Introduction
Traumatic brain injury is a trauma with high
disability and mortality. About 22% of the patients with severe
brain injury are severely disabled, 5% are in vegetative state and
40% die (1,2). An acutely severe brain injury is caused
by violent impact on the head, resulting in brain contusion, brain
edema, disturbance of consciousness and other symptoms. Most
patients are critically ill, and their conditions change rapidly;
the morbidity and mortality are quite high (3). There are currently no drugs available
for effective treatment. With the increase of accidents such as car
accidents and falling objects, the number of patients with acutely
severe brain injury is on the rise (4).
Heat shock protein 70 (Hsp70) is a stress protein
that maintains its own stability (5). In recent years, a large number of
studies have confirmed that Hsp70 is rapidly induced in the brain
damage of mammals, and has obvious protective effects on the body
and brain (6,7). In previous findings,
immunohistochemical staining was used to directly detect the
expression of Hsp70 in brain tissue, and the subjects were limited
to experimental animal models (8,9). Annexin
A1 (ANXA1), a calcium-dependent phospholipid-binding protein, is a
member of the annexin family (10).
ANXA1 has been shown to be involved in the regulation of important
endogenous factors in the blood-brain barrier of patients with
multiple sclerosis (11). ANXA1 on
either side of the cell membrane and ANXA1 out of cells interact
with actin to form FPR2 receptor as well as cytoskeleton
interactions to shape stable tight junctions between the cells,
which is of great significance for maintaining the integrity of the
blood-brain barrier (12). However,
the relationship between ANXA1 and acutely severe traumatic brain
injury remains to be elucidated.
The purpose of this study was to provide a feasible
method for early assessment of patients with acutely severe
traumatic brain injury by observing the changes of expression
levels of serum Hsp70 and ANXA1, and studying the diagnostic values
of Hsp70 and ANXA1 in the diagnosis of acute severe traumatic brain
injury.
Patients and methods
Patients
Eighty-four patients with severe traumatic brain
injury were admitted to Binzhou Center Hospital (Binzhou) from June
2014 to July 2017. These patients with severe traumatic brain
injury were the experimental group, including 49 males and 35
females, aged from 16 to 70 years, and with the mean age of
41.24±6.56 years. A further 75 healthy subjects in the same period
were the control group, including 47 males and 28 females; aged
from 18 to 70 years, and with the mean age of 43.68±7.53 years. The
mean acute physiology and chronic health evaluation II (APACHE II)
score (13) was 28.27±5.21 points.
The mean Glasgow Coma Scale (GCS) score for the severe traumatic
brain injury was 6.12±1.17 points. The inclusion criteria were: The
time from trauma to admission of patients in the experimental group
was <6 h; severe traumatic brain injury (GCS score ≤8) (14), and brain contusion, subarachnoid
hemorrhage, cerebral hemorrhage, epidural hematoma, subdural
hematoma, diffuse brain swelling, skull base fracture and cranial
bone fracture were confirmed by head CT, and the patients were
admitted to hospital within 6 h after injury. The healthy control
group was examined in the physical examination center of the
hospital and the results were normal; patients had no other type of
tumor, heart, liver, kidney or other important organ diseases and
no family members with a history of cancer.
The study was reviewed and approved by the ethics
committee of Binzhou Center Hospital, and all the patients or their
guardians signed an informed consent form. The exclusion criteria
were: Patients with combined injury; with hypoxemia after trauma;
with previous severe organic disease such as hypotension as well as
liver and kidney dysfunction; with neurodegenerative diseases,
depression, other nervous system diseases, autoimmune diseases and
severe metabolic disorders. Patients in the experimental group were
given routine treatment after admission, including maintaining
airway patency, dehydration, hemostasis, anti-infection, acid
production, nutritional cranial nerves, support and symptomatic
treatment. After admission, blood was taken on the 1st day and
centrifuged, and the supernatant was taken for use. When the
patient was discharged from hospital, they were divided according
to the Glasgow Outcome Scale (GOS). Good recovery: returning to
normal life with mild defects; mild disability: disabled but living
independently, able to work under protection; severe disability:
awake, disability, need care in daily life; vegetative state: only
minimal response (e.g., the eyes could open with the sleep/wake
cycle); or dead.
Main instruments and reagents
Hsp70 enzyme-linked immunoassay kit (Biorbyt, UK,
orb55612); ANXA1 enzyme-linked immunoassay kit (Biorbyt, UK,
orb437946); enzyme-linked immunoassay meter (Molecular Devices, US,
Spectra MaxiD5).
