Introduction
Prostate cancer is ranked as the most common
genitourinary malignancy among males worldwide, with a high
morbidity rate in most developed countries (1). Although the incidence of prostate
cancer is lower in China than that in the USA, the survival
outcomes are generally lower than those in the USA (1–3).
Currently, widespread screening for prostate-specific antigen
(PSA), as a tumor marker, has greatly helped us to diagnose
prostate cancer patients at an early stage (4). However, this method still has some
shortcomings in the metastatic setting (5). Due to the strong metastatic ability of
prostate cancer, the quality of life and prognosis of patients at
advanced stages are still unfavorable (6). Thus, there is an ongoing need for more
effective gene therapy programs for prostate cancer.
The tetraspanin family, a large evolutionarily
conserved family of proteins, is widely expressed in most cells in
multicellular organisms; these proteins contain four transmembrane
domains, short N- and C-terminal cytoplasmic domains, two
extracellular loops and a small intracellular loop (7). Tetraspanins form tetraspanin-enriched
microdomains (TEMs) in the cell membrane with their partner
proteins, such as integrins and immunoglobulins, to regulate
diverse cellular functions and play a role in tumor progression
(8,9). CD81 is a member of the
tetraspanin family that was originally identified as a target of
the antiproliferative antibody TAPA-1 (10). In addition to its important role in
the immune system, CD81 has been revealed to be involved in
the progression of most types of cancer (11,12).
CD81 expression was revealed to be increased in breast
cancer and promoted cell migration and proliferation in breast
cancer cell lines (13). In prostate
cancer, a gene expression profiling study evaluated several
differentially expressed prostate cancer-associated genes,
including CD81, in two prostate cancer cell lines (14). However, the expression of CD81
in prostate cancer tissues and other prostate cancer cell lines, as
well as its potential role, are still unclear.
The aim of the present study was to identify whether
CD81 is upregulated in prostate cancer tissues and linked to
poor prognosis. In addition, the effects of CD81 on prostate
cancer cell proliferation, migration, and invasion were
explored.
Materials and methods
Patients and tissue specimen
collection
The study was approved by the Research Ethics
Committee of Tongren Hospital, Shanghai Jiao Tong University School
of Medicine (Shanghai, China). All the patients signed written
informed consent. All specimens were handled and anonymized
according to ethical and legal standards.
Paired prostate cancer tissue specimens and adjacent
normal tissue specimens were obtained from 114 prostate cancer
patients who received the same radical prostatectomy treatment at
the hospital from February 2011 to January 2013. None of the
enrolled patients had received any androgen-deprivation treatment,
chemotherapy, or radiotherapy prior to sampling. The prostate
cancer tissues and adjacent normal tissues were snap frozen in
liquid nitrogen after collection for further usage. Moreover, the
clinicopathological information of the prostate cancer patients was
collected and summarized in Table I.
After surgery, a 5-year follow-up survey was collected and recorded
for the subsequent survival analysis.
 | Table I.Relationship between CD81
expression and clinical characteristics of prostate cancer
patients. |
Table I.
Relationship between CD81
expression and clinical characteristics of prostate cancer
patients.
|
|
| CD81
expression |
|
|---|
|
|
|
|
|
|---|
| Parameters | No. of cases
(n=114) | Low (n=55) | High (n=59) | P-value |
|---|
| Age |
|
|
| 0.731 |
|
<65 | 52 | 26 | 26 |
|
|
≥65 | 62 | 29 | 33 |
|
| PSA (ng/ml) |
|
|
| 0.660 |
|
<10 | 66 | 33 | 33 |
|
|
≥10 | 48 | 22 | 26 |
|
|
Differentiation |
|
|
| 0.207 |
|
Well-Moderate | 69 | 30 | 39 |
|
|
Poor | 45 | 25 | 20 |
|
| Gleason score |
|
|
| 0.190 |
|
<7 | 57 | 31 | 26 |
|
| ≥7 | 57 | 24 | 33 |
|
| Lymph node
metastasis |
|
|
| 0.038a |
|
Negative | 59 | 34 | 25 |
|
|
Positive | 55 | 21 | 34 |
|
| TNM stage |
|
|
| 0.008a |
|
I–II | 62 | 37 | 25 |
|
|
III–IV | 52 | 18 | 34 |
|
Cell lines and transfection
PC3, DU145, LNCaP, and 22RV1 prostate cancer cell
lines and normal prostate epithelial RWPE-1 cells were purchased
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). PC3 and DU145 cells were cultured in Ham's F-12K medium
(HyClone; GE Healthcare Life Sciences) supplemented with 10% fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.).
LNCap, 22RV1, and RWPE-1 cells were cultured in RPMI-1640 medium
(Hyclone; GE Healthcare Life Sciences) supplemented with 10% FBS.
All cells were cultured in a humidified atmosphere containing 5%
CO2 at 37°C.
