytokines, including tumor necrosis factor-α (TNF-α), IL-6,
monocyte chemoattractant protein-1 and adiponectin (31,32).
However, the effect that this HG-induced reduction in CSE
expression and H2S production has on the expression of
the NLRP3 inflammasome remains unknown. The hypothesis of the
present study was that H2S may play a role in HG
regulation of NLRP3 expression in adipocytes. To test this
hypothesis the expression of the NLRP3 inflammasome in adipocytes
exposed to HG was investigated and an H2S donor was used
to try to reverse the HG effects.
Materials and methods
Cell culture and treatment
Adipocytes were cultured at 37°C with 5%
CO2 and differentiated from 3T3-L1 cells (American Type
Culture Collection) as previously described (30). After disassociation using 0.125%
trypsin, 1×106/ml adipocytes were seeded and grouped for
treatment. To determine the effect of HG, cells were treated with
either low glucose (LG) DMEM (5.5 mmol/l glucose; HyClone; GE
Healthcare Life Sciences; cat. no. SH30021.01) or HG DMEM (25.0
mmol/l glucose; HyClone; GE Healthcare Life Sciences; cat. no.
SH30022.01) for 24 h. To determine the effect of H2S,
cells were treated with HG DMEM containing increasing
concentrations of sodium hydrosulfide (NaHS; Sigma-Aldrich; Merck
KGaA; cat. no. 161527) or HG DMEM without NaHS for 24 h. Our
previous study revealed that 10, 25 and 50 nmol/l are effective
concentrations of NaHS for treatment of adipocytes, so those
concentrations were used in the present study (30). To inhibit the activity of the NLRP3
inflammasome, cells were treated with HG DMEM containing 10 µg/ml
N-acetyl-tyrosyl-valyl- alanyl-aspartyl chloromethyl ketone
(Ac-YVAD-CMK); Sigma-Aldrich; Merck KGaA; cat. no. SML0429) or DMSO
for vehicle for 24 h.
Western blot analysis
Proteins were extracted from adipocytes using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). The protein concentration was assayed using a BCA
Protein Assay kit (Beyotime Institute of Biotechnology). Protein
samples (50 µg) were used for SDS-PAGE (4 and 10% gel) and
subsequently transferred to nitrocellulose membranes. After
blocking with 5% skim milk for 2 h at 37°C, membranes were
incubated with primary antibodies against NLRP3 (Abcam; cat. no.
ab10931; 1:1,000), apoptosis-associated speck-like protein
containing A CARD (CST Biological Reagents Co., Ltd.; cat. no.
4628; 1:1,000), caspase-1 (Santa Cruz Biotechnology, Inc.; cat. no.
SC-514, 1:1,000) and β-actin (Sigma-Aldrich; Merck KGaA; cat. no.
A5441; 1:8,000) for 12 h at 4°C. The nitrocellulose membranes were
then incubated with a secondary HRP-conjugated antibody (1:2,000).
Immunoreactive proteins were then visualized using Immobilon
Western Chemiluminescent HRP Substrate (Merck KGaA) and a Tanon
5200 Multi scanner (Tanon Science and Technology Co., Ltd.). The
band intensities were calculated by ImageJ (version 1.51b; National
Institutes of Health). Then the ratio of band intensities to
β-actin was obtained to quantify the relative protein expression
levels.
ELISA analysis of IL-1β and IL-18
release
After treatment, culture media were collected and
the concentrations of IL-1β and IL-18 released into culture media
were determined using IL-1β (cat. no. F10770) and IL-18 (cat. no.
F10920) ELISA kits (Shanghai Westang Biotech Co., Ltd.) according
to the manufacturer's instructions. All assays were performed in
duplicate.
