Introduction
Vascular dementia (VD) is one of the most common
types of dementia after Alzheimer's disease, accounting for around
15% of cases (1) and characterized
by a progressive decline in memory and learning (2). Accumulating evidence suggests that
vascular risk factors may contribute to the onset of VD (3). However, there are currently no licensed
treatments for VD and the mechanisms underlying its pathogenesis
remain unclear.
Autophagy is a cell self-degradation process that is
important for maintaining the stability of the internal environment
of the body (4) by clearing damaged
cellular components, such as mitochondria (5). However, overactivation of autophagy
triggers cell death as a result of excessive self-digestion through
the degradation of essential proteins and organelles (6). Previous studies have reported that
activation of autophagy as a result of transient ischemia promotes
neuronal damage in brain tissues (7,8),
suggesting that autophagy is a common pathway leading to cell death
in the central nervous system.
3-n-butylphthalide (NBP), initially extracted from
the seeds of Chinese celery (Apium gravelens), has been
approved for the treatment of ischemic cerebrovascular disease
(9). Based on its multi-target
therapeutic properties, NBP has demonstrated an important role in a
number of nervous system diseases, including amyotrophic lateral
sclerosis (10), Parkinson's disease
(11) and VD (12), as well as models of Alzheimer's
disease (13). Studies have also
demonstrated that multiple mechanisms are involved in the
neuroprotective effects of NBP, including anti-inflammatory
effects, suppression of oxidative stress, inhibition of platelet
aggregation and anti-apoptosis (14–17).
However, little is known about the protective role of NBP against
chronic ischemia-induced excessive autophagy in VD. The present
study aimed to investigate the effect of NBP on autophagy in the
hippocampus of a rat model of VD and to determine the signaling
pathways involved in the observed effects.
Materials and methods
Animals and groups
A total of 60 male Sprague-Dawley rats (age, 2
months; weight, 250–280 g) were purchased from the Experimental
Animal Center of China Medical University (Shenyang, China). All
rats were housed in a specific pathogen-free animal experiment room
at 24±2°C with 60% humidity under a 12-h light/dark cycle and were
allowed free access to water and food. The experiments were
approved by the China Medical University Animal Care and Use
Committee and adhered to the Chinese Academy of Science guidelines
for the care and use of laboratory animals. All rats were randomly
divided into five groups (n=12 rats/group): i) Sham (S) group; ii)
VD group; iii) NBP (N) group; iv) rapamycin (R) group; and v) NBP
and rapamycin (N+R) group. One day prior to the surgery, rats in
the R and N+R groups underwent lateral ventricle catheterization
and 50 µl rapamycin (1 mmol/ml) was injected slowly into the
lateral ventricle (2 µl/min), leaving the needle in for 5 min. With
the exception of the S group, all rats underwent vessel ligation.
VD was induced by two-vessel occlusion as previously described
(18). Sham rats were subjected to
the same procedure without ligation of the arteries. Rats in the S
and VD groups received vegetable oil, and the other groups received
60 mg/kg NBP per day. All rats were weighed daily. Four weeks after
the surgery, there were 12 rats in the S group, 10 in the VD group,
11 in the N group, 10 in the R group and 11 in the N+R group. A
total of six rats were excluded from the study due to epilepsy in
two rats and death of unexplained causes in four rats. NBP soft
capsules were purchased from Shijiazhuang Pharmaceutical Co. Ltd.
The study timeline is presented in Fig.
1.
Behavioral tests T-maze
T-maze tests can be used to assess an animal's
spatial working memory (19). T-maze
tests were performed after 4 weeks of NBP treatment (S, n=12; VD,
n=10; N, n=11; R, n=11; N+R, n=11). Each T-maze trial consisted of
a sample run and a choice run. On the sample run, rats were forced
to enter either the left or the right arm of the maze to get sugar,
while the other arm was blocked by a sliding door. On the choice
run, the blocked door was removed and the rats were allowed to
choose either arm freely. There was an interval of 10 sec between
the sample and choice runs. If the rats entered the previously
unvisited arm, they were rewarded. The delay was then prolonged to
90 and 180 sec. Each daily session consisted of five trials, and
rats performed one trial at a time with an interval of 10 min. The
number of corrections made when the rats entered the unvisited arm
of the T-maze was measured.
