Introduction
With the rising incidence rate of diabetes, the risk
of morbidity and the occurrence of diabetes complications increases
dramatically (1). Diabetic
nephropathy (DN) is one of the serious microvascular complications
of diabetes, accounting for approximately 40% of end-stage renal
disease, second only to tubal nephritis (2,3). At
present, the commonly used treatment methods mainly include
controlling blood sugar, maintaining blood pressure and inhibiting
renin angiotensin system (RAS). Due to the complicated pathogenesis
of DN, there is no clinical method to completely treat DN. Although
the occurrence of DN can be delayed, its development still cannot
be prevented or reversed (4). DN is
an inflammatory disease, therefore anti-inflammation is one of the
keys to control the disease. Inflammatory cytokines are closely
related to the disease process of DN. Inhibition of TNF-α
expressions has been proved to have protective effects on the
kidneys (5,6). Finding more effective drugs or
treatments is a top priority.
Beraprost sodium (BPS) is an oral prostacyclin
derivative, which has good antiplatelet aggregation, vasodilation
and antioxidant activities. It can inhibit the proliferation of
vascular smooth muscle cells and the production of inflammatory
cytokines, increase blood flow, effectively improve
microcirculation and increase erythrocyte deformation (7,8). Some
studies have shown that BPS can effectively repair renal
microvasculature and reduce renal interstitial fibrosis, inhibit
local RAS key factors, angiotensin II receptor 1, angiotensin
converting enzyme, angiotensinogen, promote expression of
angiotensin II receptor 2, and delay the development of chronic
renal failure (9,10). Alprostadil can increase the intimal
blood flow of body nerve cells, dilate blood vessels and reduce the
resistance of peripheral blood vessels. Moreover, alprostadil can
inhibit platelet aggregation, effectively improve renal blood flow
and reduce proteinuria (11,12). BPS and alprostadil have both been
reported to treat DN (13,14), but there are few related studies on
the combination of BPS and alprostadil, and its efficacy still
needs further verification.
Therefore, the aim of this study was to provide
references for clinical treatment of DN by investigating the
efficacy of BPS combined with alprostadil on DN and its influence
on renin angiotensin system and TNF-α.
Patients and methods
Study subjects
A total of 102 patients with type 2 diabetic kidney
disease who were admitted to Weifang People's Hospital (Weifang,
China) from July 2017 to January 2019, aged 40–60 years were
studied. Based on whether alprostadil combined with BPS was the
drug treatment, patients were divided into two groups. A total of
50 patients with alprostadil treatment were the control group and
52 patients with alprostadil combined with BPS were the combined
group. Inclusion criteria: All the patients met the WHO 1999
diagnostic criteria for type 2 diabetic nephropathy (15) and were diagnosed as DN patients for
the first time. The patients' urinary albumin/creatinine was ≥30
mg/mmol or ≤300 mg/mmol, and the urea nitrogen and creatinine
indicators were within the normal range. Relevant treatments were
not performed before serum samples were obtained, and clinical data
of patients were complete. Exclusion criteria: Patients with renal
failure, kidney stones and a medical history of digestive system
diseases in active phase, adrenocortical hyperfunction, abnormal
liver and heart-lung functions; pregnant women or breast-feeding
patients; patients with mental diseases or abnormal brain
judgment.
This study was approved by the Medical Ethics
Committee of Weifang People's Hospital. Patients who participated
in this research had complete clinical data. The signed informed
consents were obtained from the patients or the guardians.
Treatment methods
All patients had a low-salt and low-fat diet, and
according to their own blood glucose levels and kidney damage, all
patients were treated with conventional drugs: oral administration
of gliguidone or subcutaneous injection of insulin; administration
of 100 mg/day aspirin (H31021877, Shanghai Baolong Pharmaceutical
Co., Ltd.), 20 mg/day simvastatin (H20030207, Jingxin
Pharmaceutical Co., Ltd.). Patients in control group were treated
with 10 µg alprostadil + 50 ml normal saline intravenous drip for
the first 2 weeks, once a day, and the administration was stopped
after 2 weeks. Only conventional treatments were performed for the
second 2 weeks. Patients in combined group were treated with the
same drugs as the control groups for two weeks, then the
administration of alprostadil was stopped. Combined with the
conventional treatment, BPS was administered orally, 40 µg/time,
twice a day.
