Introduction
Cerebral ischemic stroke (CIS), a cerebrovascular
disease with high incidence, disability rate and recurrence rate,
seriously endangers human life and health accounting for 60–80% of
all strokes (1,2). CIS imposes a burden on the patient's
family and society (3). Therefore,
in order to promote the recovery of nerve function, reduce the
disability rate and improve the prognosis of CIS patients it is of
great significance to explore new treatment methods for CIS.
Numerous studies have demonstrated that new blood
vessels improve the blood supply of local brain tissue thereby
providing nutritional support for neurons, saving sudden death
neurons and improving the prognosis of CIS patients (4–6).
Therefore, timely and effective promotion of neovascularization in
the cerebral ischemic area has an important role in the recovery of
neurological function of ischemic brain tissue. As an effective
method to improve the blood supply of brain tissue, angiogenesis is
crucial for the prognosis of patients with CIS (7–9)
therefore has been the focus of CIS research in recent years.
Angiogenesis refers to the proliferation, migration and remodeling
of endothelial cells from original blood vessels as well as the
formation of new blood vessels by budding to meet the biological
function of local tissues (10).
Angiogenesis serves an important role in various physiological and
pathological processes such as wound healing, tissue regeneration,
tumorigenesis and ischemia (11,12).
Angiogenesis is a critical process of recovery from cerebrovascular
disease (8,9).
MicroRNAs (miRNAs), a group of small non-coding
RNAs, can regulate gene expression through binding to the
3′-untranslated regions (3′-UTRs) of the target mRNAs, and have key
roles in the regulation of cell proliferation, differentiation and
apoptosis (13,14). Currently, miRNAs have been identified
as crucial regulatory factors that may serve as diagnostic
biomarkers and/or therapeutic targets for human diseases. A large
body of evidence indicates that miRNAs have important regulatory
roles in angiogenesis (15–17). Increasing amounts of studies have
demonstrated that miRNAs can participate in the development of CIS
by regulating angiogenesis (18–20).
miR-221 has been well studied in a variety of cancers (21–24) and
has also been identified to be downregulated in CIS patients
(25,26). However, to the best of our knowledge,
the role and mechanism of miR-221 in CIS remains unclear.
In the present study, the expression pattern of
miR-221 in the plasma of patients with CIS was investigated and
in vitro experiments explored the effects and mechanisms of
miR-221 on the function of human umbilical vein endothelial cells
(HUVECs). Findings will hopefully provide a potential new target
for the treatment of CIS.
Materials and methods
Clinical samples
A total of 20 samples of peripheral blood from 20
patients with CIS (13 males and 7 females; age range, 35 to 67
years) and 20 samples of peripheral blood from 20 healthy
volunteers without any cerebrovascular diseases (12 males and 8
females; age range, 33 to 71 years) were collected at The
Affiliated Hospital of Guizhou Medical University (Guizhou, China)
from May 2016 to June 2018. Blood samples were centrifuged at 1,000
× g for 10 min at 4°C to obtain serum. The diagnosis of CIS was
confirmed by computed tomography scan (CT) or magnetic resonance
imaging scan (MRI) examinations. Inclusion criteria were as
follows: i) Presentation of subjects within 72 h of the event; ii)
National Institutes of Health Stroke Scale (NIHSS) score between 4
and 15 (27); and iii) APACHE II
score evaluation <22, Cincinnati Score positive (28) for neurological symptoms at admission
(including dysarthria and hemiparesis) and neuroimaging positive
(CT or MRI positive). Exclusion criteria were patients with severe
renal, liver or thyroid failure, acute infectious disease,
rheumatic immune or hematologic disease, cancer or they had been
taking lipid-lowering drugs within the last half of the year. The
present study was approved by The Ethical Committee of the
Affiliated Hospital of Guizhou Medical University and written
informed consent was obtained from each patient.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from American Type Culture Collection. HUVECs were grown
in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.) containing 10%
FBS (Hyclone; GE Healthcare Life Sciences) and the cells were
incubated at 37°C and 5% CO2.
Cell transfection
HUVECs were transfected with 100 nM inhibitor
control (5′-CAGUACUUUUGUGUAGUACAA-3′; Shanghai GenePharma Co.,
Ltd.), 100 nM miR-221 inhibitor (5′-GAAACCCAGCAGACAAUGUAGCU-3′;
Shanghai GenePharma Co., Ltd.), 50 nM mimic control (sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′; Shanghai GenePharma Co., Ltd.), 50 nM
miR-221 mimic (sense, 5′-AGCUACAUUGUCUGCUGGGUUUC-3′ and antisense,
5′-AACCCAGCAGACAAUGUAGCUUU-3′; Shanghai GenePharma Co., Ltd.), 1 µg
control-plasmid (cat. no. sc-437275; Santa Cruz Biotechnology,
Inc.), 1 µg phosphatase and tensin homolog (PTEN)-plasmid (cat no.
sc-400103-ACT; Santa Cruz Biotechnology, Inc.), 50 nM miR-221 mimic
+ 1 µg control-plasmid or 50 nM miR-221 mimic + 1 µg PTEN-plasmid
for 48 h using Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Transfection efficiency was detected by reverse
transcription-quantitative PCR (RT-qPCR) 48-h following
transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
To collect the total RNA from serum and cells,
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) was used according to the manufacturer's instructions. The
PrimeScript™ RT reagent kit (Takara Bio, Inc.) was used to
synthesize cDNAs following the manufacturer's instructions. The
temperature protocol for the reverse transcription reaction was as
follows: 50°C for 5 min and 80°C for 2 min. For qPCR,
SYBR® Premix Ex Taq (Takara Bio, Inc.) was performed
according to the manufacturer's protocol. The primer sequences used
were as follows: U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and
reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′; GAPDH forward,
5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse,
5′-GTAGAGGCAGGGATGATGTTCT-3′; miR-221 forward,
5′-TGCGGAGCTACATTGTCTGCTGG-3′; and reverse,
5′-CCAGTGCAGGGTCCGAGGT-3′ and PTEN forward,
5′-GTCACTGCTTGTTGTTTGC-3′ and reverse, 5′-TTCTTTGTTGATAGCCTCCAC-3′.