Cell transfection was conducted by using
Lipofectamine RNAiMax (Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. CD81 small interfering RNA
(siRNA; 5′-CACGTCGCCTTCAACTGTA-3′) and scrambled-siRNA control
(5′-AATTCTCCGAACGGTCACGT-3′) were purchased from Guangzhou RiboBio
Co., Ltd., which was used to inhibit CD81 expression or as
the negative control of CD81 siRNA, respectively. The transfection
efficiency was detected using quantitative real-time polymerase
chain reaction (qRT-PCR). Untreated cells were used as a
control.
RNA extraction and qRT-PCR
Total RNA was isolated from prostate cancer tissues
and cell lines using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
concentration and quality of RNA were confirmed using a NanoDrop
ND-1000 spectrophotometer (Thermo Fisher Scientific, Inc.). Then,
complementary DNA (cDNA) synthesis was performed using a
PrimeScript RT Reagent Kit (Takara Biotechnology Co., Ltd.).
qRT-PCR was performed using SYBR Green I Master Mix kit
(Invitrogen; Thermo Fisher Scientific, Inc.) and a 7300 Real-Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The primer sequences were as follows: CD81 forward,
5′-GGGAGTGGAGGGCTGCACCAAGTGC-3′ and reverse,
5′-GATGCCACAGCACAGCACCATGCTC-3′; GADPH forward,
5′-CCAAAATCAGATGGGGCAATGCTGG-3′ and reverse,
5′-TGATGGCATGGACTGTGGTCATTCA-3′. The relative mRNA levels of
CD81 were calculated using the 2−ΔΔCq method
(15) and normalized to
GAPDH.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) assays were used to detect the effects of
CD81 on the cell proliferation of prostate cancer cells.
Briefly, ~4×103 transfected cells/well were seeded in
96-well plates. Cell proliferation assays were assessed at 0, 24,
48, and 72 h. CCK-8 reagent (10 µl) was added to the wells at each
time-point and the absorbance value of each sample was measured at
450 nm with a microplate reader (Bio-Rad Laboratories, Inc.).
Cell migration and invasion
assays
Transwell analysis with a 24-well Transwell chamber
(Corning Life Sciences) was used to assess the effects of
CD81 on the migration and invasion capacities of prostate
cancer cells. Cells transfected with CD81 siRNA or control
vectors (3×104 cells/well) were seeded and incubated in
serum-free culture medium in the upper chamber. The lower
compartment was filled with 500 µl complete medium containing 10%
FBS. For invasion assays, the upper chambers were pre-coated with
Matrigel (BD Biosciences). After incubation for 24 h at 37°C with
5% CO2, the cells remaining on the upper membranes were
removed, and migratory or invasive cells on the lower chamber
membranes were fixed with 4% paraformaldehyde for 20 min at room
temperature and stained with 0.1% crystal violet for 30 min at room
temperature. Five random fields from each membrane were counted
with a light microscope (magnification, ×200).
Statistical analysis
All the aforementioned experiments were performed at
least three times. All of the data are presented as the mean ± SD.
Statistical analyses were performed using SPSS 20.0 (IBM Corp.) and
GraphPad 5.0 (GraphPad Software, Inc.). A Student's t-test was used
to analyze differences between the tumor and normal groups. A
χ2 test was used to analyze the association of CD81
expression and clinical characteristics of prostate cancer
patients. In addition, one-way ANOVA followed by Tukey's post hoc
test was used to compare differences in more than three groups. The
Kaplan-Meier method and Cox regression analyses were used to
perform survival analysis and determine the prognostic performance
of CD81 for prostate cancer. P-values <0.05 were
considered to indicate a statistically significant difference.