Observation indicators
Related indexes of fasting blood glucose (blood
glucose detector, Jinan Hanfang Medical Devices Co., Ltd.),
hemorheology (automatic blood hemorheology analyzer, Jinan Gelite
Technology Co., Ltd.), coagulation function (full automatic
hemagglutination analyzer, Sysmex), renal function and 24 h urinary
protein (Upro) (full automatic biochemical analyzer, Beckman
Olympus), renin angiotensin system (full automatic
chemiluminescence instrument, Wuhan Mingde Biotechnology Co.,
Ltd.), and changes of TNF-α (ELISA) were observed before and after
treatment, and the occurrence of adverse reactions of patients in
the two groups were recorded. TNF-α and ELISA kit were purchased
from Diken (Shanghai) Trading Co., Ltd., article number:
BE45471.
Statistical analysis
SPSS 19.0 (SPSS, Inc.) was used. The measurement
data were expressed by n(%), and the comparison of rates between
the two groups was performed by χ2 test. Enumeration
data were expressed by mean ± SD. Comparison between the two groups
was conducted by independent-samples t-test, and the comparison
before and after treatment was conducted by paired-samples t-test.
P<0.05 was considered as statistically significant.
Results
General information
There were 50 patients in the control group,
including 35 males (70.00%), 15 females (30%), aged 52.38±9.36
years, and 52 patients in combined group, including 34 males
(65.38%), 18 females (34.62%), aged 53.15±10.14 years. There was no
significant difference in sex ratio and age of patients between the
two groups (P>0.05), and there was no significant difference in
BMI and other data of patients between the two groups (P>0.05)
(Table I).
 | Table I.Comparison of clinical data of
patients between the two groups [n (%)] (mean ± SD). |
Table I.
Comparison of clinical data of
patients between the two groups [n (%)] (mean ± SD).
| Factors | Control group
(n=50) | Joint group
(n=52) | χ2/t
value | P-value |
|---|
| Sex |
|
| 0.248 | 0.618 |
| Male | 35 (70.00) | 34 (65.38) |
|
|
|
Female | 15 (30.00) | 18 (34.62) |
|
|
| Age (years) | 52.38±9.36 | 53.15±10.14 | 0.398 | 0.914 |
| BMI
(kg/m2) | 23.82±3.41 | 24.16±4.14 | 0.452 | 0.652 |
| Course of diabetes
(years) | 10.45±5.62 | 10.29±5.81 | 0.141 | 0.888 |
| Retinopathy |
|
| 1.178 | 0.758 |
| No | 30 (60.00) | 29 (55.77) |
|
|
| Simple
type | 17 (34.00) | 17 (32.69) |
|
|
|
Maculopathy | 1 (2.00) | 3 (5.77) |
|
|
|
Proliferation | 2 (4.0) | 3 (5.77) |
|
|
| History of
cardiovascular disease |
|
| 0.837 | 0.975 |
|
Myocardial infarction | 12 (24.00) | 15 (28.85) |
|
|
| Coronary
artery disease | 3 (6.00) | 2 (3.85) |
|
|
|
Peripheral arterial
disease | 3 (6.00) | 3 (5.77) |
|
|
| Venous
insufficiency | 4 (8.00) | 3 (5.77) |
|
|
| Stroke or
transient ischemic stroke | 2 (4.00) | 3 (5.77) |
|
|
|
Unknown | 26 (52.00) | 26 (50.00) |
|
|
| Glycosylated
hemoglobin (%) | 7.06±1.58 | 7.17±1.65 | 0.344 | 0.732 |
| Smoking |
|
| 1.912 | 0.167 |
|
Yes | 22 (44.00) | 30 (57.69) |
|
|
| No | 28 (56.00) | 22 (42.31) |
|
|
Analysis of complications of patients
after treatment in the two groups
There were no significant adverse reactions in the
groups, but mild adverse reactions still occurred. In the control
group, there were 2 cases of mild vascular pain, 3 cases of mild
nausea, 4 cases of diarrhea, and 5 cases of mild headache. The
total adverse rate was 28.00%. In the combined group, there were 1
case of mild vascular pain, 1 case of mild nausea, 2 cases of
diarrhea, and 2 cases of mild headache. The total adverse rate was
11.54%. There was a significant difference in the total adverse
rate between the two groups (P<0.05) (Table II).
 | Table II.Complications of patients after
treatment in the two groups. |
Table II.