Thermocycling conditions were as follows: 10 min at 95°C followed
by 37 cycles of 15 sec at 95°C and 40 sec at 55°C. Relative gene
expression was quantified by the 2-∆∆Ct method (29) and normalized to U6 or GAPDH. Each
experiment was performed in triplicate.
Western blot analysis
Total proteins were extracted from cells using RIPA
lysis buffer (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. To quantify the protein samples, a
bicinchoninic acid protein quantitative kit (Thermo Fisher
Scientific, Inc.) was used, according to the manufacturer's
instructions. Then an equal amount of proteins (35 µg/lane) were
separated by SDS-PAGE on 10% gels and transferred to polyvinylidene
difluoride membranes. Subsequently, the membranes were blocked with
5% non-fat milk at room temperature for 1 h and then incubated with
primary antibodies against PTEN (cat. no. 9188; 1:1,000; Cell
Signaling Technology, Inc.), Bcl-2 (cat. no. 3498; 1:1,000; Cell
Signaling Technology, Inc.), Bax (cat. no. 5023; 1:1,000; Cell
Signaling Technology, Inc.), phosphorylated (p)-AKT (cat. no. 4060;
1:1,000; Cell Signaling Technology, Inc.), AKT (cat. no. 4685;
1:1,000; Cell Signaling Technology, Inc.) and β-actin (cat. no.
4970; 1:1,000; Cell Signaling Technology, Inc.) at 4°C overnight.
Membranes were then incubated with horseradish
peroxidase-conjugated secondary antibody anti-rabbit IgG (1:2,000;
cat. no. 7074; Cell Signaling Technology, Inc.) at room temperature
for 2 h. Protein bands were visualized using an enhanced
chemiluminescence kit (EMD Millipore) and the proteins were
quantified by densitometry (Quantity One 4.5.0 software; Bio-Rad
Laboratories, Inc.) with β-actin as the loading control.
MTT assay
Following specific treatments, cell viability was
detected by MTT assay. The day before cell transfection, HUVECs
were seeded into a 96-well plate (5×103 cells per well) and
incubated at 37°C. Following transfection with or without inhibitor
control, miR-221 inhibitor, mimic control, miR-221 mimic, miR-221
mimic + control-plasmid, or miR-221 mimic + PTEN-plasmid for 48-h,
20 µl MTT reagent was added to each well. Then the cells were
incubated at 37°C for a further 4 h following which 150 µl dimethyl
sulfoxide (DMSO) was applied in each well to dissolve the purple
formazan crystals. Finally, the absorbance was detected at 490 nm
wavelength using the FLUOstar® Omega Microplate Reader
(BMG Labtech GmbH).
Flow cytometry
Following specific treatments, the apoptosis of
HUVECs was analyzed using the Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis detection kit [cat.
no.70-AP101-100; Hangzhou MultiSciences (Lianke) Biotech Co., Ltd.]
according to the manufacturer's protocol. Briefly, 48 h following
cell transfection, HUVECs were collected, washed with PBS, fixed in
70% ethanol at 4°C overnight and then stained with 5 µl Annexin
V-FITC and 5 µl PI for 30 min in the dark at room temperature. A
flow cytometer (BD FACS Aria; BD Biosciences) was used to analyze
cell apoptosis and FlowJo software (version 7.6.1; FlowJo LLC) was
used for data analysis. Experiments were repeated at least 3
times.
Transwell assay
In the present study, in vitro invasion and
migration assays were performed using Transwell plates (pore size,
8 µm; BD Biosciences) with (invasion assay) or without
Matrigel® (migration assay; BD Biosciences). The
Matrigel pre-coating procedures were carried out at 37°C for 5 h.
HUVECs (1×104 cells) diluted in serum-free DMEM were seeded into
the upper chamber of the Transwell plates. Then 600 µl DMEM medium
containing 20% FBS as the chemoattractant was added to the lower
chamber. Following 48-h incubation, cells on the upper surface of
the membrane were removed and the invasive or migratory cells on
the lower surface of the membrane were fixed with methanol at room
temperature for 30 min and then stained with 0.5% crystal violet at
room temperature for 20 min. The membrane was counted in five
randomly selected visual fields under an inverted light microscope
(magnification, ×100; Olympus Corporation) using which the mean
values were calculated.
Tube formation assay
Endothelial tube formation in HUVECs was determined
by performing the tube formation assay. At 48-h following cell
transfection, HUVECs (2×104 per well) were plated into the 96-well
plates which were first pre-coated with 10 mg/ml
Matrigel® at 37°C for 1 h (Becton, Dickinson and
Company) and incubated in fully-supplemented DMEM for 24 h. To
quantify tube formation, the tube length was calculated using
ImageJ 1.38X software (National Institutes of Health).
Dual luciferase reporter assay
To predict the potential targets of miR-221,
microRNA target site prediction software (www.microRNA.org; August 2010 Release) was used. The
binding sites between the 3′-UTR of PTEN and miR-221 were
identified. Then, a dual luciferase reporter assay was performed to
confirm the binding sites between the 3′-UTR of PTEN and miR-221.
The wild type (WT)-PTEN and mutant (MUT)-PTEN 3′-UTR of PTEN were
cloned into a pmiR-RB-Report™ dual luciferase reporter gene plasmid
vector (Guangzhou RiboBio Co., Ltd.) according to the
manufacturer's protocol. To point-mutate the miR-221 binding domain
on the 3′UTR of PTEN, a QuikChange Site-Directed Mutagenesis kit
(Stratagene; Agilent Technologies, Inc.) was performed in
accordance with the manufacturer's protocol. HUVECs were
co-transfected with 1 µg WT-PTEN or 1 µg MUT-PTEN and 50 nM miR-221
mimic or 50 nM mimic control using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) in line with the
manufacturer's protocols. Following 48 h incubation, a
dual-luciferase assay system (Promega Corporation) was used to
assess the luciferase activity. Luciferase activity was normalized
to the Renilla luciferase activity.
