Introduction
Chronic obstructive pulmonary disease (COPD) is one
of the most common lung diseases observed in clinical practice and
the third leading cause of death in the world (1,2). COPD
prevalence rate of people aged over 40 years is 10.1% in the world
and 8.2% in China (3). According to
relevant data, the death rate of COPD inpatients in the United
States was 4.8% in 2002 and the average hospitalization cost of
patients was as high as $22,187 (4).
COPD mainly manifests as airflow obstruction and lung inflammation
(5). Due to deterioration of lung
function, people with severe COPD are usually unable to participate
in normal physical activities (6).
Acute exacerbation of COPD (AECOPD) is associated with acute
inflammation and infection and increases morbidity and mortality
(7). However, little is known about
the prognostic consequences of these deterioration (8).
Serum amyloid A (SAA), a precursor protein of
amyloid fibrils (9), is an evolved
and highly conserved acute phase protein secreted mainly by
hepatocytes (10). Some studies have
pointed out that SAA may be a potential marker of lung cancer
(11). However, at present, there
are still few studies on the role of SAA in AECOPD. Interleukin-6
(IL-6) is one of the most important inflammatory cytokines
(12). It was originally described
as B-stimulating factor 2, which induces B lymphocytes to produce
immunoglobulin (13). It can be
rapidly synthesized in the case of infection and tissue damage
(14). Relevant data showed that
IL-6 plays a key role in the pathogenesis of inflammatory diseases
and the homeostasis of nerve tissue (15). COPD is characterized by local and
systemic inflammation. Because inflammation plays a key role in the
progression, course and severity of COPD, inflammatory markers have
the potential to improve current diagnosis and prognosis methods
(16). However, few studies have
systematically investigated the levels of inflammatory cytokines in
patients with AECOPD.
Therefore, we explored the expression of SAA and
IL-6 in the peripheral blood serum of AECOPD patients through
experiments to provide accurate basis for future clinical diagnosis
and treatment of AECOPD.
Patients and methods
Baseline data of patients
In this study, 51 patients with acute exacerbation
of chronic obstructive pulmonary disease (AG) admitted to Qingdao
Eighth People's Hospital (Qingdao, China) from March 2010 to
February 2015 were selected, including 31 males and 20 females,
aged 45–70 years with an average age of (56.31±6.74) years.
Fifty-one patients with stable chronic obstructive pulmonary
disease (SG) were selected, including 33 males and 18 females, aged
44–69 years and an average age of (56.62±6.49) years. The study was
approved by the Ethics Committee of Qingdao Eighth People's
Hospital. Signed informed consents were obtained from the patients
and/or guardians.
Inclusion and exclusion criteria
Inclusion criteria
The patients who met the diagnostic criteria of the
Spanish management guide of COPD in 2017 (17). The patients received treatment in the
above hospital after diagnosis and had complete case data. All the
patients agreed to cooperate with the work arrangements of the
medical staff in the hospital. Informed consent was signed by the
patient or an immediate family member.
Exclusion criteria
Died during treatment; injury of important organs;
comorbid with other cardiovascular and cerebrovascular diseases;
physical disability; pregnancy; comorbid with other autoimmune
diseases; comorbid with other chronic diseases; transferred to
another hospital; contraindications of surgery; mental diseases,
language dysfunction and diseases affecting the results of this
study.
Methods
In both groups, 3 ml of peripheral venous blood was
extracted on an empty stomach in the early morning, and the serum
was centrifuged for 10 min at 300 × g and 28°C, and then separated
and stored at −80°C for measurement. The serum IL-6 and SAA levels
were detected by enzyme-linked immunosorbent assay (ELISA). IL-6
detection kit was purchased from Shanghai Chunshi Biotechnology
Co., Ltd., item number (CS-13629E). The SAA detection kit was
purchased from Shanghai YuanMu Biological Technology Co. Ltd., item
number (YM-SX0898). The operation steps were carried out strictly
in accordance with the requirements of the manual.