Results
CD81 expression in tissue specimens
and cell lines
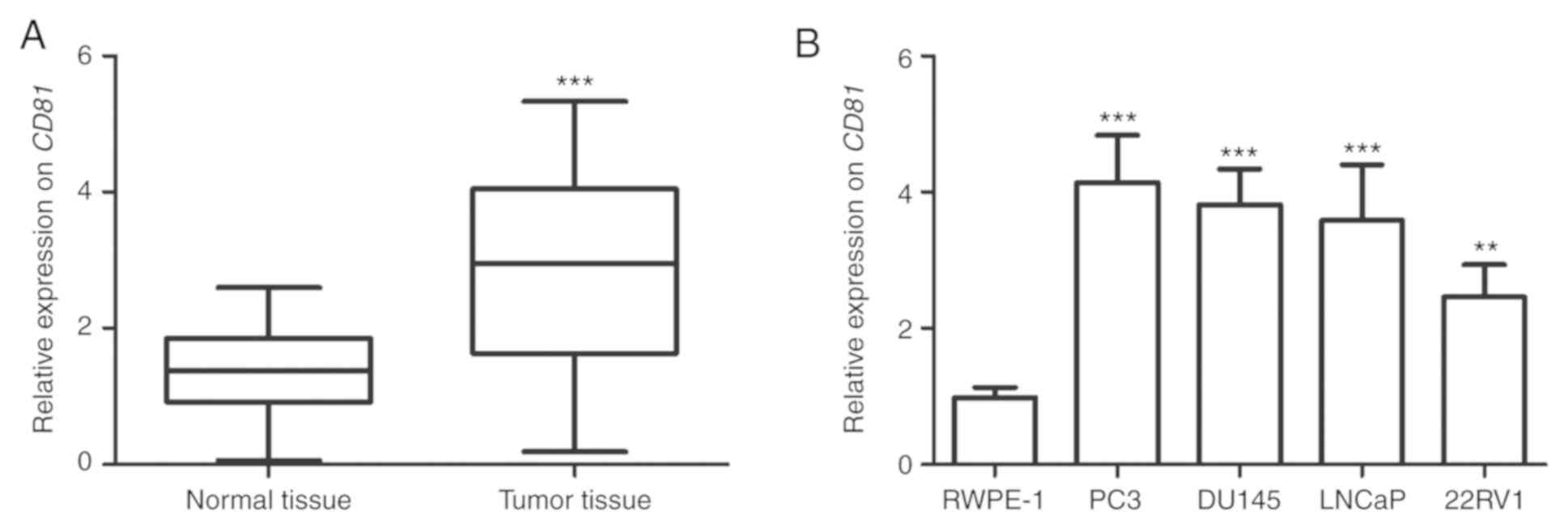
To investigate the expression pattern of CD81
in prostate cancer tissues and cell lines, qRT-PCR was performed.
The analysis results revealed that CD81 expression was
significantly higher in prostate cancer tissues than in adjacent
normal tissues (P<0.001, Fig.
1A). In addition, CD81 was markedly upregulated in all
prostate cancer cell lines compared with that in RWPE-1 cells
(P<0.01, Fig. 1B). Thus, it was
speculated that overexpression of CD81 may play an oncogenic
role in prostate cancer. Considering that the expression levels of
CD81 were relatively higher in PC3, DU145 and LNCaP cells,
these three cell lines were selected for subsequent experiments to
verify the potential role of CD81 in vitro.
In addition, the relative mean value of the
CD81 expression level (2.851) in all prostate cancer tissues
was used as the cutoff point for the grouping the patients. All
prostate cancer patients were divided into a low-CD81
expression group (n=55, based on the relative expression levels
<2.851) and high-CD81 expression group (n=59, based on
the relative expression levels >2.851).
Increased expression of CD81 in
prostate cancer tissues is associated with the clinicopathological
features of prostate cancer patients
The associations between CD81 expression and
the clinicopathological features of prostate cancer patients were
analyzed by χ2 test. As summarized in Table I, high CD81 expression in
prostate cancer tissues was significantly associated with positive
lymph node metastasis (P=0.038) and advanced TNM stage (P=0.008).
However, the expression status of CD81 was not associated
with patient age, PSA, differentiation, or Gleason score.
Increased expression of CD81 in
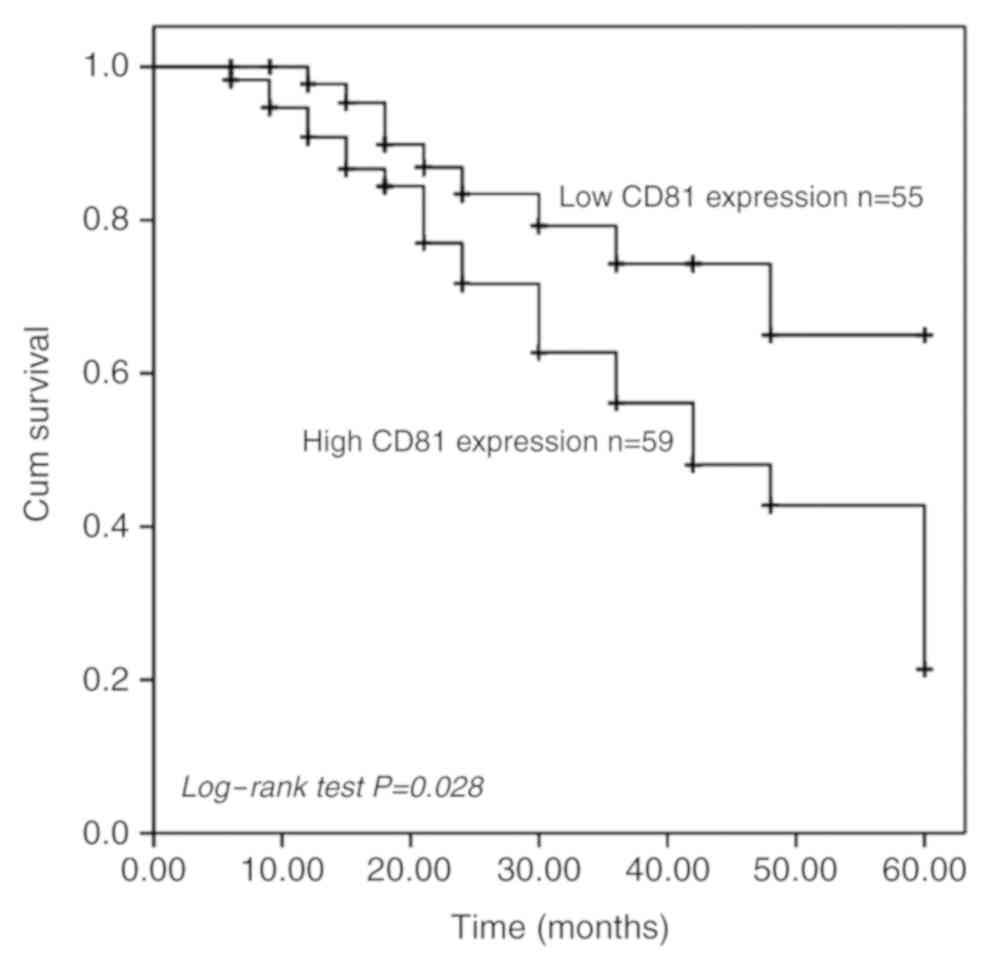
prostate cancer tissues predicts poor prognosis