Morris water maze (MWM)
The MWM test was performed in a circular pool
(diameter, 180 cm; height, 60 cm; depth, 35 cm) filled with opaque
water by stirring in milk (temperature, 26±1°C). The pool was
divided into four quadrants, and a white platform was located in
the center of one quadrant. The rat was gently placed in the water,
facing the side-wall of the maze not housing the platform. The time
to find the hidden platform (escape latency) and path length
(distance traveled) were recorded for each training trial. The rat
was given a maximum of 90 sec to find the hidden platform, any rat
who failed the mission within 90 sec would be guided to the hidden
platform and allowed to stay on the platform for 15 sec, before the
training was terminated. The rats started each trial from a
different quadrant and were trained twice a day for 5 consecutive
days with an interval of 3 h. The escape latency and swimming
distances were recorded using a video camera, and the digital
images were analyzed using water maze software (HVS Image 2020; HVS
Image Software Ltd.) Additional probe trials were conducted with
the hidden platform removed on the 6th day of the test. Swimming
speed, times of crossing the platform, time spent and swimming
distance in the target quadrant were also recorded.
Nissl staining
Rats were euthanized with an intraperitoneal
injection of sodium pentobarbital (200 mg/kg); when continuous
spontaneous breathing arrest for 2–3 min and muscle relaxation
occurred, rats were transcardially perfused with normal saline
(0.9%) followed by 4% paraformaldehyde in 0.1 M sodium phosphate
buffer (pH 7.3). The brain was then dissected, postfixed with 4%
paraformaldehyde at 4°C for three days, embedded in paraffin, cut
into 5-µm sections and stained with 1% Toluidine Blue at 60°C for
40 min. Nissl-positive cells in the hippocampus were examined to
assess neuronal survival. The population of normal neurons in the
CA1 subfield was counted using a light microscope at ×400
magnification (model, BX53; Olympus Corporation) by two independent
investigators who were blinded to the experimental conditions. The
mean number of neurons was obtained by counting 3 sections per
brain and 5 representative fields were randomly selected to count
per section.
Transmission electron microscopy
Brain tissues were prepared for electron microscopy
by fixing in 2.5% glutaraldehyde in PBS (pH 7.4) for 1 h at 4°C and
in 1% OsO4 in 0.1 mol/l cacodylate buffer (pH 7.4) for 2
h at 4°C The tissues were subsequently stained with 1% aqueous
uranyl acetate at 4°C overnight and embedded in Durcopan
(Sigma-Aldrich, Merck KGaA). The sections (50–70 nm) of the
hippocampus were cut using a Leica ultracut microtome (Leica
Microsystems, Inc.) and collected on formvar-coated copper grids.
The sections were stained with 1% aqueous uranyl acetate and lead
citrate and then imaged on a H-600/7650 transmission electron
microscope (Hitachi High-Technologies Corporation).
Western blotting
Rats were euthanized with an intraperitoneal
injection of sodium pentobarbital (200 mg/kg) and sacrificed by
transcardiac perfusion with normal saline (0.9%) followed by 4%
paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.3). The rat
(n=5/group) hippocampus was homogenized in RIPA lysis buffer (cat.
no. P0013B; Beyotime Institute of Biotechnology) containing PMSF,
and centrifuged at 12,000 × g for 10 min at 4°C. Protein
concentrations were determined using a bicinchoninic acid protein
assay kit (Beyotime Institute of Biotechnology). A total of 30 µg
of protein per lane was separated by 12% SDS-PAGE and transferred
onto PVDF membranes. The membranes were blocked with 3% bovine
serum albumin (Sigma-Aldrich; Merck KGaA) in Tris-buffered saline
at room temperature for 30 min and incubated overnight at 4°C with
primary antibodies against mammalian target of rapamycin (mTOR;
1:400; cat. no. 2972; Cell Signaling Technology, Inc.),
phosphorylated (p)-mTOR (Ser2448; 1:250; cat. no. 2971; Cell
Signaling Technology, Inc.), LC3B (1:1,000; cat. no. 2775; Cell
Signaling Technology, Inc.), Beclin 1 (1:1,000; cat. no. sc-48341;
Santa Cruz Biotechnology, Inc.) or β-actin (1:1,000, cat. no. 4967;
Cell Signaling Technology, Inc.). Following incubation with
horseradish-peroxidase conjugated anti-rabbit IgG (1:2,000; Cell
Signaling Technology, Inc.) for 1 h at room temperature. Protein
signals were detected using an enhanced chemiluminescence system
(EMD Millipore) and quantified using Quantity-One software version
4.6.3 (Bio-Rad Laboratories, Inc.). β-actin was used as a
protein-loading control.
