Introduction
Inflammation is a response of tissues to chemical
and mechanical injury or infection, which is usually caused by
various bacteria (1). The
inflammatory response or chronic infections may cause significant
damage to the host, including rheumatoid arthritis and psoriasis.
Lipopolysaccharide (LPS), a component of the outer membrane of
gram-negative bacteria, initiates a number of major cellular
responses that serve critical roles in the pathogenesis of
inflammatory responses (2). LPS may
lead to an acute inflammatory response towards pathogens. Bacterial
LPS has been extensively used to establish an inflammatory model as
it stimulates the release of inflammatory cytokines including
interleukin (IL)-8, IL-6 and IL-1β in various cell types (3,4).
Toll-like receptor 4 (TLR4) is the cell-surface
receptor for LPS. Regulation of TLR4 activation involves
glycosylphosphatidylinositol (GPI)-anchored monocyte
differentiation antigen CD14 (CD14), lymphocyte antigen 96 (MD-2),
and the lipopolysaccharide-binding protein (LBP). LBP binds to the
lipid A moiety of LPS and transfers LPS to CD14, which guarantees
and optimizes signaling through the TLR4/MD-2 complex (5). A total of 2 signaling pathways are
initiated by TLR4 activation; one leads to the activation of NF-κB
and mitogen-activated protein kinases (MAPKs) through the
recruitment and activation of myeloid differentiation primary
response protein MyD88 (MyD88) and Toll/interleukin-1 receptor
domain-containing adapter protein (TIRAP). The other pathway is
modulated by TIR domain-containing adapter molecule 2 (TRAM) and
TIR domain-containing adaptor molecule 1 (TRIF), requiring the
internalization of TLR4, which activates IκB kinase and interferon
(IFN) regulatory factor 3 (IRF3), leading to the induction of type
1 IFN genes (6). These cascaded
transcriptional reactions induce robust expressions of thousands of
genes, finally regulating the release of inflammatory cytokines and
anti-inflammatory factors. Therefore, the TLR4/NF-κB/MAPKs pathways
are considered as some of the primary signaling pathways involved
in inflammatory response (7).
Resveratrol (3,4′, 5-Trihydroxy-trans-stilbene;
Res), a type of natural phytoalexin polyphenol with marked
biological effects, is present in a number of plants. It has been
suggested that Res has a number of therapeutic properties,
including antioxidant, cardio-protective, antiviral, anti-aging and
anti-inflammatory effects (8). At
present, Res is present in food, medicine and health care products.
One of the primary ways that Res exerts its anti-inflammatory
activity is regulation of a number of signaling pathways. It has
been suggested that Res may inhibit the NF-κB activation induced by
TLR4-mediated signaling (9). In
addition, another important anti-inflammatory action of Res it the
suppression of LPS-induced TNF receptor-associated factor 6 (TRAF6)
expression and ubiquitination, consequently attenuating the
LPS-induced TLR4-TRAF6, MAPK and Akt pathways (10). Previous data suggests that Res
inhibits the inflammation via regulating the NF-κB, MAPKs, TLR4 and
AKT signaling pathways; however, to the best of our knowledge, the
combined evaluation of all these pathways following Res treatment
has not been performed. Therefore, the aim of present study was to
evaluate the association between the anti-inflammatory effect of
Res and the production of inflammatory factors and finally to
reveal the protective mechanism of Res in LPS-induced
inflammation.
Materials and methods
Reagents
Res was purchased from Beijing Solarbio Science and
Technology Co., Ltd. SP600125 (cat. no. HY-12041), BAY11-7082 (cat.
no. HY-13453) and SB203580 (cat. no. HY-10256) were purchased from
MedChemExpress. LPS (Escherichia coli 055:B5; cat. no. L2880) and
L-glutamine (cat. no. G3126) were purchased from Sigma-Aldrich;
Merck KGaA The mice mononuclear macrophage RAW264.7 cell line was
obtained from the China Center for Type Culture Collection. Fetal
bovine serum was purchased from Beijing TransGen Biotech Co., Ltd.
The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo
Molecular Technologies. Dulbecco's modified Eagle medium (DMEM) and
PBS were purchased from HyClone; GE Healthcare Life Sciences.
Revert Aid first-strand cDNA synthesis kit was purchased from
Thermo Fisher Scientific, Inc. The total protein extraction kit for
cultured cells was purchased from Wuhan Boster Biological
Technology, Ltd. (cat. no. AR0103). The BCA kit was purchased from
Beijing Solarbio Science and Technology Co., Ltd. (cat. no.
PC0020). IQ SYBR Supermix extraction reagent and TRIzol®
reagent were purchased from Bio-Rad Laboratories, Inc. Mouse tumor
necrosis factor-α (TNF-α; cat. no. ml002095), IL-6 (cat. no.
ml063159) IL-8 (cat. no. ml058632), IL-10 (cat. no. ml002285) and
IFN-β (cat. no. ml063095) ELISA kits were purchased from Shanghai
Enzyme-linked Biotechnology Co., Ltd. Anti-β-actin (cat. no.
12620), anti-NF-κB inhibitor (IκBα; cat. no.
4814), extracellular signal-regulated kinase (ERK; cat. no. 4695),
p38 MAPK (cat. no. 8690), phosphorylated (p)-IκBα (cat. no. 2859),
p-p38 MAPK (cat. no. 4511) and p-ERK (cat. no. 4370) antibodies
were purchased from Cell Signaling Technology, Inc.
Stress-activated kinases (SAPK)/c-Jun N-terminal kinase (JNK; cat.
no. ab179461), phospho-SAPK/JNK (cat. no. ab76572), IRF3 (cat. no.
ab76493), phospho-IRF3 (cat. no. ab68481) and TLR4 (cat. no.
ab13556) antibodies were purchased from Abcam. Secondary antibody
for goat anti-rabbit and anti-mouse immunoglobulin (IgG)
horseradish peroxidase (HRP) were acquired from BIOSS (cat. nos.
BS-0293G and BS-0296G).
Cell culture
RAW264.7 cells were cultured in endotoxin-tested
DMEM with 10% fetal calf serum (FCS) supplied by Beijing Transgen
Biotech Co., Ltd. in the presence of 5% CO2 at 37°C.
Prior to treatment, cells were incubated overnight at 37°C.
Cell viability assay
CCK-8 assays to measure the viability of cells.
Briefly, the cells were cultured into 96-wells plate with a density
of 5×103 cells/well and incubated overnight in the
presence of 5% CO2 at 37°C. Then the cells were washed
with fresh 1% FCS and treated with or without LPS (625, 1,500,
2,500, 5,000 and 10,000 ng/ml), Res (6.25, 12.5, 25, 50 and 100
µM), BAY11-7082 (the inhibitor of NF-κB; 2.5, 5, 10, 20 and 40 µM),
SP600125 (the broad-spectrum inhibitor of JNK; 2.5, 5, 10, 20 and
40 µM) or SB203580 (the inhibitor of p38 MAPK; 2.5, 5, 10, 20 and
40 µM) for 24 h. The cells were washed with PBS. In each well, 10
µl CCK-8 solution was added and incubated for 1 h at 37°C. The
absorbance was measured at 450 nm. The cell viability was
calculated and represented graphically.
Stimulation conditions of LPS and Res
concentration
RAW264.7 cells in 6-well (5×105 cells/ml)
plates were pretreated with 62.5–500 ng/ml LPS for 12 and 24 h. The
TNF-α and IL-6 levels were measured using ELISAs. To investigate
the optimal Res concentration against LPS-induced inflammation,
cells were pretreated with 6.25–25 µM of Res for 2 h followed by
the stimulation with LPS (500 ng/ml) for 12 h. The TNF-α and IL-6
levels were analyzed using ELISA.
Western blot analysis
RAW264.7 cells in 6-well (5×105 cells/ml)
plates were pretreated with different concentrations of Res (6.25,
12.5 and 25 µM) for 2 h prior to the addition of 500 ng/ml LPS for
12 h. After incubation for 12 h, the total proteins were extracted
following the instructions of protein extraction kit. Protein
concentrations were measured by the BCA Protein Assay kit.
Following this, equal amounts of protein (25 µg) from each sample
were heated to 95°C for 5 min with 4X Protein SDS-PAGE Loading
Buffer and then separated by SDS-PAGE (30% gel). Proteins were
transferred onto polyvinylidene fluoride membranes. Following
blocking for 1 h with 5% skim milk at room temperature, the
membranes were incubated with primary antibodies against β-actin,
IκBα, p-IκBα, p38 MAPK, p-p38 MAPK, IRF3, p-IRF3, ERK1/2, p-ERK1/2,
JNK, p-JNK overnight at 4°C. Following washing 3 times with TBST
buffer, the membranes were incubated with the aforementioned
secondary antibodies for 1 h at room temperature. The
electrochemiluminescence kit purchased from Beijing Solarbio
Science & Technology Co., Ltd. (cat. no. PE0010) was used to
detect the bands. ImageJ v1.8.0 software (National Institutes of
Health) and GraphPad Prism v.6 software (GraphPad Software, Inc.)
were used to perform the densitometric analysis.
