Introduction
Ankylosing spondylitis (AS) is a chronic autoimmune
disease (1). The morbidity of AS in
China is ~0.3%, and the onset age is at 13–31 years, with a peak at
20–30 years (2). AS mainly occurs in
spinal column, skeleton, peripheral joints and extra-articular
tissues, mainly manifested as backache, and accompanied by
peripheral arthritis, attachment point inflammation, and proctitis
(3,4). In the early stage of onset, 50–92%
patients are accompanied by osteopenia or osteoporosis (5–7), and
often accompanied by fractures and neurological complications,
which seriously affect the treatment and prognosis of AS patients.
Smith (8) considered that the
pathogenesis of AS is related to genetic, infection, immunity and
physical and chemical factors. At present, there is no cure for AS,
but timely treatment can relieve patients' pain and improve their
quality of life (9).
The drugs commonly used in clinic are infliximab and
etanercept, which are tumor necrosis factor-α (TNF-α) antagonists.
TNF-α is an important inflammatory cytokine in the pathogenesis of
AS (10). TNF-α antagonist has
significant effect in treating AS patients (11,12), and
in 30% of patients symptoms are relieved by 70–80% in short-term
treatment (13). Advanced AS
patients will be accompanied by severe joint dysfunction, so early
diagnosis is very important for AS patients. In the early stage of
AS, C-reactive protein (CRP) and erythrocyte sedimentation rate
(ESR) increase rapidly, so ESR and CRP are important evaluation
indexes for clinical auxiliary diagnosis (14,15).
Recent studies have found that biochemical changes of bone
metabolism precede systemic osteoporosis and joint stiffness
(16,17), which can also provide basis for early
diagnosis of AS (18).
In this study, various indexes were detected before
and after treatment, bone metabolism indexes, such as bone-specific
alkaline phosphatase (BALP), β-collagen special sequence (β-CTX),
C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR),
and adverse reactions and the clinical efficacy of the drugs were
evaluated to explore the effect of etanercept and infliximab on AS
patients and the effect on bone metabolism index.
Patients and methods
General information
Clinical data of 80 patients with AS admitted to
Affiliated Hospital of Hebei University of Engineering (Handan,
China) from June 2015 to March 2016 were selected into the study.
The average age of the 39 AS patients treated with etanercept was
29.68±7.24 years, with 21 males and 18 females. Further 41 AS
patients treated with infliximab were the control group with an
average age of 30.48±7.18 years, with 22 males and 19 females.
Inclusion criteria were as follows: The treatment
conformed to New York AS Diagnostic Criteria (19) revised in 1984; patients aged 20–50
years; the duration of illness did not exceed 3 years; patients
accompanied by family members on admission; patients with complete
clinical data and good compliance.
Exclusion criteria were as follows: patients unable
to cooperate with the examination due to other factors such as
aphasia and dysphoria; patients who had received etanercept and
infliximab therapy in the prior 6 months; HIV antibody positive
patients; patients participated in other clinical trials; patients
with severe organic diseases; patients had previous history of
mental illness and family history of mental illness; patients had a
history of drug dependence.
This study was approved by the Ethics Committee of
Affiliated Hospital of Hebei University of Engineering. All the
patients and their families were informed in advance and signed a
complete informed consent form.
Method
Treatment methods
Thirty-nine patients in the etanercept group
received intermittent administration for 24 weeks (etanercept;
Shenzhen Phys Biotechnology Co., Ltd., item no. 152). Subcutaneous
injection was carried out twice a week for the first eight weeks
(20 mg), once a week for the ninth to sixteenth weeks (15 mg), and
once every two weeks for the last eight weeks (10 mg). Further 41
patients in infliximab group received intermittent administration
for 24 weeks (infliximab; Shanghai Teramabs Biotechnology Co., Ltd.
item no. TM-Infl-00002_1), 100 mg in the first week, the second
week and the sixth week, and the same dose every six weeks.
Patient's rest and diet were adjusted, appropriate rehabilitation
exercise and adequate sleep were maintained.
Blood collection
A total of 4 ml of fasting peripheral blood was
collected early in the morning before treatment, 12 weeks after
treatment and at 24 weeks. Sample was put into anticoagulant tubes,
and sent to clinical laboratory to examine CRP and ESR of the
patients. After coagulation for 60 min (20–25°C), the blood sample
was centrifuged at 1,006.2 × g for 10 min with a centrifuge radius
of 10 cm and a centrifuge temperature of 4°C, the upper serum was
separated and stored for later use to avoid hemolysis and repeated
freezing and thawing. After obtaining the upper serum, the serum
bone-specific alkaline phosphatase (BALP) (BALP ELISA kit; Wuhan
Aimosaisi Technology Co., Ltd.; item no. ELA-E1091r), β-β-crosslaps
(β-CTX) (β-CTX ELISA kit; Shanghai Zhenyu Biotechnology Co., Ltd.;
item no. E-EL-H0960km-1) and the level of bone metabolism index was
detected by enzyme-linked immunosorbent assay. The detection
process was strictly carried out in accordance with the instruction
manual of the kit. The specific steps of ELISA were as follows: 100
µl of sample and standard substance were added into reagent
diluent, the plate was sealed, incubated at room temperature for 2
h, and washed; and 100 µl of detection antibody was added to each
well, the plate was sealed, incubated at room temperature for 2 h,
and washed; then 100 µl of streptavidin-HRP working diluent was
added to each well. The plate was sealed, incubated at room
temperature in the dark for 20 min, and washed. Then 100 µl of
substrate solution was added to each well, incubated at 37°C in the
dark for 20 min, 50 µl of termination solution was added and ELXS00
microplate reader was used at 450 nm within 15 min.
