Introduction
Wilms' tumor (WT), a malignant embryonic tumor
derived from nephridioblasts, accounts for ~87% of childhood kidney
tumors, of which the peak age is 3–4 years, and 80% of patients are
aged <5 years (1). Such a tumor,
first described by Rance in 1814, is named as Wilms' tumor since
Max Wilms further described its characteristics in 1899 (2). As to its treatment, surgical resection
is mainly adopted at present. Besides, WT is sensitive to
radiotherapy, postoperative radiotherapy can improve the efficacy
(3), while preoperative chemotherapy
is able to decrease the size of the tumor, reduce the risk of
surgery, and increase the rate of complete resection. The prognosis
of WT depends on histological type, tumor stage, patient age and
biological characteristics (4), and
is good in most WT patients. After systemic treatment, the 4-year
survival rate of patients with WT at any stage is ~54.8–90%
(5). However, there are no special
precautions for WT. Therefore, the detection and treatment as early
as possible are important for the improvement of the prognosis of
WT patients.
Micro ribonucleic acids (miRNAs) pair with their
messenger RNAs (mRNAs) to induce post-transcriptional inhibition on
their target genes, which are widely involved in modulating most
physiological processes such as apoptosis, proliferation, survival,
cell cycle and differentiation (6).
Considering that >50% of miRNAs are located in cancer-related
genomic regions or vulnerable sites, they are of importance in the
pathogenesis of many types of cancer, including WT. For instance,
Ludwig et al (7) identified
the miRNA expression profiles in 36 WT tissues and 4 normal kidney
tissues via microarray and discovered that miR-183, miR-301a/b and
miR-335 are upregulated in embryonic subtypes, and miR-181b,
miR-223 and miR-630 are raised in regression subtypes. Jiang et
al (8) proved that miR-1180 is
overexpressed in WT tissues, and its knockdown induces apoptosis of
SK-NEP-1 cells and reduces tumor growth in nude mice. Liu et
al (9) collected WT tissues and
adjacent normal tissues from WT patients and confirmed that the
knockdown of miR-19b evidently represses the proliferation,
invasion and migration of WT SK-NEP-1 cells, and the inhibition of
miR-19b suppresses the progression of WT by modulating the gene of
phosphate and tension homology deleted on chromosome ten
(PTEN)/phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)
signaling pathway. All of the above findings suggest that miRNAs
play important roles in the development and progression of WT.
It has been confirmed that miR-21 is overexpressed
in almost all solid tumors studied, accounting for 15–25% of total
miRNAs in tumor cells and serves as a proto-oncogene (10). Moreover, miR-21 can negatively
regulate various target genes, such as programmed cell death 4
(PDCD-4) (11). Given this, miR-21
is a vital participant promoting the proliferation and
differentiation of certain tumor cells. However, the studies on the
role of miR-21 in WT are rare. Hence, in this study, the effects of
miR-21 on the proliferation and apoptosis of WT were
investigated.
Materials and methods
Materials
Roswell Park Memorial Institute (RPMI)-1640 medium
(HyClone; GE Healthcare Life Sciences), fetal bovine serum (FBS)
(Gibco; Thermo Fisher Scientific, Inc.), M-MLV reverse
transcriptase (Promega Corporation), apoptosis assay kit
(Sigma-Aldrich; Merck KGaA), 2X Ultra SYBR Mixture kit (Takara
Bio), Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.), cell counting kit-8 (CCK-8) reagents (Dojindo), psiCHECK™
plasmids and dual luciferase kit (Promega Corporation), PTEN, Akt,
phosphorylated Akt (p-Akt) antibodies (Cell Signaling Technology,
Inc.), CFX96 sequence detection system (Bio-Rad Laboratories, Inc.)
and flow cytometer (BD Biosciences).
Cell culture and miRNA
transfection
Human WT SK-NEP-1 cell line was from American Type
Culture Collection (ATCC). The SK-NEP-1 cells were inoculated in
RPMI-1640 medium containing 10% FBS and cultured in a 5%
CO2 incubator at 37°C. miR-21 mimic and inhibitor as
well as si-PTEN were designed and synthesized by RiboBio, and then
transfected into the SK-NEP-1 cells according to the transfection
instructions of Lipofectamine 2000.
