Introduction
Systemic sclerosis (SSc) is a rare autoimmune
disease associated with a high risk of morbidity and mortality. The
intricate pathogenic mechanisms involved in SSc comprise a
substantial immune disturbance, microvascular and neural changes,
together with an excessive deposition of collagen in the tissues
subsequently leading to organ fibrosis (1,2).
The burden of symptoms in scleroderma has been shown
to derive from various manifestations including skin involvement,
cardiopulmonary and peripheral vascular changes, renal,
gastrointestinal, and musculoskeletal impairment (3,4).
Consequently, patients with SSc exhibit a considerably poorer
quality of life compared to individuals suffering from other
chronic skin conditions such as ichtyosis, atopic dermatitis,
non-melanoma skin cancer, and vitiligo (5,6).
Furthermore, studies proved that considerable loss in work ability
occurs as early as 3 years from the onset of SSc (7). Interestingly, the patient-centered
approach to the burden of symptoms often provides different
insights compared to the physicians' view on the severity of the
disease (8,9).
Vascular injury is virtually ubiquitous in
scleroderma, making the assessment of microvascular damage
imperative in these patients. Moreover, Raynaud's phenomenon marks
the onset of the disease in the majority of cases and contributes
to the appearance of functional hindrance and a decreased quality
of life (10). While the initial
trigger of vascular damage in SSc remains a matter of debate,
recent evidence indicates that autoantibody profiles, abnormal
cytotoxic T cell activity and reperfusion injury all participate in
the development of SSc-related vasculopathy (11–13). It
has also been stated that oxidative stress may play a role in the
pathomechanisms of scleroderma, similar to other pathologies
(1,14,15).
Endothelin-1 (ET-1) is produced by a wide variety of
cells such as macrophages, fibroblasts, neutrophils, cardiac muscle
and vascular endothelial cells (16,17).
Given its potent vasoconstrictor activity together with its
pro-inflammatory and pro-fibrotic effects, ET-1 has been proven to
play a central role in the pathogenesis of SSc. Endothelial cells,
fibroblasts, vascular smooth muscle cells and neutrophils express
endothelin-specific receptors (ETα and β) (16). Furthermore, ET-1 acts as a mediator
of the transforming growth factor-β (TGF-β) signaling pathway, thus
promoting the appearance and development of fibrotic changes in SSc
(18).
The impact of digital ulcers (DUs) on disability and
health-related quality of life in SSc has long been demonstrated.
Bosentan is an endothelin receptor antagonist capable of preventing
the onset of new DUs in scleroderma patients with an added effect
on fibro-proliferative vascular remodeling and pulmonary arterial
hypertension (PAH) (19–21). Recent research indicates that along
with other therapies targeting the same factor, bosentan treatment
is accompanied by an improvement in patients' quality of life
and/or functional capacity (22–24).
Our primary aim was to establish whether or not
scleroderma patients experience an improvement in functionality
during the first year of treatment with bosentan. Secondary
objectives involved the analysis of the burden of disease with
regard to Raynaud's phenomenon and DUs, as well as the
identification of factors that could influence patient-perceived
outcomes in our study group.
Materials and methods
We conducted a prospective observational study in
which we included adult individuals with SSc that underwent
Bosentan therapy between January 2016 and January 2017. The
inclusion criteria were the following: confirmed SSc diagnosis
(2013 European League Against Rheumatism criteria), elligible and
willing to begin Bosentan therapy, informed consent given by
subjects in written form before the initiation of study screening,
age over 18 years, and Body Mass Index (BMI) between 18.5 and 30
kg/m2. The exclusion criteria were: age <18 years,
patients' refusal to participate, overlap syndromes (25), scleroderma sine scleroderma (26), current treatment with Cyclosporine A
(27), Cyclophosphamide (28), phosphodiesterase type 5 inhibitors
(29,30) or prostacyclin analogs (31,32), and
severe or uncontrolled cardiac, liver or renal involvement
(33).
The onset of the disease was defined as the initial
appearance of Raynaud's phenomenon. Patients were stratified with
respect to disease phenotype as diffuse cutaneous SSc (dcSSc) or
limited cutaneous SSc (lcSSc) in accordance with the LeRoy
classification.
We determined capillaroscopic patterns utilizing a
FedMed Digitale 100N digital microscope at a magnification rate of
200X.
The study participants were asked to evaluate the
burden of Raynaud's symptoms as well as DUs with the help of a
visual analogue scale (VAS) which ranged from 0 millimeters
(best/no symptoms) to 100 mm (worst/most intense symptoms). We
assessed disease-related functional impairment using the Health
Assessment Questionnaire Disability Index (HAQ-DI). The
patient-perceived burden of symptoms and the HAQ-DI were documented
at baseline, at 3, 6, 9 months, and at 12 months post-treatment
with bosentan.
The statistical analysis of the data was performed
using Microsoft Office Excel and IBM SPSS Statistics v20 for
Windows. We applied the student t-test to compare the
characteristics of different subgroups of patients and the paired
t-test to determine the differences between evaluations (34). We used odds ratio (OR) to assess the
impact of clinical characteristics on functional impairment and
patient-reported outcomes in our study group. In the contingency
tables, we applied the chi2 test and Fisher's exact test
(where chi2 did not demonstrate statistical
consistency). In order to highlight the relationships between
different variables, we used either Pearson's or Spearman's
correlation coefficients depending on the distribution of
variables. The statistical significance was a priori set at
<0.05.
The study protocol was approved by the Ethics
Committee on Research of the Clinical Rehabilitation Hospital
(Iasi, Romania).
Results
Of the 41 patients recruited, 28 were classified as
dcSSc (68.3%) and 13 as lcSSc (31.7%). The time from diagnosis to
study enrollment ranged between 6 months and 33 years. Although
none of the patients were smokers, the majority of participants
(23, 56.1%) reported having dyspnea and regarded it as a major
burden on their general health. Dyspnea was not significantly more
frequent in elderly patients compared to the rest of the group
(Fisher's exact test, P=0.447).
The patients were treated with 62.5 mg twice daily
for 4 weeks, then 125 mg twice daily with frequent monitoring of
liver function (prior to inclusion, after the first 4 weeks, at 6
weeks, and then monthly until the end of the study), complete blood
work (monthly). All subjects were started on a personalized
rehabilitation program prior to the study and continued it over the
entire follow-up period.
The patient-reported severity of Raynaud's symptoms
(VAS-R) and DU-related discomfort (VAS-DU) received between 50 and
100 points on the VAS at the beginning of the study. Score values
did not differ across the dcSSc and lcSSc groups, with only a trend
towards significance for HAQ-DI (P=0.051). The baseline
characteristics of the study population are summarized in Table I.
 | Table I.Baseline characteristics of the study
population. |
Table I.