To assess the potential prognostic value of
CD81 as a biomarker in prostate cancer, the 5-year survival
information of prostate cancer patients was analyzed using the
Kaplan-Meier method. The Kaplan-Meier curve indicated that prostate
cancer patients with high-CD81 expression levels exhibited a
significantly shorter survival time than those with low-CD81
expression levels (P=0.028, Fig. 2).
Furthermore, the multivariate survival analysis with the Cox
proportional hazards model demonstrated that CD81 was
closely correlated with poor overall survival and could be used as
an independent prognostic factor for prostate cancer patients
(HR=2.350, 95% CI=1.038–5.318, P=0.040, Table II).
 | Table II.Multivariate Cox analysis for
CD81 in prostate cancer patients. |
Table II.
Multivariate Cox analysis for
CD81 in prostate cancer patients.
|
| Multivariate
analysis |
|---|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value |
|---|
| CD81 | 2.350 | 1.038–5.318 | 0.040a |
| Age | 1.640 | 0.801–3.359 | 0.176 |
| PSA | 0.853 | 0.398–1.830 | 0.684 |
|
Differentiation | 0.692 | 0.336–1.426 | 0.319 |
| Gleason score | 1.378 | 0.621–3.057 | 0.430 |
| Lymph node
metastasis | 1.246 | 0.548–2.837 | 0.600 |
| TNM stage | 0.445 | 0.189–1.051 | 0.065 |
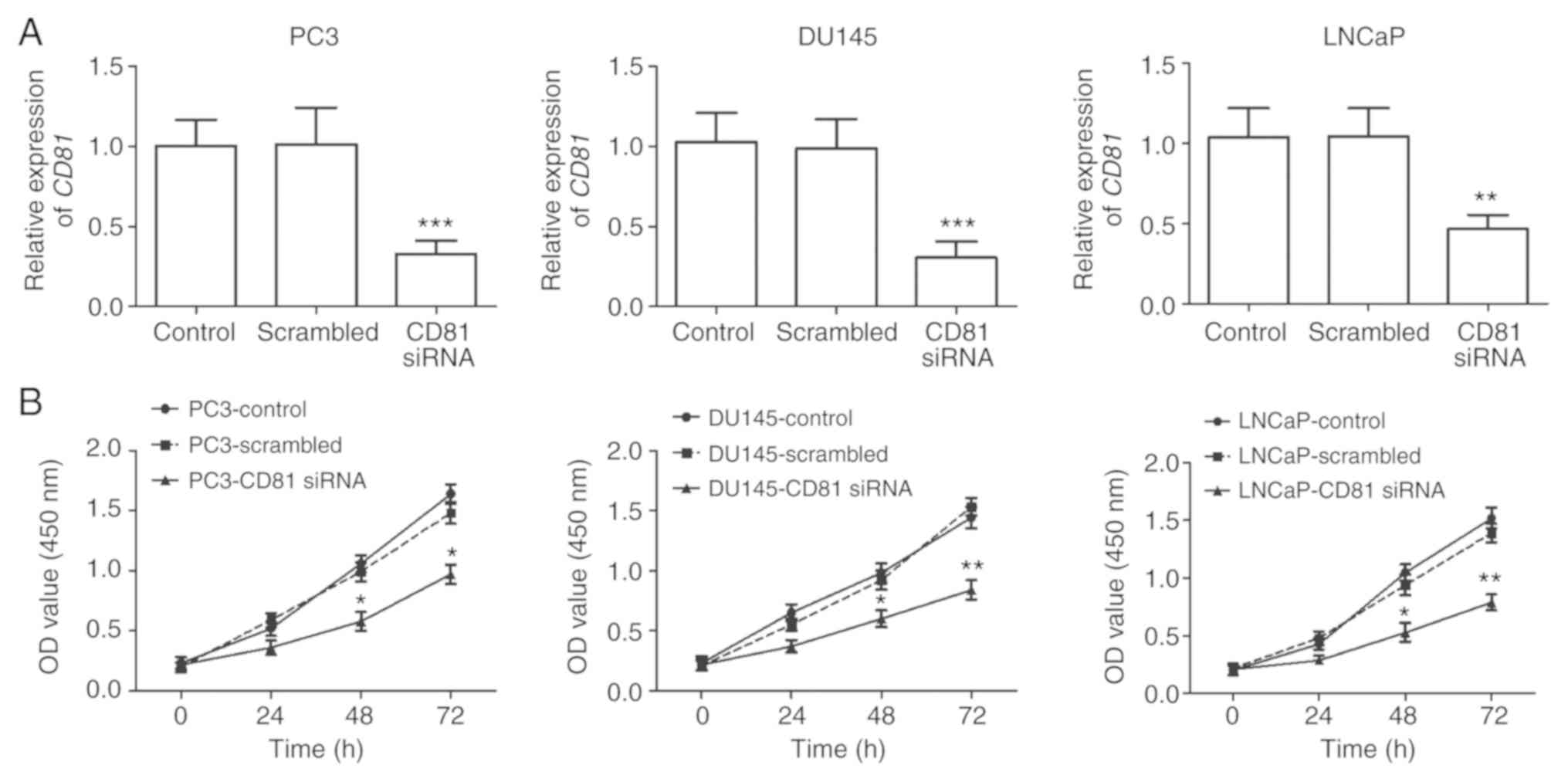
Effects of silencing CD81 on cell
proliferation, migration, and invasion in prostate cancer
cells
To investigate the biological functions of
CD81 in prostate cancer, cell viability, migratory, and
invasive capacities were determined in PC3, DU145, and LNCaP cells.
These cell lines were transfected with CD81 siRNA to
regulate the expression of CD81 in cancer cells. The results
of qRT-PCR revealed that the expression of CD81 in prostate
cancer cells transfected with CD81 siRNA was significantly
downregulated compared with that in control cells (P<0.001,
Fig. 3A). The results of the CCK-8