Cytokine assays
RAW264.7 cells in 6-well (5×105 cells/ml)
plates were pretreated with Res (25 µM), SP600125 (10 µM), SB203580
(20 µM) or BAY11-7082 (10 µM) for 2 h prior to the addition of 500
ng/ml LPS for 12 h. Then, the supernatants were collected by
centrifugation at 0.12 × g for 10 min at 4°C. Levels of TNF-α,
IL-6, IL-10, IL-8 and IFN-β were measured by ELISA kits.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RAW264.7 cells in 6-well (5×105 cells/ml)
plates were pretreated with Res (25 µM), SP600125 (10 µM), SB203580
(20 µM) or BAY11-7082 (10 µM) for 2 h prior to adding 500 ng/ml LPS
for 12 h. Then, the supernatants were harvested at 0.12 × g for 10
min at 4°C. The total RNA of RAW264.7 cells samples were extracted
using TRIzol® according to the manufacturer's protocol.
Then, RNA quality was determined by measuring the 260/280 ratio.
Samples measuring >2.0 were considered to be of sufficient
quality for further analysis. A total of ~1.5 µg total RNA was
reverse transcribed to cDNA following the Revert Aid first-strand
cDNA synthesis kit. The primer sequences are presented in Table I. RT-qPCR was used to estimate the
mRNA expression levels using the IQ SYBR Supermix extraction
reagent. Quantification cycle (Cq) values were recorded and the
relative expression level of target genes was calculated using the
2−ΔΔCq method (7,11).
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primer sequences. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primer sequences.
| Gene | Peimer
sequences |
|---|
| TNF-α | F:
5′-CCCTCACACTCAGATCATCTTCT-3′ |
|
| R:
5′-GCTACGACGTGGGCTACAG-3′ |
| IL-6 | F:
5′-TAGTCCTTCCTACCCCAATTTCC-3′ |
|
| R:
5′-TTGGTCCTTAGCCACTCCTTC-3′ |
| IL-8 | F:
5′-TGTGGGAGGCTGTGTTTGTA-3′ |
|
| R:
5′-ACGAGACCAGGAGAAACAGG-3′ |
| IFN-β | F:
5′-CAGCTCCAAGAAAGGACGAAC-3′ |
|
| R:
5′-GGCAGTGTAACTCTTCTGCAT-3′ |
| IL-10 | F:
5′-GCTCTTACTGACTGGCATGAG-3′ |
|
| R:
5′-CGCAGCTCTAGGAGCATGTG-3′ |
| MyD88 | F:
5′-ACTCGCAGTTTGTTGGATG-3′ |
|
| R:
5′-CACCTGTAAAGGCTTCTCG-3′ |
| TRAM | F:
5′-AGCCAGAAAGCAATAAGC-3′ |
|
| R:
5′-CAAACCCAAAGAACCAAG-3′ |
| TLR4 | F:
5′-GGACTCTGATCATGGCACTG-3′ |
|
| R:
5′-CTGATCCATGCATTGGTAGGT-3′ |
| GAPDH | F:
5′-GGTGAAGGTCGGTGTGAACG-3′ |
|
| R:
5′-CTCGCTCCTGGAAGATGGTG-3′ |
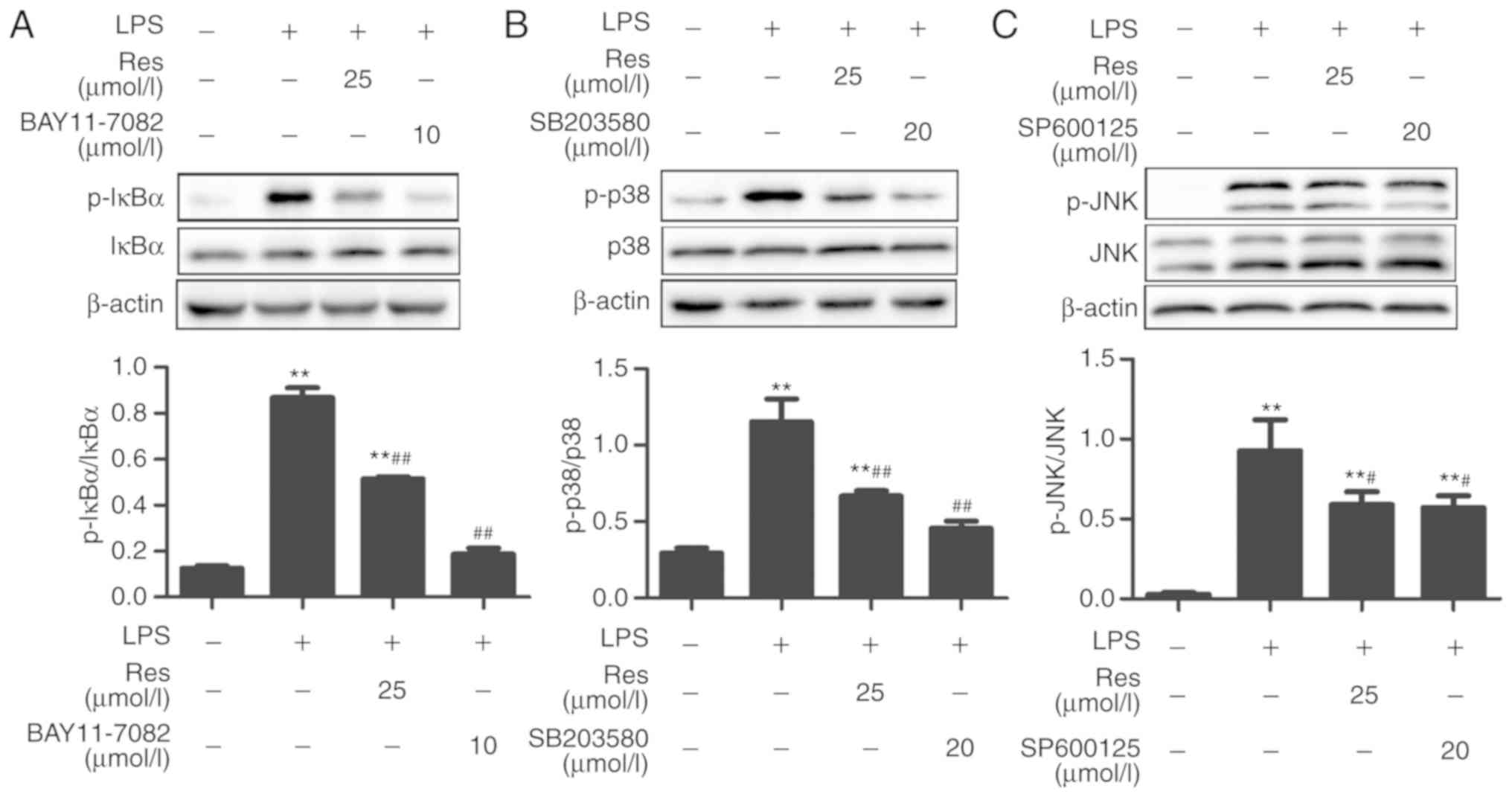
Effects of Res on LPS-induced
signaling pathway
RAW264.7 cells in 6-well (5×105 cells/ml)
plates were pretreated with Res (25 µM), SB203580 (20 µM), SP600125
(10 µM) or BAY11-7082 (10 µM) for 2 h prior to the addition of 500
ng/ml LPS for 12 h. The phosphorylation of IκBα, p38 MAPK and JNK
in the in the presence of specific inhibitors or Res were
measured.
To measure the expression of TLR4, RAW264.7 cells in
6-well (5×105 cells/ml) plates were pretreated with different
concentrations of Res (6.25, 12.5 or 25 µM) for 2 h prior to the
addition of 500 ng/ml LPS for 12 h. The expression of TLR4 was
measured.
Statistical analysis
The data were expressed as the mean ± standard
deviation. The SPSS 17.0 software was used for data analysis.