Statistical analysis
Experiments were repeated for three times. SPSS 18.0
(SPSS, Inc.) was used to perform statistical analyses. Data are
presented as the mean ± standard deviation. Comparison between two
groups was made using Student's t-test or between multiple groups
using one-way analysis of variance with Tukey's post hoc test.
P<0.05 was considered to indicate statistical significance.
Results
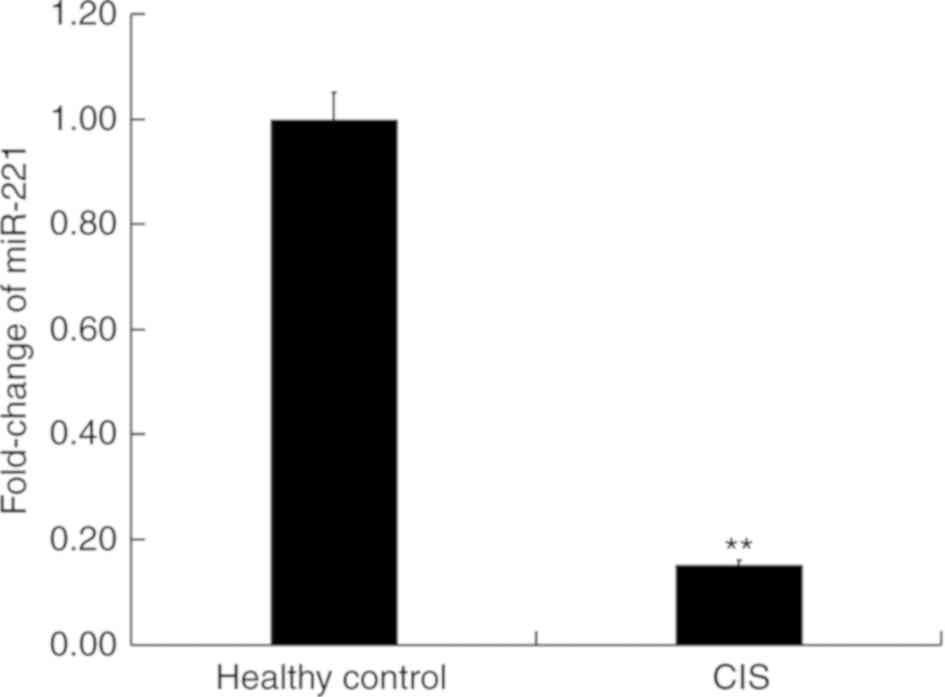
miR-221 is significantly downregulated
in the blood of patients with CIS
First, the level of miR-221 in the peripheral blood
of patients with CIS and in healthy volunteers was detected using
RT-qPCR. Results indicated that compared with the healthy
volunteers, the level of miR-221 in the peripheral blood of
patients with CIS significantly decreased (P<0.01; Fig. 1), indicating the important role of
miR-221 in CIS.
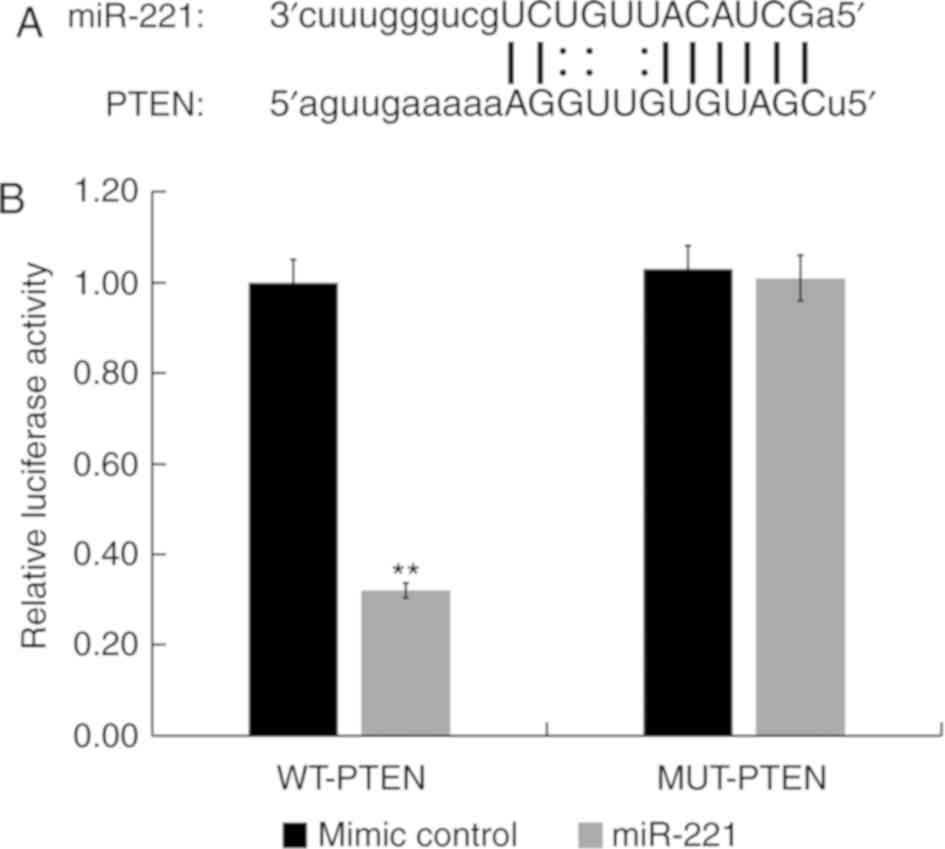
PTEN is a direct target of
miR-221
miRNA target site prediction software (microRNA.org) was used to identify the binding sites
between miR-221 and the 3′-UTR of PTEN mRNA (Fig. 2A). The dual-luciferase reporter assay
results indicated that compared with HUVECs co-transfected with
mimic control and WT-PTEN, the luciferase activity of HUVECs
co-transfected with miR-221 mimic and WT-PTEN was significantly
reduced (P<0.01; Fig. 2B).