Methods of detection
The expression levels of serum Hsp70 and ANXA1 were
detected by enzyme-linked immunosorbent assay (ELISA): The required
slats were taken out from the aluminum foil bag which was
equilibrated for 20 min at room temperature, and the remaining
slats were sealed with a ziplock bag and returned to the
refrigerator at 4°C. Standard and sample wells were set, and 50
μl standard with different expression was added to the
standard wells; 50 μl of the sample to be tested was added
into the sample well; the blank wells were not added; 100 μl
of detection antibody labeled by horseradish peroxidase was added
to each standard well and sample well, and reaction wells were
sealed with a sealing membrane, and then incubated at 37°C in an
incubator for 60 min; the liquid was discarded, and then the wells
were patted dry with absorbent paper, filled with the washing
solution (350 μl), and left to stand for 1 min. The washing
solution was removed, patted dry with absorbent paper, and the
plate washing was repeated 5 times; 50 μl of substrate A and
50 μl of substrate B were added to each well, incubated at
37°C for 15 min in the dark; 50 μl of the stopping solution
was added to each well, and the OD value of each well was measured
at a wavelength of 450 nm of the enzyme-linked immunosorbent meter
within 15 min.
Statistical analysis
Data were statistically analyzed by SPSS 22.0
statistical analysis software (IBM Corp, Armonk, NY, USA). The
enumeration data were expressed by number of cases/percentage [n
(%)], and the comparison of enumeration data between groups was
tested by the Chi-square test. The measurement data were expressed
as mean ± standard deviation (mean ± SD), and the comparison of
measurement data between groups was performed by an independent
sample t-test. One-way analysis of variance (ANOVA) was used for
the comparison between multiple groups of means, and the Tukey test
was used for subsequent pairwise comparisons. Comparison between
multiple time points was performed by repeated measures analysis of
variance, and the subsequent pairwise comparison was performed by
the Bonferroni test. The receiver operating characteristic (ROC)
curve was used to evaluate the efficacy of Hsp70 and ANXA1 in
diagnosing death from acutely severe traumatic brain injury.
P<0.05 was considered as statistically significant.
Results
Two sets of patients
There were no significant differences in the general