Statistical methods
The experimental results were calculated using
SPSS24.0 statistical (Beijing Sitron Weida Information Technology
Co., Ltd.). The image was drawn by GraphPad Prism 7. Counting data
was expressed as a percentage (%). The chi-square test was used to
compare between the two groups. The measurement data was expressed
as mean ± standard deviation (SD). t-test was used for comparison
between groups. The receiver operating characteristic (ROC) curve
was used to evaluate the diagnostic efficacy and calculate the
sensitivity, and specificity. Pearson test was used to analyze the
correlation between SAA and IL-6 in serum. Chi-square (χ2) test was
used for counting data to conduct single factor analysis of AECOPD.
The indexes of P<0.05 in single factor analysis were
incorporated into logistic multiple regression model (stepwise
regression) for multivariate analysis. Independent risk factors
related to AECOPD were screened. The difference was statistically
significant at P<0.05.
Results
Comparison of the baseline data of
patients between the two groups
The two groups of patients were comparable in
baseline data of sex, age and BMI, with no significant difference
(P>0.05) (Table I).
 | Table I.Comparison of the baseline data of
COPD patients between the two groups [n (%)]. |
Table I.
Comparison of the baseline data of
COPD patients between the two groups [n (%)].
| Items | AG (51) | SG (51) | t or
χ2 | P-value |
|---|
| Sex |
|
| 0.168 | 0.682 |
| Male | 31 (60.78) | 33 (64.71) |
|
|
|