Statistical analysis
The data are presented as the mean ± SEM and were
analyzed by one-way ANOVA followed by LSD-t test using SPSS
(version 20; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
HG significantly upregulates the
expression of NLRP3 inflammasome in adipocytes
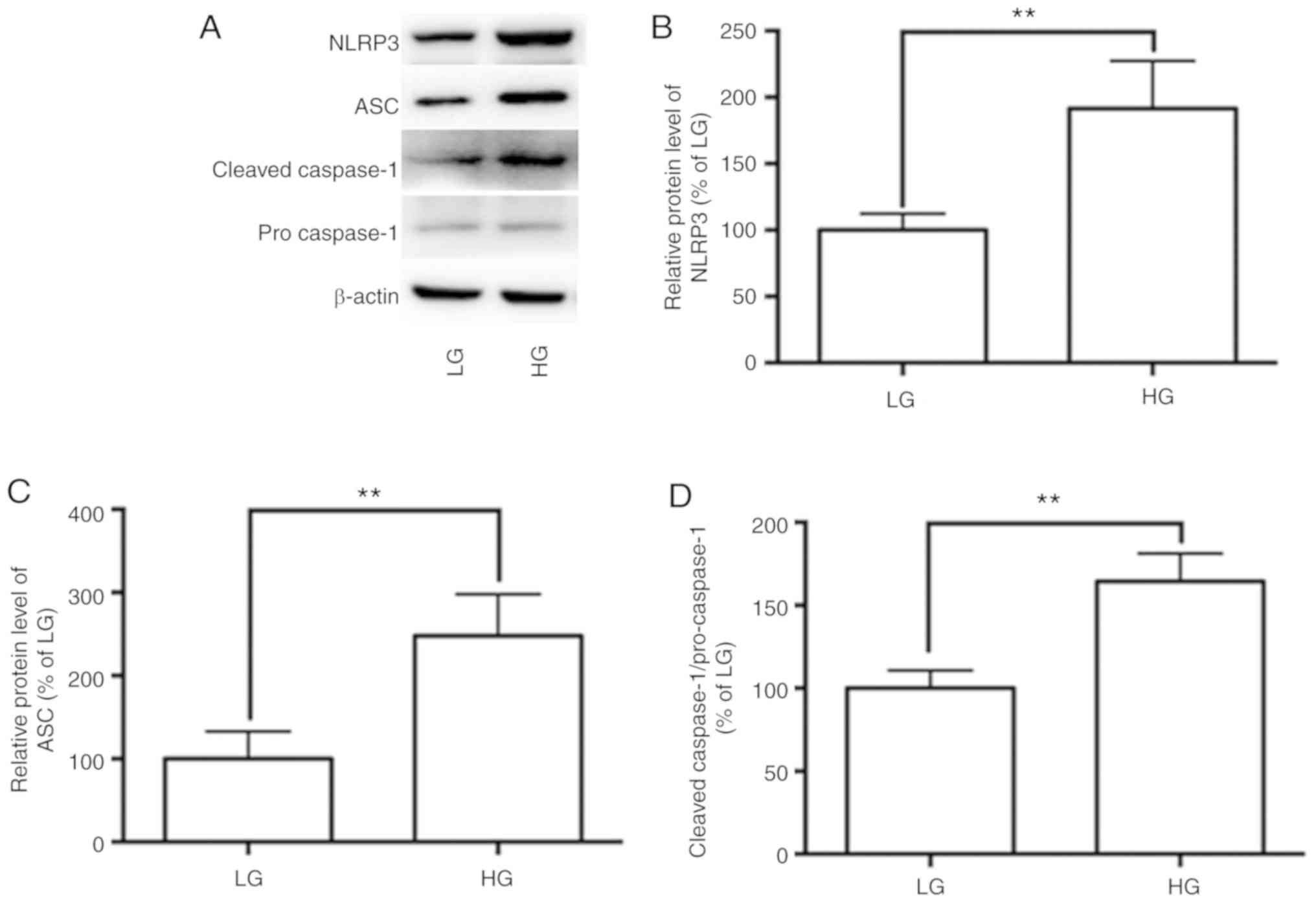
Activation of the NLRP3 inflammasome initiates
inflammation in adipose tissues and adipocytes. To investigate
whether HG is associated with activation of the NLRP3 inflammasome,
its expression in adipocytes was observed. The expression levels of
NLRP3 inflammasome components NLRP3, ASC and caspase-1 were
determined by western blot analysis. Compared with the LG group, HG
significantly increased the level of NLRP3 and ASC, and the ratio
of cleaved caspase-1 to pro-caspase-1 (Fig. 1A-D).