Observation indicators
The changes of CRP and ESR levels before and 12 and
24 weeks after treatment were observed to evaluate the effect of
etanercept and infliximab on laboratory indexes of AS patients. The
bone metabolism level (BALP, β-CTX), morning stiffness time,
Schober test time, AS activity index (BASDAI) (20), AS function index (BASFI) (21) and the clinical efficacy and adverse
reactions of the two groups of patients before and after treatment
were recorded.
Evaluation indicators
Efficacy evaluation
Efficacy evaluation (19): Cure: no morning stiffness, limited
activity, pain in trunk joints, ESR and CRP returned to normal.
Markedly effective: morning stiffness, movement restriction, trunk
joint pain, ESR and CRP decreased significantly. Effective: morning
stiffness, movement restriction, trunk joint pain, ESR and CRP
decreased. Ineffective: morning stiffness, movement restriction,
trunk joint pain, ESR and CRP did not decrease.
SPSS 20.0 (IBM Corp.) was used for all statistical
analysis of the experimental results. GraphPad Prism 7 (GraphPad
Software Co., Ltd.) was used for visualizing the results.
Enumeration data was expressed as [n(%)], and Chi-square test was
used for comparison among groups. The measurement data were
expressed as (mean ± SD), the two groups were compared by paired
t-test, the comparison of multiple time-points was analyzed by
repeated measurement variance, and LSD-t was used in back testing.