Baseline characteristics of the study
population.
|
Characteristics | Mean (± SD)/nο
(%) |
|---|
| Age, years | 58.17 (±12.1) |
|
30–39 | 3 (7.3) |
|
40–49 | 9 (22) |
|
50–59 | 7 (17.1) |
|
60–69 | 15 (36.6) |
|
70–79 | 6 (14.6) |
| 80 | 1 (2.4) |
| Disease duration,
years | 9.66 (±6.8) |
| Sex |
|
|
Female | 31 (75.6) |
|
Male | 10 (24.4) |
|
Female:Male ratio | 3:1 |
| Disease
phenotype |
|
|
dcSSc | 28 (68.3) |
|
lcSSc | 13 (31.7) |
| Capillaroscopic
pattern |
|
|
Early | 2 (4.9) |
|
Active | 20 (48.8) |
|
Late | 19 (46.3) |
| Dyspnea |
|
|
Present | 23 (56.1) |
|
Absent | 18 (43.9) |
| Treatment |
|
|
Low-dose oral steroids | 6 (14.6) |
| Calcium
channel blockers | 27 (65.9) |
|
Synthetic DMARDs |
|
|
Methotrexate | 14 (34.1) |
|
Azathioprine | 2 (4.9) |
|
Hydroxychloroquine | 8 (19.5) |
|
Mycophenolate
mofetil | 1 (2.4) |
| VAS-R | 79.15 (±16.2) |
| VAS-DU | 76.83 (±16.04) |
| HAQ-DI | 1.65 (±0.68) |
Most of the patients (95.12%) exhibited
capillaroscopic changes suggestive of either the active
(n=20, 48.8%) or the late (n=19, 46.3%) SSc patterns. The
severity of microvascular changes was positively correlated with
the duration of symptoms (R=0.39, P=0.014), and the burden of the
disease as reflected by Raynaud's syndrome (R=0.46, P=0.003), DUs
(R=0.038, P=0.017), HAQ-DI (R=0.55, P<0.001).
Increased disability index values were accompanied
by higher VAS levels for Raynaud's (R=0.55, P<0.001), and DUs
(R=0.49, P=0.002). Patients' age was also correlated with HAQ-DI
(R=0.78, P<0.001).
At baseline, the student t-test demonstrated
statistically significant differences between the
early/active and the late capillaroscopic patterns
regarding VAS-R (P<0.001), VAS-DU (P=0.005), and HAQ-DI
(P=0.001).
The dcSSc group displayed elevated odds ratio for
dyspnea (OR=7.13, 95% CI: 3.51–33.43), more severe microvascular
changes (OR=8.4, 95% CI: 2.54–46.18) and an above-mean burden of
Raynaud's symptoms (OR=3.4, 95% CI: 1.81–14.24).
Patients over 60 years of age presented with higher
odds of displaying elevated VAS-DU scores at baseline compared to
the group mean (OR=2.06, 95% CI: 1.57–7.47). Female patients were
more likely to report greater HAQ-DI (OR=2.85, 95% CI:
1.65–12.05).
Due to adverse effects manifested through an
important increase in liver enzymes, two participants (both female,
65 and 68 years old without underlying liver disease) were unable
to continue Bosentan therapy and dropped out from the study after
one month. The two excluded patients demonstrated a rapid
normalization of liver function tests after the discontinuation of
Bosentan.
The remaining 39 patients were able to continue the
treatment until the end of the study and exhibited a significant
improvement in HAQ-DI, VAS-R, and VAS-DU scores over the one-year
follow-up period (P<0.001 for all parameters) (Table II).
 | Table II.Analysis of the scores during the
12-month follow-up period. |
Table II.
Analysis of the scores during the
12-month follow-up period.
| Score | First visit (3
months) | Second visit (6
months) | Third visit (9
months) | Fourth visit (12
months) | P-value (paired
t-test) |
|---|
| VAS-R | 14.27
(9.38–19.15) | 24.61
(20.39–28.84) | 31.03
(25.67–36.38) | 38.71
(33.26–44.17) | <0.001 |
| VAS-DU | 21.34
(14.44–28.24) | 31.92
(26.69–37.15) | 41.28
(34.23–48.33) | 51.41
(44.45–58.37) | <0.001 |
| HAQ-DI | 0.47
(0.36–0.58) | 0.48
(0.32–0.64) | 0.71
(0.54–0.88) | 0.72
(0.57–0.87) | <0.001 |
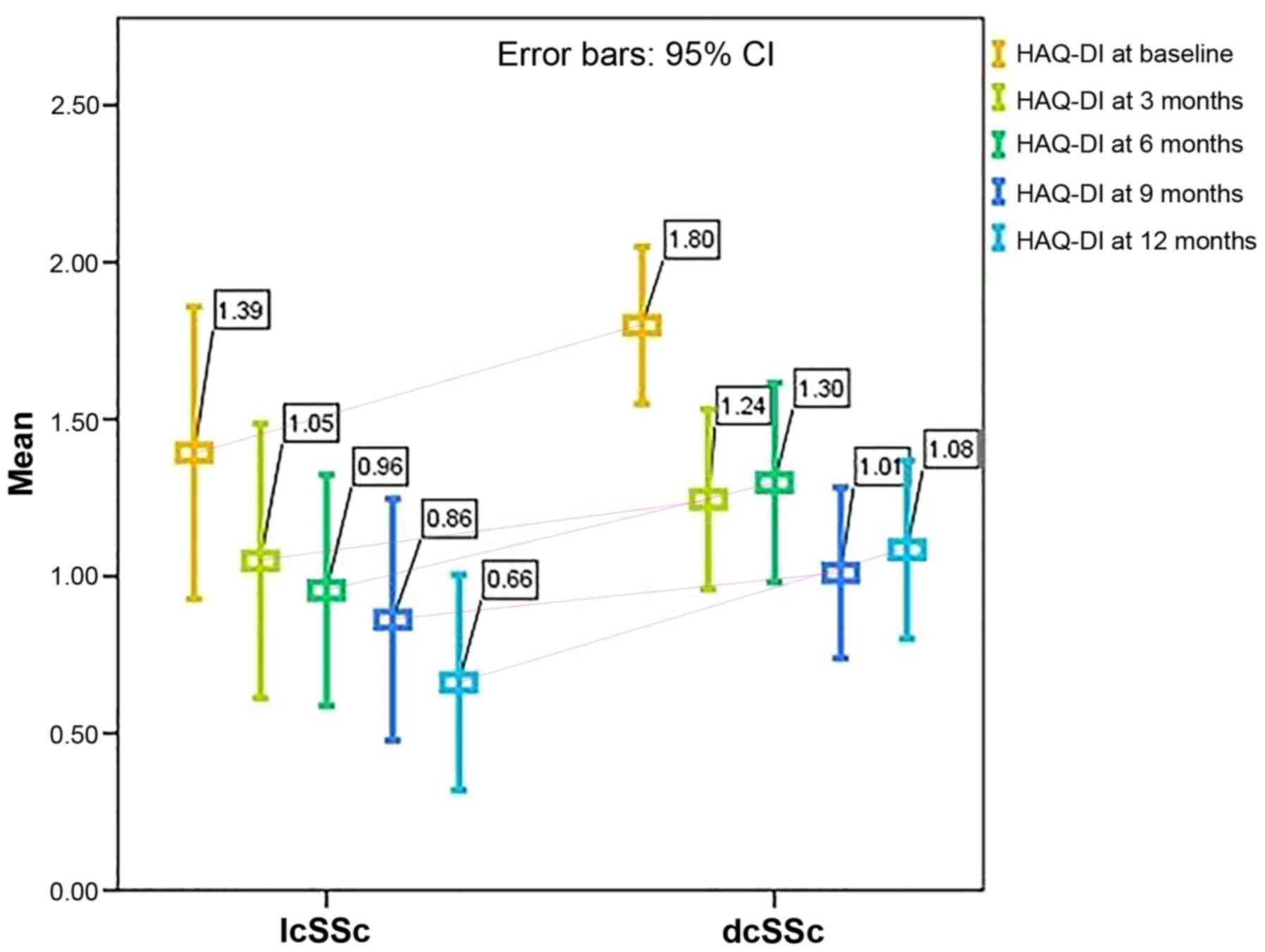
The disability index scores did not differ
substantially with regard to disease phenotype over the 12 months
of treatment (HAQ-DI at 3 months: P=0,428; HAQ-DI at 6 months:
P=0.142; HAQ-DI at 9 months: P=0.503; HAQ at 12 months: P=0.152).