assay revealed that prostate cancer cell proliferation was
suppressed in CD81 siRNA-transfected cells compared with
that in control cells (P<0.05, Fig.
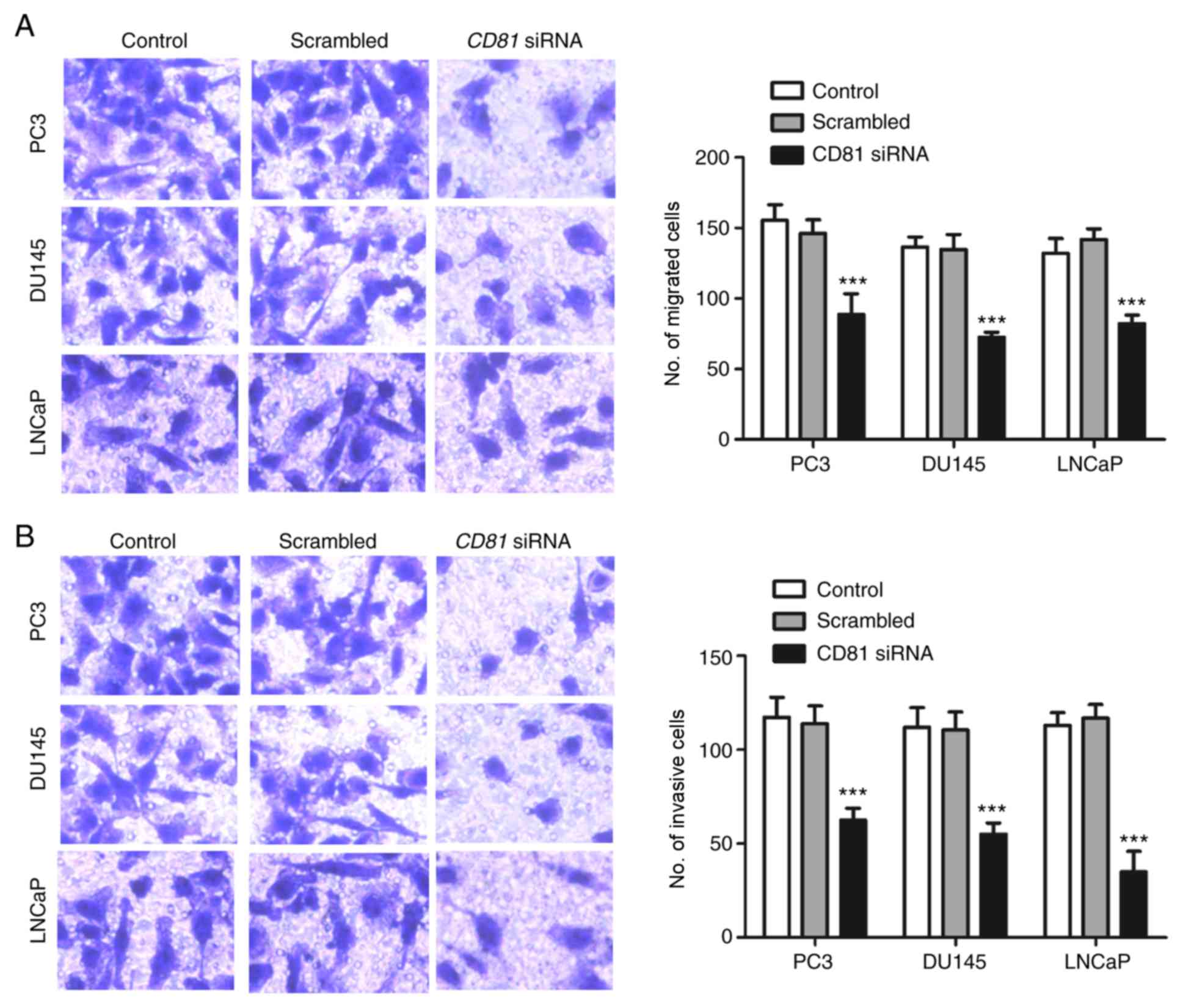
3B). In addition, Transwell migration and invasion assays were
used to examine whether CD81 is involved in metastasis. The
results presented in Fig. 4
indicated that downregulation of CD81 significantly
inhibited the migratory and invasive properties of prostate cancer
cells compared with the control (P<0.001).
Discussion
Prostate cancer is a markedly heterogeneous tumor.
Since it is the most prevalent cancer occurring in men, it has
received great attention, and the prognosis has significantly
improved in developed countries (1).
However, the overall survival of prostate cancer patients is
significantly lower in China than in some developed countries.
Although marked therapeutic method advancements have led to
efficacy improvements in patients with prostate cancer, the
prognosis of some cancer patients at advanced stages remains
unideal, and ~27–53% of patients experience biochemical recurrence
after local therapy (16,17). Therefore, prognosis improvement is
essential for prostate cancer patients. Recently, molecular
biomarkers have received considerable attention for their
diagnostic and prognostic abilities, and for their involvement in
tumor formation and progression. In the present study, the
expression pattern of CD81 was detected and it was revealed
that CD81 was higher in prostate cancer tissues than in
adjacent normal tissues and higher in prostate cancer cells than in
normal prostate epithelial RWPE-1 cells. Overexpression of
CD81 in prostate cancer tissues was identified to be
significantly associated with positive lymph node metastasis,
advanced TNM stages, and poor prognosis in prostate cancer
patients. Furthermore, cell functional analysis of PC3, DU145, and