data regarding sex, age, history of smoking and drinking between
the control and experimental groups (P>0.05) (Table I).
 | Table I.Patient data [n (%), mean ± SD]. |
Table I.
Patient data [n (%), mean ± SD].
| Classification | Control group
(n=75) | Experimental group
(n=84) | χ2/t | P-value |
|---|
| Sex |
|
| 0.311 | 0.577 |
| Male | 47 (62.67) | 49 (58.33) |
|
|
|
Female | 28 (37.33) | 35 (41.67) |
|
|
| Age (years) | 43.68±7.53 | 41.24±6.56 | 0.499 | 0.667 |
| History of
smoking |
|
| 0.161 | 0.688 |
| Yes | 36 (48.00) | 43 (51.19) |
|
|
| No | 39 (52.00) | 41 (48.81) |
|
|
| History of
drinking |
|
| 0.288 | 0.592 |
| Yes | 46 (61.33) | 48 (57.14) |
|
|
| No | 29 (38.67) | 36 (42.86) |
|
|
| BMI
(kg/m2) | 21.15±2.31 | 21.54±2.73 | 0.499 | 0.667 |
| Place of
residence |
|
| 0.392 | 0.531 |
|
Country | 43 (57.33) | 44 (52.38) |
|
|
| City | 32 (42.67) | 40 (47.62) |
|
|
| Marital status |
|
| 0.039 | 0.843 |
|
Single | 36 (48.00) | 39 (46.43) |
|
|
|
Married | 39 (52.00) | 45 (53.57) |
|
|
| Cultural degree |
|
| 1.661 | 0.198 |
| A high
school education or less | 37 (49.33) | 50 (59.52) |
|
|
| Above
senior high school education | 38 (50.67) | 34 (40.48) |
|
|
| Working
condition |
|
| 0.266 | 0.606 |
| No | 30 (40.00) | 37 (44.05) |
|
|
|
Yes | 45 (60.00) | 47 (55.95) |
|
|
| AST (U/l) | 19.23±7.08 | 18.19±7.13 | 0.921 | 0.358 |
| ALT (U/l) | 22.25±9.37 | 21.76±10.21 | 0.314 | 0.754 |
Expression levels of serum Hsp70 and
ANXA1 in the experimental group at different time points of
admission compared to the control group
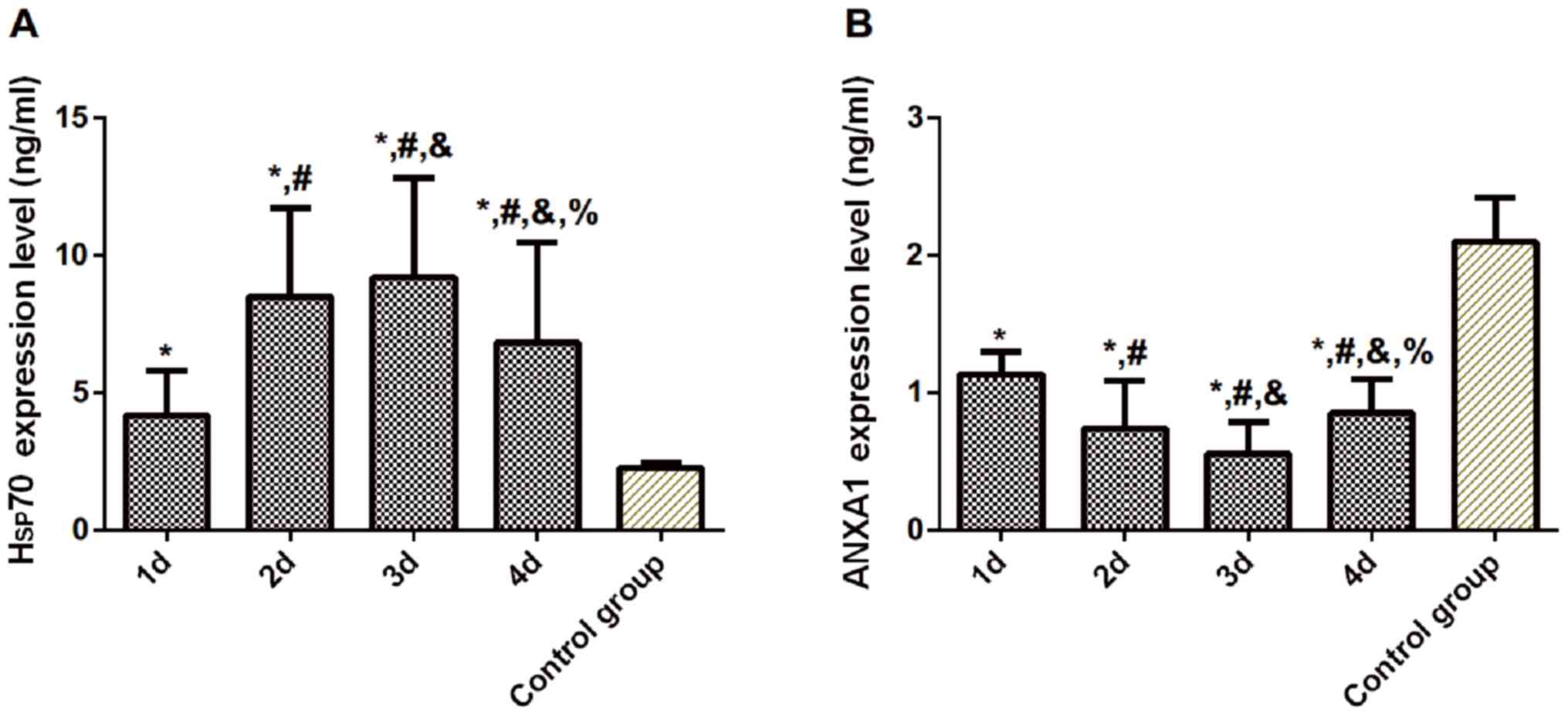
Compared with the control group, the expression of
Hsp70 in the experimental group was significantly increased on the
1st, 2nd, 3rd and 4th day after admission (P<0.05), while
expression of ANXA1 was significantly decreased (P<0.05). The
expression level of serum Hsp70 in the experimental group peaked on
the 3rd day after admission, and differences were statistically
significant compared with that on the 1st, 2nd and 4th day
(P<0.05). The expression of ANXA1 was the lowest on the 3rd day,
and differences were statistically significant compared with that
on the 1st, 2nd, and 4th day (P<0.05) (Table II and Fig. 1).
 | Table II.Comparison of levels of serum Hsp70
and ANXA1 between the two groups (mean ± SD). |
Table II.
Comparison of levels of serum Hsp70
and ANXA1 between the two groups (mean ± SD).