Real-time quantitative polymerase
chain reaction (RT-qPCR) assay
Total RNAs were extracted from the SK-NEP-1 cells
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), reverse
transcribed into complementary deoxyribonucleic acids (cDNAs) using
the M-MLV reverse transcriptase (Promega Corporation) as per the
instructions of the reverse transcription kit. RT-qPCR was carried
out on a CFX96 sequence detection system with 20 ng of cDNA using
the 2X Ultra SYBR Mixture kit. The thermal cycle: initial
denaturation at 95°C for 1 min, denaturation at 94°C for 30 sec,
annealing at 58°C for 30 sec and extension at 72°C for 10 sec for a
total of 30 cycles, and then at 72°C for 2 min and at 16°C for 5
min. The primer sequences are: miR-21: F,
5′-GCCAGGCATAGCTTATCAGACTG-3′ and R, 5′-CCACTGTCTAGCACGACACTAA-3′;
PTEN: F, 5′-AAAGGGACGAACTGGTGTAATG-3′ and R,
5′-TGGTCCTTACTTCCCCATAGAA-3′; U6: F,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and R,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; and GAPDH: F,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′.
Cell proliferation assay
The proliferation ability of SK-NEP-1 cells
transfected with miR-21 inhibitor or control was detected using the
CCK-8. The cells were seeded into a 96-well plate at a density of
2×103 cells/well and transfected for 24, 48 and 72 h as
described above. Then, the cells were added with 10 µl of CCK-8
solution and incubated in an incubator for 3 h. A microplate reader
was utilized to read the optical density (OD) value at a wavelength
of 450 nm. Colony formation assay was conducted to determine the
clonogenic capacity of the SK-NEP-1 cells transfected with miR-21
inhibitor or control. The SK-NEP-1 cells transfected for 24 h were
re-inoculated into a 6-well plate at 500 cells/well for 2 weeks of
culture. Paraformaldehyde was added to fix the viable clones,
followed by staining with 0.5% crystal violet at 37°C for 1 h.
Thereafter, viable cells were counted, and images were captured
with a digital camera.
Apoptosis assay
The SK-NEP-1 cells were seeded into a 6-well plate
for 1 day and then transfected for 48 h. Next, the cells were
trypsinized, harvested, washed twice with cold phosphate buffered
saline (PBS), and then re-suspended in 1X binding buffer.
Thereafter, the cell suspension (1×105 cells) was added
with Annexin V and propidium iodide solution at a ratio of 100 µl:
5 µl, mixed and let stand in a dark place at room temperature for
15 min. The suspension was added with 1X binding buffer and loaded
on the instrument for detection. Each assay was repeated 3 times
independently.
Western blot analysis
The SK-NEP-1 cells were collected and lysed in HEPES
lysis buffer containing protease inhibitors. The bicinchoninic acid
(BCA) protein concentration assay kit (Pierce; Thermo Fisher
Scientific, Inc.) was employed to quantify the total protein
concentration. An equal amount of 50 µg of total protein was
separated by 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene
fluoride (PVDF) membranes (EMD Millipore). The membrane with
separated proteins was blocked with 5% non-fat milk powder at room
temperature for 1 h, incubated with the corresponding primary
antibodies at 4°C overnight, washed with Tris-buffered saline with
Tween-20 (TBST) 3 times, and incubated with the secondary
antibodies at 4°C for 1 h. Target protein expression bands were
visualized through electrochemiluminescence.
Detection of luciferase activity
TargetScan was employed to predict the targets of
miR-21. In the luciferase reporter gene assay, the 3′-untranslated
region (UTR) was designed based on bioinformatics software. The
PTEN 3′-UTR sequences containing predicted miR-21 binding sites and
corresponding mutation sites were subjected to PCR amplification
and then inserted into the psiCHECK™ vector together with the
downstream luciferase genes. SK-NEP-1 cells were cultured in a
24-well plate at a density of 2.5×105 cells/wells, and
then co-transfected with PTEN wild-type (WT) or mutant (MUT)
reporter plasmids (final concentration: 10 nM) and miR-21 mimic or
control, and 48 h later, the dual luciferase assay kit was utilized
to determine the luciferase activity.
Statistical analysis
Data were expressed as mean ± standard deviation.
Differences between two groups were analyzed by using the Student's
t-test. Comparison between multiple groups was done using One-way
ANOVA test followed by post hoc test (least significant
difference). P<0.05 was considered to indicate a statistically
significant difference.