GraphPad Prism v.6 software (GraphPad Software, Inc.) was used to
analyze the cytokine concentrations, mRNA expression levels and
protein densitometry data. Comparisons between the control and
experimental groups were made using a one way analysis of variance
followed by a Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
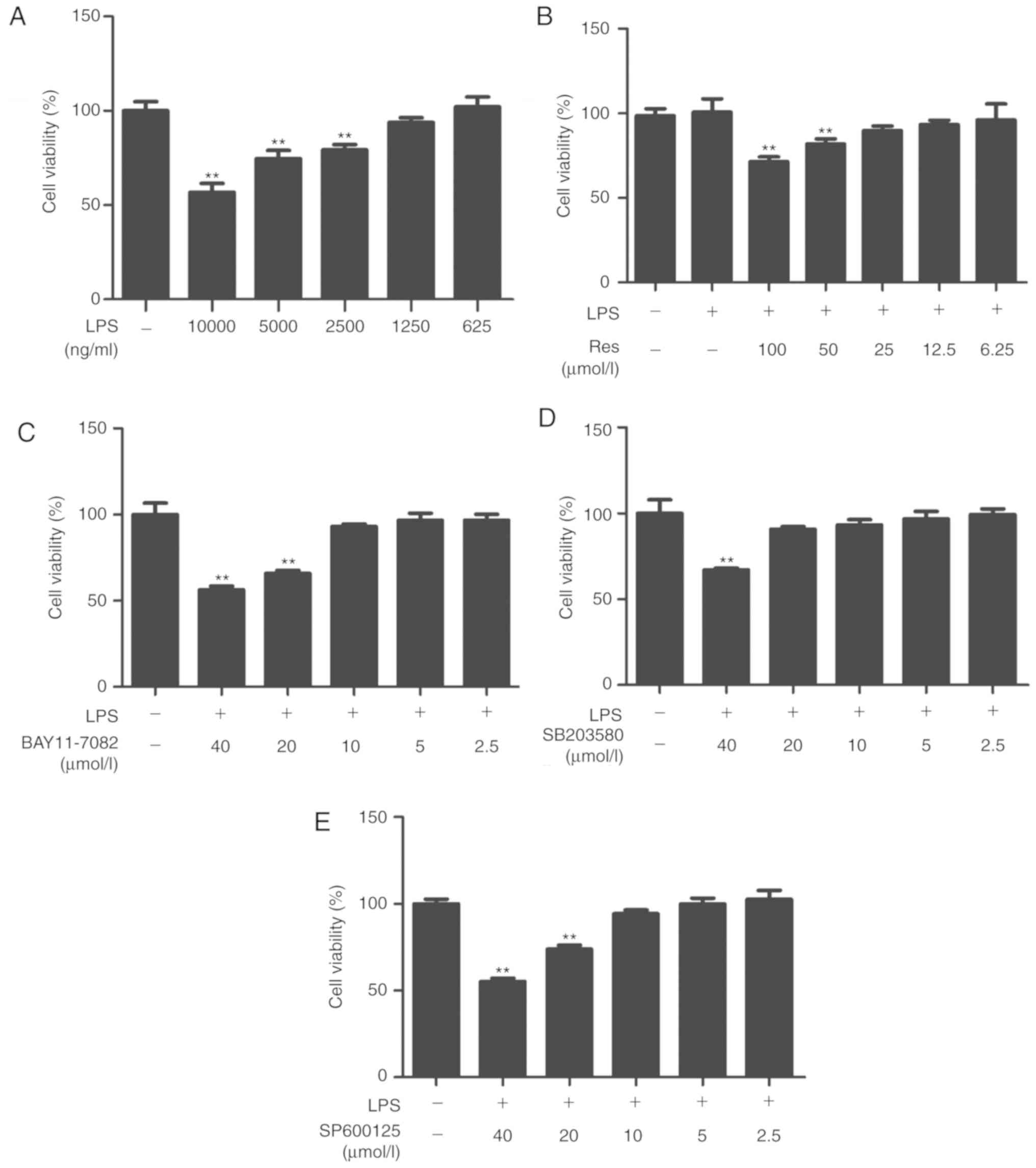
Cell viability
Res and inhibitors were examined for toxicity
against RAW264.7 cells in the absence or presence of LPS for 24 h.
The maximum non-toxic concentrations of LPS, Res, BAY11-7082,
SP600125 and SB203580 were 1250 ng/ml, 25, 10, 10 and 20 µM,
respectively (Fig. 1). Therefore, in
subsequent experiments, cells were treated with the different
compounds at nontoxic concentrations.
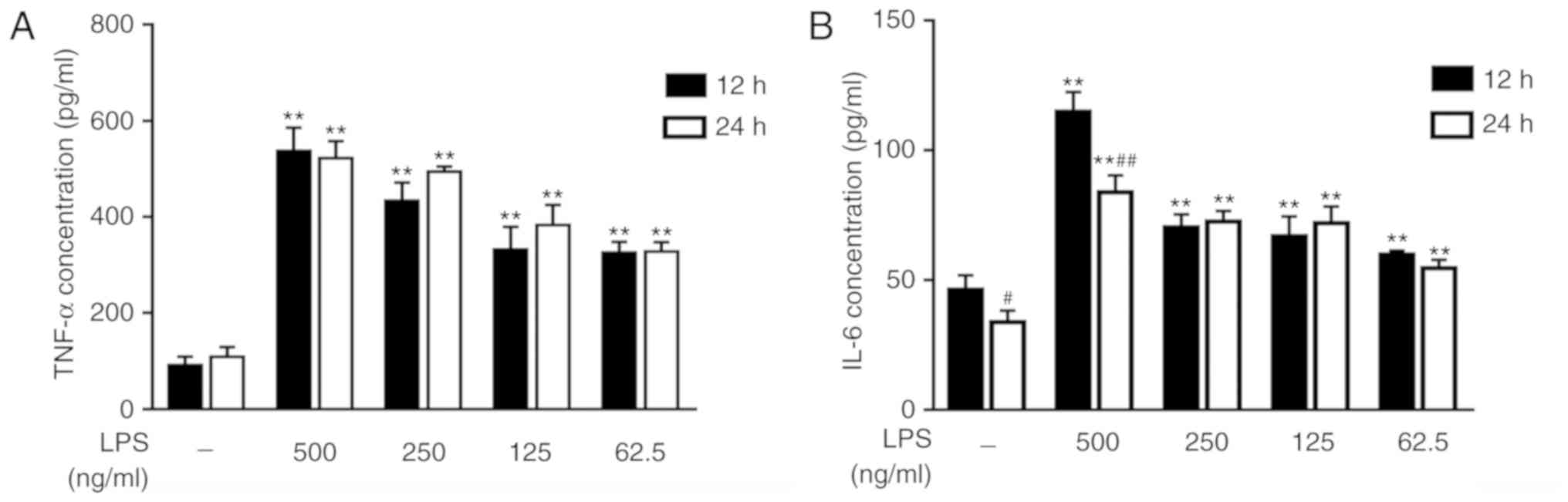
Appropriate stimulus conditions
To explore the appropriate stimulus conditions,
RAW264.7 cells were treated with different concentrations of LPS
for 12 or 24 h. ELISA was used to measure the concentrations of
TNF-α and IL-6, which are two inflammatory cytokines. The results
indicated that RAW264.7 cells, under stimulation with LPS,
expressed increased TNF-α and IL-6 levels compared with the
untreated cells. With the increase of LPS concentration, the
expression levels of TNF-α and IL-6 were increased (Fig. 2A and B). In terms of treatment time
intervals, there was no significant difference observed in the
contents of TNF-α between the groups (Fig. 2A). These results revealed that LPS
stimulated TNF-α and IL-6 expression, and suggested that adding 500
ng/ml LPS to the cells and culturing for 12 h is an appropriate
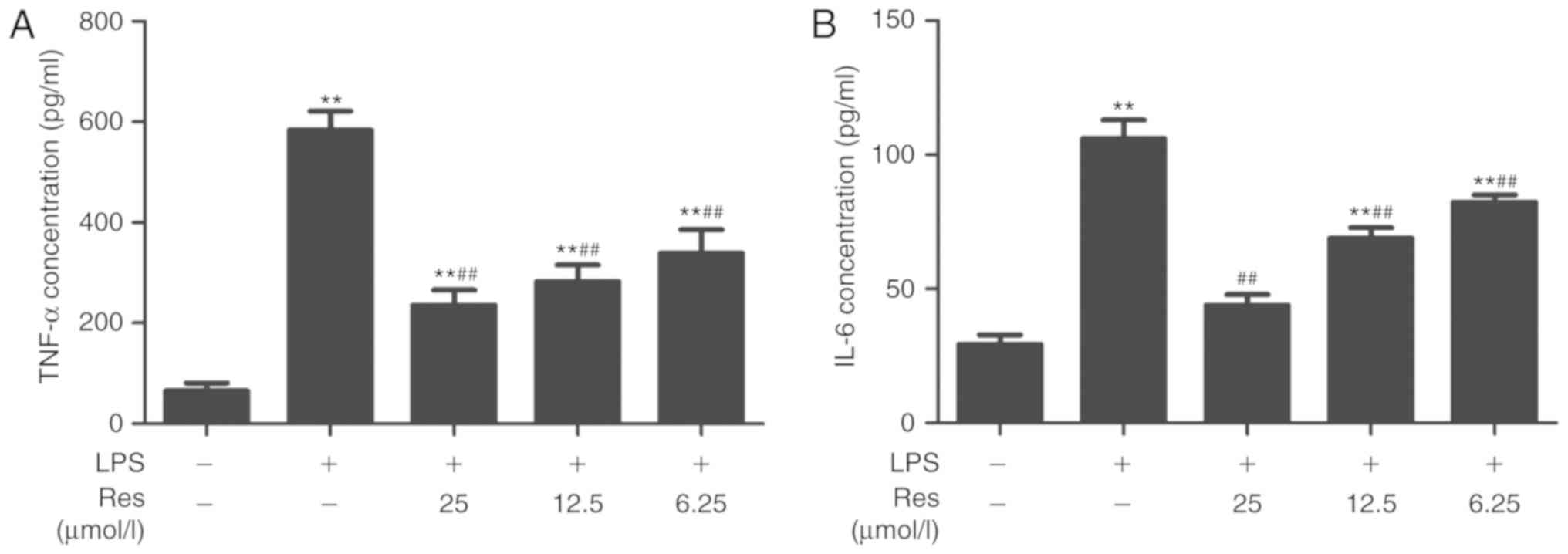
cell model of LPS-induced inflammation. Res inhibited the secretion
of TNF-α and IL-6 (Fig. 3A and B)
and effective dose was between 6.25–25 µM.