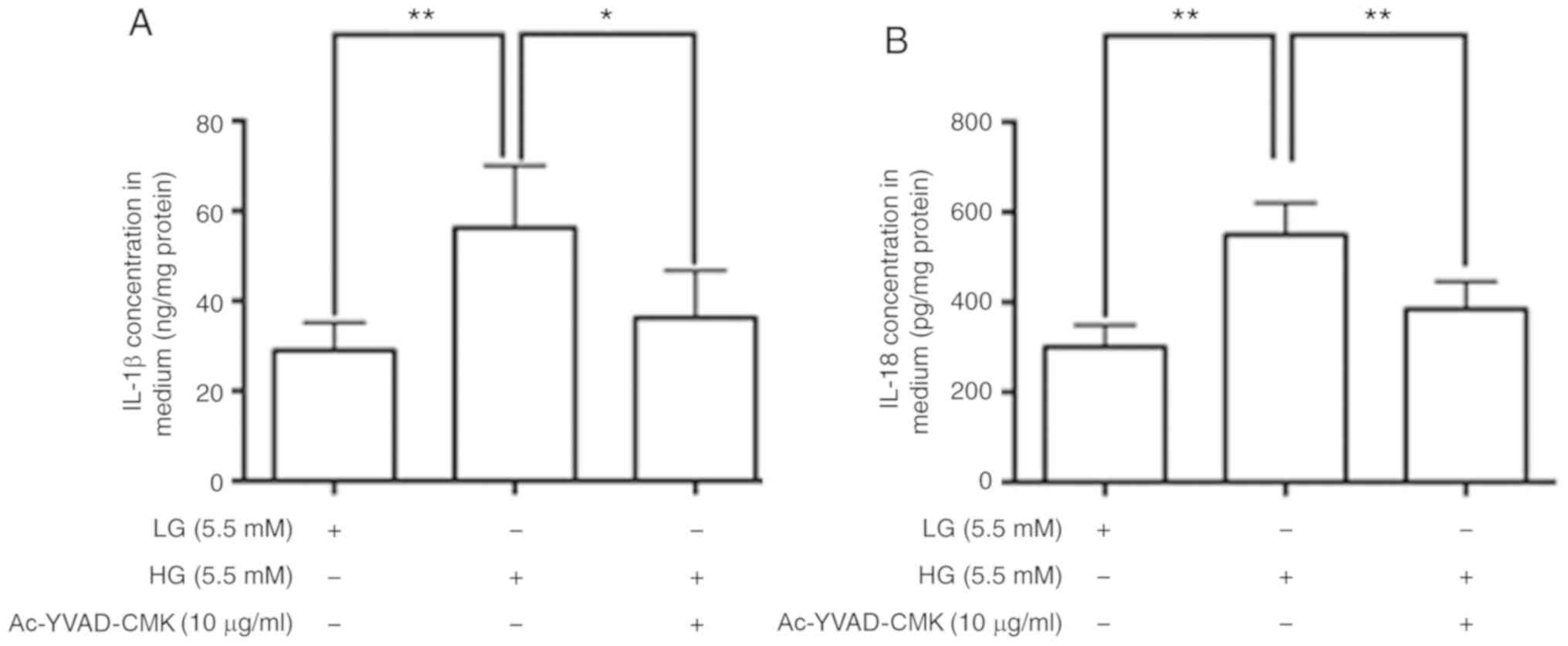
HG stimulates the release of IL-1β and
IL-18 by adipocytes via activation of the NLRP3 inflammasome
In the present study, mature IL-1β and IL-18 levels
in culture media were determined by ELISA. The data revealed that
IL-1β and IL-18 concentrations were significantly higher in the
culture media of adipocytes treated with HG compared with those
treated with LG. In order to confirm the role of NLRP3 inflammasome
activation in this HG-induced IL-1β and IL-18 release, adipocytes
were treated with Ac-YVAD-CMK. As shown in Fig. 2, the IL-1β and IL-18 concentrations
in the culture media of adipocytes treated with HG + Ac-YVAD-CMK
were significantly lower compared with HG DMEM containing
vehicle.