Results
Silencing miR-21 inhibits
proliferation of SK-NEP-1 cells
To investigate the key role of miR-21 in the
proliferation of WT cells, miR-21 inhibitor was transfected into
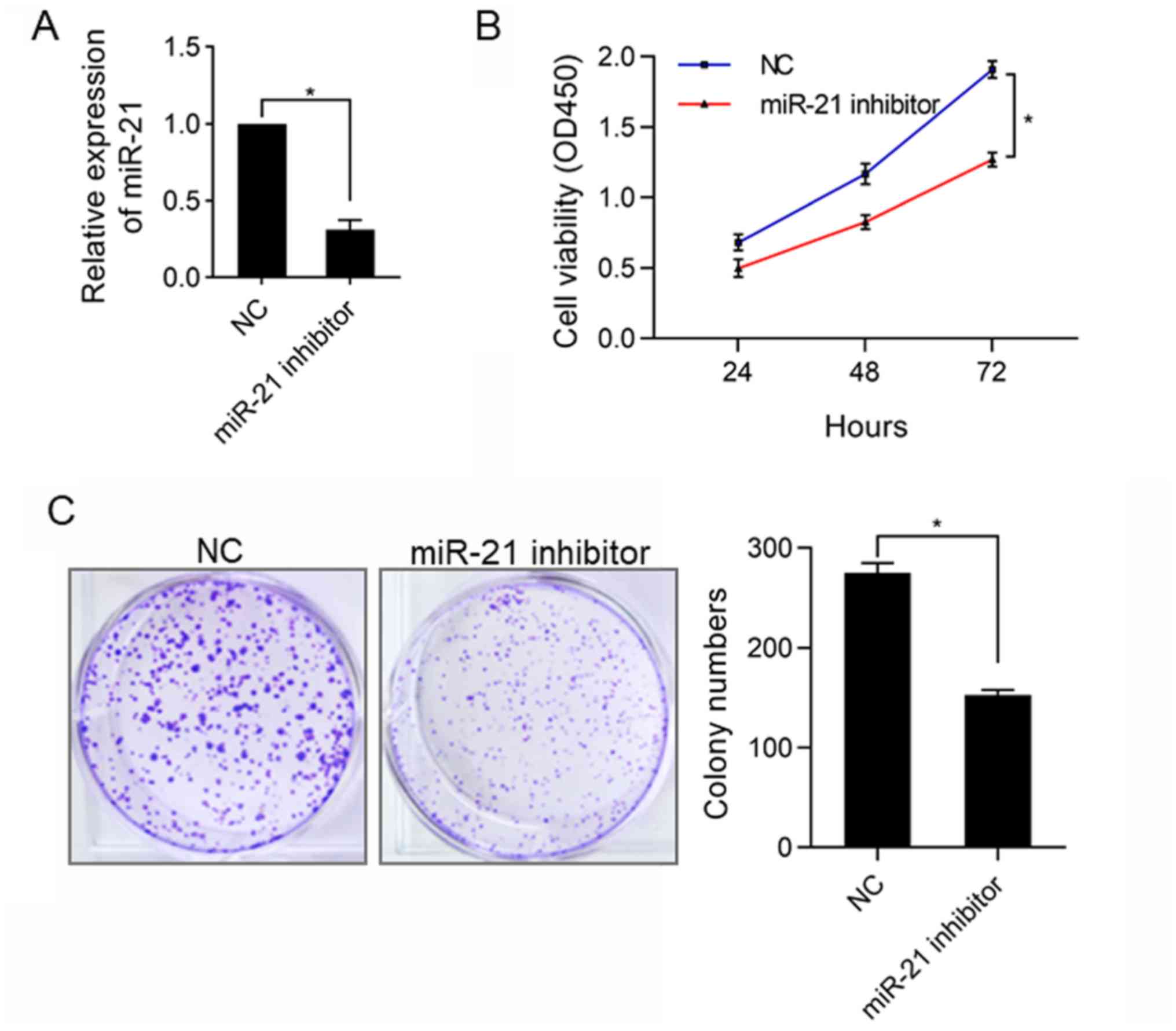
SK-NEP-1 cells to silence the expression of miR-21. The results
(Fig. 1A) of RT-qPCR analysis showed
that at 48 h after transfection, the expression of miR-21 was
remarkably decreased in SK-NEP-1 cells (P<0.05), demonstrating
that the expression of miR-21 was silenced, so in vitro
studies can be conducted. Next, CCK-8 assay was carried out to
explore the influence of silencing miR-21 on the proliferation of
WT SK-NEP-1 cells, and it was found that the proliferation ability
of SK-NEP-1 cells was clearly repressed in miR-21 inhibitor group
compared with that in normal control (NC) group (P<0.05)
(Fig. 1B), which was further
verified through colony formation assay subsequently. The results
(Fig. 1C) revealed that the number
of clones was obviously lower in miR-21 inhibitor group than that
in NC group (P<0.05).