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using SPSS 16.0 statistical software (SPSS,
Inc.). Statistical significance was determined by one-way ANOVA
followed by the least significant difference post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
NBP ameliorates autophagy-induced
learning and memory injury in rats with VD
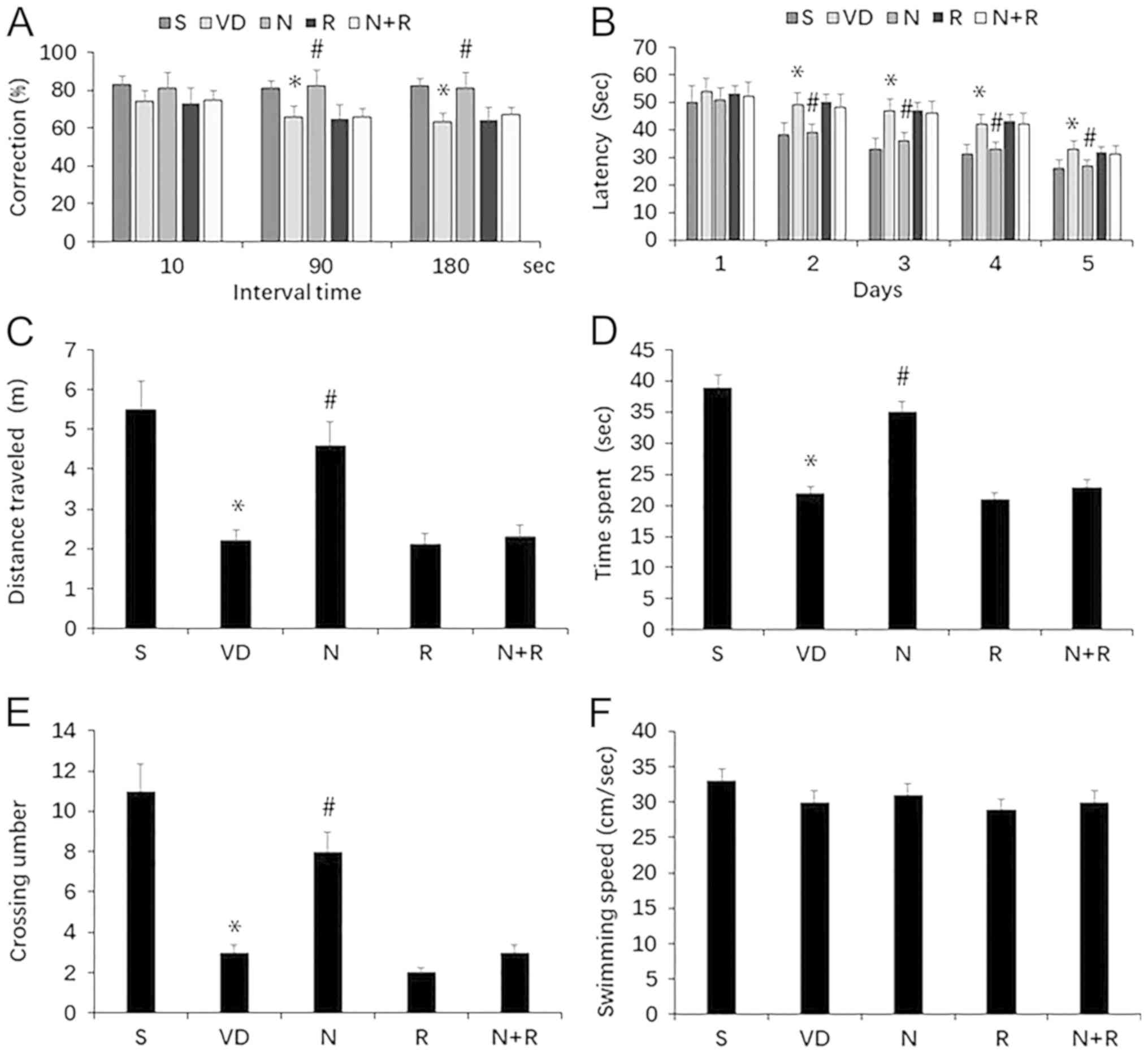
The cognitive function of rats with VD was first
assessed using the T-maze test. Rats in the VD group exhibited
impaired spontaneous alternation behavior compared with those in
the S group (P<0.05); however, NBP attenuated this impairment in
rats with VD (P<0.05; Fig. 2A).
NBP also improved the performance of VD rats when the interval
between the sample and choice runs was delayed by 90 and 180 sec
(both P<0.05). In addition, compared with the R group, rats in
the N+R group exhibited no improvement in performance in terms of
spontaneous alternation behavior, including when the interval was
delayed by 90 and 180 sec (P>0.05; Fig. 2A).