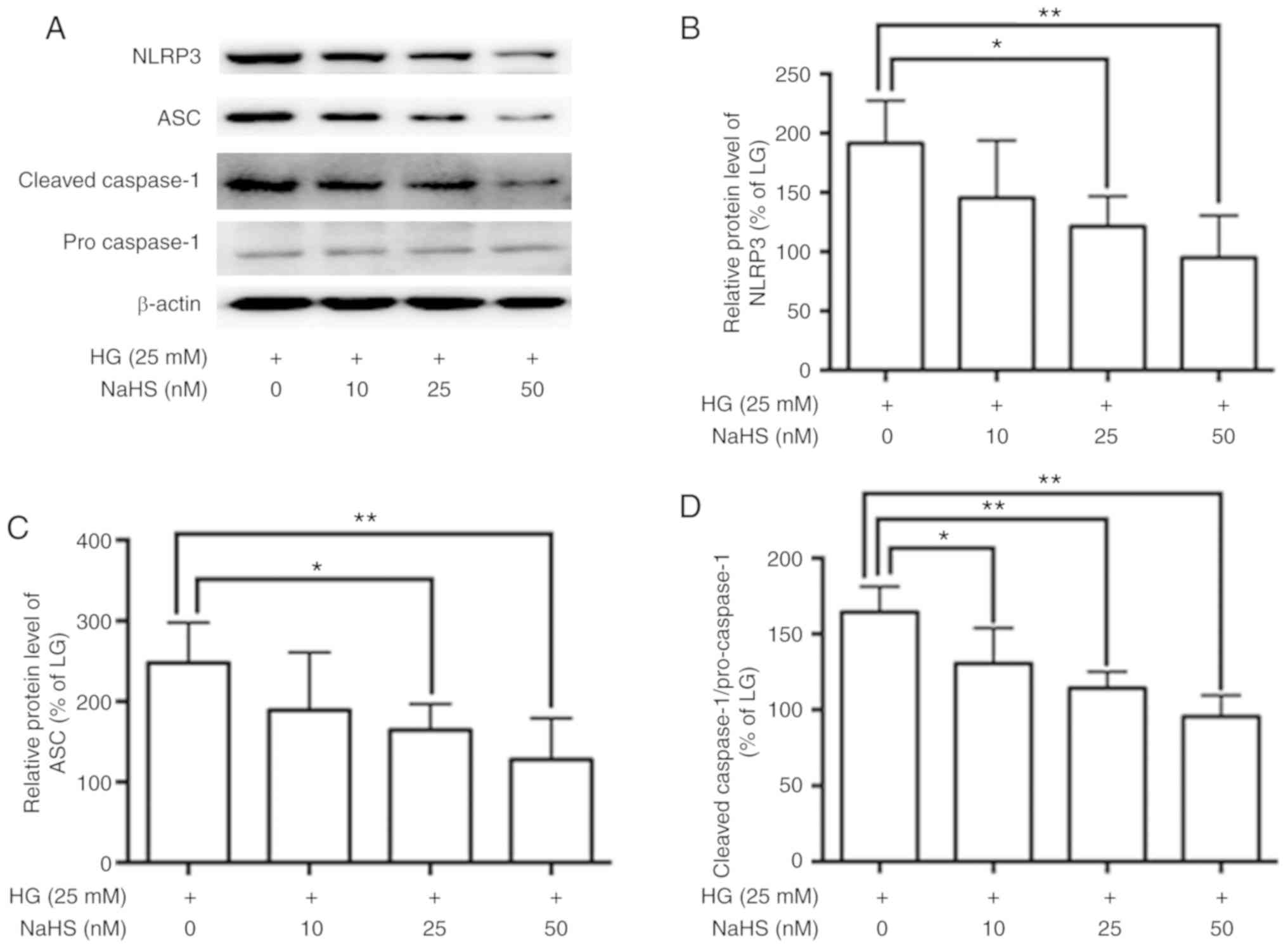
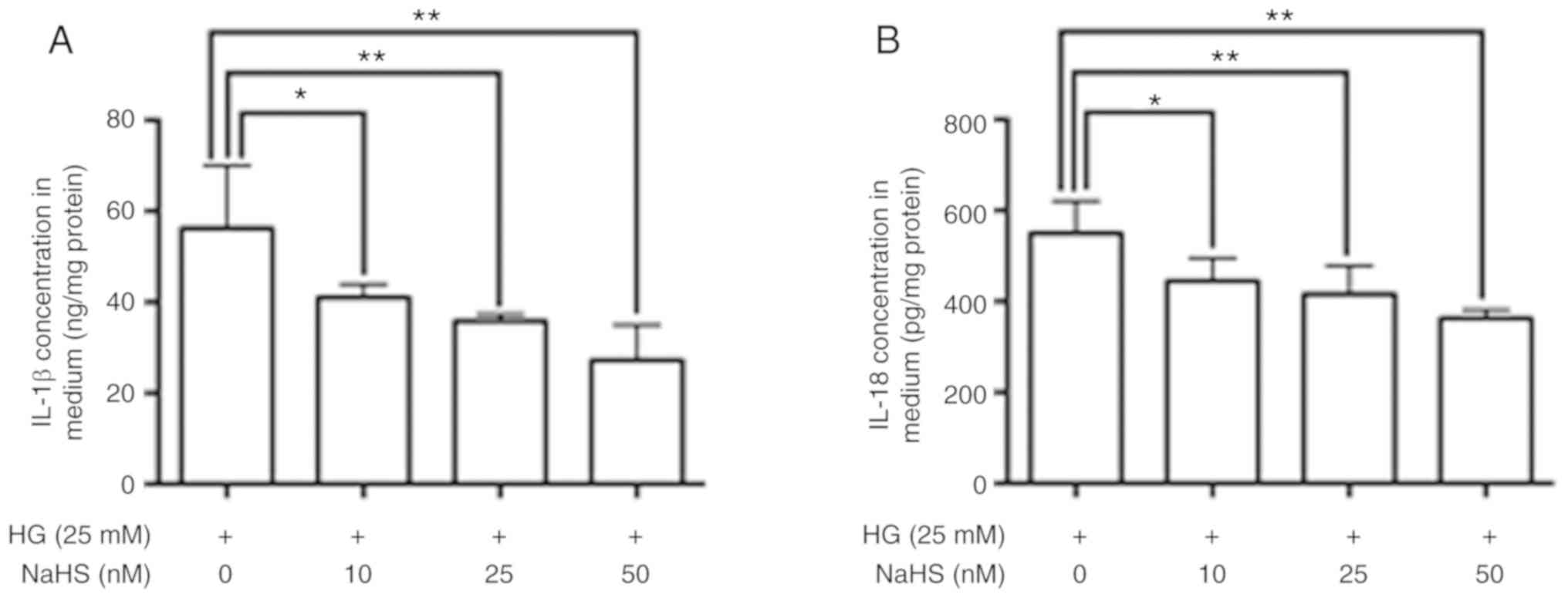
Role of H2S in the
regulation of NLRP3 inflammasome activation in adipocytes
To confirm the role of H2S in regulation
of NLRP3 inflammasome activation, adipocytes were treated with HG
DMEM containing increasing doses of the H2S donor NaHS.
The treatment of adipocytes with NaHS resulted in decreased
HG-induced expression levels of NLRP3 and ASC, a reduced cleaved
caspase-1 to pro caspae-1 ratio (Fig.
3), and a reduced HG-induced release of IL-1β and IL-18
(Fig. 4).
Discussion
The present study demonstrated that HG increased the
activity of the NLRP3 inflammasome in adipocytes. Additionally,
NaHS, an H2S donor, inhibited HG-induced expression of
the NLRP3 inflammasome and the release of IL-1 and IL-18.
The link between T2DM and inflammation is well
established. T2DM is considered to be, in part, a consequence of
subclinical chronic low-grade inflammation (1–3). Several
studies have reported that circulating inflammatory cytokines, such
as c-reactive protein, TNF-α, IL-1β, IL-6 and IL-18, are
significantly elevated in T2DM patients (33–38).
Elevated levels of these inflammatory cytokines directly induce
insulin resistance and impair glucose homeostasis (39–41).
Cytokines of the IL-1 family are critical regulators of
inflammation and control numerous inflammatory processes. Both
IL-1β and IL-18, which are classic pro-inflammatory cytokines of
the IL-1 family, contribute to insulin resistance and islet β-cell
damage in T2DM (39,42,43).
Additionally, chronic inflammation is also a major feature of
atherosclerosis. In the progression of T2DM, IL-1β and IL-18
increase the risk of microvascular and macrovascular complications
by accelerating atherosclerosis (44). However, the underlying molecular
mechanism behind the elevation of IL-1β and IL-18 levels in T2DM