Effects of miR-21 silencing on
apoptosis of SK-NEP-1 cells
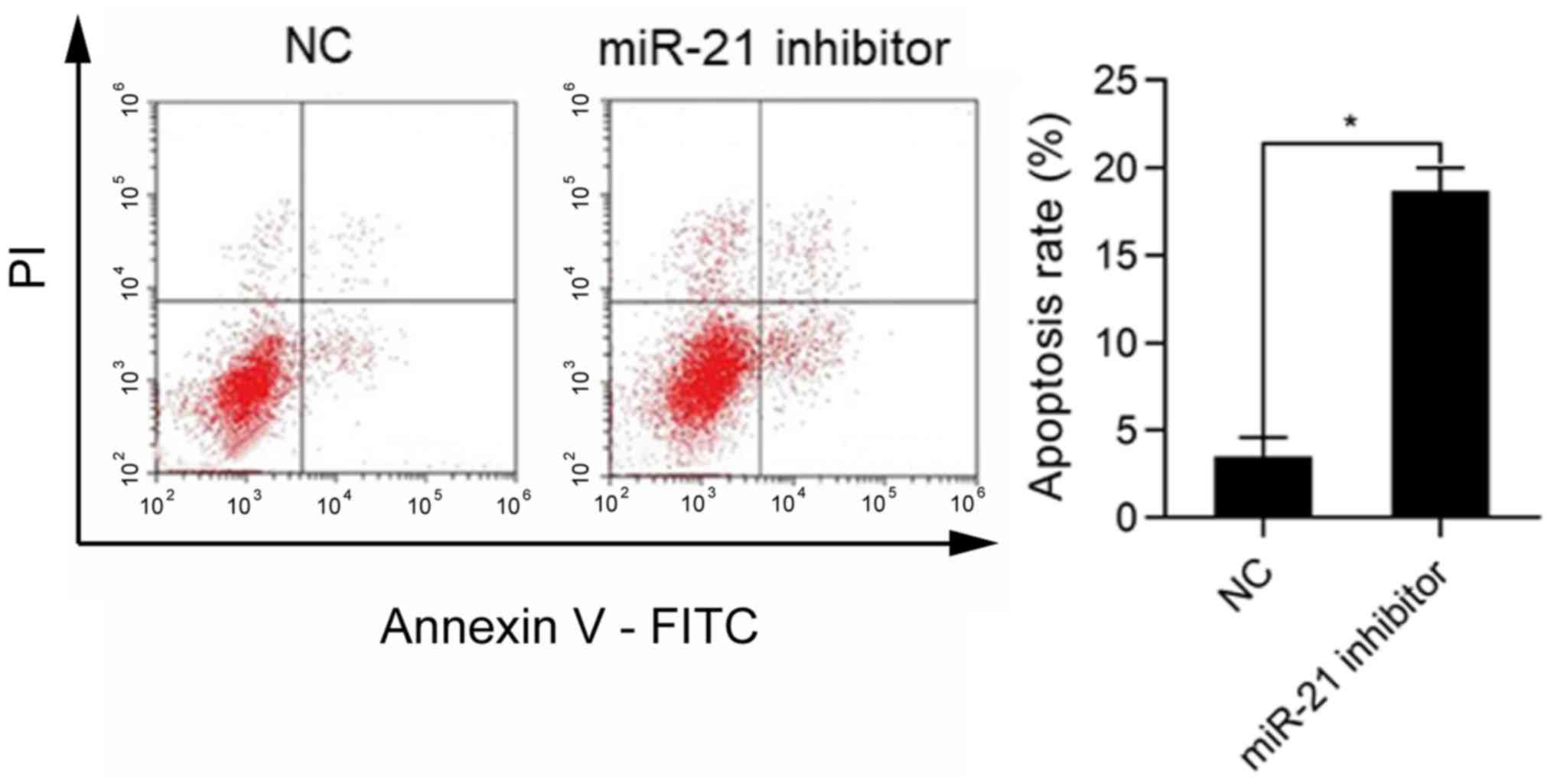
The role of miR-21 in apoptosis of WT cells was
investigated. The SK-NEP-1 cells were transfected with miR-21
inhibitor and cultured for 48 h, followed by detection of apoptosis
via flow cytometry. The results revealed that the ratio of
apoptotic cells was markedly higher in miR-21 inhibitor group than
that in NC group (P<0.05) (Fig.
2).