P<0.05 was regarded as statistically significant.
Results
Comparison of general data between the
two groups
There was no difference in general clinical data
between the two groups in terms of age, sex, body mass index,
smoking and drinking history, education level and complications
(P>0.05) (Table I).
 | Table I.Comparison of clinical general data
(mean ± SD), n[%]. |
Table I.
Comparison of clinical general data
(mean ± SD), n[%].
|
| Enbrel group
(n=39) | Infliximab group
(n=41) | χ2/t
value | P-value |
|---|
| Average age
(years) | 29.68±7.24 | 30.48±7.18 | 0.50 | 0.62 |
| Sex |
|
| 0.00 | 0.99 |
| Male | 21 (53.85) | 22 (53.66) |
|
|
|
Female | 18 (46.15) | 19 (46.34) |
|
|
| Body mass index
(kg/m2) | 21.51±3.42 | 20.89±3.58 | 0.79 | 0.43 |
| Smoking |
|
| 0.01 | 0.92 |
| Yes | 11 (28.21) | 12 (29.27) |
|
|
| No | 28 (71.79) | 29 (70.73) |
|
|
| Drinking |
|
| 0.28 | 0.59 |
| Yes | 5
(12.82) | 7
(17.07) |
|
|
| No | 34 (87.18) | 34 (82.93) |
|
|
| Educational
level |
|
| 0.03 | 0.86 |
| Junior
high school | 7
(17.95) | 8
(19.51) |
|
|
| College
degree or above | 32 (82.05) | 33 (80.49) |
|
|
| Complication |
|
Hypertension | 6
(15.38) | 8
(19.51) | 0.24 | 0.63 |
| High
blood lipid | 4
(10.26) | 3 (7.32) | 0.22 | 0.64 |
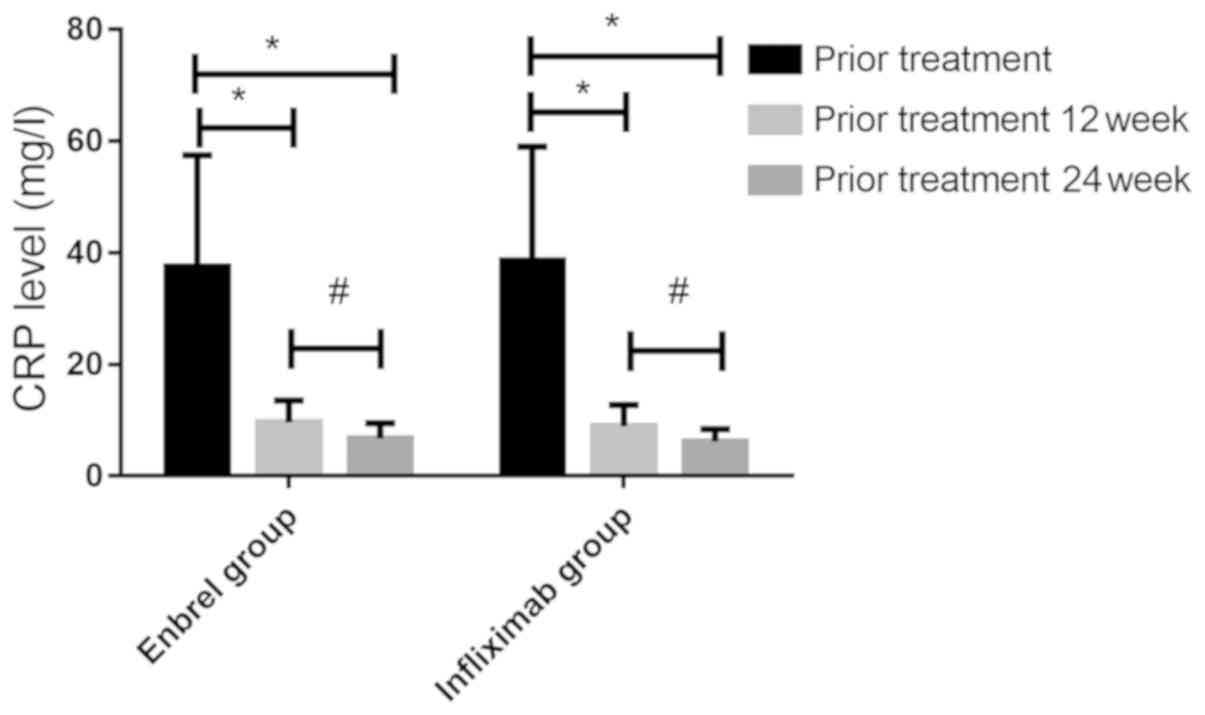
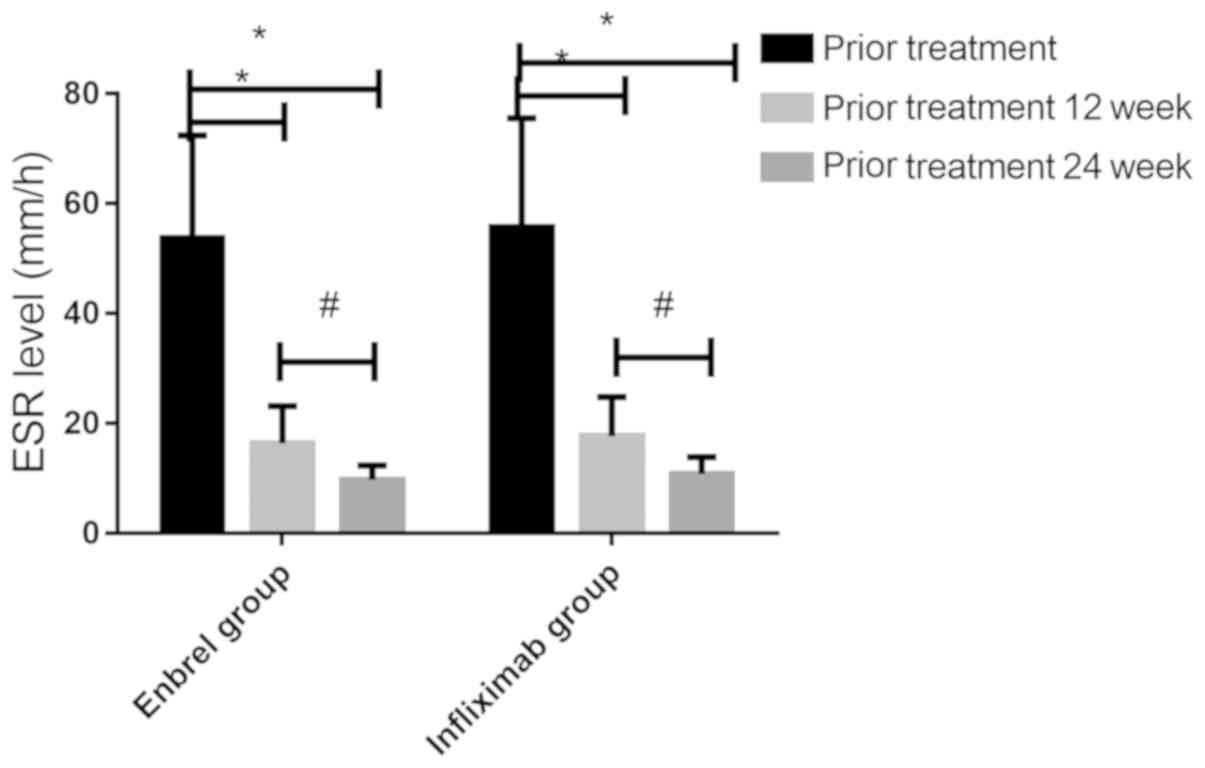
CRP and ESR levels before and after
treatment in the two groups of patients
The CRP and ESR levels between the two groups before
and after treatment were compared (Figs.