patients has not been fully elucidated.
The majority of cytokines in the IL-1 family have
been linked to obesity (14,15). Accumulating evidence has indicated
that the NLRP3 inflammasome plays a critical role in regulating
IL-1β and IL-18 production in adipose tissues (3,13). Once
NLRP3 is activated, the inflammasome recruits pro-caspase-1. The
clustering of pro-caspase-1 subunits at the inflammasome complex
results in auto-cleavage and formation of active caspase-1. Active
caspase-1 converts pro-IL-1β and pro-IL-18 into their mature forms,
IL-1β and IL-18. In the adipose tissues of obese individuals,
compared with lean individuals, activity of the NLRP3 inflammasome
and expression levels of IL-1β and IL-18 are significantly elevated
(16,45,46).
T2DM patients with higher BMI and a greater amount of adipose
tissue are found to have higher serum IL-1β and IL-18 levels, which
results in more severe insulin resistance and increased risk of
cardiovascular disease (35,47). Despite this, whether an HG
environment effects NLRP3 inflammasome expression and IL-1β and
IL-18 release in the adipose tissue of T2DM patients is unknown and
merits further investigation. Results of the present study
indicated that HG significantly increased the expression levels of
NLRP3 and ASC, and caspase-1/pro capase-1 ratio in adipocytes. The
results also suggested that HG significantly increased IL-1β and
IL-18 release in the media of cultured adipocytes. In order to
identify whether NLRP3 inflammasome activation was involved in
HG-induced IL-1β and IL-18 release, cells were treated with HG DMEM
containing an NLRP3 inflammasome inhibitor. Results indicated that
inhibition of the NLRP3 inflammasome abolished HG-induced IL-1β and
IL-18 release. These data suggest that HG increased the production
of IL-1β and IL-18 in adipocytes via activation of the NLRP3
inflammasome.