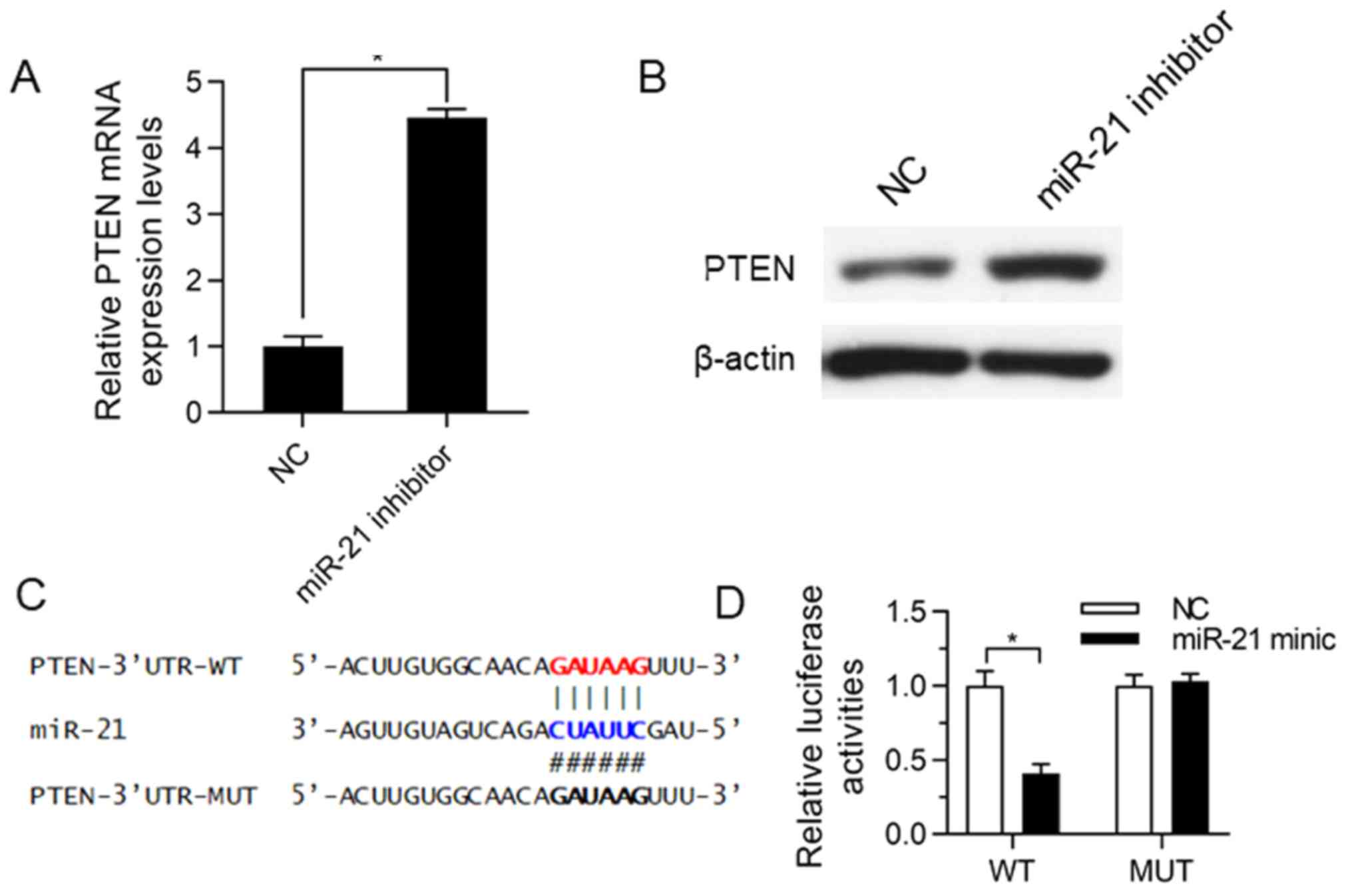
miR-21 bound to 3′-UTR of PTEN
Online TargetScan software was applied to analyze
miRNA target genes, and it was discovered that PTEN was the most
likely potential target of miR-21. The effects of miR-21 inhibition
on PTEN expression was verified in SK-NEP-1 cells. After the
SK-NEP-1 cells were transfected with miR-21 inhibitor and cultured
for 72 h, RT-qPCR assay and western blot analysis were performed to
detect the changes in PTEN at the mRNA and protein levels. The
results uncovered that repressing miR-21 expression upregulated the
PTEN expression at both the mRNA level (Fig. 3A, P<0.05) and the protein level
(Fig. 3B), implying that miR-21
negatively regulates PTEN. To confirm that PTEN is a functional
target of miR-21, the activity detection via dual luciferase
reporter gene assay was carried out using the cells co-transfected
with PTEN 3′-UTR WT/MUT and miR-21 minic (Fig. 3C). The miR-21 minic group exhibited
inhibited luciferase activity of WT plasmids but unchanged
luciferase activity of MUT plasmids, indicating that miR-21
suppresses the expression of PTEN by binding to PTEN 3′-UTR.