Female | 20 (41.18) | 18 (35.29) |
|
|
| Age/years | 56.31±6.74 | 56.62±6.49 | 0.237 | 0.813 |
| BMI
(kg/m2) | 22.47±3.83 | 22.15±4.62 | 0.381 | 0.704 |
| Place of
residence |
|
| 0.056 | 0.813 |
| City | 39 (76.47) | 40 (78.43) |
|
|
|
Rural | 12 (23.53) | 11 (21.57) |
|
|
| Ethnicity |
|
| 0.703 | 0.402 |
| Han | 45 (88.24) | 42 (82.35) |
|
|
|
Minority | 6
(11.76) | 9
(17.65) |
|
|
| Smoking history |
|
| 0.050 | 0.822 |
|
Yes | 38 (74.51) | 37 (72.55) |
|
|
| No | 13 (25.49) | 14 (27.45) |
|
|
| Drinking
history |
|
| 0.177 | 0.674 |
|
Yes | 33 (64.71) | 35 (68.63) |
|
|
| No | 18 (35.29) | 16 (31.37) |
|
|
| Education
level |
|
| 0.403 | 0.526 |
| <
High school | 15 (29.41) | 18 (35.29) |
|
|
| ≥ High
school | 36 (70.59) | 33 (64.71) |
|
|
| Marital status |
|
| 0.297 | 0.586 |
|
Married | 42 (82.35) | 44 (86.27) |
|
|
|
Unmarried | 9
(17.65) | 7
(13.73) |
|
|
| Family history |
|
| 0.159 | 0.689 |
|
Yes | 23 (45.10) | 21 (41.18) |
|
|
| No | 28 (54.90) | 30 (58.82) |
|
|
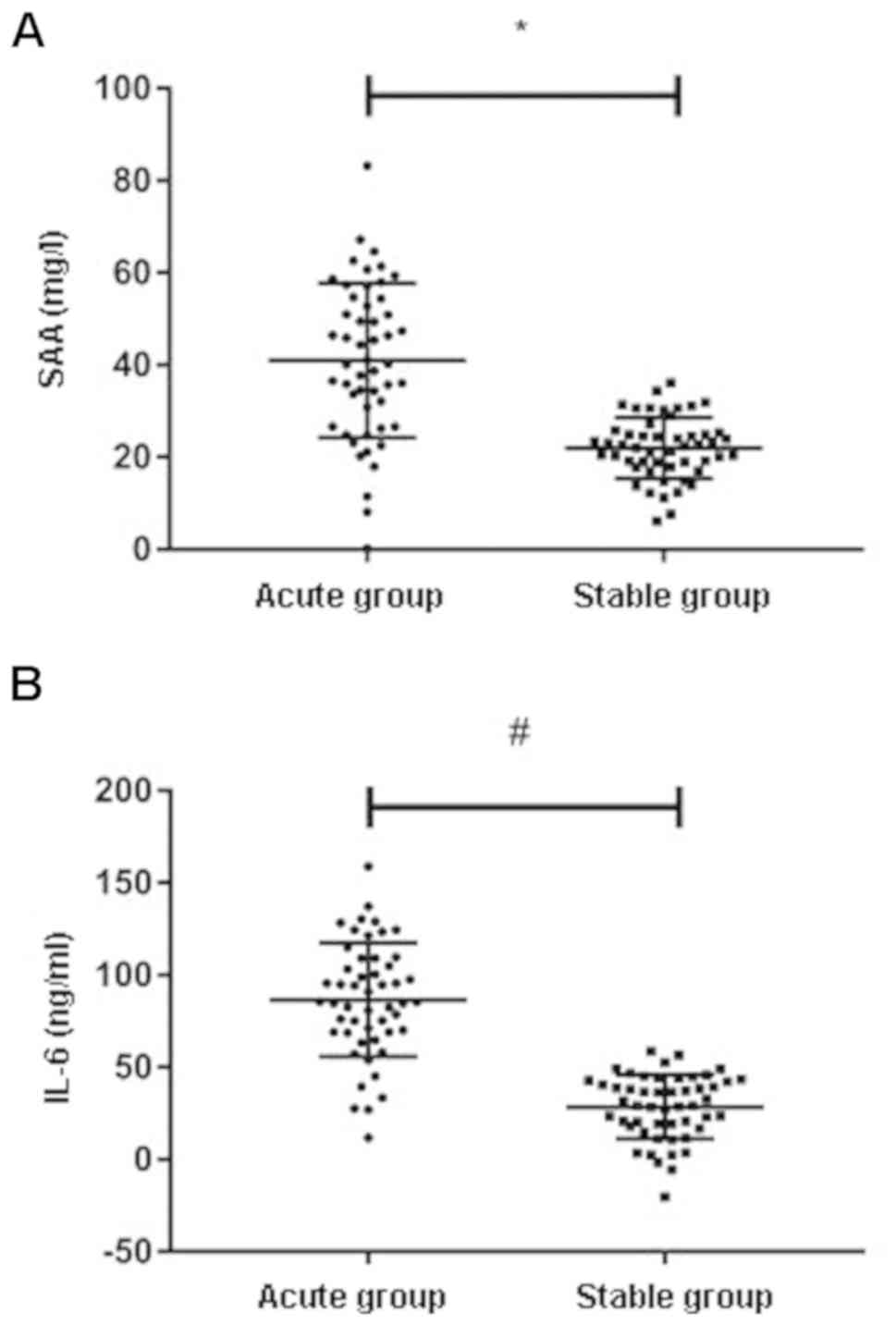
Expression levels of SAA and IL-6 in
peripheral blood of patients in the two groups
The results showed that the relative level of SAA
expression in serum of AECOPD patients (40.68±14.49) ng/ml was
significantly higher than that of patients with stable COPD
(21.58±6.91) ng/ml, P<0.001. More details are shown in Fig. 1A. The relative level of IL-6
expression in serum of AECOPD patients (80.93±33.52) ng/ml was
significantly higher than that of patients with stable COPD
(27.68±17.27) ng/ml, P<0.001 (Fig.
1B).