Complications of patients after
treatment in the two groups.
| Complications | Control group
(n=50) | Combined group
(n=52) | t value | P-value |
|---|
| Vascular pain |
| Mild
pain | 2 (4.00) | 1 (1.92) | 0.364 | 0.618 |
| Severe
pain | 0 | 0 |
|
|
| Nausea |
| Mild
nausea | 3 (6.00) | 1 (1.92) | 1.124 | 0.358 |
| Severe
nausea | 0 | 0 |
|
|
| Diarrhea |
| Mild
diarrhea | 4 (8.00) | 2 (3.85) | 0.794 | 0.432 |
| Severe
diarrhea | 0 | 0 |
|
|
| Headache |
| Mild
headache | 5 (10.00) | 2 (3.85) | 1.510 | 0.265 |
| Severe
headache | 0 | 0 |
|
|
| Total adverse
rate | 14 (28.00) | 6 (11.54) | 4.382 | 0.047 |
Changes of blood glucose after
treatment of patients in the two groups
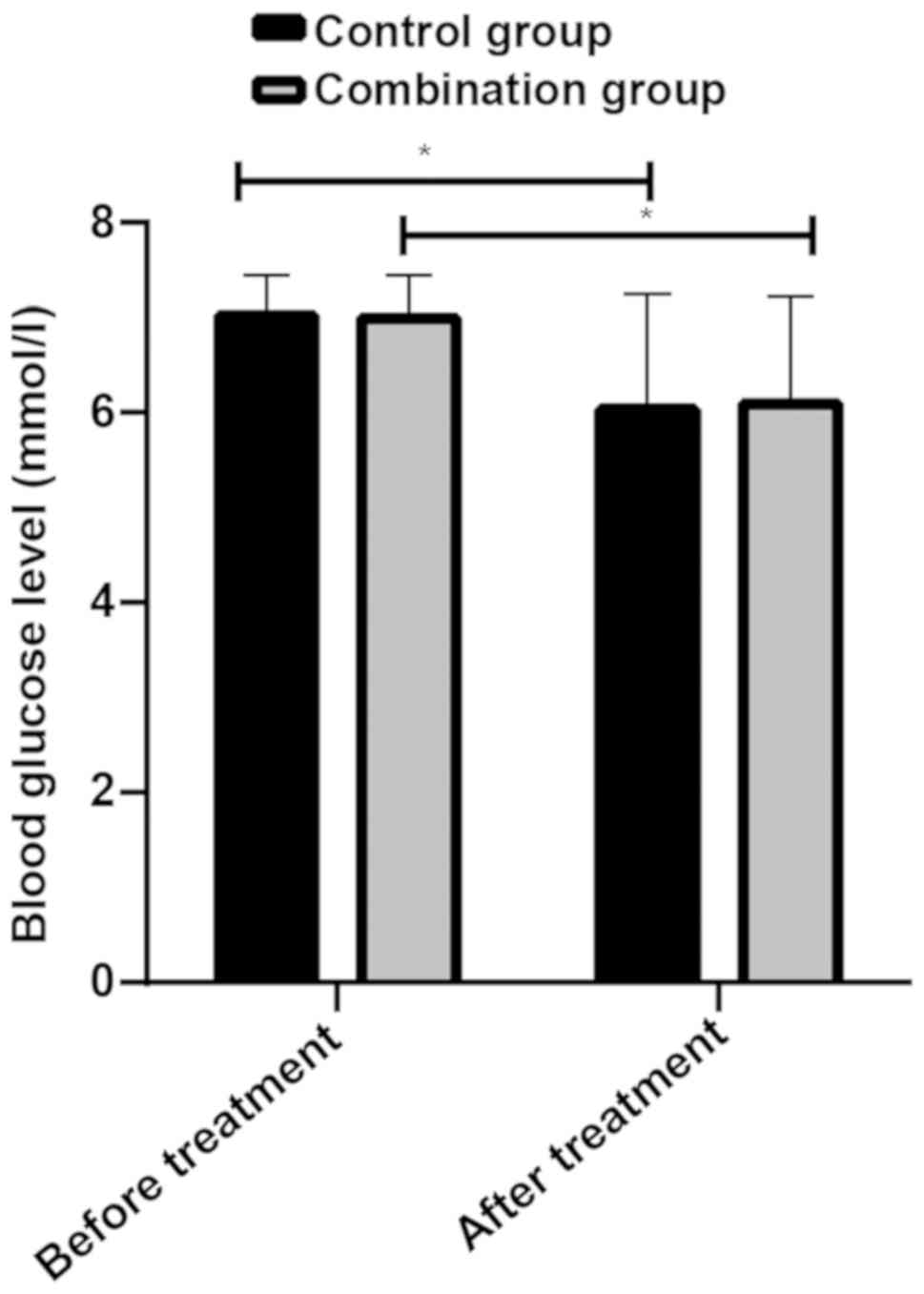
Blood glucose levels of patients in control group
before and after treatment were 7.02±0.42 mmol/l and 6.03±1.22
mmol/l, respectively, while blood glucose levels of patients in
combined group before and after treatment were 6.99±0.46 mmol/l and
6.09±1.13 mmol/l, respectively. There was no statistical difference
in fasting blood glucose of patients between the two groups before
and after treatment (P>0.05), but the fasting blood glucose of
patients in the two groups after treatment was lower than that
before treatment (P<0.05) (Fig.