H2S, a gaseous signaling transmitter, is
reportedly involved in inflammation in various tissues (17). Adipocytes have been shown to express
both CBS and CSE, and the expression of CSE and the generation of
H2S have been shown to be suppressed by HG (29,48). As
previously discussed, activation of the NLRP3 inflammasome and
release of IL-1β and IL-18 increased in adipocytes exposed to HG.
Therefore, it was hypothesized that a reduction in H2S
in HG DMEM may increase NLRP3 inflammasome expression and IL-1β and
IL-18 release. In order to verify this hypothesis, adipocytes were
treated with HG DMEM in the presence of increasing concentrations
of H2S donor NaHS. The findings indicated that NaHS
significantly suppressed NLRP3 inflammasome expression and IL-1β
and IL-18 release in adipocytes. These data suggest that exogenous
H2S can inhibit HG-induced NLRP3 inflammasome activation
in adipocytes.
In summary, the results of the present study suggest
that HG increased activation of the NLRP3 inflammasome in
adipocytes. Exogenous H2S donor NaHS significantly
inhibited NLRP3 inflammasome expression, and IL-1β and IL-18
production in adipocytes.
Acknowledgements
The authors thank Dr Xin Ni from the Department of
Physiology, Second Military Medical University (Shanghai, China)
for valuable comments on the manuscript.
Funding
This work was supported by the Natural Science
Foundation of China (grant no. 81701481) and the Health Science and
Technology Plan (General) Project of Hangzhou (grant no.
2016B56).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TXH and NNZ were involved in drafting the
manuscript. TXH, NNZ and YR collected and analyzed the data. QYT
and JW interpreted the data, and all authors gave final approval of
the version to be published. All authors reviewed the initial
manuscript and revised it critically for important intellectual
content.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Calle MC and Fernandez ML: Inflammation
and type 2 diabetes. Diabetes Metab. 38:183–191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prattichizzo F, De Nigris V, La Sala L,
Procopio AD, Olivieri F and Ceriello A: ‘Inflammaging’ as a
druggable target: A senescence-associated secretory
phenotype-centered view of type 2 diabetes. Oxid Med Cell Longev.
2016:18103272016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esser N, Legrand-Poels S, Piette J, Scheen
AJ and Paquot N: Inflammation as a link between obesity, metabolic
syndrome and type 2 diabetes. Diabetes Res Clin Pract. 105:141–150.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Assar ME, Angulo J and Rodriguez-Manas L:
Diabetes and ageing-induced vascular inflammation. J Physiol.
594:2125–2146. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bessueille L and Magne D: Inflammation: A
culprit for vascular calcification in atherosclerosis and diabetes.
Cell Mol Life Sci. 72:2475–2489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kelsey MM, Forster JE, Van Pelt RE, Reusch
JE and Nadeau KJ: Adipose tissue insulin resistance in adolescents
with and without type 2 diabetes. Pediatr Obes. 9:373–380. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee J: Adipose tissue macrophages in the
development of obesity-induced inflammation, insulin resistance and
type 2 diabetes. Arch Pharm Res. 36:208–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Silaghi CA, Silaghi H, Crăciun AE, Fărcaș
A, Colosi HA, Cosma DT, Pais R, Hâncu N and Georgescu CE: Age,
abdominal obesity, and glycated hemoglobin are associated with
carotid atherosclerosis in type 2 diabetes patients with
nonalcoholic fatty liver disease. Med Ultrason. 17:300–307. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung CH, Kim BY, Kim KJ, Jung SH, Kim CH,
Kang SK and Mok JO: Contribution of subcutaneous abdominal fat on
ultrasonography to carotid atherosclerosis in patients with type 2
diabetes mellitus. Cardiovasc Diabetol. 13:672014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McGown C, Birerdinc A and Younossi ZM:
Adipose tissue as an endocrine organ. Clinics Liver Dis. 18:41–58.
2014. View Article : Google Scholar
|
|
11
|
Coelho M, Oliveira T and Fernandes R:
Biochemistry of adipose tissue: An endocrine organ. Arch Med Sci.