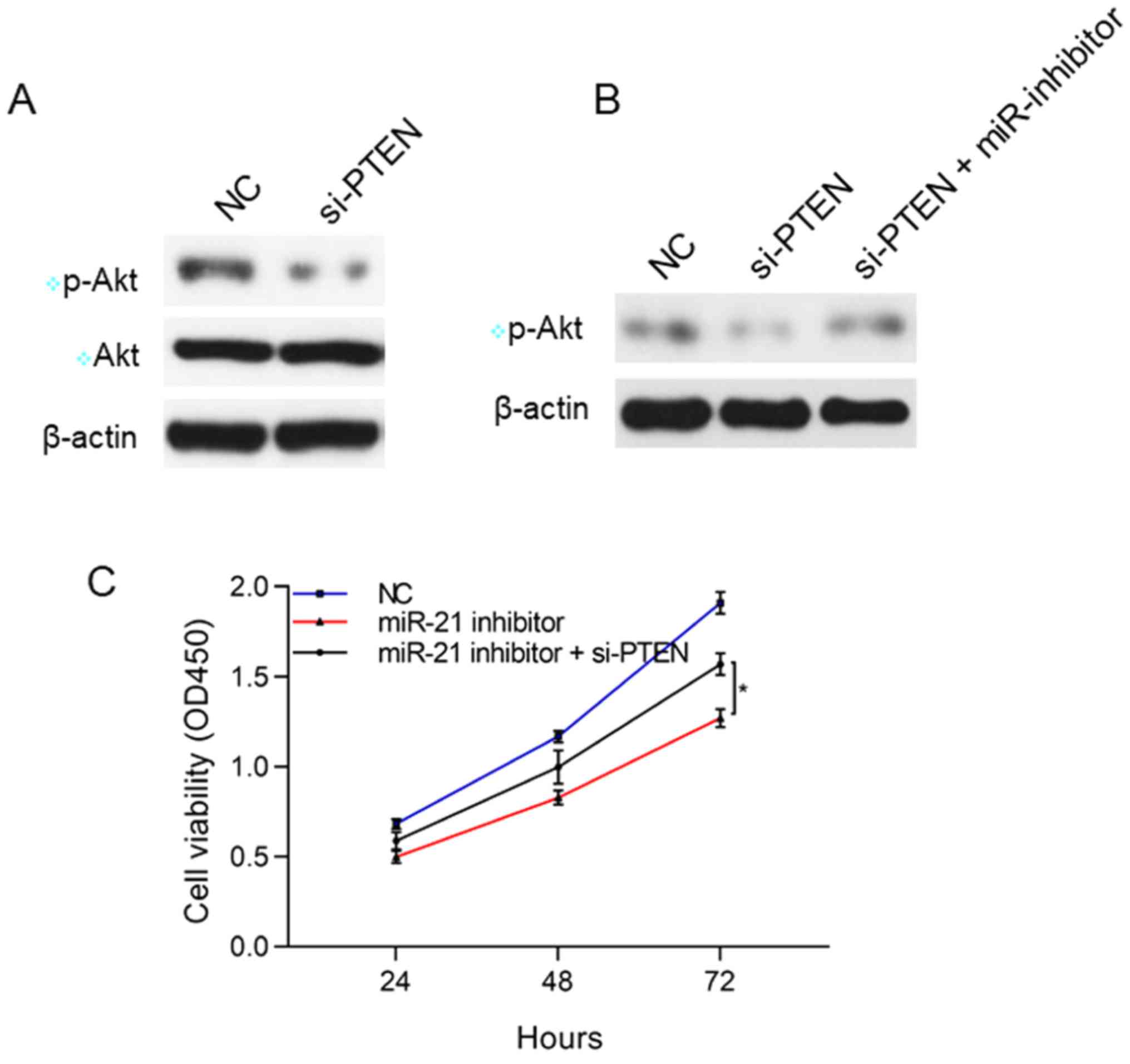
Correlation of miR-21 with PTEN and
Akt pathway
The suppression of PTEN expression may have a
relation to the Akt pathway. Therefore, the associations of miR-21
with PTEN and Akt were studied. The results showed that after
silencing PTEN, the p-Akt level rose in SK-NEP-1 cells, but the Akt
protein level had no obvious changes, suggesting that PTEN
negatively modulates p-Akt. After silencing both PTEN and miR-21,
the lower p-Akt was reversed, which thus reversed the inhibitory
effect of miR-21 on the proliferation of SK-NEP-1 cells
(P<0.05), implying that miR-21 inhibits the proliferation of the
WT SK-NEP-1 cells through the PTEN/Akt pathway (Fig. 4).