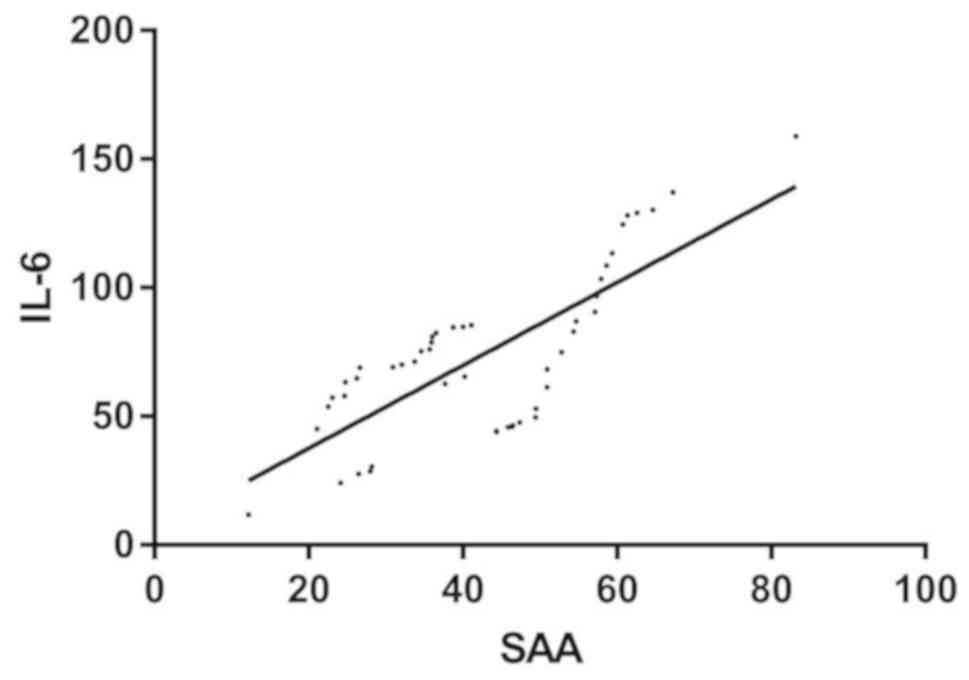
Correlation analysis of SAA and IL-6
in AG
Pearson correlation analysis showed that SAA level
in serum of patients in AG was positively correlated with IL-6
(r=0.765, P<0.001) (Table II and
Fig. 2).
 | Table II.Correlation analysis of SAA and
IL-6. |
Table II.
Correlation analysis of SAA and
IL-6.
| Items | Value |
|---|
| r | 0.765 |
| 95% CI | 0.6202–0.8594 |
| P-value | <0.001 |
Predictive value of SAA and IL-6 for
AECOPD progression
When the cut-off value was 10.06 ng/ml, the
sensitivity of SAA to AECOPD progression was 82.35% and the
specificity was 68.63%. When the cut-off value was 15.69 ng/ml, the
sensitivity of IL-6 to AECOPD progression was 88.24% and the
specificity was 49.02% (Table III
and Fig. 3A and B).
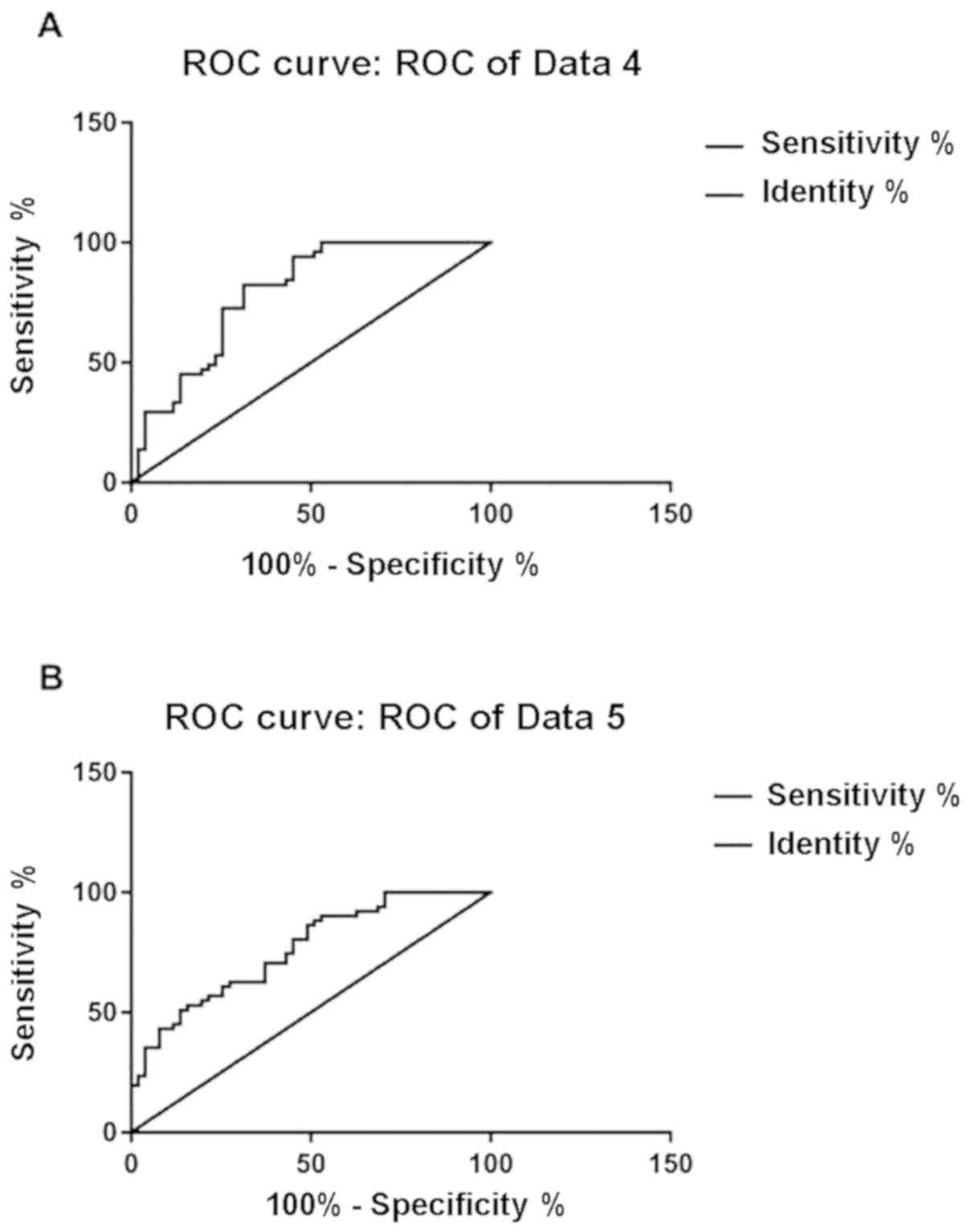 | Figure 3.Diagnostic value of SAA and IL-6 for
AECOPD. (A) ROC curve of SAA prediction of AECOPD. Cut-off value
was 10.06, AUC was 0.789, SE was 0.045, 95% CI was 0.7002–0.8777,
sensitivity was 82.35% and specificity was 68.63%, P<0.001. (B)
ROC curve of SAA prediction of AECOPD. Cut-off value was 15.69, AUC
was 0.762, SE was 0.046, 95% CI was 0.678–0.859, sensitivity was
88.24% and specificity was 49.02%, P<0.001. SAA, serum amyloid
A; IL-6, interleukin-6; AECOPD, acute exacerbation of chronic
obstructive pulmonary disease; ROC, receiver operating
characteristic; SE, standard error. |
 | Table III.Predictive value of SAA and IL-6 for