However, when analyzed separately, both dcSSc and lcSSc patients
exhibited significant improvements in all the scores tested
(P<0.001). The mean HAQ-DI levels with respect to disease
phenotype are shown in Fig. 1.
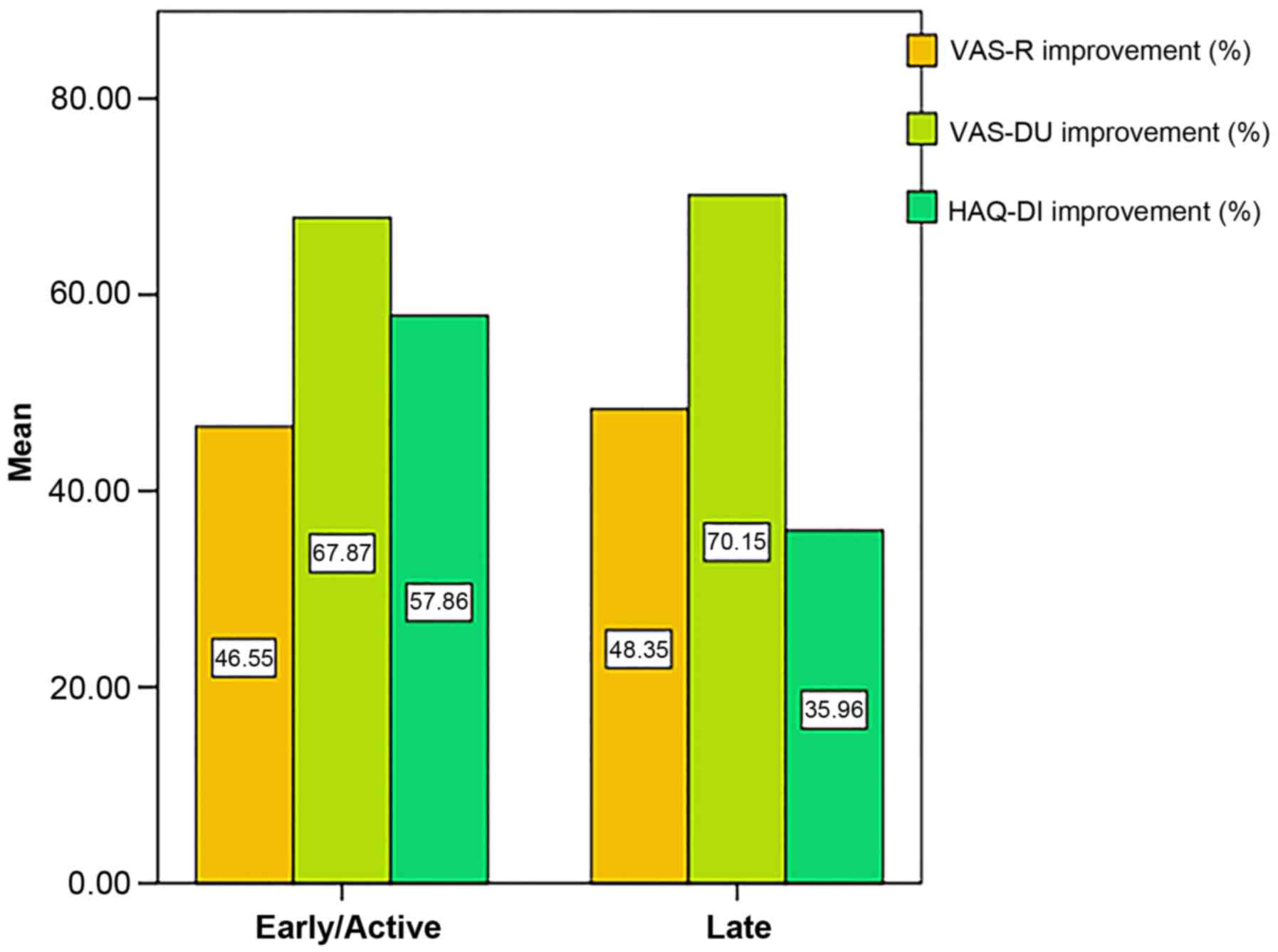
The post-treatment amelioration of VAS-R, VAS-DU and
HAQ-DI was not found to be significantly different across the
capillaroscopic pattern subgroups at 12 months (P>0.05; Fig. 2).
The values of VAS-DU, VAS-R, and HAQ-DI as well as
response rates were similar in female and male participants. The
post-treatment improvement in VAS-DU levels was associated with
better outcomes in HAQ-DI (R=0.44, P=0.005). This relationship was
not proven for VAS-R (R=0.24, P=0.137).