LNCaP human prostate cancer cells revealed that CD81
functions as an oncogene in prostate cancer by promoting cell
proliferation, migration, and invasion. These findings indicated
that CD81 functions as an oncogene in prostate cancer and
may be a potential prognostic biomarker for human prostate
cancer.
In recent years, an increasing number of studies
have focused on the development of accurate molecular biomarkers
for better detection, diagnosis, prognosis, and treatment (18–20). For
instance, forkhead transcription factor (FoxM1) functions as
an oncogene in the initiation, development, and progression of
cancer, and its neoplastic functions can be used as a strong
biomarker for the diagnosis and treatment of cancer (21). In prostate cancer, numerous
prognostic biomarkers were also investigated (22–24). For
instance, minichromosome maintenance 10 replication initiation
factor (MCM10) was revealed to be significantly upregulated
in prostate cancer patients, and overexpression of MCM10
promoted cell proliferation and predicted poor prognosis in
prostate cancer (25). Another study
revealed that ribosome binding protein 1 (RRBP1) was
upregulated in prostate cancer tissues and significantly associated
with T stage, lymph node metastasis, PSA, Gleason score, and
shorter survival time in prostate cancer patients, thus,
RRBP1 may serve as a potential biomarker in prostate cancer
(26). The aforementioned studies
revealed the pivotal role of cancer-related molecules in cancer
diagnosis, prognostication, and treatment.
The present findings as well as those from other
studies indicate that CD81 is an oncogene in various
cancers, including prostate cancer. In the present study,
CD81 was upregulated in prostate cancer tissues and cell
lines. Moreover, high expression of CD81 was revealed to be
significantly associated with lymph node metastasis and TNM stage.