AECOPD progression. |
Table III.
Predictive value of SAA and IL-6 for
AECOPD progression.
| Items | SAA | IL-6 |
|---|
| AUC | 0.789 | 0.762 |
| SE | 0.045 | 0.046 |
| 95% CI | 0.7002–0.8777 | 0.6721–0.8527 |
| P-value | <0.001 | <0.001 |
| Cut-off | 10.06 ng/ml | 15.69 ng/ml |
| Sensitivity
(%) | 82.35 | 88.24 |
| Specificity
(%) | 68.63 | 49.02 |
Analysis of single risk factors in the
two groups
The indicators with differences in the above results
were taken as the results of single factor analysis and assigned
values (Table IV). SPSS was used to
select LR: forward for multivariate regression analysis. The
results showed that diabetes was an independent risk factor for
affecting AECOPD (OR, 1.234; 95% CI, 2.281–19.694). Respiratory
failure was an independent risk factor for affecting AECOPD (OR,
5.020; 95% CI, 1.080–23.324). SAA was an independent risk factor
for affecting AECOPD (OR, 3.227; 95% CI, 1.124–9.256). IL-6 was an
independent risk factor for affecting AECOPD (OR, 2.019; 95% CI,
1.267–3.218) (Table V).
 | Table IV.Assignments. |
Table IV.
Assignments.
| Index | Assignment |
|---|
| Diabetes
mellitus | No=0; Yes=1 |
| Respiratory
failure | No=0; Yes=1 |
| SAA (mg/l) | The data conformed
to continuous variables and were analyzed with original data. |
| IL-6 (ng/l) | The data conformed
to continuous variables and were analyzed with original data. |
 | Table V.Multivariate regression analysis. |
Table V.