Effects of Res on LPS-induced NF-κB
signaling pathway
The IκB/NF-κB signaling pathway has been suggested
to regulate a number of the genes involved in the inflammatory
response and the production of inflammatory cytokines and
pro-inflammatory enzymes (2,12). To investigate whether Res inhibited
the NF-κB signaling pathway, the effects of Res on IkB
phosphorylation were first investigated. As demonstrated in
Fig. 4A, the protein level of the
p-IκBα in the LPS group was increased compared with that in the
blank control group. Compared with the untreated cells, LPS-induced
phosphorylation of IκB was significantly decreased in the
Res-treated cells in a concentration-dependent manner.
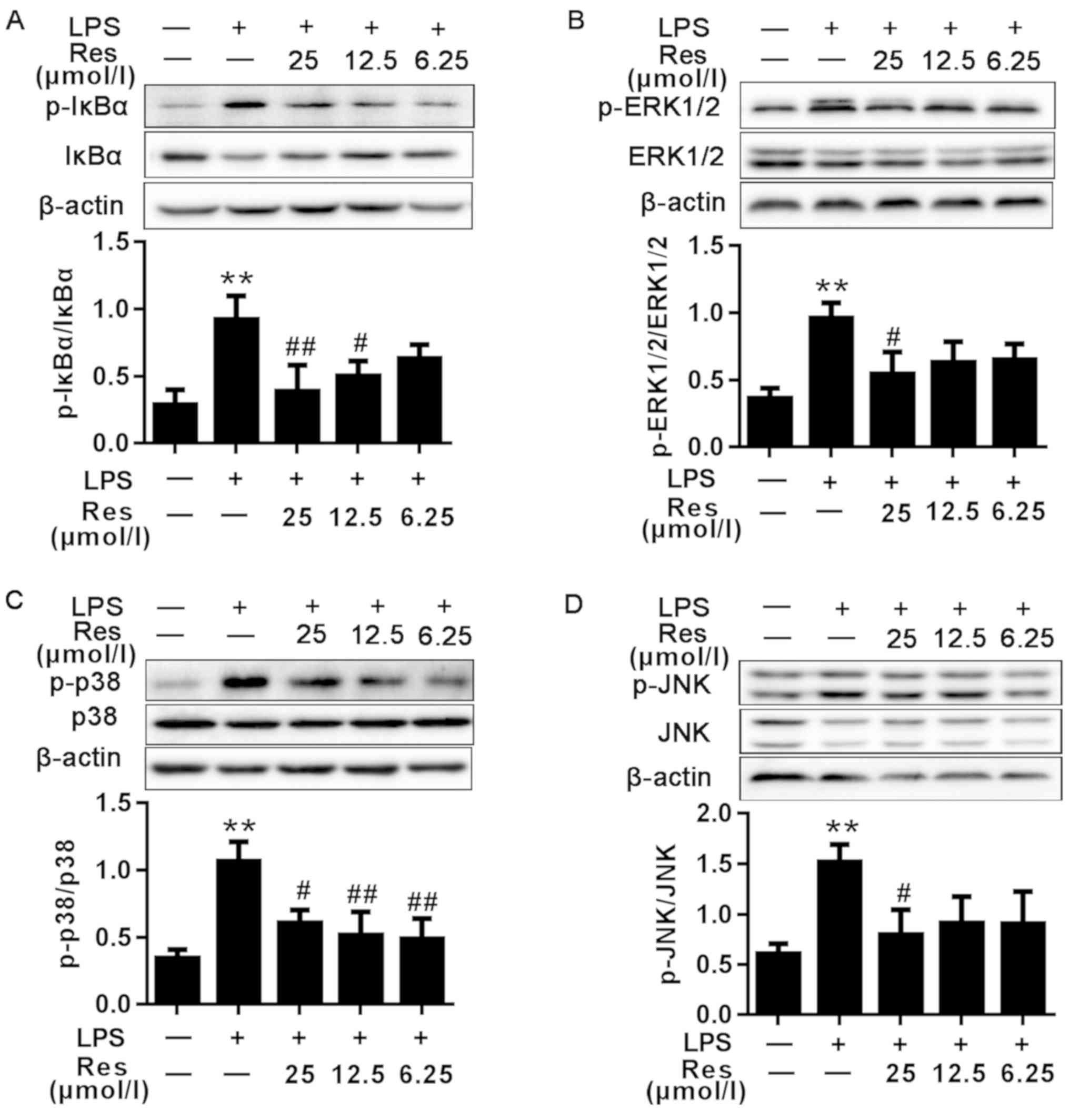 | Figure 4.Effects of different concentrations
of Res on LPS-induced NF-κB, MAPKs and IRF3 signaling pathway.
Protein samples were analyzed by western blot analysis with
specific antibodies. (A) The expression levels of the total and
phosphorylated proteins associated with the NF-κB signaling
pathway. (B-E) The expression levels of the total and
phosphorylated proteins associated with the (B-D) MAPKs and (E)
IRF3 signaling pathways. Data are presented as the mean ± standard
error of the mean (n=3). **P<0.01 vs. control; #P<0.05 and
##P<0.01 vs. LPS. Res, resveratrol; LPS, lipopolysaccharide;
MAPKs, mitogen-activated protein kinases; IκBα, NF-κB inhibitor;
ERK1/2, extracellular signal-regulated kinase; p38, p38 MAPK; JNK,
c-Jun N-terminal kinase; p-, phosphorylated; IRF3, interferon
regulatory factor 3. |
Effects of Res on LPS-induced MAPKs
induction
The MAPK pathway serves a key role in the
LPS-induced inflammatory (7).
Therefore, whether Res inhibited inflammation through the MAPK
pathway was determined. As indicated in Fig. 4B-D, LPS was able to stimulate the
phosphorylation of ERK, p38 MAPK and JNK, whereas the
phosphorylation of these proteins was inhibited by pretreatment
with Res.
Effects of Res on LPS-induced IRF3
signaling pathway
IRF3 has been demonstrated to regulate cell
proliferation, apoptosis, inflammation, innate immune responses and
insulin resistance (13–16). It can be activated by LPS.
TRIF-mediated signaling in response to LPS challenge may activate
IRF3, resulting in the production of IFN-β, IP-10 and other
IRF-3-dependent genes (17). As
shown in Fig. 4E, compared with the
blank group, the phosphorylated protein level of IRF3 in the
LPS-only group was increased. However, following treatment with
Res, the expression levels of the phosphorylated proteins were
decreased compared with the LPS-only group.
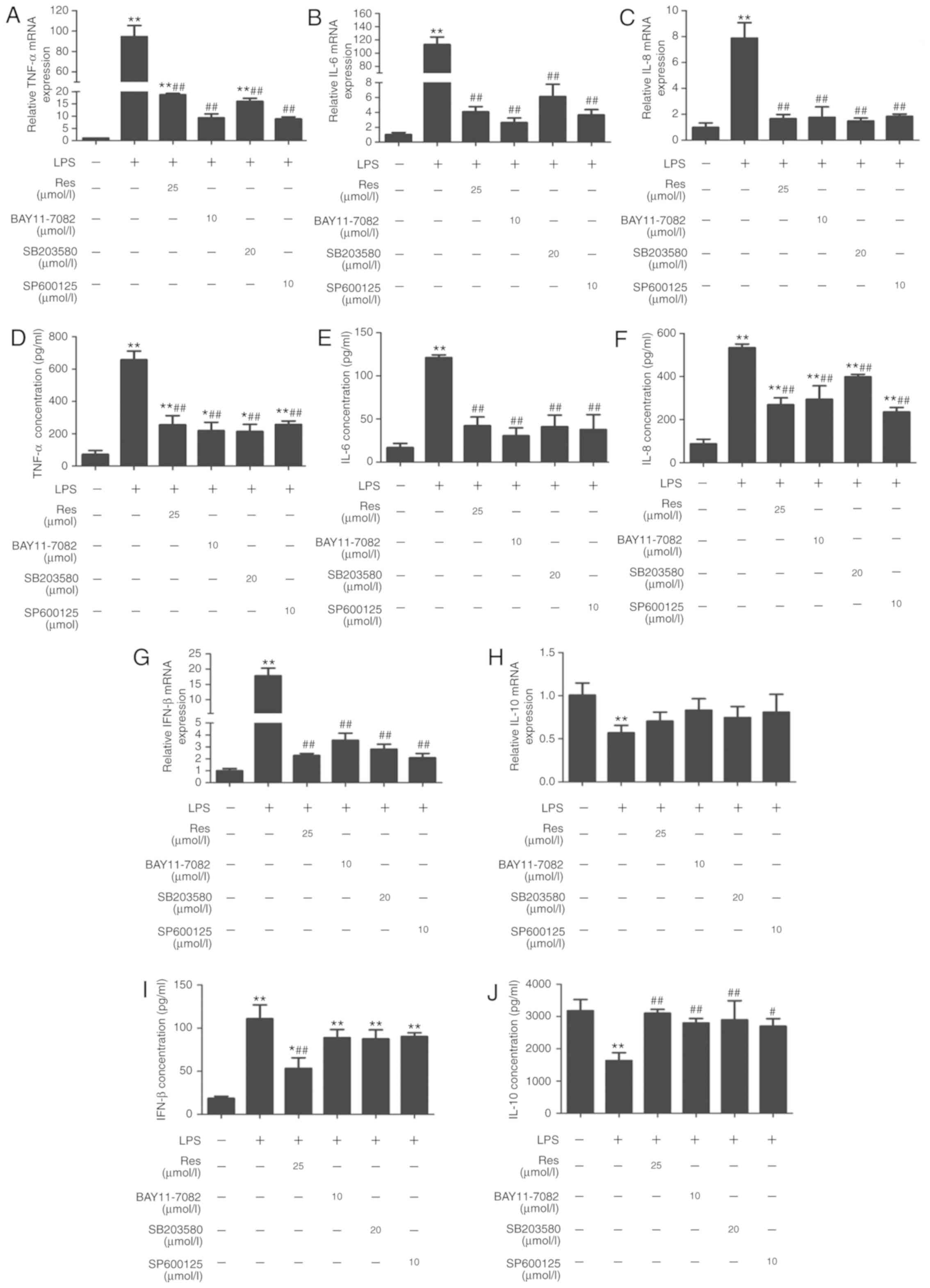
Res inhibits the LPS-induced
pro-inflammatory cytokines production
The pro-inflammatory cytokines IL-1β, TNF- α, IL-6,
IL-8 and IL-10 serve pivotal roles in inflammation progression as a
result of monocyte activation (18).