However, compared with HUVECs co-transfected with mimic control and
MUT-PTEN, there was no significant difference in the luciferase
activity of HUVECs co-transfected with miR-221 mimic and MUT-PTEN
(Fig. 2B). These results indicated
that PTEN was a direct target of miR-221 in HUVECs.
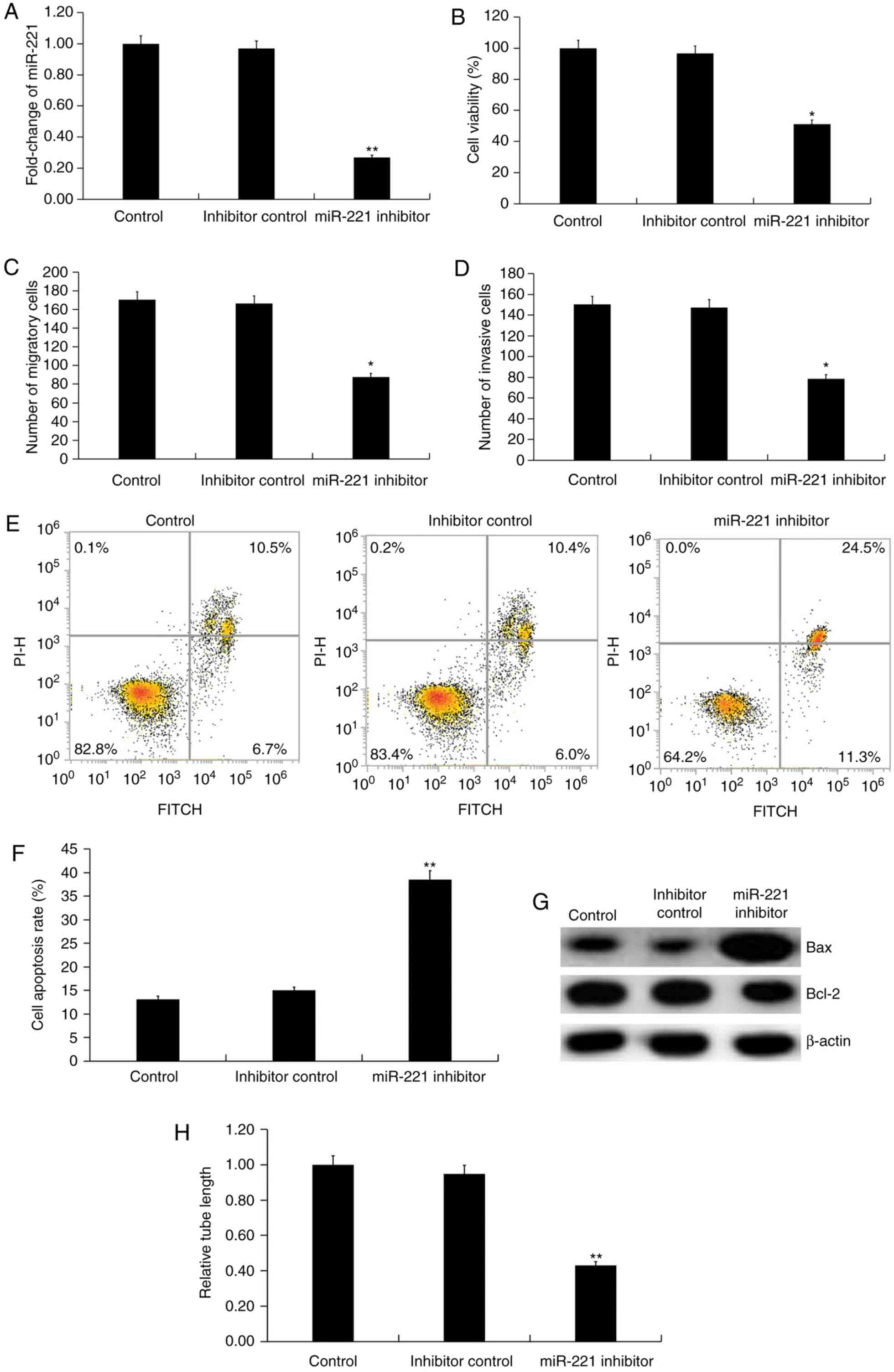
miR-221 downregulation inhibits the
function of HUVECs
The effect of miR-221 downregulation on HUVECs was
investigated using miR-221 inhibitor. Transfection with miR-221
inhibitor for 48 h significantly decreased the level of miR-221 in
HUVECs (P<0.01; Fig. 3A). Further
analysis indicated that compared with the inhibitor control,
miR-221 inhibitor significantly decreased the cell viability
(P<0.05; Fig. 3B), migration
(P<0.05; Fig. 3C) and invasion
ability (P<0.05; Fig. 3D) of
HUVECs. Inhibition of miR-221 also induced cell apoptosis compared
with the inhibitor control (P<0.01; Fig. 3E and F). The protein level of Bcl-2
was decreased by miR-221 inhibitor, while Bax protein expression
was markedly enhanced in miR-221 inhibitor-transfected HUVECs
compared with inhibitor control (Fig.
3G). To investigate the effect of miR-221 on the angiogenic
abilities of endothelial cells, the tube formation of HUVECs was
examined with findings demonstrating that miR-221 inhibitor
repressed tube formation of HUVECs compared with the inhibitor
control. (P<0.01; Fig. 3H).
miR-221 upregulation promotes the
function of HUVECs
The effect of miR-221 upregulation on HUVECs was
also investigated in the present study. HUVECs were transfected
with control-plasmid, PTEN-plasmid, miR-221 mimic, miR-221 mimic +
control-plasmid, or miR-221 mimic + PTEN-plasmid for 48 h. miR-221
mimic significantly increased the level of miR-221 in HUVECs
compared with the mimic control group (P<0.01; Fig. 4A). PTEN-plasmid significantly
enhanced the mRNA level of PTEN in HUVECs compared with the
control-plasmid group (P<0.01; Fig.