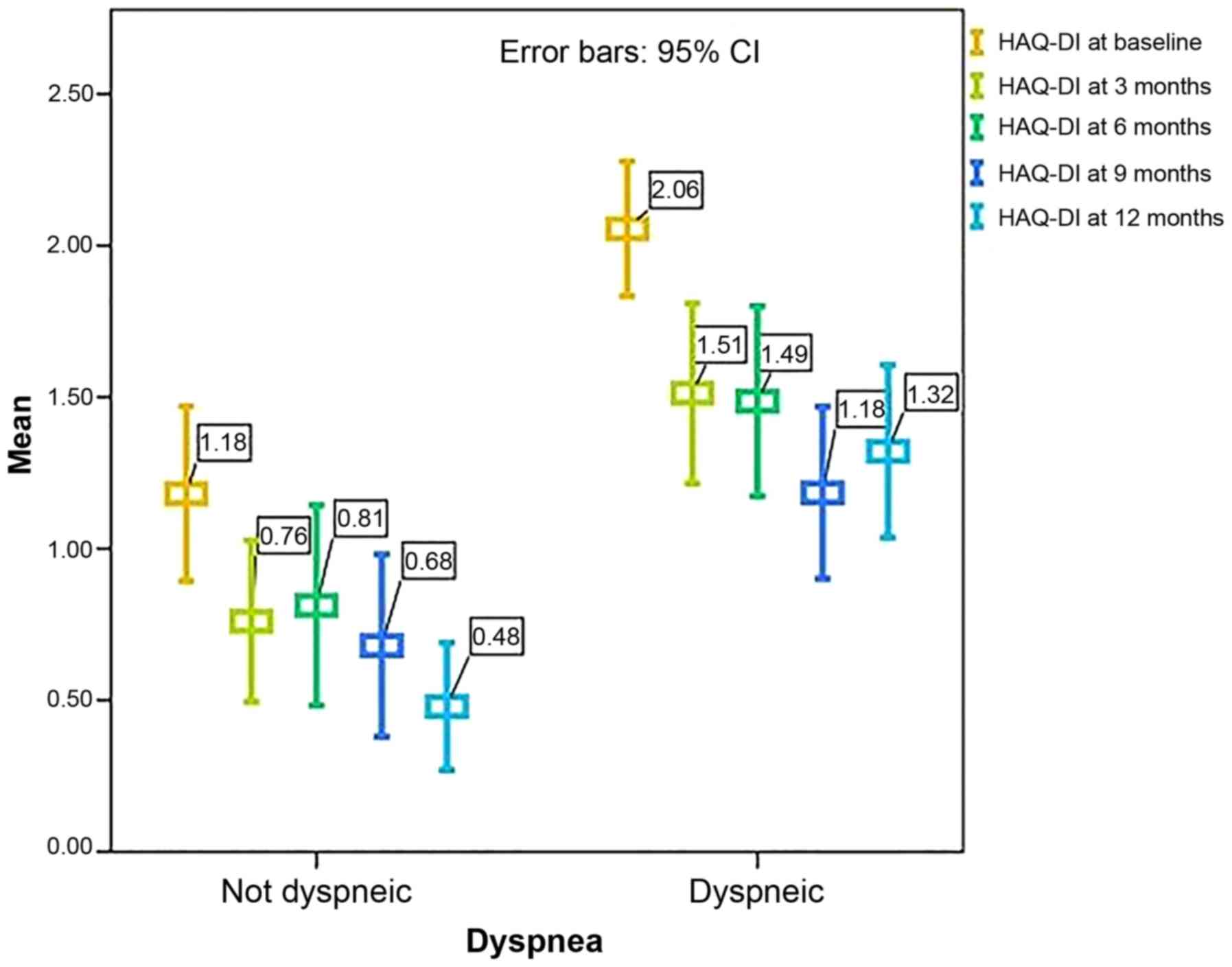
Throughout the follow-up period, patients with
dyspnea presented with significantly higher HAQ-DI levels (Fig. 3) (P<0.001 at baseline, at 3
months, and 12 months; P=0.004 at 6 months, and P=0.015 at 9
months, respectively).
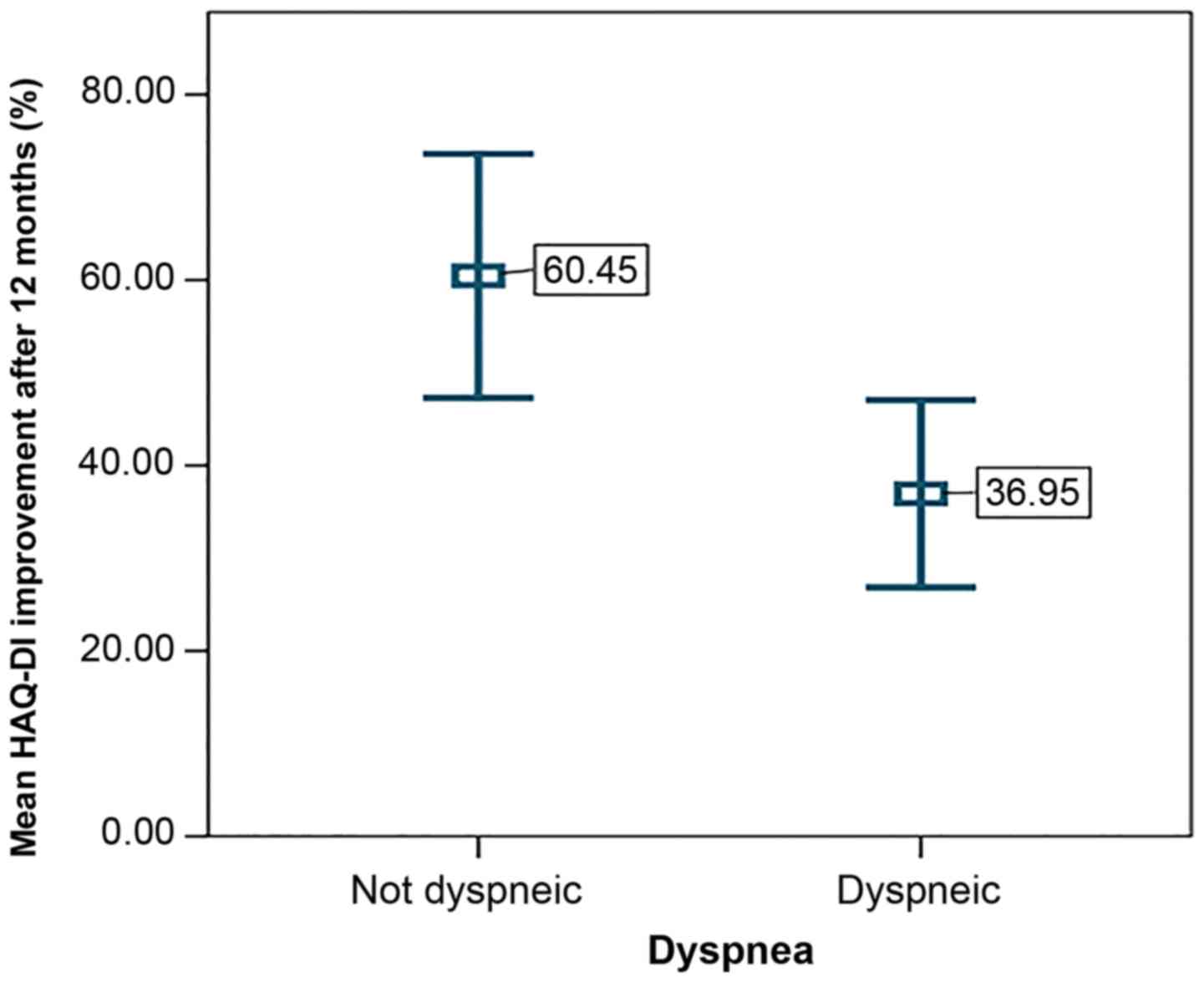
Moreover, dyspneic participants were prone to
experiencing inferior results with respect to HAQ-DI amelioration
(P=0.005) (Fig. 4). The risk of
exhibiting a below-mean improvement was increased by 41% in these
patients.
Higher scores at baseline (above the mean value for
the entire group) predicted weaker results after one year of
treatment, the risk being increased by 220% for VAS-R, 116% for
VAS-DU, but not HAQ-DI. Patients with elevated disability index
score values at baseline experienced more dramatic improvements
post-treatment (R=0.48, P=0.002).
The late capillaroscopic pattern was
associated with a weaker amelioration of the scores tested, with a
109% risk elevation for VAS-DU, 43% for VAS-R, and 24% for HAQ-DI.
Patients with a disease duration of over 5 years displayed an
increased risk for poor outcome by 104% for VAS-R and 64% for
HAQ-DI.