Multivariate regression analysis.
| Items | Β | SE | Wald | P-value | OR | 95% CI |
|---|
| Diabetes
mellitus | 1.922 | 0.431 | 18.832 | 0.020 | 1.234 | 2.281–19.694 |
| Respiratory
failure | 1.613 | 0.784 | 4.238 | 0.040 | 5.020 | 1.080–23.324 |
| SAA | 1.172 | 0.536 | 4.744 | 0.028 | 3.227 | 1.124–9.256 |
| IL-6 | 0.701 | 0.239 | 8.705 | 0.002 | 2.019 | 1.267–3.218 |
Discussion
COPD is a common, preventable and treatable disease,
which is caused by airway and/or alveolar abnormalities and is
usually caused by exposure to harmful particles or gases (18,19).
Moreover, frequent hospitalization leads to a significant decrease
in the quality of life of patients (20) and severe acute exacerbation also
predicts a poor prognosis, with mortality rates exceeding,
respectively, 20 and 50% in 1 and 5 years (21).
SAA is a major acute plasma protein, which can
regulate innate immunity and cholesterol homeostasis (22). SAA has a significant relationship
with acute phase reaction. Serum level rises up to 1,000 times
within 24 h (23). This is the same
as C-reactive protein (CRP). SAA can be used as a diagnostic,
prognostic or therapeutic follow-up marker for many diseases
(24,25). Cytokines are effective inducers of
SAA in hepatocytes (26). Relevant
literature shows that the synthesis of SAA is regulated by IL-6
(27). IL-6 is a multi-effect
cytokine with known multiple functions in immune regulation,
inflammation and tumorigenesis (28,29).
COPD is an inflammatory and irreversible lung disease (30). Biological medicines for inflammatory
cytokine IL-6 are increasingly considered as treatment methods for
chronic diseases and cancers (31).
However, the role of IL-6 in AECOPD is still less elaborated.
Therefore, analyzing the impact of SAA and IL-6 on AECOPD, is not
only of great significance for future clinical screening of AECOPD,
but also provides new ideas for potential therapeutic targets of
AECOPD in the future.
The results of this study showed that the expression
levels of SAA and IL-6 were significantly up-regulated in COPD
patients, suggesting that SAA and IL-6 may participate in the
development and progression of AECOPD, which is consistent with the
research results of Li et al (32) and Chen et al (33) and support the results of this study.
According to Pearson correlation analysis, SAA level in serum of
patients in AG was positively correlated with IL-6 (r= 0.765,
P<0.001), which shows serious tissue damage in AECOPD
progression. At this time, the content of pro-inflammatory cytokine
IL-16 increases significantly and regulates the accelerated
secretion of SAA. By drawing ROC curve of SAA and IL-6, we found
SAA AUC=0.789, 95% CI, 0.7002–0.8777, while IL-6 AUC=0.762, 95% CI,
0.6721–0.8527. This showed that SAA and IL-6 have a very good
predictive value in the prediction of AECOPD progress. Moreover,
the study of Landi et al (34) showed that IL-16 has a good predictive
value for myogenic myelitis. Previous studies also confirmed that
SAA has a good predictive effect for postoperative infection of
osteosarcoma and ovarian cancer (35,36),
which also confirms our viewpoint. Through multivariate regression
analysis, diabetes, respiratory failure, SAA and IL-6 are
independent risk factors for affecting AECOPD, suggesting that in
clinical situation of AECOPD patients should be paid attention
during treatment in clinic. Patients with longer course and more
severe disease should be paid more attention during the treatment
process.
The purpose of this study was to explore and analyze
the role of SAA and IL-6 in AECOPD. However, the number of cases is
limited, and the experimental period is short. Thus, further study
is required.