1 and 2). There was no
significant difference in CRP levels between the two groups before
treatment (37.58±19.89 and 38.62±20.41 mg/l), 12 weeks after
treatment (9.65±3.94 and 8.94±3.81 mg/l) or 24 weeks after
treatment (6.78±2.72 and 6.21±2.25 mg/l) (P>0.05). CRP levels in
both groups decreased significantly before treatment, 12 and 24
weeks after treatment (P<0.05), and there was a downward trend
from 12 to 24 weeks after treatment (P<0.05). There was no
significant difference in ESR level between the two groups before
treatment (53.67±18.75 and 55.71±19.87 mm/h), 12 weeks after
treatment (16.43±6.74 and 17.68±7.12 mm/h) or 24 weeks after
treatment (9.74±2.65 and 10.81±3.10 mm/h) (P>0.05). CRP levels
in both groups decreased significantly before treatment, 12 and 24
weeks after treatment (P<0.05), and there was a downward trend
from 12 to 24 weeks after treatment (P<0.05).
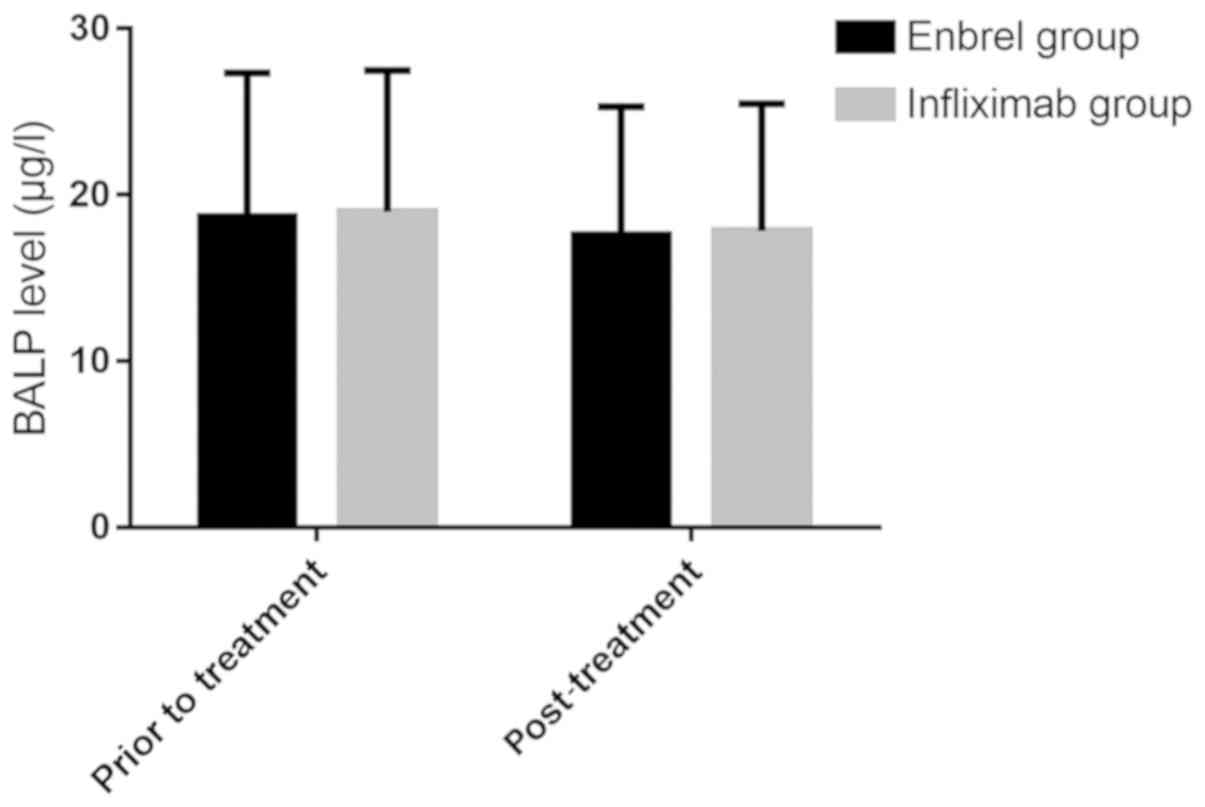
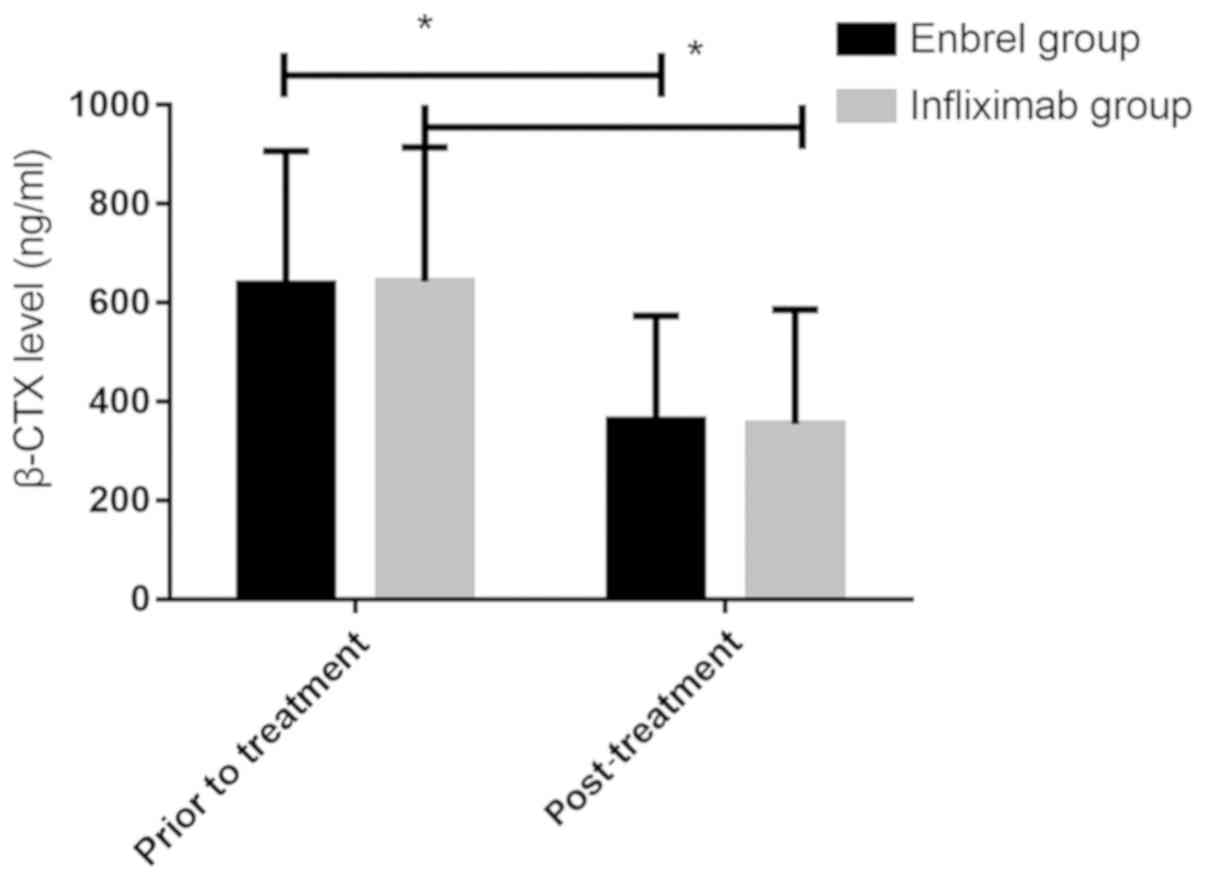
Comparison of bone metabolism levels
before and after treatment between the two groups
The bone metabolism levels between the two groups
before and after treatment were compared (Figs. 3 and 4). There was no significant difference in
BALP level between Enbrel group and Infliximab group before
treatment (18.72±8.62 and 18.98±8.51 µg/l) and after treatment
(17.59±7.71 and 17.84±7.64 µg/l) (P>0.05), and there was no
significant difference between the two groups before and after