The risk of experiencing a below-mean improvement in
functionality index scores was increased by 64% in patients over 60
years of age. However, this category was more likely to exhibit
better outcomes by 30% in terms of Raynaud's symptoms and by 18% in
VAS-DU scores.
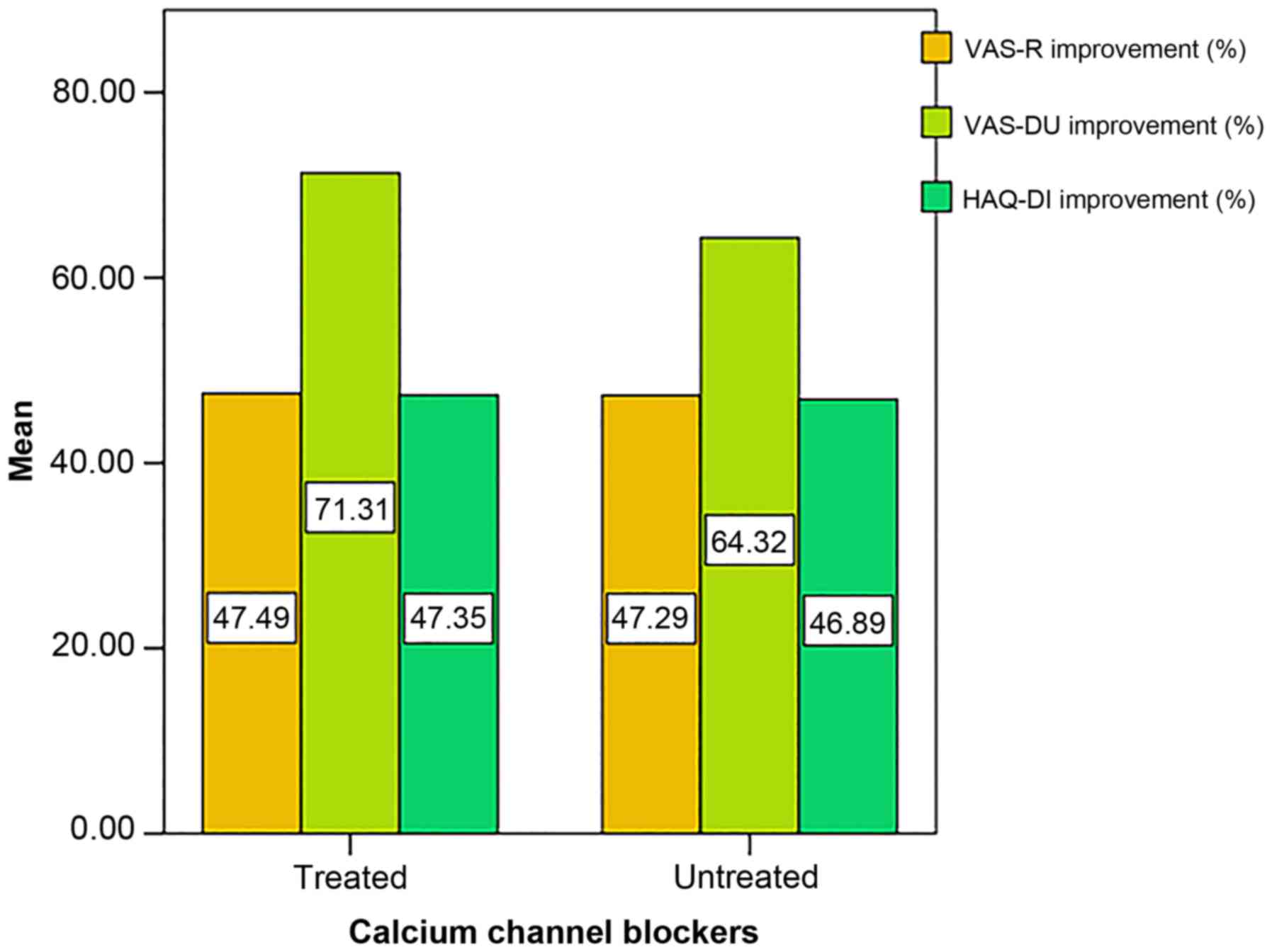
At 12 months, 26 patients were under treatment with
calcium channel blockers (66.7%). Nevertheless, concomitant
treatment with calcium channel blockers did not result in a
significantly greater amelioration of VAS-R (P=0.971), VAS-DU
(P=0.479) or HAQ-DI (P=0.960) compared to the rest of the group
(Fig. 5).
The use of synthetic DMARDs did not exhibit a
notable influence on functional outcomes, VAS-R or VAS-DU at 12
months of Bosentan therapy (P=0.148, P=0.128, P=0.691). Moreover,
we found no association between concomitant treatment with low-dose
glucocorticoids and the amelioration of VAS-R, VAS-DU or HAQ-DI
scores in our study population (P=0.384, P=0.816, P=0.508).
Discussion
SSc is a chronic disease accompanied by an elevated
risk of disability and a high mortality rate despite currently
available treatment (35,36). It has been shown that multiple
factors contribute to the potentially severe disease-related
manifestations and the hindrance of patients' functional capacity
(37,38). Together with an extended fibrosis of
the skin, joint contractures, sarcopenia, gastrointestinal and
cardiopulmonary involvement, the presence of Raynaud's phenomenon
and DUs have an important impact on daily life activities and
work-related endeavors (8,38).
Ischemic DUs are a common and serious health issue
in scleroderma, often leading to considerable hand disability and
pain in these patients (35,39), which is especially relevant since
chronic pain constitutes a leading cause of life quality decrease
frequently requiring therapeutic management (40–42). In
our study population, the burden of Raynaud's phenomenon and
DU-related symptoms as assessed by patients (subjective) were
correlated with the capillaroscopic anomalies at baseline
(objective). The severity of microvascular findings also influenced
the patient-perceived outcome in terms of DU-related discomfort
post-treatment. These results support the association between
objectively measured severity of microvascular damage and the
patients' view on the burden of symptoms. Admitting that physicians
habitually place their focus on the objective assessment of
disease-related impairment, findings are frequently disparate from
the patients' perspective (39,43,44).
Our research concentrated mainly on patient-reported
outcomes during the first year of endothelin-1 receptor antagonist
therapy. The majority of patients who required treatment with
bosentan for active DUs presented with the diffuse phenotype. The
latter were more likely to have dyspnea, greater HAQ-DI scores at
baseline (P=0.051) and more severe capillaroscopic changes. Recent
studies have reported more severe organ manifestations, higher
mortality rates in patients with dcSSc compared to lcSSc (45,46).
However, the post-treatment disability index scores were not
substantially different with respect to disease phenotype in our
cohort.
The patient-centered approach to disability in
scleroderma leads to the conclusion that SSc-related dyspnea often
constitutes one of the main factors involved in the decrement of
life quality as well as disability (47–49).
According to recent data from the DeSScipher project within the
EUSTAR (European Scleroderma Trials and Research) group, dyspnea
and the presence of DUs emerge as major determinants of functional
impairment in SSc patients (8).