9:191–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adamczak M and Wiecek A: The adipose
tissue as an endocrine organ. Semin Nephrol. 33:2–13. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin Z, Deng T, Peterson LE, Yu R, Lin J,
Hamilton DJ, Reardon PR, Sherman V, Winnier GE, Zhan M, et al:
Transcriptome analysis of human adipocytes implicates the NOD-like
receptor pathway in obesity-induced adipose inflammation. Mol Cell
Endocrinol. 394:80–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kursawe R, Dixit VD, Scherer PE, Santoro
N, Narayan D, Gordillo R, Giannini C, Lopez X, Pierpont B, Nouws J,
et al: A role of the inflammasome in the low storage capacity of
the abdominal subcutaneous adipose tissue in obese adolescents.
Diabetes. 65:610–618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murphy AM, Lyons CL, Finucane OM and Roche
HM: Interactions between differential fatty acids and inflammatory
stressors-impact on metabolic health. Prostaglandins Leukot Essent
Fatty Acids. 92:49–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bando S, Fukuda D, Soeki T, Nishimoto S,
Uematsu E, Matsuura T, Ise T, Tobiume T, Yamaguchi K, Yagi S, et
al: Expression of NLRP3 in subcutaneous adipose tissue is
associated with coronary atherosclerosis. Atherosclerosis.
242:407–414. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Renga B: Hydrogen sulfide generation in
mammals: The molecular biology of cystathionine-β-synthase (CBS)
and cystathionine-gamma-lyase (CSE). Inflamm Allergy Drug Targets.
10:85–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tripatara P, Patel NS, Collino M,
Gallicchio M, Kieswich J, Castiglia S, Benetti E, Stewart KN, Brown
PA, Yaqoob MM, et al: Generation of endogenous hydrogen sulfide by
cystathionine gamma-lyase limits renal ischemia/reperfusion injury
and dysfunction. Lab Invest. 88:1038–1048. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Panthi S, Chung HJ, Jung J and Jeong NY:
Physiological importance of hydrogen sulfide: Emerging potent
neuroprotector and neuromodulator. Oxid Med Cell Longev.
2016:90497822016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng XM, Huang X, Zhang CM, Liu DH, Lu HL,
Kim YC and Xu WX: Hydrogen sulfide-induced enhancement of gastric
fundus smooth muscle tone is mediated by voltage-dependent
potassium and calcium channels in mice. World J Gastroenterol.
21:4840–4851. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Das A, Samidurai A, Hoke NN, Kukreja RC
and Salloum FN: Hydrogen sulfide mediates the cardioprotective
effects of gene therapy with PKG-Iα. Basic Res Cardiol. 110:422015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vandiver M and Snyder SH: Hydrogen
sulfide: A gasotransmitter of clinical relevance. J Mol Med (Berl).
90:255–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang XH, Wang F, You SJ, Cao YJ, Cao LD,
Han Q, Liu CF and Hu LF: Dysregulation of cystathionine γ-lyase
(CSE)/hydrogen sulfide pathway contributes to ox-LDL-induced
inflammation in macrophage. Cell Signal. 25:2255–2262. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirata I, Naito Y, Takagi T, Mizushima K,
Suzuki T, Omatsu T, Handa O, Ichikawa H, Ueda H and Yoshikawa T:
Endogenous hydrogen sulfide is an anti-inflammatory molecule in
dextran sodium sulfate-induced colitis in mice. Dig Dis Sci.
56:1379–1386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu XH, Cui LB, Wu K, Zheng XL, Cayabyab
FS, Chen ZW and Tang CK: Hydrogen sulfide as a potent
cardiovascular protective agent. Clin Chim Acta. 437:78–87. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu S, Liu Z and Liu P: Targeting hydrogen
sulfide as a promising therapeutic strategy for atherosclerosis.
Int J Cardiol. 172:313–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsai CY, Peh MT, Feng W, Dymock BW and
Moore PK: Hydrogen sulfide promotes adipogenesis in 3T3L1 cells.
PKLoS One. 10:e01195112015. View Article : Google Scholar
|
|
28
|
Beltowski J: Endogenous hydrogen sulfide
in perivascular adipose tissue: Role in the regulation of vascular
tone in physiology and pathology. Can J Physiol Pharmacol.
91:889–898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan Z, Wang H, Liu Y, Yu C, Zhang Y, Chen
J, Wang X and Guan Q: Involvement of CSE/H2S in high glucose
induced aberrant secretion of adipokines in 3T3-L1 adipocytes.
Lipids Health Dis. 13:1552014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu TX, Wang G, Wu W, Gao L, Tan QY and
Wang J: Hydrogen sulfide inhibits high glucose-induced sFlt-1
production via decreasing ADAM17 expression in 3T3-L1 adipocytes.