treatment (P>0.05). After treatment, β-CTX levels in Enbrel
group and Infliximab group (362.58±211.45 and 354.74±231.52 ng/ml)
were significantly lower than those before treatment (638.52±268.74
and 642.75±271.66 ng/ml) (P<0.0001), but there was no
significant difference between the two groups before and after
treatment (P>0.05).
Curative effect of the two groups
before and after treatment
Statistical analysis of the clinical efficacy of the
two groups of patients after treatment (Table II) showed that the total effective
rate of Enbrel group (89.74%) and Infliximab group (90.24%) had no
significant difference (P>0.05).
 | Table II.Comparison of clinical efficacy
n[%]. |
Table II.
Comparison of clinical efficacy
n[%].
|
| Cure | Markedly effect | Effective | Ineffective | Total efficiency |
|---|
| Enbrel group
(n=39) | 11 (28.20) | 12 (30.77) | 12 (30.77) | 4
(10.26) | 35 (89.74) |
| Infliximab group
(n=41) | 12 (29.27) | 13 (31.70) | 12 (29.27) | 4 (9.76) | 37 (90.24) |
| χ2
value | – | – | – | – | 0.01 |
| P-value | – | – | – | – | 0.94 |
Comparison of adverse reactions
between the two groups of patients
The adverse reactions of the two groups of patients
after treatment were compared (Table
III). The adverse reactions of the two groups of patients were
relieved after symptomatic treatment. The adverse reaction rate of
Infliximab group (21.95%) was higher than that of Enbrel group
(5.13%) (P>0.05).
 | Table III.Comparison of postoperative adverse
reactions n[%]. |
Table III.