1).
Hemorheological changes of patients in
the two groups after treatment
Before treatment, there was no difference in blood
viscosity, plasma viscosity and erythrocyte deformation exponent of
patients between the two groups (P>0.05). After treatment, blood
viscosity, plasma viscosity and erythrocyte deformation exponent of
patients between the two groups decreased (P<0.05), and the
combined group was lower than the control group (P<0.05)
(Table III).
 | Table III.Hemorheological changes of patients
after treatment in the two groups. |
Table III.
Hemorheological changes of patients
after treatment in the two groups.
| Hemorheological
changes | Control group
(n=50) | Joint group
(n=52) | t value | P-value |
|---|
| Blood viscosity
(m/pas) |
| Before
treatment | 21.03±2.21 | 20.96±2.18 | 0.616 | 0.842 |
| After
treatment |
13.52±2.65a |
11.67±2.53a | 3.607 | 0.001 |
| Plasma viscosity
(m/pas) |
| Before
treatment | 3.75±0.52 | 3.71±0.54 | 0.381 | 0.704 |
| After
treatment |
2.11±0.71a |
1.82±0.47a | 2.441 | 0.016 |
| Erythrocyte
deformation exponent |
| Before
treatment | 8.68±1.47 | 8.66±1.53 | 0.067 | 0.947 |
| After
treatment |
6.08±2.12a |
4.05±2.24a | 4.697 | <0.001 |
Changes of coagulation function of
patients after treatment in the two groups
There was no difference in the average volume of
plasma fibrinogen (FIB), D dimer and platelet of patients between
the two groups before treatment (P>0.05). After treatment, the
average volume of FIB, D dimer and platelet of patients in the two
groups decreased (P<0.05), and the combined group was lower than
the control group (P<0.05) (Table
IV).
 | Table IV.Changes of coagulation function of
patients after treatment in the two groups. |
Table IV.
Changes of coagulation function of
patients after treatment in the two groups.
| Coagulation
function changes | Control group
(n=50) | Joint group
(n=52) | t value | P-value |
|---|
| FIB (g/l) |
| Before
treatment | 5.12±0.82 | 5.15±0.71 | 0.198 | 0.844 |
| After
treatment |
4.88±0.61a |
4.54±0.67a | 2.677 | 0.009 |
| D dimer (µg/l) |
| Before
treatment | 1.86±0.64 | 1.84±0.82 | 0.137 | 0.891 |
| After
treatment |
1.91±0.64a |
1.37±0.77a | 3.844 | <0.001 |
| Average platelet
volume (fl) |
| Before
treatment | 12.24±1.95 | 12.25±1.88 | 0.026 | 0.979 |
| After
treatment |
10.93±1.92a |
9.72±1.12a | 3.906 | <0.001 |
Changes of renal function of patients
after treatment in the two groups
There was no difference in UACR, CysC, β2-MG and
α1-MG of patients between the two groups before treatment
(P>0.05). After treatment, UACR, CysC, β2-MG and α1-MG of
patients decreased in the two groups (P<0.05), and the combined
group was lower than the control group (P<0.05) (Table V).
 | Table V.Changes of renal function of patients
after treatment in the two groups. |
Table V.
Changes of renal function of patients
after treatment in the two groups.
| Renal function
changes | Control group
(n=50) | Joint group
(n=52) | t value | P-value |
|---|
| UACR (µmol/l) |
| Before
treatment | 31.11±4.13 | 29.96±3.37 |
1.544 | 0.126 |
| After
treatment |
19.73±2.42a |
12.12±1.88a | 17.775 | <0.001 |
| CysC (mg/l) |
| Before
treatment | 2.21±0.34 | 2.27±0.37 |
0.852 | 0.396 |
| After
treatment |
1.53±0.21a |
0.93±0.22a | 14.079 | <0.001 |
| β2-MG (mg/l) |
| Before
treatment | 3.92±0.61 | 3.93±0.51 |
0.090 | 0.929 |
| After
treatment |
2.84±0.47a |
1.93±0.36a | 11.004 | <0.001 |
| α1-MG (mg/l) |
| Before
treatment | 16.23±2.14 | 16.37±2.27 |
0.320 | 0.750 |
| After
treatment |
12.74±1.82a |
8.43±1.18a | 14.246 | <0.001 |
| Upro (mg/day) |
| Before
treatment | 132.52±8.97 | 130.64±7.61 |
1.143 | 0.256 |
| After
treatment | 62.14±6.39 | 58.68±5.91 |
2.841 | 0.006 |
Changes of related indexes of renin
angiotensin system of patients after treatment in the two
groups
Before treatment, there was no difference in renin
and angiotensin II of patients between the two groups (P>0.05).