To explore the effect of Res on the expression levels of these
inflammatory mediators, LPS-stimulated cells were treated with Res
(25 µM) and inhibitors including SP600125 (10 µM), BAY11-7082 (10
µM), SB203580 (20 µM) for 2 h. Then, the mRNA levels and serum
concentrations of TNF-α, IL-6, IL-8, IFN-β and IL-10 were detected
by RT-qPCR and ELISA, respectively. Res and the pathway-specific
inhibitors successfully inhibited the expression and secretion of
TNF-α, IL-6, IL-8 and IFN-β, which were increased by LPS treatment
(Fig. 5A-G and I). By contrast, the
level of IL-10 was decreased following LPS-stimulation, and was
recovered following Res treatment (Fig.
5H and J).
Res serves an anti-inflammatory effect through
the MAPK and NF-κB pathway. The above experiments
investigated whether Res had an impact on the expression of
phosphorylated proteins including IκBα, p38 MAPK, JNK, ERK1/2 and
IRF3. The results revealed that Res inhibited IκBα, p38 MAPK and
JNK activation through the MAPK and NF-κB pathways. In order to
reveal the protective mechanism of Res on LPS-induced RAW264.7
cells inflammation, the RAW264.7 cells were pretreated with 25 µM
Res and inhibitors, including SP600125 (10 µM), BAY11-7082 (10 µM)
and SB203580 (20 µM) for 2 h, followed by treatment with LPS for 12
h.
For IκBα, the expression level of its phosphorylated
form in the LPS group was increased compared with the control
group, but it was significantly decreased following treatment with
Res. Similar results were obtained when Res was replaced by
BAY11-7082, which is a selective inhibitor of NF-κB kinase
(Fig. 6A). There was an increased
expression of p-p38 MAPK in the LPS-treated group as compared with
the control group. However, when RAW264.7 cells were pretreated
with SB203580, a specific p38 MAPK signaling inhibitor, this
increase was markedly inhibited, which was similar to the effects
of Res treatment (Fig. 6B). Similar
results were obtained when the inhibitor was replaced by SP600125,
which is a broad-spectrum inhibitor of JNK (Fig. 6C).
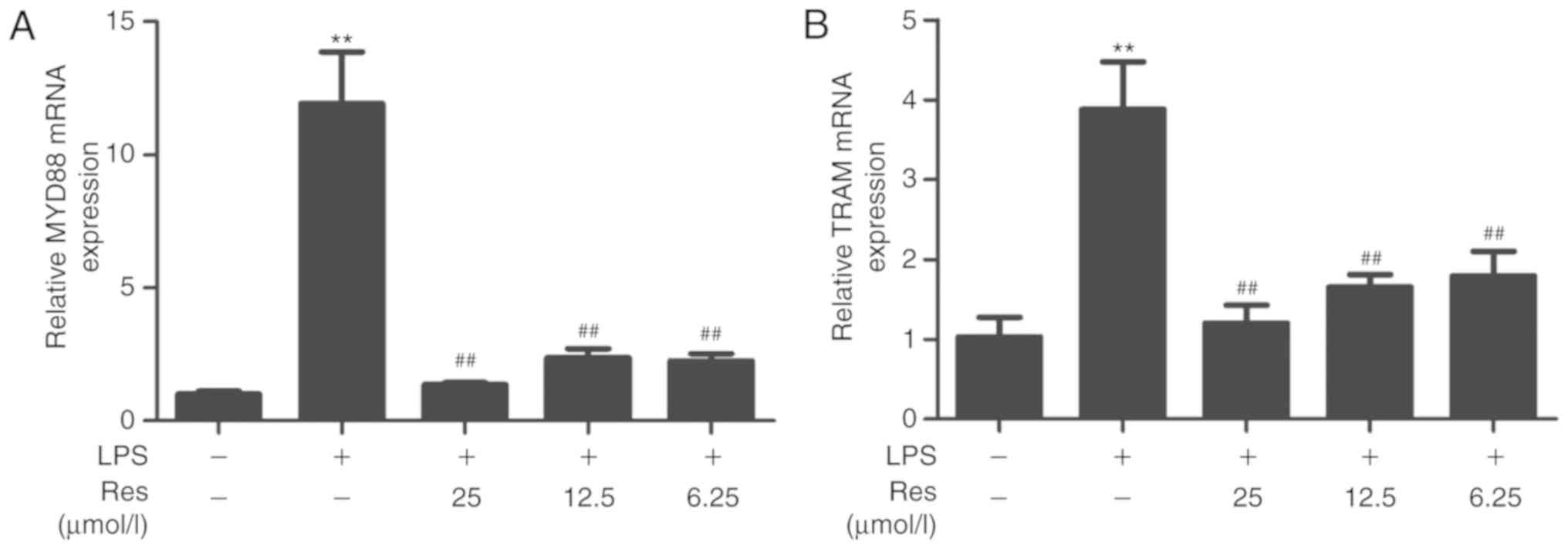
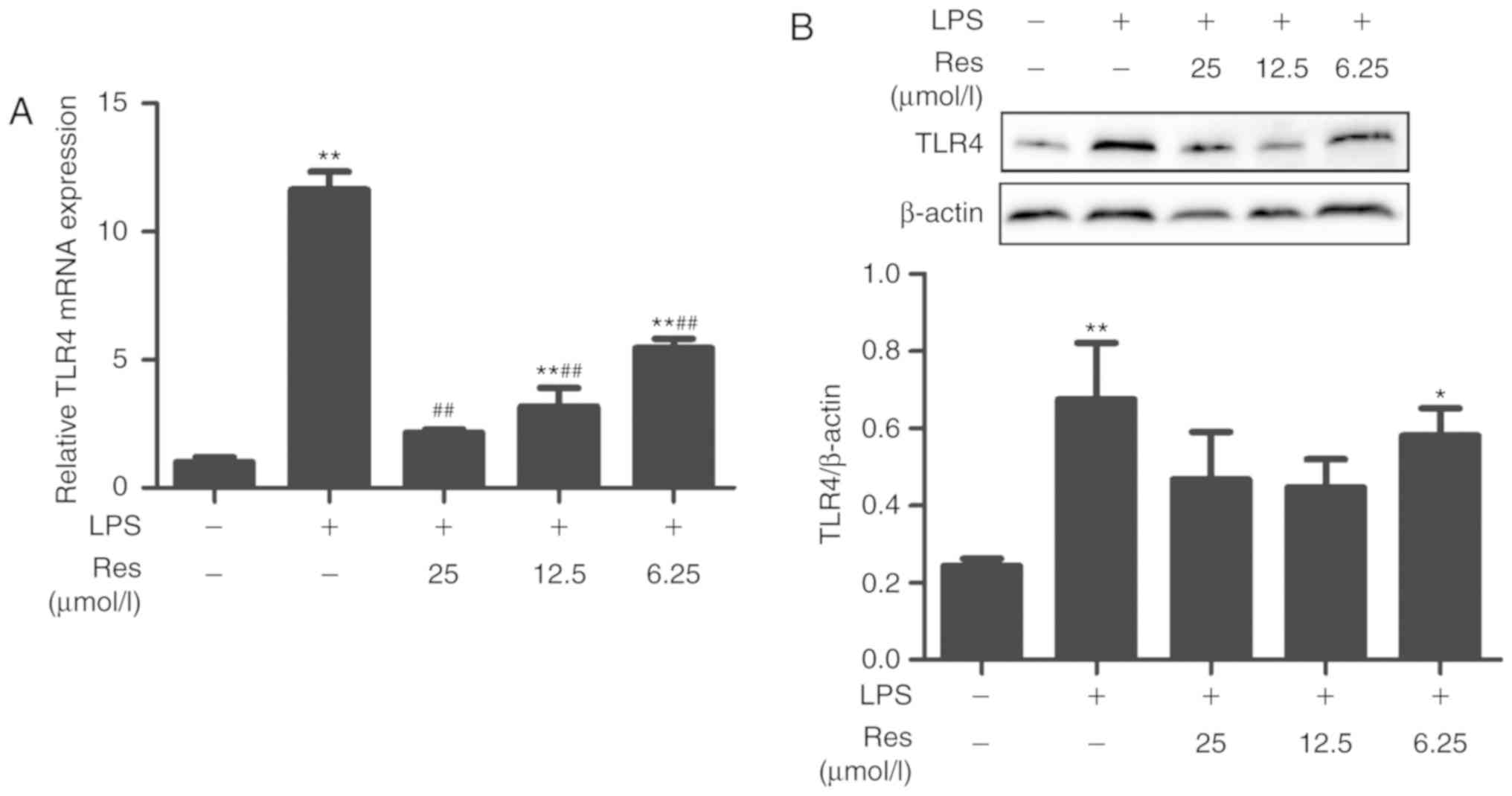
TLR4 is the target protein of Res
MYD88 and TRAM are two upstream proteins of the TLR4
pathway. Whether Res affected the TLR4 pathway through the MYD88 or
TRIF-dependent pathways is not known. Therefore, the effects of Res
on the transcription levels of TRAM, MYD88 and TLR4 and the
expression of TLR4 protein were investigated. Res exhibited an
inhibitory effect on the transcription levels of TRAM and MYD88 and
the expression level of TLR4 protein (Figs. 7 and 8). The results of the current study
indicated that Res suppressed the release of TNF-α, IL-6, IL-8 and
IFN-β and increased the release of IL-10 through inhibiting the
TLR4-NF-κB/MAPKs/IRF3 signaling pathway (Fig. 9).