CD81 was considered to function as an oncogene in prostate
cancer. Previous studies have revealed differential expression of
CD81 in various types of cancers, such as classic vs.
variant hairy cell leukemia, breast cancer, and gastric cancer
(13,27,28). A
study by Vences-Catalan et al indicated that CD81,
which is a promoter of tumor growth and metastasis, is widely
expressed in most tissues and on the majority of tumor cells
(11). In breast cancer, CD81
was also revealed to be upregulated in tumor tissues, associated
with poor overall survival, and promoted tumor cell proliferation
and migration (13). In plasma cell
myeloma, it was revealed that CD81 was an independent factor
affecting the overall survival and progression-free survival of
patients, and CD81 positivity predicted poor prognosis
(29). The aforementioned studies
demonstrated that CD81 also has prognostic value in cancers.
In the present study, the clinical significance of CD81 in
prostate cancer was also investigated. Kaplan-Meier survival curves
revealed that patients with high-CD81 expression levels had
shorter survival times than those with low-CD81 expression
levels, indicating that increased CD81 expression was
correlated with poor overall survival. The multivariate Cox
analysis results indicated that CD81 expression was an
independent prognostic factor for prostate cancer patients.
Several tetraspanins have been studied for their
essential role in tumor cell growth, migration, invasion, and
metastasis, including CD81 (12,30,31). In
the present study, downregulation of CD81 significantly
inhibited cell proliferation, migration, and invasion in
transfected PC3, DU145, and LNCaP metastatic cell lines.
Vences-Catalan et al revealed that tetraspanin CD81
promotes tumor growth and metastasis by modulating the functions of
T regulatory and myeloid-derived suppressor cells (32). In melanoma, upregulation of
CD81 was revealed to increase melanoma cell motility by
upregulating metalloproteinase MT1-MMP expression through
the AKT-dependent Sp1 activation signaling pathway, leading to
increased cell invasion and metastasis (33). Overexpression of CD81 in
different types of cancers could potentially induce greater
susceptibility to antibody binding and may thus represent a
promising tumor target for immunotherapy due to its unique
properties (33,34). Although the exact mechanism of action
has not been clarified, it is clear that CD81 has an
important function in cancer. Further studies are required to
assess the precise molecular mechanisms underlying the role of
CD81 in prostate cancer.
Collectively, the data in the present study revealed
that CD81, a member of the tetraspanin family, was
significantly upregulated in prostate cancer tissues and cell lines
compared with that in controls, respectively. To the best of our
knowledge, these findings provide the first evidence that
CD81 may be a potential prognostic biomarker and therapeutic
target for prostate cancer and correlated with the progression of
prostate cancer cells.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YZ conducted the experiments, analyzed the data, and
wrote the manuscript. HQ conceived the study, and revised the
manuscript critically for important intellectual content. AX and GY
made substantial contributions to patient and tissue specimen
collection and data interpretation. All authors read and approved
the final version and agree to be accountable for all aspects of
the research in ensuring that the accuracy or integrity of any part
of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Tongren Hospital, Shanghai Jiao Tong University
School of Medicine (Shanghai, China). All the patients signed
written informed consent. All specimens were handled and anonymized
according to ethical and legal standards.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000–14 (CONCORD-3): Analysis of individual records for 37 513 025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abu-Bakr El-Bayoumy AS, Hessien Keshta AT,
Sallam KM, Ebeid NH, Elsheikh HM and Bayoumy BE: Extraction,
purification of prostate-specific antigen (PSA), and establishment
of radioimmunoassay system as a diagnostic tool for prostate
disorders. J Immunoassay Immunochem. 39:12–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caram ME, Skolarus TA and Cooney KA:
Limitations of prostate-specific antigen testing after a prostate
cancer diagnosis. Eur Urol. 70:209–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amiya Y, Yamada Y, Sugiura M, Sasaki M,
Shima T, Suzuki N, Nakatsu H, Murakami S and Shimazaki J: Treatment
of locally advanced prostate cancer (Stage T3). Jpn J Clin Oncol.
47:257–261. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levy S and Shoham T: The tetraspanin web
modulates immune-signalling complexes. Nat Rev Immunol. 5:136–148.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hemler ME: Tetraspanin functions and
associated microdomains. Nat Rev Mol Cell Biol. 6:801–811. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bassani S and Cingolani LA: Tetraspanins:
Interactions and interplay with integrins. Int J Biochem Cell Biol.
44:703–708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oren R, Takahashi S, Doss C, Levy R and
Levy S: TAPA-1, the target of an antiproliferative antibody,
defines a new family of transmembrane proteins. Mol Cell Biol.
10:4007–4015. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vences-Catalan F, Rajapaksa R, Srivastava
MK, Marabelle A, Kuo CC, Levy R and Levy S: Tetraspanin CD81, a
modulator of immune suppression in cancer and metastasis.