| Groups | n | Time after
admission (days) | Hsp70 (ng/ml) | ANXA1 (ng/ml) |
|---|
| Control | 75 | – | 2.261±0.188 | 2.095±0.321 |
| Experimental | 84 | 1 |
4.177±1.623a |
1.137±0.158a |
|
|
| 2 |
8.497±3.206a,b |
0.736±0.351a,b |
|
|
| 3 |
9.194±3.614a–c |
0.553±0.236a–c |
|
|
| 4 |
6.822±3.642a–d |
0.852±0.243a–d |
| F-value | – | – | 42.64 | 76.62 |
| P-value | – | – | <0.001 |
<0.001 |
Expression levels of serum Hsp70 and
ANXA1 in groups relating to survival
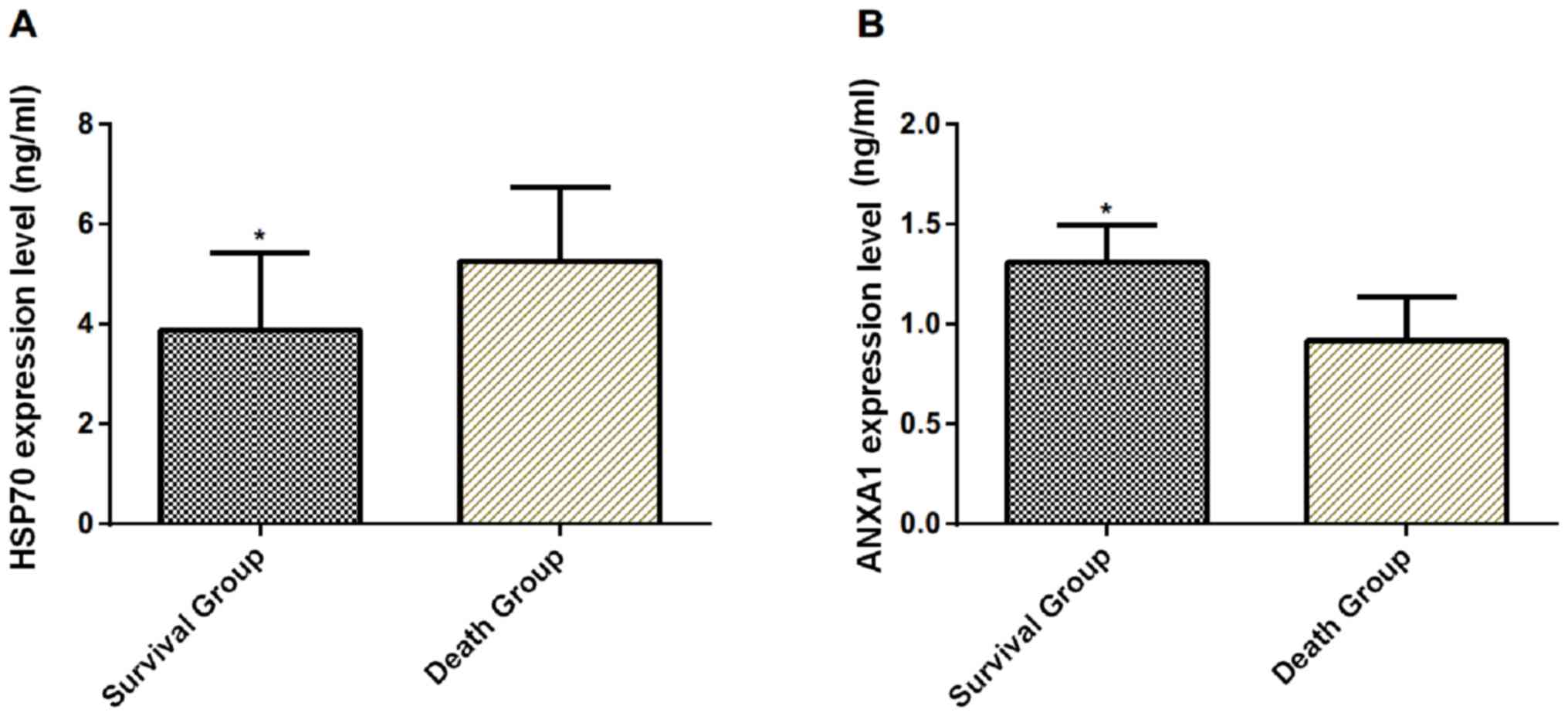
A total of 47 patients survived (survival group) and
37 patients died (death group) in the experimental group during the
treatment. The serum Hsp70 in the survival group was significantly
lower than that in the death group on the 1st day after admission
(P<0.001), while the expression level of ANXA1 was significantly
higher than that in the death group (P<0.001) (Table III and Fig. 2).
 | Table III.Comparison of results of expression
levels of serum Hsp70 and ANXA1 between the survival and the death
group on the 1st day after admission (mean ± SD). |
Table III.