After treatment, renin and angiotensin II of patients decreased in
both groups (P<0.05), and the combined group was lower than the
control group (P<0.05) (Table
VI).
 | Table VI.Changes of related indexes of renin
angiotensin system of patients after treatment in the two
groups. |
Table VI.
Changes of related indexes of renin
angiotensin system of patients after treatment in the two
groups.
| Related index
changes | Control group
(n=50) | Joint group
(n=52) | t value | P-value |
|---|
| Renin (pg/ml) |
| Before
treatment | 21.15±4.26 | 21.24±4.33 | 0.106 | 0.916 |
| After
treatment |
17.26±5.13a |
13.41±3.79a | 4.323 | <0.001 |
| Angiotensin II
(pg/ml) |
| Before
treatment | 261.42±53.88 | 275.73±54.61 | 1.332 | 0.186 |
| After
treatment |
217.79±58.68a |
157.27±40.79a | 6.068 | <0.001 |
| ACE (U/l) |
| Before
treatment | 62.25±6.71 | 63.94±7.58 | 1.191 | 0.237 |
| After
treatment | 45.62±5.41 | 42.87±4.73 | 2.736 | 0.007 |
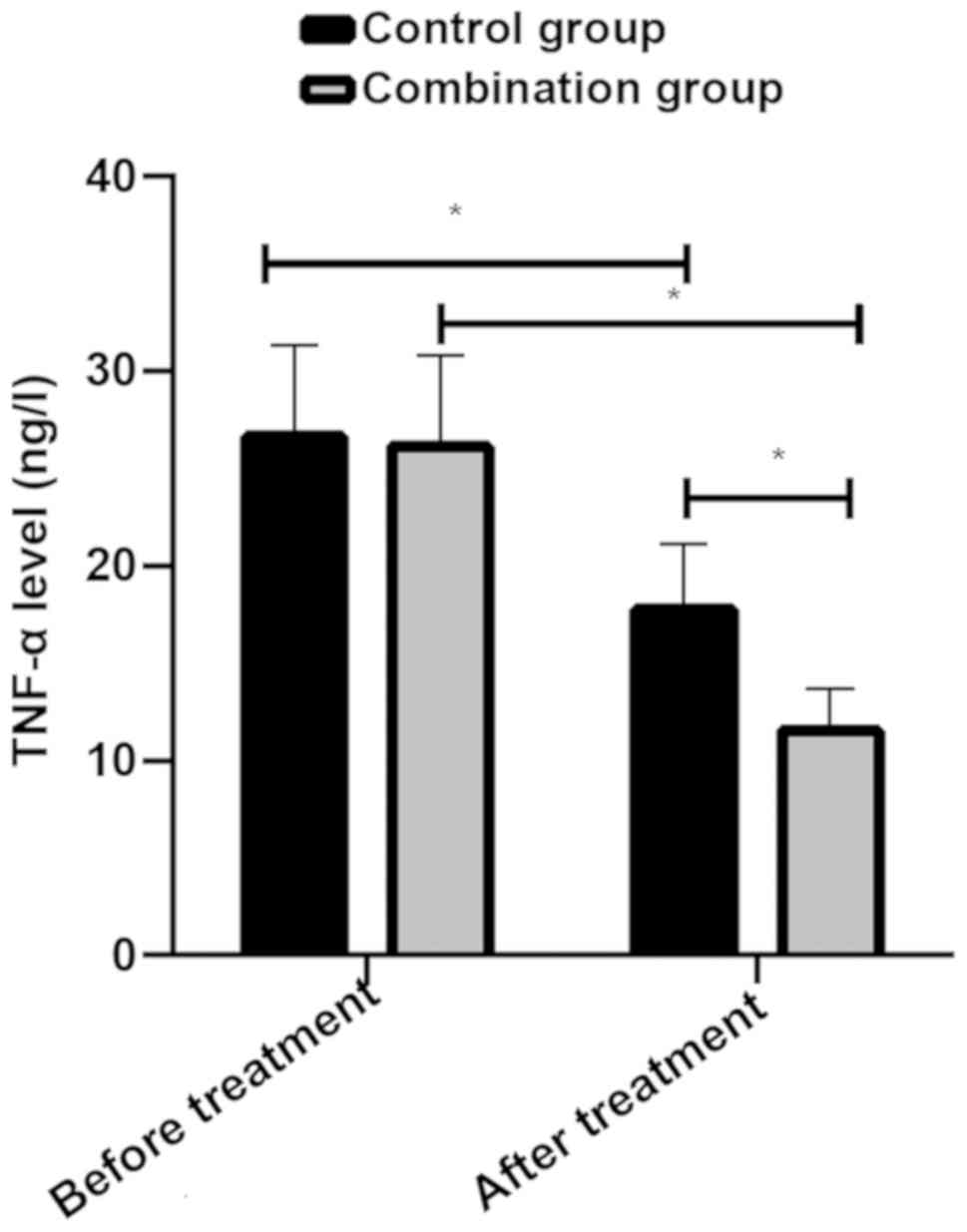
Changes of TNF-α after treatment in the two groups.