4B). Compared with the mimic control group, miR-221 mimic
significantly increased the cell viability (P<0.05; Fig. 4C), migration (P<0.05; Fig. 4D) and invasion ability (P<0.05;
Fig. 4E) of HUVECs, and reduced cell
apoptosis (P<0.01; Fig. 4F). In
addition, miR-221 mimic markedly enhanced the protein level of
Bcl-2 and inhibited Bax protein expression in HUVECs (Fig. 4G). In addition, miR-221 mimic
significantly promoted the tube formation of HUVECs compared with
the mimic control (P<0.01; Fig.
4H). More importantly, all the effects of miR-221 mimic on
HUVECs were reversed by PTEN overexpression (Fig. 4).
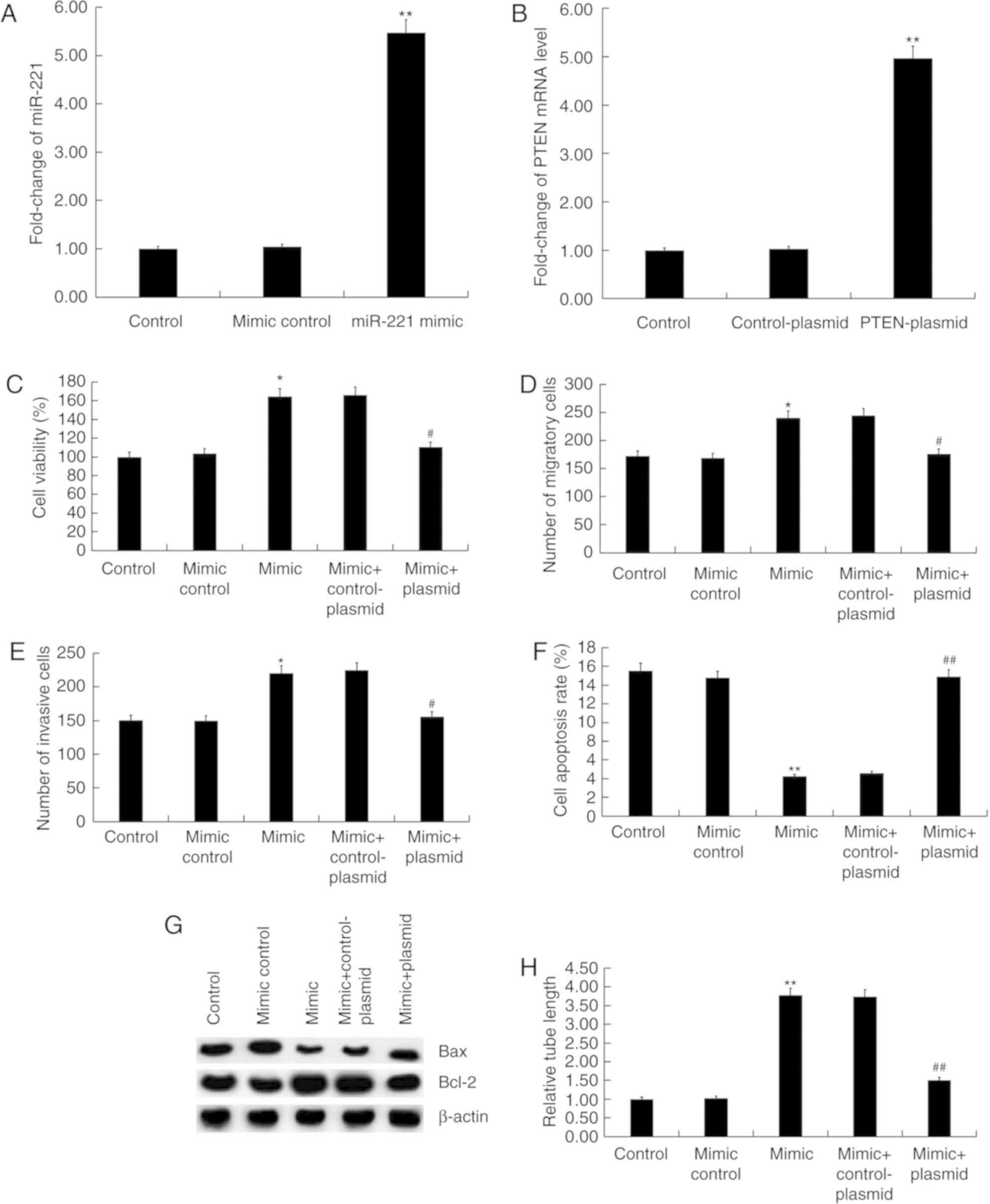 | Figure 4.Effect of miR-221 mimic on HUVECs.