Comparison of results of expression
levels of serum Hsp70 and ANXA1 between the survival and the death
group on the 1st day after admission (mean ± SD).
| Groups | n | Hsp70 (ng/ml) | ANXA1 (ng/ml) |
|---|
| Survival | 47 | 3.884±1.545 | 1.309±0.187 |
| Death | 37 | 5.264±1.476 | 0.919±0.217 |
| t-value |
|
4.280 |
4.247 |
| P-value |
| <0.001 | <0.001 |
Diagnostic value of Hsp70 and ANXA1 in
the diagnosis of acutely severe traumatic brain injury
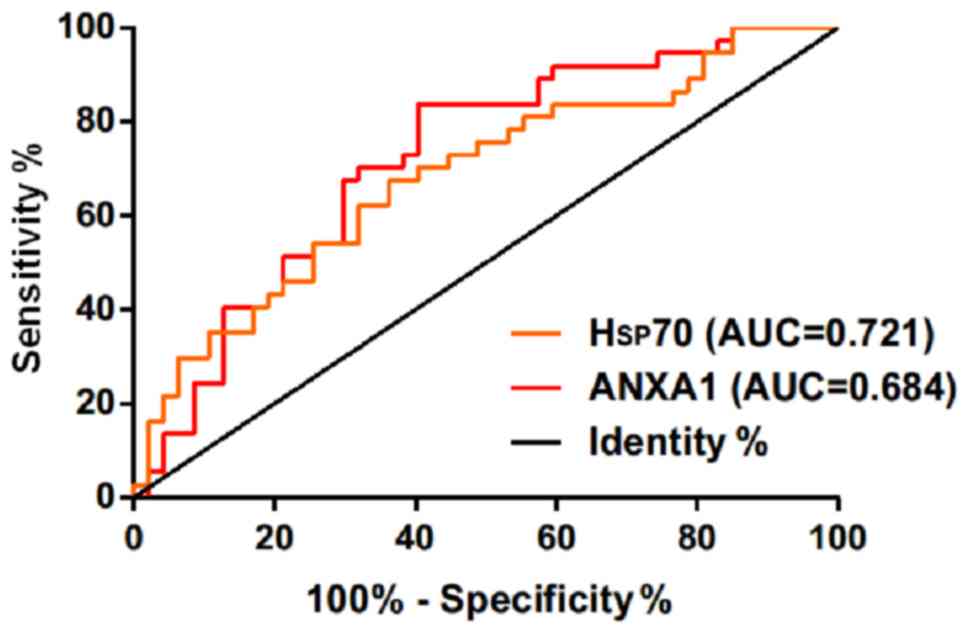
The ROC curves of expression of serum Hsp70 and
ANXA1 in the diagnosis of acutely severe traumatic brain injury
were drawn. The area under the curve (AUC) of serum Hsp70 in the
diagnosis of death of patients with acutely severe traumatic brain
injury was 0.721 (95% CI: 0.611–0.829), and the cut-off value was
4.235 ng/ml; the diagnostic sensitivity was 83.78%, and the
specificity was 57.45%. The AUC of serum ANXA1 in the diagnosis of
death of patients with acutely severe traumatic brain injury was
0.684 (95% CI: 0.569–0.799), and the cut-off value was 1.163 ng/ml;
the diagnostic sensitivity was 64.86%, and the specificity was
63.83% (Table IV and Fig. 3).
 | Table IV.Diagnostic value of serum Hsp70 and
ANXA1 for death in acutely severe traumatic brain injury. |
Table IV.
Diagnostic value of serum Hsp70 and
ANXA1 for death in acutely severe traumatic brain injury.
| Diagnosis
index | AUC | 95% CI | Standard error | Cut-off value | Sensitivity
(%) | Specificity
(%) |
|---|
| Hsp70 | 0.721 | 0.611–0.829 | 0.056 | 4.235 (ng/ml) | 83.78 | 57.45 |
| ANXA1 | 0.684 | 0.569–0.799 | 0.059 | 1.163 (ng/ml) | 64.86 | 63.83 |
Relationship between relative
expression levels of Hsp70 and ANXA1 in APACHE II score
Serum Hsp70 and ANXA1 expression was significantly
different in patients with different APACHE II scores (P<0.05).
Compared with the APACHE II score <10 points, the serum Hsp70
expression level of patients with APACHE II scores of 11–20, 20–30,
and >30 was significantly increased (P<0.05), and the serum
ANXA1 expression level was significantly decreased (P<0.05).
Compared with the group of APACHE II score of 11–20 points, the
serum ANXA1 level of APACHE II score 21–30 group was significantly
lower than that of APACHE II score 11–20 group (P<0.05), while
Hsp70 was not significantly different between the two groups
(P>0.05). The expression level of serum Hsp70 in APACHE II score
>30 group was significantly higher than other groups
(P<0.05), and the serum ANXA1 expression level was significantly
lower than the other groups (P<0.05) (Table V).
 | Table V.Relationship between the relative
expression levels of serum Hsp70 and ANXA1 and the APACHE II score
in patients with acute severe traumatic brain injury (mean ±
SD). |
Table V.