Levels of TNF-α before and after treatment of patients in control
group were 26.63±4.73 ng/l and 17.74±3.35 ng/l, respectively, while
levels of TNF-α before and after treatment of patients in combined
group were 26.14±4.68 ng/l and 11.54±2.17 ng/l, respectively. There
was no difference in TNF-α of patients between the two groups
before treatment (P>0.05). Levels of TNF-α of patients in the
two groups decreased after treatment (P<0.05), and levels in
combined group were lower than those in control group (P<0.05)
(Fig. 2).
Discussion
The morbidity of diabetes is expected to increase to
7.7% in the world by 2030 (16).
About one third of diabetic patients will be affected by DN. DN has
become the main cause of end-stage renal disease in developed
countries, with extremely high morbidity and mortality among
diabetic patients (17,18). At present, there is no cure for DN.
It is necessary to find the best treatment scheme to delay the
development of early DN. This study analyzed therapeutic effects of
BPS combined with alprostadil in DN and its influences on renin
angiotensin system and TNF-α, providing theoretical reference for
clinical treatment.
BPS and alprostadil have both been reported for the
treatment of DN. BPS is reported to be used in combination with RAS
inhibitor to effectively prevent the progress of DN (19). Alprostadil can improve renal blood
supply of DN patients, effectively delay the occurrence of fibrosis
and protect renal function (20),
but reports of combined application of the two in DN are few.
Mathiesen et al (21)
reported that BPS combined with alprostadil can protect renal
function of DN patients, reduce proteinuria, improve glomerular
filtration function and microcirculation disturbance, and inhibit
platelet activation. BPS combined with alprostadil in the treatment
of chronic renal failure has also been reported indicating that
this treatment method can improve glomerular filtration rate,
reduce urinary albumin excretion rate, slow down the increase of
serum creatinine, reduce levels of FIB and D-dimer, thus delaying
the progress of chronic renal failure caused by chronic
glomerulonephritis, and with good safety (22). Results of the present study show that
BPS combined with alprostadil can more effectively inhibit platelet
and repair glomerular filtration, thereby improving hemodynamics,
coagulation and renal function in patients with DN, which are
similar to the previously reported results (21,22), and
effects of combination and alprostadil alone on blood glucose are
similar. At present, there is no study confirming that BPS or
alprostadil can affect glucose metabolism in human body. Our study
found no adverse reaction of patients in the two groups, which may
be related to the duration of the treatment. Based on the above
results, BPS combined with alprostadil has better efficacy on DN,
and will not increase the occurrence of adverse reactions in the
short-term.
RAS exists in the circulatory system and is a
humoral regulation system composed of hormones and enzymes, mainly
including renin and angiotensin. It is of great significance to
maintain the balance of body blood pressure, water, electrolyte and
the stability of internal environment (23,24). Our
results show that BPS combined with alprostadil has another
advantage in that it can effectively inhibit RAS, which is of great
significance for maintaining the blood pressure of patients. In
1979, alprostadil was found to inhibit
renin-angiotensin-aldosterone system (25). In subsequent studies, BPS derivative
of alprostadil was also found to inhibit expression of RAS-related
factors in mice, thus delaying the development of chronic renal
failure (8), but influences of
alprostadil on RAS are still uncertain.
Changes of TNF-α after treatment were analyzed.
TNF-α changes glomerular hemodynamics and promotes the increase of
vascular endothelial permeability. It can also promote infiltration
of inflammatory cells, new formation of extracellular matrix,
production of reactive oxygen species and blood flow disorders.
Moreover, overexpression levels of TNF-α are closely related to the
occurrence of proteinuria (26,27).