Other studies have reported similar findings regarding the
importance of dyspnea in SSc-related quality of life (50,51). Our
results highlight the role of dyspnea in the persistent functional
hindrance amongst the study group, dyspneic patients presenting
with significantly higher HAQ-DI scores at every visit despite all
subjects following a personalized rehabilitation program (52,53).
Furthermore, dyspneic patients in our cohort were more likely to
report poorer improvement in HAQ-DI after one year of bosentan
therapy compared to the non-dyspneic subgroup.
Current therapeutic options for SSc are limited and
thought of as insufficient (54,55),
patients often using over-the-counter drugs for symptom management
(56). It has been demonstrated that
calcium channel blockers exert a positive effect on vascular
changes in scleroderma (57,58). However, the association of Bosentan
with calcium channel blockers did not result in better outcomes in
our study population.
Although research shows that immunosuppressive
agents have beneficial effects on SSc patients' outcomes (59–63),
concomitant treatment with synthetic DMARDs did not influence the
results after one year of Bosentan treatment. The same was true for
low-dose oral glucocorticoids in our cohort (64,65).
Bosentan has been proven to diminish the appearance
of new DUs in SSc patients (66).
For the entire study cohort, HAQ-DI scores were significantly
improved after one year of treatment with Bosentan. Hereof, our
results were similar to those obtained by Mouthon et al
(22) which indicated a lower level
of disability and improved hand function at 12 months.
During the follow-up period, Bosentan therapy was
proven beneficial in our study population irrespective of disease
phenotype, HAQ-DI improvement correlating with that of VAS-DU but
not VAS-R, thus suggesting the important role of DU-related
symptoms in scleroderma patients' functional impairment (22). However, the currently available
literature reports discrepant results in this respect.
Matucci-Cerinic et al (66)
did not obtain functional improvement in patients treated with
Bosentan versus placebo at 24 weeks. In contrast to these results,
Nguyen et al (67) reported a
significant amelioration of functional scores but not Raynaud's
phenomenon in their study cohort. Nevertheless, more research is
needed in order to analyze long-term effects.
SSc is associated with a considerable decrease in
functionality and quality of life. Nonetheless, the
patient-perceived burden of the disease is seldom superposed to
physicians' view on the severity of organ impairment. Along with
the limited therapeutic options, the potentially severe multisystem
involvement that accompanies this diagnosis leads to a negative
prognosis for SSc patients, especially if they exhibit a pronounced
burden of symptoms at the moment of diagnosis. The present
observational study faces certain limitations such as the absence
of a control group, the small number of patients included and the
short follow-up period. However, Bosentan treatment led to a
significant decrease in VAS-R, VAS-DU as well as HAQ-DI scores
irrespective of disease phenotype suggesting a positive effect on
the patient-perceived severity of vascular involvement and
functional hindrance. Future research addressing patient-reported
outcomes may provide further insight on the effect of long-term
endothelin-1 receptor antagonist therapy in scleroderma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ER, AMB, GRZ and AC conceived and designed this
article, performed the experiments and analysis, and wrote the
manuscript. BG, TAS and CR interpreted the data, drafted the
manuscript and made critical revisions. All authors researched the
literature, discussed the results and reviewed and approved the
final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee on Research of the Clinical Rehabilitation Hospital
(Iasi, Romania). All patients were required to provide informed
consent beforehand.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pattanaik D, Brown M, Postlethwaite BC and
Postlethwaite AE: Pathogenesis of systemic sclerosis. Front
Immunol. 6:2722015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi MY and Fritzler MJ: Progress in
understanding the diagnostic and pathogenic role of autoantibodies
associated with systemic sclerosis. Curr Opin Rheumatol.
28:586–594. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Crincoli V, Fatone L, Fanelli M, Rotolo
RP, Chialà A, Favia G and Lapadula G: Orofacial manifestations and
temporomandibular disorders of systemic scleroderma: An
observational study. Int J Mol Sci. 17:11892016. View Article : Google Scholar
|
|
4
|
Paik JJ, Wigley FM, Mejia AF and Hummers
LK: Independent association of severity of muscle weakness with
disability as measured by the health assessment questionnaire
disability index in scleroderma. Arthritis Care Res (Hoboken).
68:1695–1703. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bretterklieber A, Painsi C, Avian A, Wutte
N and Aberer E: Impaired quality of life in patients with systemic
sclerosis compared to the general population and chronic
dermatoses. BMC Res Notes. 7:5942014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nguyen C, Ranque B, Baubet T, Bérezné A,
Mestre-Stanislas C, Rannou F, Papelard A, Morell-Dubois S, Revel M,
Moro MR, et al Groupe Français de Recherche sur la Sclérodermie, :
Clinical, functional and health-related quality of life correlates
of clinically significant symptoms of anxiety and depression in
patients with systemic sclerosis: A cross-sectional survey. PLoS
One. 9:e904842014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sandqvist G, Hesselstrand R, Petersson IF
and Kristensen LE: Work disability in early systemic sclerosis: A
longitudinal population-based cohort study. J Rheumatol.
42:1794–1800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaeger VK, Distler O, Maurer B, Czirják L,
Lóránd V, Valentini G, Vettori S, Del Galdo F, Abignano G, Denton
C, et al: Functional disability and its predictors in systemic
sclerosis: A study from the DeSScipher project within the EUSTAR
group. Rheumatology (Oxford). 57:441–450. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ziemek J, Man A, Hinchcliff M, Varga J,
Simms RW and Lafyatis R: The relationship between skin symptoms and
the scleroderma modification of the health assessment
questionnaire, the modified Rodnan skin score, and skin pathology
in patients with systemic sclerosis. Rheumatology (Oxford).
55:911–917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morrisroe K, Stevens W, Huq M, Prior D,
Sahhar J, Ngian GS, Celermajer D, Zochling J, Proudman S and
Nikpour M; Australian Scleroderma Interest Group (ASIG), : Survival
and quality of life in incident systemic sclerosis-related
pulmonary arterial hypertension. Arthritis Res Ther. 19:1222017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Denton CP: Systemic sclerosis: From
pathogenesis to targeted therapy. Clin Exp Rheumatol. 33 (Suppl
92):S3–S7. 2015.PubMed/NCBI
|
|
12
|
Denton CP: Advances in pathogenesis and
treatment of systemic sclerosis. Clin Med (Lond). 16:55–60. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burlui A, Cardoneanu A, Macovei LA, Arhire
L, Graur M and Rezus E: Is there a place for anti-nucleosome
antibody assessment in scleroderma? Rom J Rheumatol. 27:169–173.