Relationship between the relative
expression levels of serum Hsp70 and ANXA1 and the APACHE II score
in patients with acute severe traumatic brain injury (mean ±
SD).
| APACHE II score
(points) | n | Hsp70 (ng/ml) | ANXA1 (ng/ml) |
|---|
| <10 | 4 | 2.49±1.17 | 1.865±0.233 |
| 11–20 | 28 |
5.67±2.32a |
1.326±0.419a |
| 21–30 | 31 |
6.68±2.98a |
1.027±0.259a,b |
| >30 | 21 |
8.55±3.63a–c |
0.556±0.184a–c |
| F-value |
| 6.744 | 34.880 |
| P-value |
| <0.001 | <0.001 |
Relationship between the relative
expression levels of Hsp70 and ANXA1 and the GOS score
Hsp70 and ANXA1 expression of serum was
significantly different in patients with different GOS assessment
results (P<0.05). Compared to patients with good recovery, serum
Hsp70 expression levels were significantly increased in patients
with mild disability, in vegetative state, and the dead
(P<0.05); serum ANXA1 expression levels were significantly
decreased (P<0.05). Compared to patients with mild disability,
the serum ANXA1 levels in the patients in vegetative state and the
dead were significantly lower (P<0.05), while the serum Hsp70
and ANXA1 expression levels were not significantly different
between the patients in vegetative state and the dead (P>0.05)
(Table VI).
 | Table VI.GOS scores. |
Table VI.
GOS scores.
| GOS assessment
results | n | Hsp70 (ng/ml) | ANXA1 (ng/ml) |
|---|
| Good recovery | 7 | 1.942±0.852 | 1.873±0.199 |
| Mild
disability | 23 |
3.398±1.459a |
1.405±0.324a |
| Vegetative
state | 17 |
4.571±2.372a |
1.029±0.305a,b |
| Death | 37 |
5.264±1.476a,b |
0.919±0.217a,b |
| F-value |
| 11.36 | 34.33 |
| P-value |
| <0.001 | <0.001 |
Discussion
Patients with acutely severe traumatic brain injury
have obvious oxidative stress and inflammatory response. Excessive
activation of inflammatory response leads to increased cerebral
vascular permeability, increased leukocyte release, and complement
activation, resulting in a cascade of inflammatory factors and
aggravating secondary damage of brain tissue (15). The support and functional testing of
all organ systems is a way to improve the success rate of severe
traumatic brain injury and reduce the death of clinical patients
(16,17).
Previous studies have shown that Hsp70 expression
was increased in the process of injury tissue cells and has
protective effects on cells (18–20).
Zhang et al (21) found that
Hsp70 signaling pathway can regulate TLR4-Trif-Stat3 signaling,
thereby inhibiting NOX3 induction and reducing oxidative damage in
the lung. Xia et al (22)
found that Hsp70 can prevent brain ischemia-reperfusion injury and
protect the nerve. Ren et al (23) found that expression level of serum
Hsp70 can reflect the extent of cell damage by detecting the
expression of Hsp70 in three time periods after trauma, and that
expression level of serum Hsp70 was significantly higher in
patients with mild, moderate, and severe injury than in healthy
patients, and expression levels of serum Hsp70 in the severely
injured group were significantly higher than those in the lightly
injured group in 1 to 6 h after trauma. However, we observed that
expression level of serum Hsp70 in experimental group reached the
peak 3 days after admission, which was statistically significant
compared with that on the 1st, 2nd, and 4th day, and greatly higher
than those of the control group. We speculate that the change of
Hsp70 level reflects the degree of craniocerebral injury to some
extent. The damaged brain tissue has strong stress and anti-injury
ability within 3 days, so it can secrete inflammatory factors and
promote the body to produce a large amount of Hsp70 for anti-injury
and repair. The increased expression may be beneficial to the
repair of nerve cell damage. da Rocha et al (24) measured Hsp70 levels in 20 male
patients with traumatic brain injury at the time of admission, 24 h
and 7 days after admission. Compared with the control group, serum
Hsp70 concentration was significantly increased in patients with
severe traumatic brain injury. The serum Hsp70 concentration
reached the peak at the time of admission, but they did not measure
the concentration on the 3rd and 5th day of admission and the
concentrations of female patients. Therefore, there is difference
in the peak time of Hsp in their study and ours, which may be
caused by the difference in measurement time and subjects. This is
similar to studies of Ren et al (23). ANXA1 regulates the function of the
blood-brain barrier, which plays an important role in regulating
the integrity of the blood-brain barrier by promoting the
restoration of polarity of cerebrovascular skin cells and
cytoskeletal integrity. Capraz et al (25) found that ANXA1 disappeared in the
cerebrovascular and ependymal hypoxia injury within 24 h and
induced up-regulation after injury of microglial cells in 72 h,
showing that extracellular vesicles with ANXA1 can alleviate the
damage of blood-brain barrier caused by ischemia and hypoxia. Sen
et al (26) showed that ANXA1
may regulate blood-brain barrier function by promoting the recovery
of cerebrovascular skin cells. Luo et al (27) found that ANXA1 can also exert
neuroprotective effects on brain damage by polarizing microglia
cells into beneficial phenotypes. The results of the present study
showed that the expression of serum ANXA1 in the experimental group
was the lowest on the 3rd day, which was statistically significant
compared with that on the 1st, 2nd and 4th day, and significantly
higher than that of the control group. Wang et al (28) found that the expression of ANXA1
decreased after cerebral hemorrhage, and the increase of the
expression of ANXA1 can improve conditions of necrosis in
cerebrovascular skin cells and neurons, and reduce brain edema
after cerebral hemorrhage, which was similar to our study. Our
study found that patients with different APACHE II scores and GOS
assessment results had different levels of Hsp70 and ANXA1
expression. Hsp70 and ANXA1 may be involved in the development of
patients with acutely severe traumatic brain injury, which may be
related to poor prognosis. Therefore, we further evaluated the ROC
curve of poor prognosis in patients with acutely severe traumatic
brain injury by serum Hsp70 and ANXA1 on the first day after
admission, and the results showed that serum Hsp70 and ANXA1 have
certain value in the diagnosis of death in patients with acutely
severe traumatic brain injury. Detecting the expression levels of
Hsp70 and ANXA1 in the serum of patients with acutely severe
traumatic brain injury has certain predictive value for the
diagnosis of death of patients.