Collectively, the evidence suggests that TNF-α plays an important
role in the pathogenesis of DN. It is also suggested that improving
TNF-α level is important for treating DN. Our results show that BPS
combined with alprostadil can reduce TNF-α level in patients'
peripheral blood more effectively. In other disease-related
studies, alprostadil has been shown to reduce TNF-α level in
patients' serum and urinary protein is also reduced. Previous
studies (28,29) have shown that BPS and alprostadil can
reduce the level of TNF-α and achieve protection for the human
body. In blood glucose metabolism, insulin activates glucose
transporter 4 (GLUT4) translocation to the cell membrane to reduce
blood glucose levels (30), while
AKT phosphorylation promotes the translocation of GLUT4 and
down-regulates TNF-α (31).
Prostaglandins promote AKT phosphorylation (32), while BPS promotes AKT expression
(10). Therefore, BPS combined with
proproterol in the treatment of renal injury can control blood
glucose levels by activating AKT and inhibit TNF-α-induced
apoptosis and autophagy.
However, because this study is a prospective
analysis, there will inevitably be some selectivity bias and
Hawthorne effect. Thus, further study is required. A multi-center
clinical randomized controlled study will be conducted in the
future. The analysis on safety still needs to be verified, but we
will continue to track patients and record their future
changes.
Compared with alprostadil therapy, BPS combined with
alprostadil can more effectively improve hemodynamics, coagulation
function and renal function of DN patients, and inhibit expression
of RAS related factors and TNF-α, which is a more effective method
to treat DN.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX wrote the manuscript, analyzed and interpreted
the patient general data. XP performed chemiluminescence and ELISA.
SL was responsible for the analysis of observation indicators. All
the authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Medical Ethics
Committee of Weifang People's Hospital (Weifang, China). Patients
who participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mayer-Davis EJ, Lawrence JM, Dabelea D,
Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S,
Pettitt DJ, et al SEARCH for Diabetes in Youth Study, : Incidence
trends of type 1 and type 2 diabetes among youths, 2002–2012. N
Engl J Med. 376:1419–1429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
John S: Complication in diabetic
nephropathy. Diabetes Metab Syndr. 10:247–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Keri KC, Samji NS and Blumenthal S:
Diabetic nephropathy: Newer therapeutic perspectives. J Community
Hosp Intern Med Perspect. 8:200–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Magee C, Grieve DJ, Watson CJ and Brazil
DP: Diabetic nephropathy: A tangled web to unweave. Cardiovasc
Drugs Ther. 31:579–592. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Donate-Correa J, Martín-Núñez E,
Muros-de-Fuentes M, Mora-Fernández C and Navarro-González JF:
Inflammatory cytokines in diabetic nephropathy. J Diabetes Res.
2015:9484172015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeo ES, Hwang JY, Park JE, Choi YJ, Huh KB
and Kim WY: Tumor necrosis factor (TNF-alpha) and C-reactive
protein (CRP) are positively associated with the risk of chronic
kidney disease in patients with type 2 diabetes. Yonsei Med J.
51:519–525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen S, Xie S, He W, Wei D, Li S and Chen
W: Beneficial effect of beraprost sodium plus aspirin in the
treatment of acute ischemic stroke. Med Sci Monit. 23:4401–4407.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka S, Akaike T, Wu J, Fang J, Sawa T,
Ogawa M, Beppu T and Maeda H: Modulation of tumor-selective
vascular blood flow and extravasation by the stable prostaglandin
12 analogue beraprost sodium. J Drug Target. 11:45–52. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li S, Wang Y, Chen L, Wang Z, Liu G, Zuo
B, Liu C and Sun D: Beraprost sodium mitigates renal interstitial
fibrosis through repairing renal microvessels. J Mol Med (Berl).
97:777–791. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang XF, Zhang BH, Lu XQ and Wang P:
Beraprost sodium, a stable analogue of PGI2, inhibits the
renin-angiotensin system in the renal tissues of rats with chronic
renal failure. Kidney Blood Press Res. 43:1231–1244. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei W, An XR, Jin SJ, Li XX and Xu M:
Inhibition of insulin resistance by PGE1 via autophagy-dependent
FGF21 pathway in diabetic nephropathy. Sci Rep. 8:92018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang JZ, Kang XJ, Gao Y, Zheng YY, Wu TT,
Li L, Liu F, Yang YN, Li XM, Ma YT, et al: Efficacy of alprostadil
for preventing of contrast-induced nephropathy: A meta-analysis.
Sci Rep. 7:10452017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goya K, Otsuki M, Xu X and Kasayama S:
Effects of the prostaglandin I2 analogue, beraprost sodium, on
vascular cell adhesion molecule-1 expression in human vascular
endothelial cells and circulating vascular cell adhesion molecule-1
level in patients with type 2 diabetes mellitus. Metabolism.