Oncoimmunology. 5:e11203992015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vences-Catalan F, Duault C, Kuo CC,
Rajapaksa R, Levy R and Levy S: CD81 as a tumor target. Biochem Soc
Trans. 45:531–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang N, Zuo L, Zheng H, Li G and Hu X:
Increased expression of CD81 in breast cancer tissue is associated
with reduced patient prognosis and increased cell migration and
proliferation in MDA-MB-231 and MDA-MB-435S human breast cancer
cell lines in vitro. Med Sci Monit. 24:5739–5747. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bettin A, Reyes I and Reyes N: Gene
expression profiling of prostate cancer-associated genes identifies
fibromodulin as potential novel biomarker for prostate cancer. Int
J Biol Markers. 31:e153–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mottet N, Bellmunt J, Bolla M, Briers E,
Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau
S, et al: EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1:
Screening, diagnosis, and local treatment with curative intent. Eur
Urol. 71:618–629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cornford P, Bellmunt J, Bolla M, Briers E,
De Santis M, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, et al:
EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of
relapsing, metastatic, and castration-resistant prostate cancer.
Eur Urol. 71:630–642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Costa-Pinheiro P, Montezuma D, Henrique R
and Jeronimo C: Diagnostic and prognostic epigenetic biomarkers in
cancer. Epigenomics. 7:1003–1015. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Narayan VM, Konety BR and Warlick C: Novel
biomarkers for prostate cancer: An evidence-based review for use in
clinical practice. Int J Urol. 24:352–360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang E, Zhang X, Wang Q and Wang D:
Identification of prostate cancer hub genes and therapeutic agents
using bioinformatics approach. Cancer Biomark. 20:553–561. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nandi D, Cheema PS, Jaiswal N and Nag A:
FoxM1: Repurposing an oncogene as a biomarker. Semin Cancer Biol.
52:74–84. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao SG, Cheng HH and Lei Y: C-reactive
protein is a prognostic marker for patients with
castration-resistant prostate cancer. Oncol Res Treat. 39:266–271.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma X, Du T, Zhu D, Chen X, Lai Y, Wu W,
Wang Q, Lin C, Li Z, Liu L and Huang H: High levels of glioma tumor
suppressor candidate region gene 1 predicts a poor prognosis for
prostate cancer. Oncol Lett. 16:6749–6755. 2018.PubMed/NCBI
|
|
24
|
Mu J, Fan L, Liu D and Zhu D:
Overexpression of shugoshin1 predicts a poor prognosis for prostate
cancer and promotes metastasis by affecting epithelial-mesenchymal
transition. OncoTargets Ther. 12:1111–1118. 2019. View Article : Google Scholar
|
|
25
|
Cui F, Hu J, Ning S, Tan J and Tang H:
Overexpression of MCM10 promotes cell proliferation and predicts
poor prognosis in prostate cancer. Prostate. 78:1299–1310. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li T, Wang Q, Hong X, Li H, Yang K, Li J
and Lei B: RRBP1 is highly expressed in prostate cancer and
correlates with prognosis. Cancer Manag Res. 11:3021–3027. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salem DA, Scott D, McCoy CS, Liewehr DJ,
Venzon DJ, Arons E, Kreitman RJ, Stetler-Stevenson M and Yuan CM:
Differential expression of CD43, CD81, and CD200 in classic versus
variant hairy cell leukemia. Cytometry B Clin Cytom. 96:275–282.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoo TH, Ryu BK, Lee MG and Chi SG: CD81 is
a candidate tumor suppressor gene in human gastric cancer. Cell
Oncol (Dordr). 36:141–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen F, Hu Y, Wang X, Fu S, Liu Z and
Zhang J: Expression of CD81 and CD117 in plasma cell myeloma and
the relationship to prognosis. Cancer Med. 7:5920–5927. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang X, Zhang J and Huang Y: Tetraspanins
in cell migration. Cell Adh Migr. 9:406–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Malla RR, Pandrangi S, Kumari S, Gavara MM
and Badana AK: Exosomal tetraspanins as regulators of cancer
progression and metastasis and novel diagnostic markers. Asia Pac J
Clin Oncol. 14:383–391. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vences-Catalan F, Rajapaksa R, Srivastava
MK, Marabelle A, Kuo CC, Levy R and Levy S: Tetraspanin CD81
promotes tumor growth and metastasis by modulating the functions of
T regulatory and myeloid-derived suppressor cells. Cancer Res.
75:4517–4526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hong IK, Byun HJ, Lee J, Jin YJ, Wang SJ,
Jeoung DI, Kim YM and Lee H: The tetraspanin CD81 protein increases
melanoma cell motility by up-regulating metalloproteinase MT1-MMP
expression through the pro-oncogenic Akt-dependent Sp1 activation
signaling pathways. J Biol Chem. 289:15691–15704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luo RF, Zhao S, Tibshirani R, Myklebust
JH, Sanyal M, Fernandez R, Gratzinger D, Marinelli RJ, Lu ZS, Wong
A, et al: CD81 protein is expressed at high levels in normal
germinal center B cells and in subtypes of human lymphomas. Hum
Pathol. 41:271–280. 2010. View Article : Google Scholar : PubMed/NCBI
|