In summary, Hsp70 and ANXA1 may be involved in the
occurrence and progression of acutely severe traumatic brain
injury. The detection of serum Hsp70 and ANXA1 has certain
diagnostic value for the death of patients with acutely severe
traumatic brain injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and TW analyzed and interpreted the patient
general data. QL performed ELISA. NZ was responsible for the
analysis of the observation indicators. JZ wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Binzhou Center Hospital (Binzhou). Patients who participated in
this research, signed an informed consent and had complete clinical
data. Signed informed consents were obtained from the patients or
the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sun Z, Zuo H, Yuan D, Sun Y, Zhang K, Cui
Z and Wang J: Predictors of prognosis in patients with temporal
lobe epilepsy after anterior temporal lobectomy. Exp Ther Med.
10:1896–1902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Memarian N, Kim S, Dewar S, Engel J Jr and
Staba RJ: Multimodal data and machine learning for surgery outcome
prediction in complicated cases of mesial temporal lobe epilepsy.
Comput Biol Med. 64:67–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen F, Xu C and Zhang C: Effect of
indwelling nasointestinal tube for enteral nutrition support in
patients with severe craniocerebral trauma undergoing mechanical
ventilation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 30:57–60.
2018.(In Chinese). PubMed/NCBI
|
|
4
|
Xu G, Hu B, Chen G, Yu X, Luo J, Lv J and
Gu J: Analysis of blood trace elements and biochemical indexes
levels in severe craniocerebral trauma adults with Glasgow Coma
Scale and injury severity score. Biol Trace Elem Res. 164:192–197.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benjamin IJ and McMillan DR: Stress (heat
shock) proteins: Molecular chaperones in cardiovascular biology and
disease. Circ Res. 83:117–132. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang T, Yu DR, Huang J, Liu Q, Wang DX,
Luo N, Jia H, Fan HF and Liu QB: Multimodal rehabilitation program
promotes motor function recovery of rats after ischemic stroke by
upregulating expressions of GAP-43, SYN, HSP70, and C-MYC. J Stroke
Cerebrovasc Dis. 27:2829–2839. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yurinskaya MM, Funikov SY, Evgen'ev MB and
Vinokurov MG: Exogenous heat shock protein HSP70 reduces response
of human neuroblastoma cells to lipopolysaccharide. Dokl Biochem
Biophys. 469:239–243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng CY, Kao ST and Lee YC: Ferulic acid
exerts anti-apoptotic effects against ischemic injury by activating
HSP70/Bcl-2- and HSP70/autophagy-mediated signaling after permanent
focal cerebral ischemia in rats. Am J Chin Med. 47:39–61. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Y, Niu LJ, Qi FM and Guo L: Effect of
3-n-butylphthalide pretreatment on expression of the HSP70 after
brain ischemia/reperfusion. Zhongguo Ying Yong Sheng Li Xue Za Zhi.
31:136–140. 2015.(In Chinese). PubMed/NCBI
|
|
10
|
Grewal T, Wason SJ, Enrich C and Rentero
C: Annexins - insights from knockout mice. Biol Chem.
397:1031–1053. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cristante E, McArthur S, Mauro C, Maggioli
E, Romero IA, Wylezinska-Arridge M, Couraud PO, Lopez-Tremoleda J,
Christian HC, Weksler BB, et al: Identification of an essential
endogenous regulator of blood-brain barrier integrity, and its
pathological and therapeutic implications. Proc Natl Acad Sci USA.