(A) HUVECs were transfected with mimic control or miR-221 mimic for
48 h then the level of miR-221 was detected using RT-qPCR. (B)
HUVECs were transfected with control-plasmid or PTEN-plasmid for 48
h, then the mRNA level of PTEN was detected using RT-qPCR. (C)
HUVECs were transfected with mimic control, miR-221 mimic, miR-221
mimic + control-plasmid or miR-221 mimic + PTEN-plasmid for 48 h
then cell viability was measured by an MTT assay. (D) Cell
migration and (E) invasion were determined using Transwell and
Matrigel assays, respectively. (F) Flow cytometry was used to
analyze cell apoptosis. (G) Protein level of Bax and Bcl-2 was
detected by western blot analysis. (H) Tube formation assay was
used to determine cell tube formation ability. Data are presented
as the mean ± standard deviation. *P<0.05, **P<0.01 vs. Mimic
control; #P<0.05, ##P<0.01 vs. Mimic +
control-plasmid. miR, microRNA; HUVECs, human umbilical vein
endothelial cells; RT-qPCR, reverse transcription-quantitative PCR;
PTEN, phosphatase and tensin homolog. |
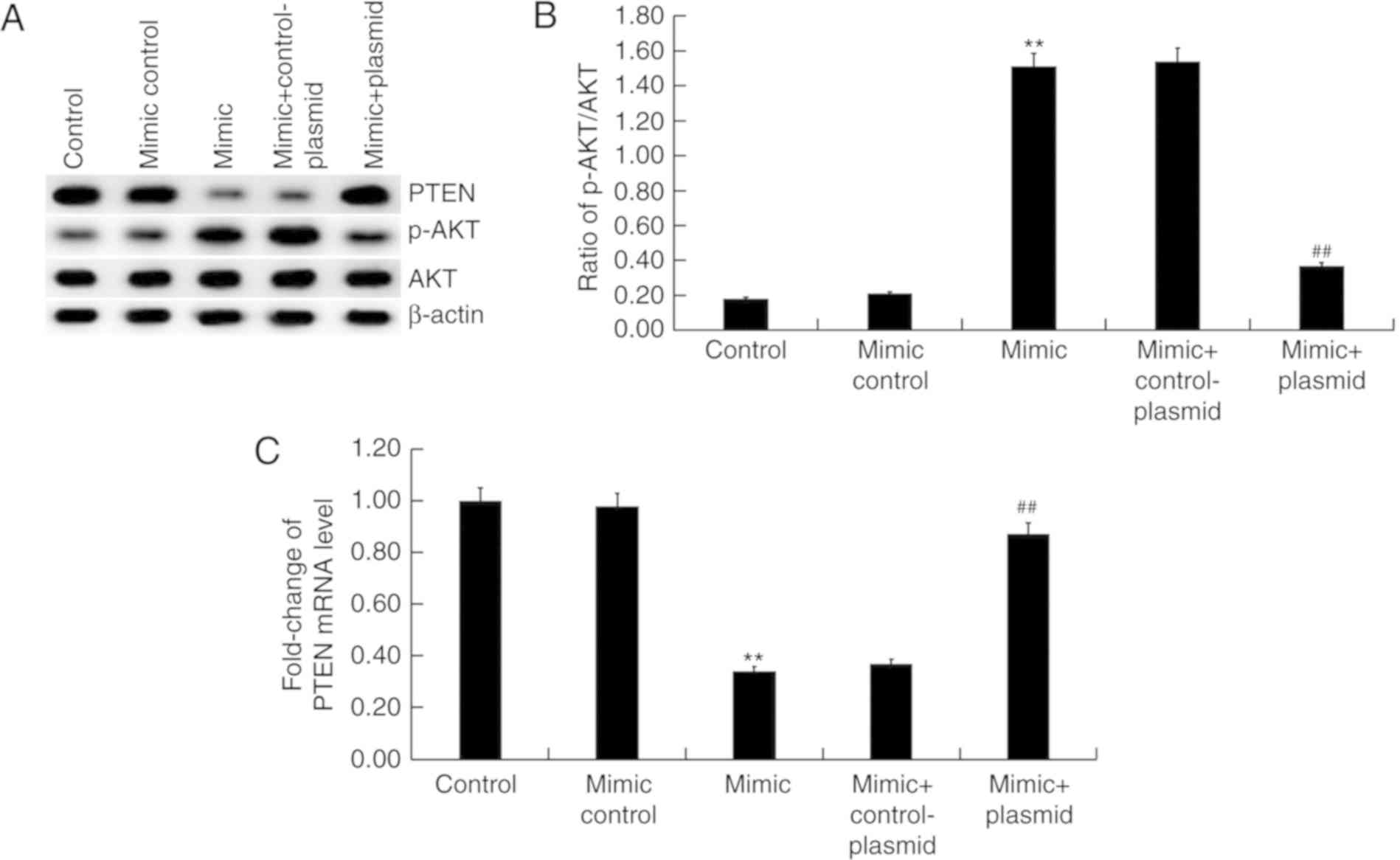
Effect of miR-221 on PTEN/PI3K/AKT
pathway in HUVECs
Finally, the effect of miR-221 on PTEN/PI3K/AKT
pathway was investigated. The results suggested that miR-221 mimic
markedly inhibited PTEN protein expression and enhanced the level
of AKT phosphorylation (Fig. 5A)
compared with the mimic control group. Transfection with the
miR-221 mimic significantly enhanced the ratio of p-AKT/AKT
(Fig. 5B) compared with the mimic
control group, in addition to significantly increasing the levels
PTEN mRNA expression compared with the mimic control group
(Fig. 5C). All the effects of
miR-221 mimic on the expression of PTEN and p-AKT were reversed by
PTEN overexpression (P<0.01; Fig.
5).
Discussion
The present study demonstrated that miR-221 was
significantly downregulated in patients with CIS. Downregulation of
miR-221 significantly inhibited the function of HUVECs as evidenced
by the decreased cell viability, migration and invasion ability and
tube formation with increased cell apoptosis. By contrast, miR-221
upregulation promoted cell viability, migration, invasion and tube
formation of HUVECs and reduced cell apoptosis. In addition,
miR-221 upregulation significantly inhibited PTEN expression and
enhanced the phosphorylation of AKT in HUVECs. All the effects of
miR-221 upregulation on HUVECs were eliminated by PTEN
overexpression. The present findings indicated that miR-221
promoted the function of HUVECs and angiogenesis. Therefore,
miR-221 might be a novel treatment target for CIS.
Currently, CIS seriously endangers human life and
health (1,2). CIS recovery is a complex process,
including inhibition of apoptosis, neuroinflammation, neurogenesis
and angiogenesis (30,31). The angiogenic process of endothelial
cells is critical for new blood vessel formation and blood flow
recovery (9). In recent years,
research on miRNA and angiogenesis in CIS has received increasing
attention (16,32,33).
miR-221, which belongs to the miR-221/222 cluster (34,35), has
been well studied in a variety of cancers including hepatocellular
carcinoma, prostate adenocarcinoma and colorectal carcinoma
(21–22). miR-221 has also been identified to
have an important role in tumor angiogenesis (24). Tsai et al (25) reported that miR-221 is significantly
decreased in the serum of stroke patients and there is a 10.4-fold
increase of stroke risk when miR-221 levels decrease. miR-221 has
also been found to be downregulated in the cerebrospinal fluid of
patients with CIS (26).