In conclusion, the levels of SAA and IL-6 are
significantly increased during AECOPD and they are positively
correlated. They may participate in the development and progression
of AECOPD and can effectively predict and affect the progress of
AECOPD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW wrote the manuscript. YW and SW conceived and
designed the study. YW and DW were responsible for the collection
and analysis of the experimental data. SW and CL interpreted the
data and drafted the manuscript. DW and CL revised the manuscript
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Qingdao Eighth People's Hospital (Qingdao, China). Patients who
participated in this research had complete clinical data. Signed
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Santibáñez M, Garrastazu R, Ruiz-Nuñez M,
Helguera JM, Arenal S, Bonnardeux C, León C and García-Rivero JL:
Predictors of hospitalized exacerbations and mortality in chronic
obstructive pulmonary disease. PLoS One. 11:e01587272016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wain LV, Shrine N, Artigas MS,
Erzurumluoglu AM, Noyvert B, Bossini-Castillo L, Obeidat M, Henry
AP, Portelli MA, Hall RJ, et al Understanding Society Scientific
Group; Geisinger-Regeneron DiscovEHR Collaboration, : Genome-wide
association analyses for lung function and chronic obstructive
pulmonary disease identify new loci and potential druggable
targets. Nat Genet. 49:416–425. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Y, Zhong NS, Li X, Chen S, Zheng J,
Zhao D, Yao W, Zhi R, Wei L, He B, et al: Tiotropium in early-stage
chronic obstructive pulmonary disease. N Engl J Med. 377:923–935.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jinjuvadia C, Jinjuvadia R, Mandapakala C,
Durairajan N, Liangpunsakul S and Soubani AO: Trends in outcomes,
financial burden, and mortality for acute exacerbation of chronic
obstructive pulmonary disease (COPD) in the United States from 2002
to 2010. COPD14. 72–79. 2017. View Article : Google Scholar
|
|
5
|
Cebron Lipovec N, Beijers RJ, van den
Borst B, Doehner W, Lainscak M and Schols AM: The prevalence of
metabolic syndrome in chronic obstructive pulmonary disease: A
systematic review. COPD. 13:399–406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
US Preventive Services Task Force
(USPSTF), ; Siu AL, Bibbins-Domingo K, Grossman DC, Davidson KW,
Epling JW Jr, García FA, Gillman M, Kemper AR, Krist AH, et al:
Screening for chronic obstructive pulmonary disease: US Preventive
Services Task Force recommendation statement. JAMA. 315:1372–1377.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tan DBA, Armitage J, Teo TH, Ong NE, Shin
H and Moodley YP: Elevated levels of circulating exosome in COPD
patients are associated with systemic inflammation. Respir Med.
132:261–264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soler-Cataluña JJ, Martínez-García MA,
Román Sánchez P, Salcedo E, Navarro M and Ochando R: Severe acute
exacerbations and mortality in patients with chronic obstructive
pulmonary disease. Thorax. 60:925–931. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takase H, Tanaka M, Yamamoto A, Watanabe
S, Takahashi S, Nadanaka S, Kitagawa H, Yamada T and Mukai T:
Structural requirements of glycosaminoglycans for facilitating
amyloid fibril formation of human serum amyloid A. Amyloid.
23:67–75. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siegmund SV, Schlosser M, Schildberg FA,
Seki E, De Minicis S, Uchinami H, Kuntzen C, Knolle PA, Strassburg
CP and Schwabe RF: Serum amyloid a induces inflammation,
proliferation and cell death in activated hepatic stellate cells.
PLoS One. 11:e01508932016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sung HJ, Ahn JM, Yoon YH, Rhim TY, Park
CS, Park JY, Lee SY, Kim JW and Cho JY: Identification and
validation of SAA as a potential lung cancer biomarker and its
involvement in metastatic pathogenesis of lung cancer. J Proteome
Res. 10:1383–1395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schaper F and Rose-John S: Interleukin-6:
Biology, signaling and strategies of blockade. Cytokine Growth
Factor Rev. 26:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M,
Shahveranov A, Ye DW and Tian YK: Interleukin-6: An emerging
regulator of pathological pain. J Neuroinflammation. 13:1412016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jordan SC, Choi J, Kim I, Wu G, Toyoda M,
Shin B and Vo A: Interleukin-6, a cytokine critical to mediation of
inflammation, autoimmunity and allograft rejection: Therapeutic
implications of IL-6 receptor blockade. Transplantation. 101:32–44.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rothaug M, Becker-Pauly C and Rose-John S:
The role of interleukin-6 signaling in nervous tissue. Biochim
Biophys Acta 1863A. 1218–1227. 2016. View Article : Google Scholar
|
|
16
|
Lopez-Campos JL, Calero-Acuña C,
Lopez-Ramirez C, Abad-Arranz M, Márquez-Martín E, Ortega-Ruiz F and
Arellano E: Implications of the inflammatory response for the
identification of biomarkers of chronic obstructive pulmonary
disease. Biomark Med. 10:109–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miravitlles M, Soler-Cataluña JJ, Calle M,
Molina J, Almagro P, Quintano JA, Trigueros JA, Cosío BG, Casanova
C, Antonio Riesco J, et al: Spanish Guidelines for Management of
Chronic Obstructive Pulmonary Disease (GesEPOC) 2017.