110:832–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiong J and Feng Y: Effect of
dl-3-N-butylphthalide on the expression of hsp70 mRNA and c-fos in
transient cerebral ischemic and reperfused rat brain. Yao Xue Xue
Bao. 33:401–406. 1998.(In Chinese). PubMed/NCBI
|
|
13
|
Brennan PM, Murray GD and Teasdale GM:
Simplifying the use of prognostic information in traumatic brain
injury. Part 1: The GCS-Pupils score: An extended index of clinical
severity. J Neurosurg. 128:1612–1620. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Godinjak A, Iglica A, Rama A, Tančica I,
Jusufović S, Ajanović A and Kukuljac A: Predictive value of SAPS II
and APACHE II scoring systems for patient outcome in a medical
intensive care unit. Acta Med Acad. 45:97–103. 2016.PubMed/NCBI
|
|
15
|
Ziebell JM and Morganti-Kossmann MC:
Involvement of pro- and anti-inflammatory cytokines and chemokines
in the pathophysiology of traumatic brain injury.
Neurotherapeutics. 7:22–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim J, Kim SH, Lim SC, Kim W and Shon YM:
Clinical characteristics of patients with benign nonlesional
temporal lobe epilepsy. Neuropsychiatr Dis Treat. 12:1887–1891.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deane CAS and Brown IR: Knockdown of heat
shock proteins HSPA6 (Hsp70B′) and HSPA1A (Hsp70-1) sensitizes
differentiated human neuronal cells to cellular stress. Neurochem
Res. 43:340–350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan J, Liang X, Zhang Y, Li Y, Cao X and
Gao J: Cloning of three heat shock protein genes (HSP70, HSP90α and
HSP90β) and their expressions in response to thermal stress in
loach (Misgurnus anguillicaudatus) fed with different levels
of vitamin C. Fish Shellfish Immunol. 66:103–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bidmon-Fliegenschnee B, Lederhuber HC,
Csaicsich D, Pichler J, Herzog R, Memaran-Dadgar N, Huber WD,
Aufricht C and Kratochwill K: Overexpression of Hsp70 confers
cytoprotection during gliadin exposure in Caco-2 cells. Pediatr
Res. 78:358–364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miova B, Dinevska-Kjovkarovska S,
Esplugues JV and Apostolova N: Heat stress induces extended plateau
of Hsp70 accumulation - a possible cytoprotection mechanism in
hepatic cells. J Cell Biochem. 116:2365–2374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Shan P, Srivastava A, Jiang G,
Zhang X and Lee PJ: An endothelial Hsp70-TLR4 axis limits Nox3
expression and protects against oxidant injury in lungs. Antioxid
Redox Signal. 24:991–1012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia M, Ding Q, Zhang Z and Feng Q: Remote
limb ischemic preconditioning protects rats against cerebral
ischemia via HIF-1α/AMPK/HSP70 pathway. Cell Mol Neurobiol.
37:1105–1114. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren B, Zou G, Huang Y, Xu G, Xu F, He J,
Zhu H and Yu P: Serum levels of HSP70 and other DAMP proteins can
aid in patient diagnosis after traumatic injury. Cell Stress
Chaperones. 21:677–686. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
da Rocha AB, Zanoni C, de Freitas GR,
André C, Himelfarb S, Schneider RF, Grivicich I, Borges L,
Schwartsmann G, Kaufmann M and Regner A: Serum Hsp70 as an early
predictor of fatal outcome after severe traumatic brain injury in
males. J Neurotrauma. 22:966–977. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Capraz IY, Kurt G, Akdemir Ö, Hirfanoglu
T, Oner Y, Sengezer T, Kapucu LO, Serdaroglu A and Bilir E: Annexin
A1 as neuroprotective determinant for blood-brain barrier integrity
in neonatal hypoxic-ischemic encephalopathy. Seizure. 29:63–68.
2015.PubMed/NCBI
|
|
26
|
Sen A, Capelli V and Husain M: Cognition
and dementia in older patients with epilepsy. Brain. 141:1592–1608.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo ZZ, Gao Y, Sun N, Zhao Y, Wang J, Tian
B and Shi J: Enhancing the interaction between annexin-1 and formyl
peptide receptors regulates microglial activation to protect
neurons from ischemia-like injury. J Neuroimmunol. 276:24–36. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Chen Z, Yang J, Yang Z, Yin J, Zuo
G, Duan X, Shen H, Li H and Chen G: Identification of two
phosphorylation sites essential for annexin A1 in blood-brain
barrier protection after experimental intracerebral hemorrhage in
rats. J Cereb Blood Flow Metab. 37:2509–2525. 2017. View Article : Google Scholar : PubMed/NCBI
|