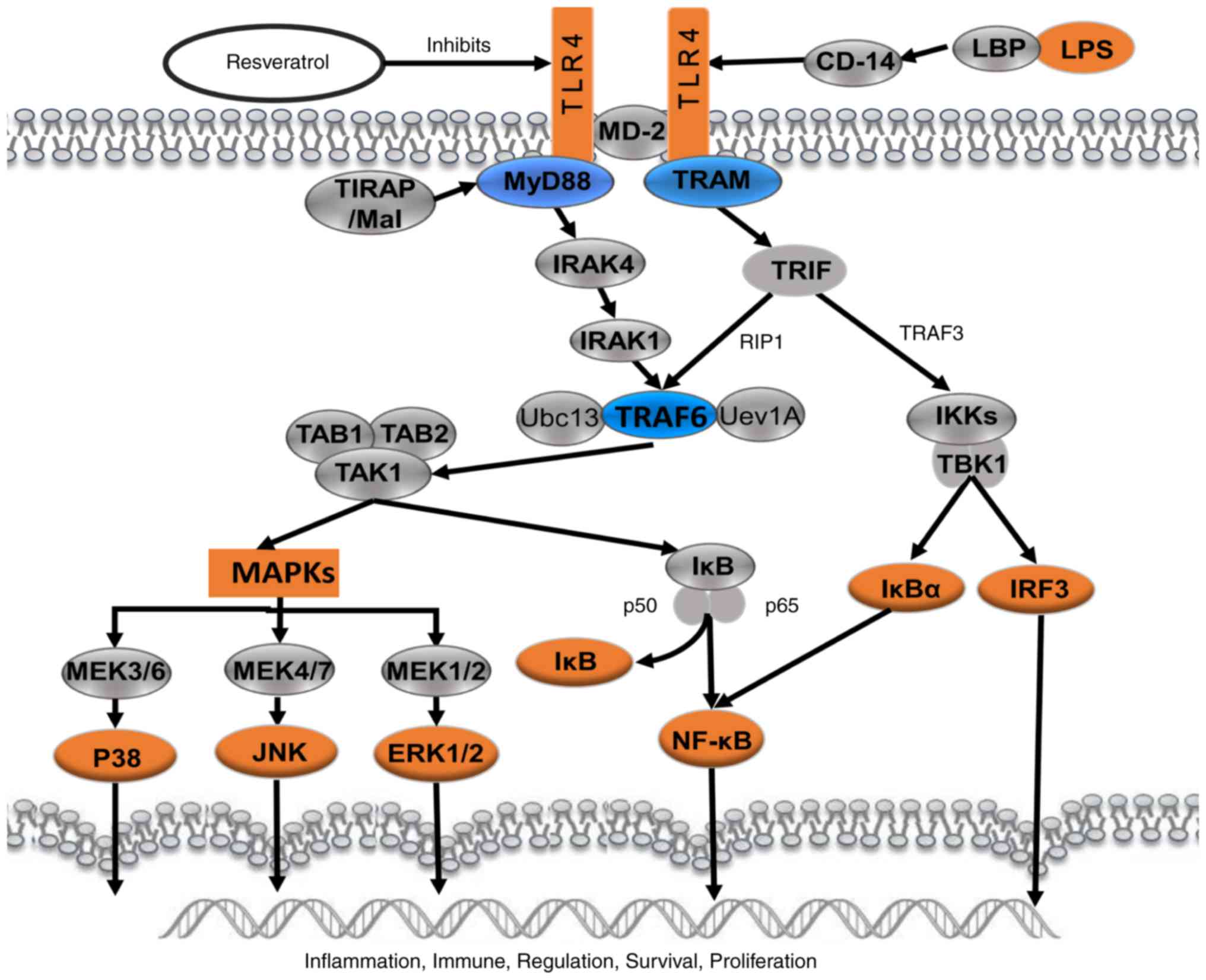 | Figure 9.Schematic presentation of
resveratrol-mediated effect on LPS-induced inflammation through
suppressing the signaling cascades of TLR4-NF-κB/MAPKs/IRF3. LPS,
lipopolysaccharide; TLR4, Toll-like receptor 4; MD-2, lymphocyte
antigen 96; MAPKs, mitogen-activated protein kinases; IRF3, IRF3,
interferon regulatory factor 3; CD14, monocyte differentiation
antigen CD14; LBP, lipopolysaccharide binding protein; TIRAP,
Toll/interleukin-1 receptor domain-containing adapter protein;
MyD88, myeloid differentiation primary response MyD88; TRAM, TIR
domain-containing adapter molecule 2; IRAK4, IL-1
receptor-associated kinase 4; TRIF, TIR domain-containing adapter
molecule 1; RIP1, receptor-interacting serine/threonine-protein
kinase 1; TRAF, TNF receptor-associated factor 6; TAB, transforming
growth factor-β-activated kinase 1-binding protein; TAK1,
mitogen-activated protein kinase kinase kinase 7; Ubc13,
ubiquitin-conjugating enzyme E2 13; Uev1A, ubiquitin-conjugating
enzyme E2 variant 1A; IKK, IκB kinase; TBK1,
serine/threonine-protein kinase TBK1; IκBα, NF-κB inhibitor; p50,
nuclear factor NF-κB p105 subunit; p65, transcription factor p65;
MEK, mitogen-activated protein kinase kinase; JNK, c-Jun N-terminal
kinase; ERK, extracellular signal-regulated kinase. |
Discussion
Inflammation, which presents with the classical
features of swelling, redness, heat and often pain is a key defense
response to injury, tissue ischemia, autoimmune responses or
infectious agents. Inflammation is also a major contributing factor
to the damage observed in autoimmune diseases (19). It can induct or activate the
production of the inflammatory mediators such as kinins,
cyclooxygenases and cytokines. The mouse macrophage RAW264.7 cell
line is a type of monocyte macrophage in mice with leukemia, which
is commonly used in biological experiments investigating
inflammation. For example, a previous study used RAW264.7 cells to
examine the mechanism of how mono- (2-ethylhexyl) phthalate
aggravates inflammatory response (20). In the present study, an inflammatory
model was successfully established using LPS stimulation in
RAW264.7 cells. LPS was demonstrated to promote the secretion of
TNF-α and IL-6 in a dose-dependent manner (Fig. 2). TNF-α is the earliest endogenous
mediator of an inflammatory reaction, and IL-6 is a major
pro-inflammatory cytokine that serves an important role in the
acute-phase response of inflammation (1). These inflammatory factors can be used
as markers of the degree of inflammation.
Firstly, overactivation of the NF-κB and MAPKs
pathways were observed in LPS-induced inflammation (7,21).
NF-κB, a critical regulator of cytokine production, cell activation
and proliferation, serves an important role in regulating
inflammation and immune responses to extracellular stimulus
(22). The present study identified
that LPS-induced inflammation was associated with the activation of
the NF-κB pathway, likely involving the disruption of the
interaction with IκBα. In addition, Res was demonstrated to
decrease IκBα overexpression in the present study. These results
were similar to those of our previous study, which revealed that
Res mitigated LPS-mediated acute inflammation in rats by inhibiting
the TLR4/NF-κBp65/MAPKs signaling cascade (7). MAPKs, including p38 MAPK, ERK and JNK,
are members of a ubiquitous protein serine/threonine kinase family
responsible for signal transduction in eukaryotic organisms
(23). It has been well established
that MAPK activation is implicated in the production of
LPS-stimulated inflammatory mediators (24–26).