Discussion
WT is usually detected in children aged <5 years,
with an average age of onset of 38 months (2). WT is the most common malignant solid
tumor in children, but it is widely regarded as one of the most
treatable tumors due to modern comprehensive treatments in which
analyzing sensitive molecular biomarkers to guide treatment and
follow-up is playing a crucial role (4,12).
Currently, extensive research has manifested that
miRNAs can act as powerful cancer biomarkers (e.g., cancer type,
prognosis and response to treatment) to play an important role in
cell differentiation, proliferation, cell cycle control and
apoptosis. More than 50% of human miRNA genes are located in
vulnerable sites and regions and often associated with the
development of cancers (13), which
demonstrates the potential importance of miRNAs in cancers. In
addition, miRNAs have been verified to serve as oncogenes or tumor
suppressors and key molecules in the development and progression of
cancers (14). As one of the
overexpressed miRNAs most commonly found in solid tumors (10), miR-21 may act as an oncogene in the
development and progression of cancers.
Increasing number of studies have proved that
(15,16) miR-21 is an attractive target in the
multi-regulation of tumor genetics and pharmacology. Overexpression
of miR-21 in pancreatic endocrine and acinar tumors is closely
correlated with high Ki67 (a cell proliferation index) and liver
metastasis (17). Analysis of the
effects of anticancer chemotherapeutic agents such as
5-fluorouracil and gemcitabine on miRNAs has demonstrated that
repressing miR-21 increases the sensitivity to gemcitabine-induced
apoptosis, whereas miR-21 expression is increased after responding
to the process with 5-fluorouracil (18). Besides, miR-21 is also detected in
biological fluids such as serum. In comparison with healthy control
samples, serum samples from patients with diffuse large B-cell
lymphoma exhibit highly expressed miR-21. Moreover, the miR-21
expression in disease samples has an association with
recurrence-free survival, but it is not related to overall
survival. These methods highlight the potential application of
miRNAs in cancers as non-invasive diagnostic markers (19). In this study, it was discovered that
silencing miR-21 inhibited the proliferation of WT SK-NEP-1 cells
and induced apoptosis.
PTEN was originally discovered to participate in the
development of various diseases as a suppressor gene. PTEN
knockdown is able to accelerate cell proliferation. Researchers
have found that PTEN inhibits Akt activity by repressing PI3K
activity. Akt participates in angiogenesis and metastasis and
partially prolongs survival signals (20). The lack of PTEN may result in the
sustained activation of the signaling pathway, thus losing the
control of cell growth. Given this, PTEN upregulation is capable of
promoting cardiomyocyte apoptosis, while its inactivation can
reduce apoptosis (21). Previous
studies have reported that apoptosis can eliminate harmful
substances in cells, accordingly respond to cell invasion, provide
energy for subcellular structure production and metabolism, and
even maintain cell stability. The activated PI3K/Akt signaling
pathway has important effects on the differentiation, proliferation
and apoptosis of smooth muscle cells and vascular fibroblasts
(22). A recent study (23) showed that miR-21 may target the key
proteins of the PTEN/PI3K/Akt signaling pathway to mediate the
proliferation, apoptosis, migration and invasion of human
esophageal cancer cells and cell cycle progression. In this study,
it was confirmed through the dual luciferase reporter gene assay
that miR-21 bound to PTEN 3′-UTR to inhibit PTEN expression. Next,
the associations of miR-21 with PTEN and PI3K/Akt signaling pathway
were investigated, and it was uncovered that silencing PTEN
elevated the p-Akt level, but had no great impact on the Akt
protein level, suggesting that PTEN negatively modulates p-Akt.
After silencing both PTEN and miR-21, the decrease in p-Akt was
reversed.
In conclusion, the results of this study suggest
that miR-21 mediates the proliferation repression in the WT
SK-NEP-1 cells through the PTEN/Akt pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XZ and LG designed the study and performed the
experiments, XZ and CL collected the data, LG and HL analyzed the
data, XZ and LG prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Liu G, Zhang Y, Fu K, Hu J, Zhao Z, Fu W
and Liu G: Meta-analysis of the effect of preoperative chemotherapy
on Wilms' tumor. J BUON. 23:211–217. 2018.PubMed/NCBI
|
|
2
|
Davidoff AM: Wilms' tumor. Curr Opin
Pediatr. 21:357–364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu CL, Wang WH, Sun YL, Zhuang HW, Xu M,
Chen HF and Liu JX: miR-144-3p inhibits the proliferation and
metastasis of pediatric Wilms' tumor cells by regulating Girdin.