2018.
|
|
14
|
Ilie AC, Alexa ID, Moroșanu AI, Covic A
and Cepoi V: Effects of oxidative stress and pharmacologycal
treatment on geriatric syndromes in the hospitalised elderly
patients. Farmacia. 64:588–593. 2016.
|
|
15
|
Rezuș E, Cardoneanu A, Burlui A, Luca A,
Codreanu C, Tamba BI, Stanciu GD, Dima N, Bădescu C and Rezuș C:
The Link Between Inflammaging and Degenerative Joint Diseases. Int
J Mol Sci. 20:6142019. View Article : Google Scholar
|
|
16
|
Shiwen X, Leask A, Abraham DJ and Fonseca
C: Endothelin receptor selectivity: Evidence from in vitro and
pre-clinical models of scleroderma. Eur J Clin Invest. 39 (Suppl
2):19–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ortega Mateo A and de Artiñano AA:
Highlights on endothelins: A review. Pharmacol Res. 36:339–351.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi-Wen X, Rodríguez-Pascual F, Lamas S,
Holmes A, Howat S, Pearson JD, Dashwood MR, du Bois RM, Denton CP,
Black CM, et al: Constitutive ALK5-independent c-Jun N-terminal
kinase activation contributes to endothelin-1 overexpression in
pulmonary fibrosis: Evidence of an autocrine endothelin loop
operating through the endothelin A and B receptors. Mol Cell Biol.
26:5518–5527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mouthon L, Mestre-Stanislas C, Bérezné A,
Rannou F, Guilpain P, Revel M, Pagnoux C, Guillevin L, Fermanian J
and Poiraudeau S: Impact of digital ulcers on disability and
health-related quality of life in systemic sclerosis. Ann Rheum
Dis. 69:214–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W and Frech TM: The critical need for
accurately defining digital ulcers in scleroderma. J Scleroderma
Relat Disord. 2:69–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schieir O, Thombs BD, Hudson M, Boivin JF,
Steele R, Bernatsky S, Hanley J and Baron M; Canadian Scleroderma
Research Group, : Prevalence, severity, and clinical correlates of
pain in patients with systemic sclerosis. Arthritis Care Res
(Hoboken). 62:409–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mouthon L, Carpentier PH, Lok C, Clerson
P, Gressin V, Hachulla E, Bérezné A, Diot E, Van Kien AK, Jego P,
et al ECLIPSE study investigators, : Controlling the digital
ulcerative disease in systemic sclerosis is associated with
improved hand function. Semin Arthritis Rheum. 46:759–766. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bose N, Bena J and Chatterjee S:
Evaluation of the effect of ambrisentan on digital microvascular
flow in patients with systemic sclerosis using laser Doppler
perfusion imaging: A 12-week randomized double-blind placebo
controlled trial. Arthritis Res Ther. 17:442015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rezuş E, Floria M, Grigoriu A, Tamba BI
and Rezus C: Cardiovascular Risk Factors in Chronic Inflammatory
Rheumatic Diseases: Modern Assessment and Diagnosis. Curr Vasc
Pharmacol. 13:716–724. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Avouac J, Walker U, Tyndall A, Kahan A,
Matucci-Cerinic M, Allanore Y, Miniati I, Muller A, Iannone F,
Distler O, et al EUSTAR, : Characteristics of joint involvement and
relationships with systemic inflammation in systemic sclerosis:
Results from the EULAR Scleroderma Trial and Research Group
(EUSTAR) database. J Rheumatol. 37:1488–1501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diab S, Dostrovsky N, Hudson M, Tatibouet
S, Fritzler MJ, Baron M and Khalidi N; Canadian Scleroderma
Research Group, : Systemic sclerosis sine scleroderma: A
multicenter study of 1417 subjects. J Rheumatol. 41:2179–2185.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oldfield V and Lyseng-Williamson KA:
Bosentan: A review of its use in pulmonary arterial hypertension
and systemic sclerosis. Am J Cardiovasc Drugs. 6:189–208. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nadashkevich O, Davis P, Fritzler M and
Kovalenko W: A randomized unblinded trial of cyclophosphamide
versus azathioprine in the treatment of systemic sclerosis. Clin
Rheumatol. 25:205–212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Badesch DB, Hill NS, Burgess G, Rubin LJ,
Barst RJ, Galiè N and Simonneau G; SUPER Study Group, : Sildenafil
for pulmonary arterial hypertension associated with connective
tissue disease. J Rheumatol. 34:2417–2422. 2007.PubMed/NCBI
|
|
30
|
Galiè N, Brundage BH, Ghofrani HA, Oudiz
RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth
A, et al Pulmonary Arterial Hypertension and Response to Tadalafil
(PHIRST) Study Group, : Tadalafil therapy for pulmonary arterial
hypertension. Circulation. 119:2894–2903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barst RJ, McGoon M, McLaughlin V, Tapson
V, Rich S, Rubin L, Wasserman K, Oudiz R, Shapiro S, Robbins IM, et
al Beraprost Study Group, : Beraprost therapy for pulmonary
arterial hypertension. J Am Coll Cardiol. 41:2119–2125. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Galiè N, Humbert M, Vachiéry JL, Vizza CD,
Kneussl M, Manes A, Sitbon O, Torbicki A, Delcroix M, Naeije R, et
al Arterial Pulmonary Hypertension and Beraprost European
(ALPHABET) Study Group, : Effects of beraprost sodium, an oral
prostacyclin analogue, in patients with pulmonary arterial
hypertension: A randomized, double-blind, placebo-controlled trial.
J Am Coll Cardiol. 39:1496–1502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vacca A, Meune C, Gordon J, Chung L,
Proudman S, Assassi S, Nikpour M, Rodriguez-Reyna TS, Khanna D,
Lafyatis R, et al Scleroderma Clinical Trial Consortium Cardiac
Subcommittee, : Cardiac arrhythmias and conduction defects in
systemic sclerosis. Rheumatology (Oxford). 53:1172–1177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gheorghevici TS, Veliceasa B, Puha B,
Toader S, Alexa ID and Alexa O: Preoperative Hemoglobin Dynamics in
Patients with Trochanteric Fractures. A multivariate analysis. Rev
Chim Buchar. 69:3320–3324. 2018.
|
|
35
|
Khanna D, Hays RD and Furst DE: Functional
disability and other health-related quality-of-life domains: Points
to consider for clinical trials in systemic sclerosis. Rheumatology
(Oxford). 56 (Suppl 5):v17–v22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baron M, Hudson M, Tatibouet S, Steele R,
Lo E, Gravel S, Gyger G, El Sayegh T, Pope J, Fontaine A, et al:
The Canadian systemic sclerosis oral health study: Orofacial
manifestations and oral health-related quality of life in systemic
sclerosis compared with the general population. Rheumatology
(Oxford). 53:1386–1394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Burlui A, Graur M, Gherasim A, Cardoneanu
A and Rezuş E: The role of adipokines in inflammation and
connective tissue diseases: Can we face the challenge? Int J Med
Dent. 8:26–33. 2018.
|
|
38
|
Siegert E, March C, Otten L, Makowka A,
Preis E, Buttgereit F, Riemekasten G, Müller-Werdan U and Norman K:
Prevalence of sarcopenia in systemic sclerosis: Assessing body
composition and functional disability in patients with systemic
sclerosis. Nutrition. 55-56:51–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
De Cata A, Inglese M, Molinaro F, De Cosmo
S, Rubino R, Bernal M and Mazzoccoli G: Digital ulcers in
scleroderma patients: A retrospective observational study. Int J
Immunopathol Pharmacol. 29:180–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ughi N, Crotti C and Ingegnoli F:
Effectiveness and safety of oxycodone/naloxone in the management of
chronic pain in patients with systemic sclerosis with recurrent
digital ulcers: Two case reports. Clin Interv Aging. 11:307–311.