In the present study, consistent with the literature
(25,26), it was identified that, the level of
miR-221 in the peripheral blood of patients with CIS significantly
decreased compared with the healthy volunteers, indicating that
miR-221may serve a role during CIS. Consistent with previous
studies (36–38), PTEN was confirmed to be a direct
target of miR-221. PTEN is a dual lipid/protein phosphatase that
has key roles in cell growth, migration and angiogenesis (39–41).
However, contrary to the present results, Urbich et al
(42) determined that miR-221
inhibits endothelial cell activity in vitro through
indirectly regulating endothelial nitric oxide synthase expression
(42). The reason for this
discrepancy requires further research to elucidate the mechanisms.
PTEN regulates the PI3K signaling pathway (40). A previous study revealed that human
aortic smooth muscle cell-derived exosomes of miR-221 inhibit
autophagy in HUVECs by modulating the PTEN/Akt signaling pathway
(43). Therefore, to explore the
mechanism by which miR-221 functioned on HUVECs, the effect of
miR-221 on the PTEN/PI3K/AKT pathway was investigated in the
present study. It was determined that miR-221 upregulation
significantly inhibited the expression of PTEN in HUVECs, and as
expected, the phosphorylation of AKT was significantly enhanced.
All the effects of miR-221 mimic on HUVECs were reversed by PTEN
overexpression.
This is only a preliminary study of the role of
miR-221 in CIS therefore, further in-depth research should be
performed to confirm its role. For example, the role of PTEN in
angiogenesis should be investigated. The correlation between the
expression levels of miR-221 and PTEN and the clinicopathological
parameters of CIS patients needs to be explored. Finally, the
function of miR-221 in CIS should be investigated in vivo.
Future work will focus on these research areas.
In summary, the present study verified that miR-221
expression was downregulated in CIS patients (25,26).
However, to the best of our knowledge, the present study was the
first to demonstrate that miR-221 promoted the function of HUVECs
by regulating the PTEN/PI3K/AKT pathway, thus promoting
angiogenesis. Therefore, miR-221 might be a promising and novel
therapeutic target for CIS treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Foundation of China (grant no. H0928).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HP designed the current study, collected and
analyzed the data, performed statistical analysis, searched the
literature, and prepared the manuscript. HY and XX contributed to
data collection and data interpretation. SHL contributed to
statistical analyzes and interpreted the data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethical Committee of
the Affiliated Hospital of Guizhou Medical University, and written
informed consent was obtained from by each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Middleton LE, Corbett D, Brooks D, Sage
MD, Macintosh BJ, McIlroy WE and Black SE: Physical activity in the
prevention of ischemic stroke and improvement of outcomes: A
narrative review. Neurosci Biobehav Rev. 37:133–137. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Doyle KP, Simon RP and Stenzel-Poore MP:
Neuropharmacology-special issue on cerebral ischemia mechanisms of
ischemic brain damage-review article. Neuropharmacology.
55:310–318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dąbrowska-Bender M, Milewska M, Gołąbek A,
Duda-Zalewska A and Staniszewska A: The impact of ischemic cerebral
stroke on the quality of life of patients based on clinical, social
and psychoemotional factors. J Stroke Cerebrovasc Dis. 26:101–107.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu XF, Rao ML, Peng J, et al: Dynamis
observed morphologic change of neuron and microcirculation in focal
cerebral ischemia and reperfusion of rat. Chin J Clin Rehabil.
6:1904–1905. 2002.(In Chinese).
|
|
5
|
Chen J and Choop M: Neurorestorative
treatment of stroke: Cell and pharmacological approaches. NeuroRx.
3:466–473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marti HJ, Bernaudin M, Bellail A, Schoch
H, Euler M, Petit E and Risau W: Hypoxia-induced vascular
endothelial growth factor expression precedes neovascularization
after cerebral ischemia. Am J Patho1. 156:965–976. 2000. View Article : Google Scholar
|
|
7
|
Krupinski J, Kaluza J, Kumar P, Kumar S
and Wang JM: Role of angiogenesis in patients with cerebral
ischemic stroke. Stroke. 25:1794–1798. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ergul A, Alhusban A and Fagan SC:
Angiogenesis: A harmonized target for recovery after stroke.
Stroke. 43:2270–2274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Velazquez OC, Snyder R, Liu ZJ, Fairman RM
and Herlyn M: Fibroblast-dependent differentiation of human
microvaseular endothelial cells into capillary-like 3-dimensional
networks. FASEB J. 16:1316–1318. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hillen F and Griffioen AW: Tumour
vascularization: Sprouting angiogenesis and beyond. Cancer
Metastasis Rev. 26:489–502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagy JA, Benjamin L, Zeng H, Dvorak AM and
Dvorak HF: Vascular permeability, vasculax hyperpermeability and
angiogenesis. Angiogenesis. 11:109–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ranganathan K and Sivasankar V:
MicroRNAs-Biology and clinical applications. J Oral Maxillofac
Pathol. 18:229–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng N, Wang Z, Zhang Z, He X, Wang C and
Zhang L: miR-487b promotes human umbilical vein endothelial cell
proliferation, migration, invasion and tube formation through
regulating THBS1. Neurosci Lett. 591:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuehbacher A, Urbich C and Dimmeler S:
Targeting microRNA expression to regulate angiogenesis. Trends
Pharmacol Sci. 29:12–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu F, Yang Z and Li G: Role of specific
microRNAs for endothelial function and angiogenesis. Biochem
Biophys Res Commun. 386:549–553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li LJ, Huang Q, Zhang N, Wang GB and Liu
YH: miR-376b-5p regulates angiogenesis in cerebral ischemia. Mol
Med Rep. 10:527–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Zhang Y, Zhang X, Zhang Y, Jiang
Y, Xiao X, Tan J, Yuan W and Liu Y: MicroRNA-433 inhibits the
proliferation and migration of HUVECs and neurons by targeting
hypoxia-inducible factor 1 alpha. J Mol Neurosci. 61:135–143. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang Z, Chi YJ, Lin GQ, Luo SH, Jiang QY
and Chen YK: MiRNA-26a promotes angiogenesis in a rat model of
cerebral infarction via PI3K/AKT and MAPK/ERK pathway. Eur Rev Med
Pharmacol Sci. 22:3485–3492. 2018.PubMed/NCBI
|
|
21
|
Liu M, Liu J, Wang L, Wu H, Zhou C, Zhu H,
Xu N and Xie Y: Association of serum microRNA expression in
hepatocellular carcinomas treated with transarterial
chemoembolization and patient survival. PLoS One. 9:e1093472014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng Q, Peskoe SB, Ribas J, Rafiqi F,
Kudrolli T, Meeker AK, De Marzo AM, Platz EA and Lupold SE:
Investigation of miR-21, miR-141 and miR-221 expression levels in
prostate adenocarcinoma for associated risk of recurrence after
radical prostatectomy. Prostate. 74:1655–1662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tao K, Yang J, Guo Z, Hu Y, Sheng H, Gao H
and Yu H: Prognostic value of miR-221-3p, miR-342-3p and miR-491-5p
expression in colon cancer. Am J Transl Res. 6:391–401.