52:192–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin L, Qin W, Wang J and Lin L: Combined
treatment of diabetic nephropathy with alprostadil and calcium
dobesilate. Exp Ther Med. 14:5012–5016. 2017.PubMed/NCBI
|
|
15
|
Gabir MM, Hanson RL, Dabelea D, Imperatore
G, Roumain J, Bennett PH and Knowler WC: Plasma glucose and
prediction of microvascular disease and mortality: Evaluation of
1997 American Diabetes Association and 1999 World Health
Organization criteria for diagnosis of diabetes. Diabetes Care.
23:1113–1118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shaw JE, Sicree RA and Zimmet PZ: Global
estimates of the prevalence of diabetes for 2010 and 2030. Diabetes
Res Clin Pract. 87:4–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanabe K, Maeshima Y, Sato Y and Wada J:
Antiangiogenic therapy for diabetic nephropathy. BioMed Res Int.
2017:57240692017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Atkins RC: The epidemiology of chronic
kidney disease. Kidney Int Suppl. 94:S14–S18. 2005. View Article : Google Scholar
|
|
19
|
Shima A, Miyamoto M, Kubota Y, Takagi G
and Shimizu W: Beraprost sodium protects against diabetic
nephropathy in patients with arteriosclerosis obliterans: A
prospective, randomized, open-label study. J Nippon Med Sch.
82:84–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo C, Li T, Zhang C, Chen Q, Li Z, Liu J
and Wang Y: Therapeutic effect of alprostadil in diabetic
nephropathy: Possible roles of angiopoietin-2 and IL-18. Cell
Physiol Biochem. 34:916–928. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mathiesen ER, Hommel E, Olsen UB and
Parving HH: Elevated urinary prostaglandin excretion and the effect
of indomethacin on renal function in incipient diabetic
nephropathy. Diabet Med. 5:145–149. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Wan JX, Jiang DW, Fu BB, Cui J and
Li GF: Clinical efficacy and safety of sequential treatment with
alprostadil and beraprost sodium for chronic renal failure induced
by chronic glomerulonephritis. Nan Fang Yi Ke Da Xue Xue Bao.
33:1521–1524. 2013.(In Chinese). PubMed/NCBI
|
|
23
|
Santos RA, Ferreira AJ, Verano-Braga T and
Bader M: Angiotensin-converting enzyme 2, angiotensin-(1–7) and
Mas: New players of the renin-angiotensin system. J Endocrinol.
216:R1–R17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Raebel MA: Hyperkalemia associated with
use of angiotensin-converting enzyme inhibitors and angiotensin
receptor blockers. Cardiovasc Ther. 30:e156–e166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyamori I, FitzGerald GA, Brown MJ and
Lewis PJ: Prostacyclin stimulates the renin angiotensin aldosterone
system in man. J Clin Endocrinol Metab. 49:943–944. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Navarro-González JF and Mora-Fernández C:
The role of inflammatory cytokines in diabetic nephropathy. J Am
Soc Nephrol. 19:433–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Navarro-González JF, Jarque A, Muros M,
Mora C and García J: Tumor necrosis factor-alpha as a therapeutic
target for diabetic nephropathy. Cytokine Growth Factor Rev.
20:165–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu CP and Yu ZJ: Study on
L-ornithine-L-aspartate in the treatment of acute-on-chronic liver
failure. Zhonghua Gan Zang Bing Za Zhi. 19:63–64. 2011.(In
Chinese). PubMed/NCBI
|
|
29
|
Deng J, Feng J, Liu T, Lu X, Wang W, Liu
N, Lv Y, Liu Q, Guo C and Zhou Y: Beraprost sodium preconditioning
prevents inflammation, apoptosis, and autophagy during hepatic
ischemia-reperfusion injury in mice via the P38 and JNK pathways.
Drug Des Devel Ther. 12:4067–4082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ho Y: Glucose metabolism and diabetes.
Patient-Specific Controller for an Implantable Artificial Pancreas.
Springer; Singapore: pp. 11–17. 2019, View Article : Google Scholar
|
|
31
|
Nitulescu GM, Van De Venter M, Nitulescu
G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis
A, Tsoukalas D, et al: The Akt pathway in oncology therapy and
beyond (Review). Int J Oncol. 53:2319–2331. 2018.PubMed/NCBI
|
|
32
|
Takenaga M, Ishihara T, Niimi J, Hamaguchi
A, Asano T, Tsuchiya R, Ohta Y, Mizushima T and Yudoh K: Nano PGE1
enhances phosphorylation of ERK1/2 and Akt to promote recovery from
motor dysfunction and muscle atrophy induced by sciatic nerve
injury. Preprints 2019. https://www.preprints.org/manuscript/201901.0250/v1
|