MAPKs are a group of signaling molecules that appear to serve key
roles in inflammatory processes (27). In the present study, the results
demonstrated that LPS-induced inflammation stimulated the
phosphorylation of JNK, ERK and p38 MAPK, suggesting that MAPKs are
involved in LPS-induced inflammation. It was also revealed that Res
treatment efficiently downregulated the LPS-induced expression
levels of p38 MAPK, JNK and ERK1/2 in RAW264.7 cells. In addition,
it was identified that the IRF3 pathway was overactivated and the
levels of IFN-β were significantly increased in the LPS group
compared with that of the control group. The levels of p-IRF3 were
decreased when treated with Res. These results suggested that Res
may relieve LPS-induced inflammation through inhibiting the
activation of the TLR4-NF-κB/MAPKs/IRF3 signaling pathway and
downregulating the phosphorylation of IκBα, p38 MAPK, JNK, IRF3 and
ERK1/2. A number of previous studies have indicated that cytokines
including IL-1, IL-12, IFN-β and IL-10 serve important roles in the
process of inflammatory diseases (28). Inhibition the production of
inflammatory cytokines and their mediators serves as a key
mechanism in the control of inflammation.
In the present study, the production of the
pro-inflammatory cytokines including TNF-α, IL-6, IL-8 and IFN-β
was significantly inhibited by Res. In addition, the LPS-stimulated
mRNA expression levels of TNF-α, IL-6, IL-8 and IFN-β were also
decreased by Res treatment, suggesting that Res suppressed the
production of TNF-α, IL-6, IL-12, IFN-β and IL-1β through the
downregulation of their gene expression.
Concomitantly, Res markedly increased the release of
IL-10 and its mRNA expression level in LPS-stimulated RAW264.7
cells. TNF-α is a pro-inflammatory cytokine that exerts multiple
biological effects. NF-κB signaling may regulate the transcription
of certain inflammatory genes, including inducible nitric oxide
synthase, prostaglandin G/H synthase 2, TNF-α, IL-1β and IL-6. The
pathogenesis of a number of inflammatory processes are associated
with the pathological stimuli and dysregulation of the NF-κB
pathway (29). The MAPKs signaling
pathway primarily regulates the production of IL-12 through the p38
MAPK and SAPK/JNK signaling pathways (30), and ERK1/2 negatively regulates the
signaling pathways leading to IL-12 synthesis (31,32). The
expression of IFN-β may be induced by the phosphorylation of IRF. A
number of gram-positive pathogenic bacteria activate IFN-β
production in immune cells (33).
Previous data indicated that IRF3 or IRF7 function complementarily
to induce the expression of IFN-β (34). IL-10 is considered primarily an
inhibitory cytokine, which is crucial in maintaining an adequate
balance between inflammatory and immunopathological responses
(35). The results from the present
study indicated that Res elicits its protective effect against
LPS-induced inflammation through regulating the production of the
cytokines that correspond with the NF-κB, MAPK and IRF3 signal
pathways. Consistent with these data, similar results have
indicated that Res inhibited the LPS-induced production of IL-1β
and TNF-α (36,37). The NF-κB, MAPK and IRF3 pathways may
be involved in the process of Res-based alleviation of LPS-induced
inflammation (38–40). Furthermore, Res regulated the
secretion of inflammatory factors through inhibiting the associated
NF-κB, MAPK and IRF3 signaling pathways.
For further confirmation of the regulation of Res on
these signaling pathways, the inhibitors of JNK, NF-κB and p38 MAPK
were used and it was identified that Res has a similar effect as
these inhibitors.
The TLR4-NF-κB/MAPK pathways are considered to be
pivotal in the inflammatory response (41–43).
TLR4 can be activated by LPS. Then, the TLR4/MD-2 complex can
transmit the signal to two pairs of adaptor proteins such as
TIRAP/MyD88 and TRAM/TRIF. MyD88 recruits the IL-1
receptor-associated kinase (IRAK)4 and IRAK2 (or IRAK1) kinases
(44–46), which leads to the activation of NF-κB
and MAPK and the production of cytokines. Conversely, TRIF promotes
the secretion of type I IFN and IFN-inducible chemokines, such as
IL-10, through stimulating the IRF3 transcription factor (47). In the present study, Res was
demonstrated to inhibit the mRNA expression of MYD88 and TRAM in
LPS-stimulated RAW264.7 cells, suggesting that Res alleviates
inflammation via inhibiting the MYD88- and TRIF-dependent pathways,
which are 2 upstream proteins in the TLR4 pathway. TRAM and MYD88,
or their upstream protein, may be the target protein of Res that
was responsible for the inhibition of the LPS-induced inflammation
observed in the present study; it was also identified that Res
inhibited TLR4 mRNA transcription and protein expression in
LPS-stimulated RAW264.7 cells, suggesting that Res inhibited
inflammation through downregulating TLR4 expression.
In conclusion, Res is a potential anti-inflammatory
agent. The anti-inflammatory mechanisms of Res attribute to a
suppression of the release of TNF-α, IL-6, IL-8 and IFN-β and an
increase in the release of IL-10 through inhibiting the
TLR4-NF-κB/MAPKs/IRF3 signaling pathways. TLR4 may be the target
protein of Res, which inhibited LPS-induced inflammation in
RAW264.7 cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key research
and development projects of Sichuan science and Technology
Department (grant no. 8ZDYF3246), the Agricultural Technology
Research and Development Project of Chengdu (grant no.
2015-NY02-00266-NC) and Sichuan Veterinary Medicine and Drug
Innovation Group of China Agricultural Research System
(CARS-SVDIP).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The manuscript was written through contributions of
all authors. All authors have given approval to the final version
of the manuscript. WT, XC, XS and ZY designed the experiments,
wrote the manuscript and prepared the figures. YC performed part of
the experiments. RJ, YZ, LL, CH, XL and LY analyzed the
experimental results. GY, CL and JL analyzed part of the data. All
authors reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu K, Yang Y, Tu Q, Luo Y and Ma R:
Alpinetin inhibits LPS-induced inflammatory mediator response by
activating PPAR-γ in THP-1-derived macrophages. Eur J Pharmacol.
721:96–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang YP, Wu Y, Li LY, Zheng J, Liu RG,
Zhou JP, Yuan SY, Shang Y and Yao SL: Aspirin-triggered lipoxin A 4
attenuates LPS-induced pro-inflammatory responses by inhibiting
activation of NF-κB and MAPKs in BV-2 microglial cells. J
Neuroinflammation. 8:952011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meng F and Lowell CA: Lipopolysaccharide
(LPS)-induced macrophage activation and signal transduction in the
absence of Src-family kinases Hck, Fgr and Lyn. J Exp Med.
185:1661–1670. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou S, Chen G, Qi M, El-Assaad F, Wang Y,
Dong S, Chen L, Yu DS, Weaver JC, Beretov J, et al: Gram negative
bacterial inflammation ameliorated by the plasma protein beta
2-glycoprotein I. Sci Rep. 6:336562016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garay-Malpartida HM, Mourão RF, Mantovani
M, Santos IA, Sogayar MC and Goldberg AC: Toll-like receptor 4
(TLR4) expression in human and murine pancreatic beta-cells affects
cell viability and insulin homeostasis. BMC Immunol. 12:182011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JJ and Sears DD: TLR4 and insulin
resistance. Gastroenterol Res Pract. 2010(pii):
2125632010.PubMed/NCBI
|
|
7
|
Wang G, Hu Z, Fu Q, Song X, Cui Q, Jia R,
Zou Y, He C, Li L and Yin Z: Resveratrol mitigates
lipopolysaccharide-mediated acute inflammation in rats by
inhibiting the TLR4/NF-κBp65/MAPKs signaling cascade. Sci Rep.
7:450062017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Holthoff JH, Woodling KA, Doerge DR, Burns
ST, Hinson JA and Mayeux PR: Resveratrol, a dietary polyphenolic
phytoalexin, is a functional scavenger of peroxynitrite. Biochem
Pharmacol. 80:1260–1265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Youn HS, Lee JY, Fitzgerald KA, Young HA,
Akira S and Hwang DH: Specific inhibition of MyD88-independent
signaling pathways of TLR3 and TLR4 by resveratrol: Molecular
targets are TBK1 and RIP1 in TRIF complex. J Immunol.
175:3339–3346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jakus PB, Kalman N, Antus C, Radnai B,
Tucsek Z, Gallyas F Jr, Sumegi B and Veres B: TRAF6 is functional
in inhibition of TLR4-mediated NF-κB activation by resveratrol. J
Nutr Biochem. 24:819–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)). Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tseng CK, Lin CK, Chang HW, Wu YH, Yen FL,
Chang FR, Chen WC, Yeh CC and Lee JC: Aqueous extract of Gracilaria
tenuistipitata suppresses LPS-induced NF-κB and MAPK activation in
RAW264.7 and rat peritoneal macrophages and exerts hepatoprotective
effects on carbon tetrachloride-treated rat. PLoS One.