2014.PubMed/NCBI
|
|
24
|
Yang F, Wang W, Zhou C, Xi W, Yuan L, Chen
X, Li Y, Yang A, Zhang J and Wang T: MiR-221/222 promote human
glioma cell invasion and angiogenesis by targeting TIMP2. Tumour
Biol. 36:3763–3773. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsai PC, Liao YC, Wang YS, Lin HF, Lin RT
and Juo SH: Serum microRNA-21 and microRNA-221 as potential
biomarkers for cerebrovascular disease. J Vasc Res. 50:346–354.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sørensen SS, Nygaard AB, Nielsen MY,
Jensen K and Christensen T: miRNA expression profiles in
cerebrospinal fluid and blood of patients with acute ischemic
stroke. Transl Stroke Res. 5:711–718. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Heldner MR, Zubler C, Mattle HP, Schroth
G, Weck A, Mono ML, Gralla J, Jung S, El-Koussy M, Lüdi R, et al:
National Institutes of Health stroke scale score and vessel
occlusion in 2152 patients with acute ischemic stroke. Stroke.
44:1153–1157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Powers WJ, Rabinstein AA, Ackerson T,
Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk
BM, Hoh B, et al: 2018 Guidelines for the early management of
patients with acute ischemic stroke: A guideline for healthcare
professionals from the American heart association/American stroke
association. Stroke. 49:e46–e110. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Attanasio S and Schaer G: Therapeutic
angiogenesis for the management of refractory angina: Current
concepts. Cardiovasc Ther. 29:e1–e11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chopp M, Zhang ZG and Jiang Q:
Neurogenesis, angiogenesis, and MRI indices of functional recovery
from stroke. Stroke. 38 (2 Suppl):S827–S831. 2007. View Article : Google Scholar
|
|
32
|
Yuan Y, Zhang Z, Wang Z and Liu J:
MiRNA-27b regulates angiogenesis by targeting ampk in mouse
ischemic stroke model. Neuroscience. 398:12–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin KJ, Hamblin M and Chen YE:
Angiogenesis-regulating microRNAs and ischemic stroke. Curr Vasc
Pharmacol. 13:352–365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen WX, Hu Q, Qiu MT, Zhong SL, Xu JJ,
Tang JH and Zhao JH: miR-221/222: Promising biomarkers for breast
cancer. Tumour Biol. 34:1361–1370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu S, Sun X, Wang M, Hou Y, Zhan Y, Jiang
Y, Liu Z, Cao X, Chen P, Liu Z, et al: A microRNA 221-and
222-mediated feedback loop maintains constitutive activation of
NFκB and STAT3 in colorectal cancer cells. Gastroenterology.
147:847–859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gong ZH, Zhou F, Shi C, Xiang T, Zhou CK,
Wang QQ, Jiang YS and Gao SF: miRNA-221 promotes cutaneous squamous
cell carcinoma progression by targeting PTEN. Cell Mol Biol Lett.
24:92019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ren Y, Yang M, Ma R, Gong Y, Zou Y, Wang T
and Wu J: Microcystin-LR promotes migration via the cooperation
between microRNA-221/PTEN and STAT3 signal pathway in colon cancer
cell line DLD-1. Ecotoxicol Environ Saf. 167:107–113. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cortés-Garcia JD, Briones-Espinoza MJ,
Vega-Cárdenas M, Ruíz-Rodríguez VM, Mendez-Mancilla A, Gómez-Otero
AE, Vargas Morales JM, García-Hernández MH and Portales-Pérez DP:
The inflammatory state of adipose tissue is not affected by the
anti-inflammatory response of the A2a-adenosine system and
miR-221/PTEN. Int J Biochem Cell Biol. 100:42–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hopkins BD, Hodakoski C, Barrows D, Mense
SM and Parsons RE: PTEN function: The long and the short of it.
Trends Biochem Sci. 39:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Serra H, Chivite I, Angulo-Urarte A, Soler
A, Sutherland JD, Arruabarrena-Aristorena A, Ragab A, Lim R,
Malumbres M, Fruttiger M, et al: PTEN mediates Notch-dependent
stalk cell arrest in angiogenesis. Nat Commun. 6:79352015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Urbich C, Kuehbacher A and Dimmeler S:
Role of microRNAs in vascular diseases, inflammation, and
angiogenesis. Cardiovasc Res. 79:581–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li L, Wang Z, Hu X, Wan T, Wu H, Jiang W
and Hu R: Human aortic smooth muscle cell-derived exosomal
miR-221/222 inhibits autophagy via a PTEN/Akt signaling pathway in
human umbilical vein endothelial cells. Biochem Biophys Res Commun.
479:343–350. 2016. View Article : Google Scholar : PubMed/NCBI
|