Comparison of postoperative adverse
reactions n[%].
|
| Skin allergy | Hot flashes | Infection | Respiratory tract
reaction | Gastrointestinal
tract reaction | Skin reaction at
injection site | Incidence of
adverse reactions |
|---|
| Enbrel group
(n=39) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (2.56) | 1 (2.56) | 2 (5.13) |
| Infliximab group
(n=41) | 2 (4.88) | 0 (0.00) | 2 (4.88) | 2 (4.88) | 3 (7.32) | 0 (0.00) | 9
(21.95) |
| χ2
value | – | – | – | – | – | – | 4.77 |
| P-value | – | – | – | – | – | – | 0.03 |
Comparison of various indexes between
the two groups before and after treatment
The various indexes between the two groups before
and after treatment were compared (Table IV). The morning stiffness time,
BASDAI, BASFI and Schober tests of the two groups were basically
the same before treatment, and there was no difference between the
two groups (P>0.05). After treatment, the morning stiffness
time, BASDAI and BASFI indexes of the two groups were significantly
lower than before treatment (P<0.0001). Schober test
significantly increased (P<0.0001). BASDAI in Infliximab group
was lower than that in etanercept group (P<0.05).
 | Table IV.Comparison of various indexes of two
groups of patients before and after treatment (mean ± SD). |
Table IV.
Comparison of various indexes of two
groups of patients before and after treatment (mean ± SD).
|
| Stiffness in the
morning (min) | Schober test | BASDAI | BASFI |
|---|
| Enbrel group
(n=39) |
| Before
treatment | 74.14±21.65 | 2.21±1.12 | 3.91±1.56 | 32.58±10.56 |
| After
treatment | 35.45±16.21 | 5.05±1.54 |
1.65±0.48a | 9.68±3.04 |
| t
value | 8.93 | 9.31 | 8.65 | 13.01 |
|
P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Infliximab group
(n=41) |
| Before
treatment | 72.56±20.48 | 2.35±1.34 | 3.87±1.34 | 33.98±11.47 |
| After
treatment | 34.28±15.67 | 5.62±1.47 |
1.04±0.51a | 8.77±3.12 |
| t
value | 9.51 | 10.53 | 12.64 | 13.58 |
|
P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Discussion
The monoclonal antibody infliximab formed by
chimeric mouse and human, is a combined soluble TNF-α and
transmembrane TNF-α receptor, thus blocking the pathological effect
and signal conduction by TNF-α (22,23).
Etanercept with receptor-immunoglobulin fusion technology is
composed of the extracellular ligand binding site of human tumor
necrosis factor receptor 2 (TNF-2/p75) and Fc fragment of human
IgG1. The fusion protein is expressed in vitro. Soluble
TNF-α in plasma and on the surface of cell membrane is highly
compatible with this fusion protein, which is neutralized by
etanercept, resulting in loss of biological activity of TNF-α and
achieving inhibition of abnormal immune response and inflammatory
process mediated by receptor (24),
thus effectively treating AS.
Bone metabolism markers are divided into bone
formation markers, bone turnover markers, bone absorption markers
and osteoporosis-related hormone markers. β-CTX is an index of bone
resorption, and some researches have shown that it is a valuable
and reliable index for evaluating bone resorption (25,26).
However, in this study, there was no significant difference in the
level of BALP before and after treatment between Enbrel group and
Infliximab group (P>0.05), and there was no significant
difference in the same group before and after treatment. Consistent
with the results of a previous study (27), it was presumed that bone metabolism
index BALP has little effect on AS. In this study, the morning
stiffness time, BASDAI and BASFI indexes of the two groups of
patients after treatment were significantly lower than before
treatment (P<0.0001), and Schober test was significantly higher
(P<0.0001). Consistent with the reduction of morning stiffness
time in the clinical efficacy of etanercept in the treatment of AS
by Liu et al (28).
According to the observation of the therapeutic
effect of infliximab on AS (29),
the indexes of BASDAI and BASFI were significantly lower than those
before treatment (P<0.0001), which indicates that etanercept and
infliximab can effectively cure AS patients. However, it is also
found that the BASDAI score of infliximab group is lower than that
of Enbrel group (P<0.05), and infliximab can reduce pain more
than etanercept. However, the total effective rate of Enbrel group
(89.74%) and Infliximab group (90.24%) had no significant
difference (P>0.05), which is consistent with the clinical
efficacy of McLeod et al (30) in the treatment of juvenile AS with
etanercept and infliximab. It was also concluded that CRP and ESR
levels in Enbrel group and Infliximab group decreased significantly
(P<0.05) before and at 12 and 24 weeks after treatment, and
showed a downward trend (P<0.05) at 24 weeks after treatment
compared with 12 weeks after treatment, indicating that infliximab
and etanercept can control the laboratory indexes well and have a
good therapeutic effect on AS patients. However, the adverse
reaction rate of Infliximab group (21.95%) was higher than that of
Enbrel group (5.13%), which is consistent with previous results
(30). Further research is needed
because there are scarce data, and in our study the results caused
by occult diseases cannot be excluded. In this study, the β-CTX
level of Enbrel group and Infliximab group decreased significantly
after treatment compared with that before treatment (P<0.0001).