Int J Endocrinol. 2017:95017922017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grosick R, Alvarado-Vazquez PA,
Messersmith AR and Romero-Sandoval EA: High glucose induces a
priming effect in macrophages and exacerbates the production of
pro-inflammatory cytokines after a challenge. J Pain Res.
11:1769–1778. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Briones L, Andrews M, Pizarro F and
Arredondo-Olguin M: Expression of genes associated with
inflammation and iron metabolism in 3T3-L1 cells induced with
macrophages-conditioned medium, glucose and iron. Biometals.
31:595–604. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akbarzadeh M, Eftekhari MH, Dabbaghmanesh
MH, Hasanzadeh J and Bakhshayeshkaram M: Serum IL-18 and hsCRP
correlate with insulin resistance without effect of calcitriol
treatment on type 2 diabetes. Iran J Immunol. 10:167–176.
2013.PubMed/NCBI
|
|
34
|
Mir M, Rostami A and Hormozi M: Comparison
of serum levels of IL-18 in peripheral blood of patients with type
II diabetes with nephropathy clinical protests and patients with
type II diabetes without nephropathy clinical protests. Diabetes
Metab Syndr. 11:245–250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Herder C, Dalmas E, Boni-Schnetzler M and
Donath MY: The IL-1 pathway in type 2 diabetes and cardiovascular
complications. Trends Endocrinol Metab. 26:551–563. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Banerjee M and Saxena M: Interleukin-1
(IL-1) family of cytokines: Role in type 2 diabetes. Clin Chim
Acta. 413:1163–1170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Daniele G, Guardado Mendoza R, Winnier D,
Fiorentino TV, Pengou Z, Cornell J, Andreozzi F, Jenkinson C,
Cersosimo E, Federici M, et al: The inflammatory status score
including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and
adiponectin underlies whole-body insulin resistance and
hyperglycemia in type 2 diabetes mellitus. Acta Diabetol.
51:123–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hussain G, Rizvi SA, Singhal S, Zubair M
and Ahmad J: Serum levels of TNF-α in peripheral neuropathy
patients and its correlation with nerve conduction velocity in type
2 diabetes mellitus. Diabetes Metab Syndr. 7:238–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hardaway AL and Podgorski I: IL-1β, RAGE
and FABP4: Targeting the dynamic trio in metabolic inflammation and
related pathologies. Future Med Chem. 5:1089–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dinarello CA: Interleukin-18 and the
pathogenesis of inflammatory diseases. Semin Nephrol. 27:98–114.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee CC, Lorenzo C, Haffner SM, Wagenknecht
LE, Festa A, Goodarzi MO, Stefanovski D, Olson NC, Norris JM,
Rewers MJ and Hanley AJ: The association of inflammatory and
fibrinolytic proteins with 5 year change in insulin clearance: The
Insulin resistance atherosclerosis study (IRAS). Diabetologia.
56:112–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Maedler K, Dharmadhikari G, Schumann DM
and Storling J: Interleukin-1 beta targeted therapy for type 2
diabetes. Expert Opin Biol Ther. 9:1177–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bosch M, Lopez-Bermejo A, Vendrell J,
Musri M, Ricart W and Fernandez-Real JM: Circulating IL-18
concentration is associated with insulin sensitivity and glucose
tolerance through increased fat-free mass. Diabetologia.
48:1841–1843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Frostegard J: Immune mechanisms in
atherosclerosis, especially in diabetes type 2. Front Endocrinol
(Lausanne). 4:1622013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vandanmagsar B, Youm YH, Ravussin A,
Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM and Dixit
VD: The NLRP3 inflammasome instigates obesity-induced inflammation
and insulin resistance. Nat Med. 17:179–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Esser N, L'Homme L, De Roover A, Kohnen L,
Scheen AJ, Moutschen M, Piette J, Legrand-Poels S and Paquot N:
Obesity phenotype is related to NLRP3 inflammasome activity and
immunological profile of visceral adipose tissue. Diabetologia.
56:2487–2497. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zilverschoon GR, Tack CJ, Joosten LA,
Kullberg BJ, van der Meer JW and Netea MG: Interleukin-18
resistance in patients with obesity and type 2 diabetes mellitus.
Int J Obes (Lond). 32:1407–1414. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Feng X, Chen Y, Zhao J, Tang C, Jiang Z
and Geng B: Hydrogen sulfide from adipose tissue is a novel insulin
resistance regulator. Biochem Biophys Res Commun. 380:153–159.
2009. View Article : Google Scholar : PubMed/NCBI
|