Pharmacological treatment of stable phase. Arch Bronconeumol.
53:324–335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vogelmeier CF, Criner GJ, Martínez FJ,
Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M,
Fabbri LM, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive lung disease 2017 report.
GOLD executive summary. Am J Respir Crit Care Med. 195:557–582.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dalal AA, Christensen L, Liu F and Riedel
AA: Direct costs of chronic obstructive pulmonary disease among
managed care patients. Int J Chron Obstruct Pulmon Dis. 5:341–349.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seemungal TA, Donaldson GC, Paul EA,
Bestall JC, Jeffries DJ and Wedzicha JA: Effect of exacerbation on
quality of life in patients with chronic obstructive pulmonary
disease. Am J Respir Crit Care Med. 157:1418–1422. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dransfield MT, Kunisaki KM, Strand MJ,
Anzueto A, Bhatt SP, Bowler RP, Criner GJ, Curtis JL, Hanania NA,
Nath H, et al COPD Gene Investigators, : Acute exacerbations and
lung function loss in smokers with and without chronic obstructive
pulmonary disease. Am J Respir Crit Care Med. 195:324–330.
2017.PubMed/NCBI
|
|
22
|
Frame NM and Gursky O: Structure of serum
amyloid A suggests a mechanism for selective lipoprotein binding
and functions: SAA as a hub in macromolecular interaction networks.
FEBS Lett. 590:866–879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sack GH Jr: Serum amyloid A - a review.
Mol Med. 24:462018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Buck M, Gouwy M, Wang JM, Van Snick J,
Opdenakker G, Struyf S and Van Damme J: Structure and expression of
different serum amyloid A (SAA) variants and their
concentration-dependent functions during host insults. Curr Med
Chem. 23:1725–1755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun L and Ye RD: Serum amyloid A1:
Structure, function and gene polymorphism. Gene. 583:48–57. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Buck M, Gouwy M, Wang JM, Van Snick J,
Proost P, Struyf S and Van Damme J: The cytokine-serum amyloid
A-chemokine network. Cytokine Growth Factor Rev. 30:55–69. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jensen LE and Whitehead AS: Regulation of
serum amyloid A protein expression during the acute-phase response.
Biochem J. 334:489–503. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Heo TH, Wahler J and Suh N: Potential
therapeutic implications of IL-6/IL-6R/gp130-targeting agents in
breast cancer. Oncotarget. 7:15460–15473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmidt-Arras D and Rose-John S: IL-6
pathway in the liver: From physiopathology to therapy. J Hepatol.
64:1403–1415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang Z and Zhu L: Update on molecular
mechanisms of corticosteroid resistance in chronic obstructive
pulmonary disease. Pulm Pharmacol Ther. 37:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Jones GW, Choy EH and Jones SA: The
biology behind interleukin-6 targeted interventions. Curr Opin
Rheumatol. 28:152–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li W, Zhou Y and Zhang T: Expressions of
serum TSLP, SAA, and CRP in COPD patients. J Chin Physician.
20:46–49. 2018.
|
|
33
|
Chen YWR, Leung JM and Sin DD: A
systematic review of diagnostic biomarkers of COPD exacerbation.
PLoS One. 11:e01588432016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Landi A, Broadhurst D, Vernon SD, Tyrrell
DL and Houghton M: Reductions in circulating levels of IL-16, IL-7
and VEGF-A in myalgic encephalomyelitis/chronic fatigue syndrome.
Cytokine. 78:27–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Flores RJ: The use of circulating SAA and
CXCL4 to predict outcome of osteosarcoma at diagnosis. Cancer Res.
76:4592016.
|
|
36
|
Fu G-Q, Xia J and Wang G: Clinical values
of SAA and hs-CRP in the diagnosis of postoperative infection in
patients with ovarian tumor. Zhongguo Jiceng Yiyao. 24:1069–1072.
2017.(In Chinese).
|