Korczowska et al (31) also
found potential clinical value in detecting bone metabolism indexes
in AS patients and the differences of bone metabolism indexes
expressed by AS patients, especially β-CTX with obvious change in
characteristics in AS patients, CRP and ESR were combined for
detection. It can provide a certain basis for early diagnosis and
differentiation of AS. The study also concluded that there was no
significant difference in β-CTX levels between the two groups
before and after treatment (P>0.05), further demonstrating that
the clinical efficacy of infliximab and etanercept are basically
the same.
The present study evaluated the effect of etanercept
and infliximab on bone metabolism indexes of AS patients by
detecting bone metabolism index (BALP, β-CTX) levels, CRP and ESR
before and after treatment, recording adverse reactions of the two
groups of patients after treatment and evaluating the clinical
efficacy of the two groups of drugs.
Collectively, etanercept and infliximab improved the
therapeutic effect on AS patients. All indexes are decreased,
effectively reducing bone metabolism indexes, which is worthy of
clinical promotion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW wrote the manuscript. CW and WL conceived and
designed the study. CW was responsible for the collection and
analysis of the experimental data. WL interpreted the data and
drafted the manuscript. CW and WL revised the manuscript critically
for important intellectual content. Both authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Affiliated Hospital of Hebei University of Engineering (Handan,
China). Patients who participated in this research had complete
clinical data. Signed informed consents were obtained from the
patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biasi D, Carletto A, Caramaschi P, Pacor
ML, Maleknia T and Bambara LM: Efficacy of methotrexate in the
treatment of ankylosing spondylitis: a three-year open study. Clin
Rheumatol. 19:114–117. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quaden DH, De Winter LM and Somers V:
Detection of novel diagnostic antibodies in ankylosing spondylitis:
An overview. Autoimmun Rev. 15:820–832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stolwijk C, Essers I, van Tubergen A,
Boonen A, Bazelier MT, De Bruin ML and de Vries F: The epidemiology
of extra-articular manifestations in ankylosing spondylitis: A
population-based matched cohort study. Ann Rheum Dis. 74:1373–1378.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davey-Ranasinghe N and Deodhar A:
Osteoporosis and vertebral fracture in ankylosing spondylitis. Curr
Opin Rheumatol. 25:509–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van der Weijden MA, Claushuis TA, Nazari
T, Lems WF, Dijkmans BA and van der Horst-Bruinsma IE: High
prevalence of low bone mineral density in patients within 10 years
of onset of ankylosing spondylitis: A systematic review. Clin
Rheumatol. 31:1529–1535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh HJ, Nimarpreet K, Ashima, Das S,
Kumar A and Prakash S: Study of bone mineral density in patients
with ankylosing spondylitis. J Clin Diagn Res. 7:2832–2835.
2013.PubMed/NCBI
|
|
8
|
Smith JA: Update on ankylosing
spondylitis: Current concepts in pathogenesis. Curr Allergy Asthma
Rep. 15:4892015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Machado MA, Moura CS, Ferré F, Bernatsky
S, Rahme E and Acurcio Fde A: Treatment persistence in patients
with rheumatoid arthritis and ankylosing spondylitis. Rev Saude
Publica. 50:502016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davis JC: Understanding the role of tumor
necrosis factor inhibiton in ankylosing spondylitis. Semin
Arthritis Rheum. 34:268–278. 2005. View Article : Google Scholar
|
|
11
|
Prince DS, McUuigan LE and McGirr EE:
Working life and physical activity in ankylosing spondylit is pre
and post antitumor necrosis factor-alpha therapy. Int J Rheum Dis.
17:165–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maxwell LJ, Zochling J, Boonen A, Singh
JA, Veras MM, Tanjong Ghogomu E, Benkhalti Jandu M, Tugwell P and
Wells GA: TNF-alpha inhibitors for ankylosing spondylitis. Cochrane
Database Syst Rev. Apr 18–2015.(Epub ahead of print). doi:
10.1002/14651858.CD005468.pub2. View Article : Google Scholar
|
|
13
|
Poddubnyy DA, Song IH and Sieper J: The
safety of celecoxib in ankylosing spondylitis treatment. Expert
Opin Drug Saf. 7:401–409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rauner M, Thiele S, Fert I, Araujo LM,
Layh-Schmitt G, Colbert RA, Hofbauer C, Bernhardt R, Bürki A,
Schwiedrzik J, et al: Loss of bone strength in HLA-B27 transgenic
rats is characterized by a high bone turnover and is mainly
osteoclast-driven. Bone. 75:183–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rostom S, Dougados M and Gossec L: New
tools for diagnosing spondyloarthropathy. Joint Bone Spine.