9:e865572014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian WL, Jiang ZX, Wang F, Guo R, Tang P,
Huang YM and Sun L: IRF3 is involved in human acute myeloid
leukemia through regulating the expression of miR-155. Biochem
Biophys Res Commun. 478:1130–1135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guinn Z, Lampe AT, Brown DM and Petro TM:
Significant role for IRF3 in both T cell and APC effector functions
during T cell responses. Cell Immunol. 310:141–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumari M, Wang X, Lantier L, Lyubetskaya
A, Eguchi J, Kang S, Tenen D, Roh HC, Kong X, Kazak L, et al: IRF3
promotes adipose inflammation and insulin resistance and represses
browning. J Clin Invest. 126:2839–2854. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen PG, Guan YJ, Zha GM, Jiao XQ, Zhu HS,
Zhang CY, Wang YY and Li HP: Swine IRF3/IRF7 attenuates
inflammatory responses through TLR4 signaling pathway. Oncotarget.
8:61958–61968. 2017.PubMed/NCBI
|
|
17
|
Rajaiah R, Perkins DJ, Ireland DD and
Vogel SN: CD14 dependence of TLR4 endocytosis and TRIF signaling
displays ligand specificity and is dissociable in endotoxin
tolerance. Proc Natl Acad Sci USA. 112:8391–8396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pham TH, Kim MS, Le MQ, Song YS, Bak Y,
Ryu HW, Oh SR and Yoon DY: Fargesin exerts anti-inflammatory
effects in THP-1 monocytes by suppressing PKC-dependent AP-1 and
NF-ĸB signaling. Phytomedicine. 24:96–103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lucas SM, Rothwell NJ and Gibson RM: The
role of inflammation in CNS injury and disease. Br J Pharmacol. 147
(Suppl 1):S232–S240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park MH, Gutiérrez-García AK and Choudhury
M: Mono-(2-ethylhexyl) phthalate aggravates inflammatory response
via sirtuin regulation and inflammasome activation in RAW264.7
cells. Chem Res Toxicol. 32:935–942. 2019.PubMed/NCBI
|
|
21
|
Zhao X, Cui Q, Fu Q, Song X, Jia R, Yang
Y, Zou Y, Li L, He C, Liang X, et al: Antiviral properties of
resveratrol against pseudorabies virus are associated with the
inhibition of IκB kinase activation. Sci Rep. 7:87822017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Liu H, Xu QS, Du YG and Xu J:
Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of
NF-κB and endothelial inflammatory response. Carbohydr Polym.
99:568–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li D, Hu J, Wang T, Zhang X, Liu L, Wang
H, Wu Y, Xu D and Wen F: Silymarin attenuates cigarette smoke
extract-induced inflammation via simultaneous inhibition of
autophagy and ERK/p38 MAPK pathway in human bronchial epithelial
cells. Sci Rep. 6:377512016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang C, Jacobson K and Schaller MD: MAP
kinases and cell migration. J Cell Sci. 117:4619–4628. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Renda T, Baraldo S, Pelaia G, Bazzan E,
Turato G, Papi A, Maestrelli P, Maselli R, Vatrella A, Fabbri LM,
et al: Increased activation of p38 MAPK in COPD. Eur Respir J.
31:62–69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gaffey K, Reynolds S, Plumb J, Kaur M and
Singh D: Increased phosphorylated p38 mitogen-activated protein
kinase in COPD lungs. Eur Respir J. 42:28–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tambuyzer BR, Ponsaerts P and Nouwen EJ:
Microglia: Gatekeepers of central nervous system immunology. J
Leukoc Biol. 85:352–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Veres B, Radnai B, Gallyas F Jr, Varbiro
G, Berente Z, Osz E and Sumegi B: Regulation of kinase cascades and
transcription factors by a poly(ADP-ribose) polymerase-1 inhibitor,
4-hydroxyquinazoline, in lipopolysaccharide-induced inflammation in
mice. J Pharmacol Exp Ther. 310:247–255. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim L, Del Rio L, Butcher BA, Mogensen TH,
Paludan SR, Flavell RA and Denkers EY: p38 MAPK autophosphorylation
drives macrophage IL-12 production during intracellular infection.
J Immunol. 174:4178–4184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng GJ, Goodridge HS, Harnett MM, Wei XQ,
Nikolaev AV, Higson AP and Liew FY: Extracellular signal-related
kinase (ERK) and p38 mitogen-activated protein (MAP) kinases
differentially regulate the lipopolysaccharide-mediated induction
of inducible nitric oxide synthase and IL-12 in macrophages:
Leishmania phosphoglycans subvert macrophage IL-12 production by
targeting ERK MAP kinase. J Immunol. 163:6403–6412. 1999.PubMed/NCBI
|
|
32
|
Ropert C, Almeida IC, Closel M, Travassos
LR, Ferguson MA, Cohen P and Gazzinelli RT: Requirement of
mitogen-activated protein kinases and IκB phosphorylation for
induction of proinflammatory cytokines synthesis by macrophages
indicates functional similarity of receptors triggered by
glycosylphosphatidylinositol anchors from parasitic protozoa and
bacterial lipopolysaccharide. J Immunol. 166:3423–3331. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Monroe KM, McWhirter SM and Vance RE:
Induction of type I interferons by bacteria. Cell Microbiol.
12:881–890. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Weiss G, Maaetoft-Udsen K, Stifter SA,
Hertzog P, Goriely S, Thomsen AR, Paludan SR and Frøkiær H: MyD88
drives the IFN-β response to Lactobacillus acidophilus in dendritic
cells through a mechanism involving IRF1, IRF3 and IRF7. J Immunol.
189:2860–2868. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cavalcanti YV, Brelaz MC, Neves JK, Ferraz
JC and Pereira VR: Role of TNF-alpha, IFN-gamma and IL-10 in the
development of pulmonary tuberculosis. Pulm Med. 2012:7454832012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Byun EB, Sung NY, Park JN, Yang MS, Park
SH and Byun EH: Gamma-irradiated resveratrol negatively regulates
LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages.
Int Immunopharmacol. 25:249–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zong Y, Sun L, Liu B, Deng YS, Zhan D,
Chen YL, He Y, Liu J, Zhang ZJ, Sun J and Lu D: Resveratrol
inhibits LPS-induced MAPKs activation via activation of the
phosphatidylinositol 3-kinase pathway in murine RAW264.7 macrophage
cells. PLoS One. 7:e441072012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vartanian KB, Stevens SL, Marsh BJ,
Williams-Karnesky R, Lessov NS and Stenzel-Poore MP: LPS
preconditioning redirects TLR signaling following stroke: TRIF-IRF3
plays a seminal role in mediating tolerance to ischemic injury. J
Neuroinflammation. 8:1402011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang G, Chang CC, Yang Y, Yuan L, Xu L, Ho
CT and Li S: Resveratrol alleviates rheumatoid arthritis via
reducing ROS and inflammation, inhibiting MAPK signaling pathways
and suppressing angiogenesis. J Agric Food Chem. 66:12953–12960.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chiang MC, Nicol CJ and Cheng YC:
Resveratrol activation of AMPK-dependent pathways is
neuroprotective in human neural stem cells against
amyloid-beta-induced inflammation and oxidative stress. Neurochem
Int. 115:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Santos SH, Andrade JM, Fernandes LR,
Sinisterra RD, Sousa FB, Feltenberger JD, Alvarez-Leite JI and
Santos RA: Oral angiotensin-(1–7) prevented obesity and hepatic
inflammation by inhibition of resistin/TLR4/MAPK/NF-κB in rats fed
with high-fat diet. Peptides. 46:47–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng Z, Yan C, Deng Q, Gao DF and Niu XL:
Curcumin inhibits LPS-induced inflammation in rat vascular smooth
muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways.
Acta Pharmacol Sin. 34:901–911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang C, Lin G, Wan W, Li X, Zeng B, Yang
B and Huang C: Resveratrol, a polyphenol phytoalexin, protects
cardiomyocytes against anoxia/reoxygenation injury via the
TLR4/NF-κB signaling pathway. Int J Mol Med. 29:557–563. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cochet F and Peri F: The role of
carbohydrates in the lipopolysaccharide (LPS)/Toll-like receptor 4
(TLR4) signalling. Int J Mol Sci. 18:E23182017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wright SD, Ramos RA, Tobias PS, Ulevitch
RJ and Mathison JC: CD14, a receptor for complexes of
lipopolysaccharide (LPS) and LPS binding protein. Science.
249:1431–1443. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ulevitch RJ and Tobias PS: Recognition of
gram-negative bacteria and endotoxin by the innate immune system.
Curr Opin Immunol. 11:19–22. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jiang Q, Akashi S, Miyake K and Petty HR:
Cutting edge: Lipopolysaccharide induces physical proximity between
CD14 and Toll-like receptor 4 (TLR4) prior to nuclear translocation
of NF-kappa. J Immunol. 165:3541–3544. 2000. View Article : Google Scholar : PubMed/NCBI
|