2016.PubMed/NCBI
|
|
41
|
Merz EL, Assassi S and Malcarne VL: Pain
and Its Management in Systemic Sclerosis. Curr Treatm Opt
Rheumatol. 4:255–267. 2018. View Article : Google Scholar
|
|
42
|
Alexa T, Marza A, Voloseniuc T and Tamba
B: Enhanced analgesic effects of tramadol and common trace element
coadministration in mice. J Neurosci Res. 93:1534–1541. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Luca FA, Ioan CA, Sasu C and Luca AC: The
Impact of Public Health Care Services on the Patients' Perception
as Regards the Health Institutions Brand on the Background of the
Health Reform in Romania. Rev Res Soc Intervention. 49:802015.
|
|
44
|
Carausu EM, Paris S, Burlea LS, Tucmeanu
AI and Antohe I: The Crisis Impact on the Romanian Health System
and Population Health. Rev Res Soc Intervention. 57:120–137.
2017.
|
|
45
|
Peytrignet S, Denton CP, Lunt M,
Hesselstrand R, Mouthon L, Silman A, Pan X, Brown E, Czirják L,
Distler JHW, et al: Disability, fatigue, pain and their associates
in early diffuse cutaneous systemic sclerosis: The European
Scleroderma Observational Study. Rheumatology (Oxford). 57:370–381.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Herrick AL, Pan X, Peytrignet S, Lunt M,
Hesselstrand R, Mouthon L, Silman A, Brown E, Czirják L, Distler
JHW, et al: Treatment outcome in early diffuse cutaneous systemic
sclerosis: The European Scleroderma Observational Study (ESOS). Ann
Rheum Dis. 76:1207–1218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lambova S: Cardiac manifestations in
systemic sclerosis. World J Cardiol. 6:993–1005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wallace B, Kafaja S, Furst DE, Berrocal
VJ, Merkel PA, Seibold JR, Mayes MD and Khanna D: Reliability,
validity and responsiveness to change of the Saint George's
Respiratory Questionnaire in early diffuse cutaneous systemic
sclerosis. Rheumatology (Oxford). 54:1369–1379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Delcroix M and Howard L: Pulmonary
arterial hypertension: The burden of disease and impact on quality
of life. Eur Respir Rev. 24:621–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Almeida C, Almeida I and Vasconcelos C:
Quality of life in systemic sclerosis. Autoimmun Rev. 14:1087–1096.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Park EH, Strand V, Oh YJ, Song YW and Lee
EB: Health-related quality of life in systemic sclerosis compared
with other rheumatic diseases: A cross-sectional study. Arthritis
Res Ther. 21:612019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mugii N, Hasegawa M, Matsushita T, Kondo
M, Orito H, Yanaba K, Komura K, Hayakawa I, Hamaguchi Y, Ikuta M,
et al: The efficacy of self-administered stretching for finger
joint motion in Japanese patients with systemic sclerosis. J
Rheumatol. 33:1586–1592. 2006.PubMed/NCBI
|
|
53
|
Volkmann ER and Tashkin DP: Treatment of
Systemic Sclerosis-related Interstitial Lung Disease: A Review of
Existing and Emerging Therapies. Ann Am Thorac Soc. 13:2045–2056.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Young A, Namas R, Dodge C and Khanna D:
Hand impairment in systemic sclerosis: Various manifestations and
currently available treatment. Curr Treatm Opt Rheumatol.
2:252–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hughes M, Ong VH, Anderson ME, Hall F,
Moinzadeh P, Griffiths B, Baildam E, Denton CP and Herrick AL:
Consensus best practice pathway of the UK Scleroderma Study Group:
Digital vasculopathy in systemic sclerosis. Rheumatology (Oxford).
54:2015–2024. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Alexa ID, Pancu AG, Morosanu AI, Ghiciuc
CM, Lupusoru C, Prada GI and Cepoi V: The impact of self-medication
with NSAIDS/analgesics in a North-Eastern region of Romania.
Farmacia. 62:1164–1170. 2014.
|
|
57
|
Finch MB, Dawson J and Johnston GD: The
peripheral vascular effects of nifedipine in Raynaud's syndrome
associated with scleroderma: A double blind crossover study. Clin
Rheumatol. 5:493–498. 1986.PubMed/NCBI
|
|
58
|
Thompson AE, Shea B, Welch V, Fenlon D and
Pope JE: Calcium-channel blockers for Raynaud's phenomenon in
systemic sclerosis. Arthritis Rheum. 44:1841–1847. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kowal-Bielecka O, Fransen J, Avouac J,
Becker M, Kulak A, Allanore Y, Distler O, Clements P, Cutolo M,
Czirjak L, et al EUSTAR Coauthors, : Update of EULAR
recommendations for the treatment of systemic sclerosis. Ann Rheum
Dis. 76:1327–1339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Omair MA, Alahmadi A and Johnson SR:
Safety and effectiveness of mycophenolate in systemic sclerosis. A
systematic review. PLoS One. 10:e01242052015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Nihtyanova SI, Brough GM, Black CM and
Denton CP: Mycophenolate mofetil in diffuse cutaneous systemic
sclerosis--a retrospective analysis. Rheumatology (Oxford).
46:442–445. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Stratton RJ, Wilson H and Black CM: Pilot
study of anti-thymocyte globulin plus mycophenolate mofetil in
recent-onset diffuse scleroderma. Rheumatology (Oxford). 40:84–88.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vanthuyne M, Blockmans D, Westhovens R,
Roufosse F, Cogan E, Coche E, Nzeusseu Toukap A, Depresseux G and
Houssiau FA: A pilot study of mycophenolate mofetil combined to
intravenous methylprednisolone pulses and oral low-dose
glucocorticoids in severe early systemic sclerosis. Clin Exp
Rheumatol. 25:287–292. 2007.PubMed/NCBI
|
|
64
|
Takehara K: Treatment of early diffuse
cutaneous systemic sclerosis patients in Japan by low-dose
corticosteroids for skin involvement. Clin Exp Rheumatol. 22 (Suppl
33):S87–S89. 2004.PubMed/NCBI
|
|
65
|
Sharada B, Kumar A, Kakker R, Adya CM,
Pande I, Uppal SS, Pande JN, Sunderam KR and Malaviya AN:
Intravenous dexamethasone pulse therapy in diffuse systemic
sclerosis. A randomized placebo-controlled study. Rheumatol Int.
14:91–94. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Matucci-Cerinic M, Denton CP, Furst DE,
Mayes MD, Hsu VM, Carpentier P, Wigley FM, Black CM, Fessler BJ,
Merkel PA, et al: Bosentan treatment of digital ulcers related to
systemic sclerosis: Results from the RAPIDS-2 randomised,
double-blind, placebo-controlled trial. Ann Rheum Dis. 70:32–38.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Nguyen VA, Eisendle K, Gruber I, Hugl B,
Reider D and Reider N: Effect of the dual endothelin receptor
antagonist bosentan on Raynaud's phenomenon secondary to systemic
sclerosis: A double-blind prospective, randomized,
placebo-controlled pilot study. Rheumatology (Oxford). 49:583–587.
2010. View Article : Google Scholar : PubMed/NCBI
|