77:108–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gerdhem P, Ivaska KK, Alatalo SL, Halleen
JM, Hellman J, Isaksson A, Pettersson K, Väänänen HK, Akesson K and
Obrant KJ: Biochemical markers of bone metabolism and prediction of
fracture in elderly women. J Bone Miner Res. 19:386–393. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garnero P and Delmas PD: Contribution of
bone mineral density and bone turnover markers to the estimation of
risk of osteoporotic fracture in postmenopausal women. J
Musculoskelet Neuronal Interact. 4:50–63. 2004.PubMed/NCBI
|
|
18
|
Garnero P, Ferreras M, Karsdal MA,
Nicamhlaoibh R, Risteli J, Borel O, Qvist P, Delmas PD, Foged NT
and Delaissé JM: The type I collagen fragments ICTP and CTX reveal
distinct enzymatlc pathways of bone collagen degradation. J Bone
Miner Res. 18:859–867. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–268. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song J, Zhou L, Chen L and Wu X:
Comparison of ASDAS, RAPID3 and BASDAI in assessing disease
activity of patients with ankylosing spondylitis. Acad J Second Mil
Med Univ. 36:909–913. 2015. View Article : Google Scholar
|
|
21
|
Garrett S, Jenkinson T, Kennedy LG,
Whitelock H, Gaisford P and Calin A: A new approach to defining
disease status in AS: The Bath Ankylosing Spondylitis Disease
Activity Index (BASDAI). J Rheumatol. 21:2286–2291. 1994.PubMed/NCBI
|
|
22
|
Neubauer S, Cifaldi M, Mittendorf T,
Ganguli A, Wolff M and Zeidler J: Biologic TNF inhibiting agents
for treatment of rheumatoid arthritis persistence and dosing
patterns in Germany. Health Econ Rev. 4:322014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fleischmann RM, Cohen SB, Moreland LW,
Schiff M, Mease PJ, Smith DB, Keenan G and Kremer JM; iRAMT Study
Group, : Methotrexate dosage reduction in patients with rheumatoid
arthritis beginning therapy with infliximab: the Infliximab
Rheumatoid Arthritis Methotrexate Tapering (iRAMT) trial. Curr Med
Res Opin. 21:1181–1190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Zhang YM and Zhang JL: Efficacy
of etanercept in patients with ankylosing spondylitis: A
double-blind, randomized, placebo controlled trial. Zhongguo Xin
Yao Za Zhi. 18:1846–1849, 1881. 2009.(In Chinese).
|
|
25
|
Chu B, Lu MQ, Wu MQ, Shi L, Fu LN, Gao S,
Fang LJ, Xiang QQ and Bao L: Clinical characteristics of bone
disease in multiple myeloma and clinical significance of monitoring
bone metabolic markers. Zhonghua Yi Xue Za Zhi. 96:1424–1429.
2016.(In Chinese). PubMed/NCBI
|
|
26
|
Fang Z, Li Y, Jiang Y and Yang F:
Significance of dynamic monitoring of bone metabolites in patients
with ankylosing spondylitis. Zhong Guo Yi Yuan Xie Hui. 4:447–449.
2014.(In Chinese).
|
|
27
|
Speden DJ, Calin AI, Ring FJ and Bhalla
AK: Bone mineral density, calcaneal ultrasound, and bone turnover
markers in women with ankylosing spondylitis. J Rheumatol.
29:516–521. 2002.PubMed/NCBI
|
|
28
|
Liu YF, Dong H, Tu SH, Zheng CH, Liu PL
and Hu YH: Etanercept in the treatment of ankylosing spondylitis: A
systematic review and meta-analysis. Exp Ther Med. 8:1585–1592.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marakli SS, Uzun S, Ozbek S and Tuncer I:
Dermatitis herpetiformis in a patient receiving infliximab for
ankylosing spondylitis. Eur J Dermatol. 18:88–89. 2008.PubMed/NCBI
|
|
30
|
McLeod C, Bagust A, Boland A, Dagenais P,
Dickson R, Dundar Y, Hill RA, Jones A, Mujica Mota R and Walley T:
Adalimumab, etanercept and infliximab for the treatment of
ankylosing spondylitis: a systematic review and economic
evaluation. Health Technol Assess. 11:1–158. 2007. View Article : Google Scholar
|
|
31
|
Korczowska I, Przepiera-Bedzak H, Brzosko
M, Lacki JK, Trefler J and Hrycaj P: Bone tissue metabolism in men
with ankylosing spondylitis. Adv Med Sci. 56:264–269. 2011.
View Article : Google Scholar : PubMed/NCBI
|