Eur Rev Med Pharmacol Sci. 22:7671–7678. 2018.PubMed/NCBI
|
|
4
|
Grundy PE, Breslow NE, Li S, Perlman E,
Beckwith JB, Ritchey ML, Shamberger RC, Haase GM, D'Angio GJ,
Donaldson M, et al National Wilms Tumor Study Group, : Loss of
heterozygosity for chromosomes 1p and 16q is an adverse prognostic
factor in favorable-histology Wilms tumor: A report from the
National Wilms Tumor Study Group. J Clin Oncol. 23:7312–7321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rivera MN and Haber DA: Wilms' tumour:
Connecting tumorigenesis and organ development in the kidney. Nat
Rev Cancer. 5:699–712. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gammell P: MicroRNAs: recently discovered
key regulators of proliferation and apoptosis in animal cells:
Identification of miRNAs regulating growth and survival.
Cytotechnology. 53:55–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ludwig N, Werner TV, Backes C, Trampert P,
Gessler M, Keller A, Lenhof HP, Graf N and Meese E: Combining miRNA
and mRNA expression profiles in Wilms tumor subtypes. Int J Mol
Sci. 17:4752016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang X and Li H: miR-1180-5p regulates
apoptosis of Wilms' tumor by targeting p73. OncoTargets Ther.
11:823–831. 2018. View Article : Google Scholar
|
|
9
|
Liu GL, Yang HJ, Liu B and Liu T: Effects
of microRNA-19b on the proliferation, apoptosis, and migration of
Wilms' tumor cells via the PTEN/PI3K/AKT signaling pathway. J Cell
Biochem. 118:3424–3434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krichevsky AM and Gabriely G: miR-21: A
small multi-faceted RNA. J Cell Mol Med. 13:39–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gratias EJ, Jennings LJ, Anderson JR, Dome
JS, Grundy P and Perlman EJ: Gain of 1q is associated with inferior
event-free and overall survival in patients with favorable
histology Wilms tumor: A report from the Children's Oncology Group.
Cancer. 119:3887–3894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Selcuklu SD, Donoghue MT and Spillane C:
miR-21 as a key regulator of oncogenic processes. Biochem Soc
Trans. 37:918–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng S, Calin GA, Croce CM, Coukos G and
Zhang L: Mechanisms of microRNA deregulation in human cancer. Cell
Cycle. 7:2643–2646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren Y, Zhou X, Mei M, Yuan XB, Han L, Wang
GX, Jia ZF, Xu P, Pu PY and Kang CS: MicroRNA-21 inhibitor
sensitizes human glioblastoma cells U251 (PTEN-mutant) and LN229
(PTEN-wild type) to taxol. BMC Cancer. 10:272010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal
K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the
PTEN tumor suppressor gene in human hepatocellular cancer.
Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roldo C, Missiaglia E, Hagan JP, Falconi
M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, et
al: MicroRNA expression abnormalities in pancreatic endocrine and
acinar tumors are associated with distinctive pathologic features
and clinical behavior. J Clin Oncol. 24:4677–4684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rossi L, Bonmassar E and Faraoni I:
Modification of miR gene expression pattern in human colon cancer
cells following exposure to 5-fluorouracil in vitro. Pharmacol Res.
56:248–253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lawrie CH: MicroRNAs and haematology:
Small molecules, big function. Br J Haematol. 137:503–512. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Panigrahi AR, Pinder SE, Chan SY, Paish
EC, Robertson JF and Ellis IO: The role of PTEN and its signalling
pathways, including AKT, in breast cancer; an assessment of
relationships with other prognostic factors and with outcome. J
Pathol. 204:93–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mocanu MM and Yellon DM: PTEN, the
Achilles' heel of myocardial ischaemia/reperfusion injury? Br J
Pharmacol. 150:833–838. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gregorian C, Nakashima J, Le Belle J, Ohab
J, Kim R, Liu A, Smith KB, Groszer M, Garcia AD, Sofroniew MV, et
al: Pten deletion in adult neural stem/progenitor cells enhances
constitutive neurogenesis. J Neurosci. 29:1874–1886. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu YR, Qi HJ, Deng DF, Luo YY and Yang SL:
MicroRNA-21 promotes cell proliferation, migration, and resistance
to apoptosis through PTEN/PI3K/AKT signaling pathway in esophageal
cancer. Tumour Biol. 37:12061–12070. 2016. View Article : Google Scholar : PubMed/NCBI
|