Cognitive function was determined using the MWM
test. During training, significant differences in the performance
of the five rat groups were observed from day 2; rats in the VD
group exhibited a higher escape latency compared with the S group
(P<0.05), and the escape latency was shortened by treatment with
NBP (P<0.05; Fig. 2B). In the
probe test, the time spent swimming and the distance in the target
quadrant, as well as the number of platform crossings were
significantly lower in the VD group compared with the S group (all
P<0.05). NBP increased the time spent swimming, the distance in
the target quadrant and the number of platforms crossed compared
with the VD group (all P<0.05). However, the N+R group exhibited
no change in time spent swimming, distance in the target quadrant
or the number of platform crossings compared with the R group (all
P>0.05; Fig. 2C-E). No difference
was observed in swimming speed among the groups (P>0.05;
Fig. 2F). These results indicated
that NBP ameliorated the impairment of learning and memory induced
by ischemia, but did not improve rapamycin-aggravated learning and
memory deficits in rats with VD (Fig.
2).
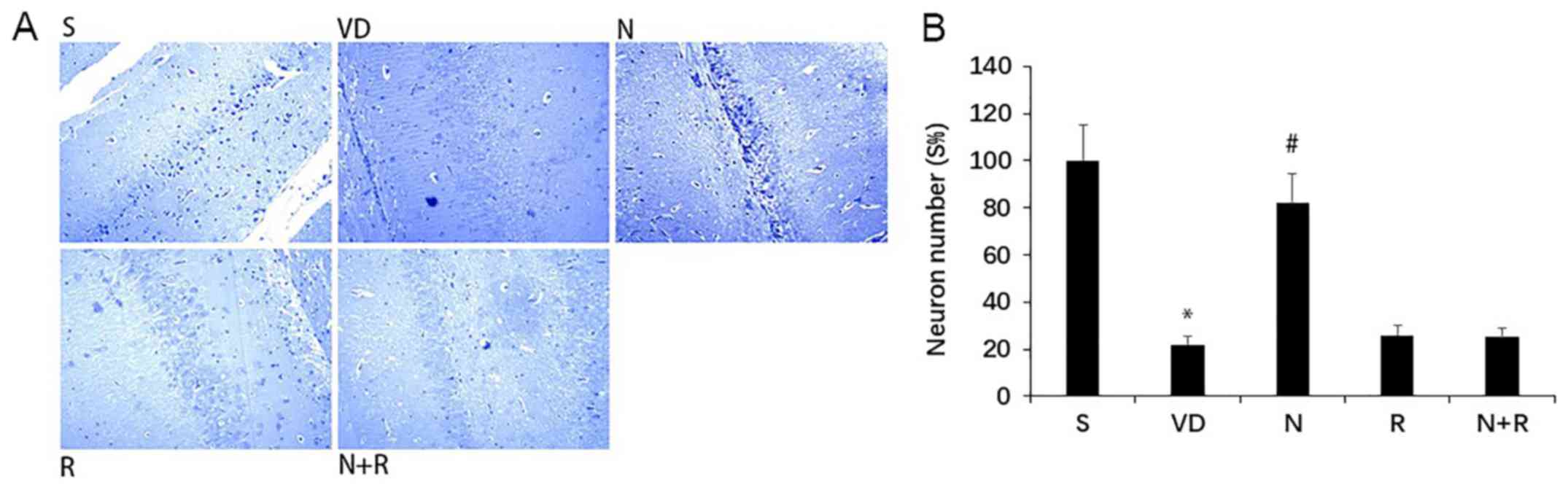
NBP promotes neuronal survival in rats
with VD
No histopathological abnormalities were observed in
the hippocampus in rats in the S group, but VD rats exhibited
marked neuronal loss and degeneration of neuronal structure in the
CA1 region (Fig. 3). By contrast,
NBP attenuated this loss and reversed the structural injury,
reflected by the density and morphology of neurons with Nissl
bodies (P<0.05). Histopathological abnormalities detected by
Nissl staining were similar in the N+R and R groups (P>0.05).
These results suggested that NBP may inhibit hippocampal neuron
death in rats with VD, but could not reverse the injury in
rapamycin-treated rats.
NBP decreases autophagosome formation
in the CA1 area of the hippocampus in rats with VD
No autophagosomes were visible in the hippocampal
CA1 region of rats in the S group under transmission electron
microscopy (Fig. 4). By contrast,
round autophagosomes were located next to the nuclei in the VD
group, and there were abundant primary lysosomes, which indicated
autophagy. Fewer autophagosomes were observed in the NBP group.
Similarly to the VD group, autophagosomes and lysosomes were
abundant in the R and N+R groups. These results suggested that NBP
may inhibit autophagosome formation in rats with VD (Fig. 4).
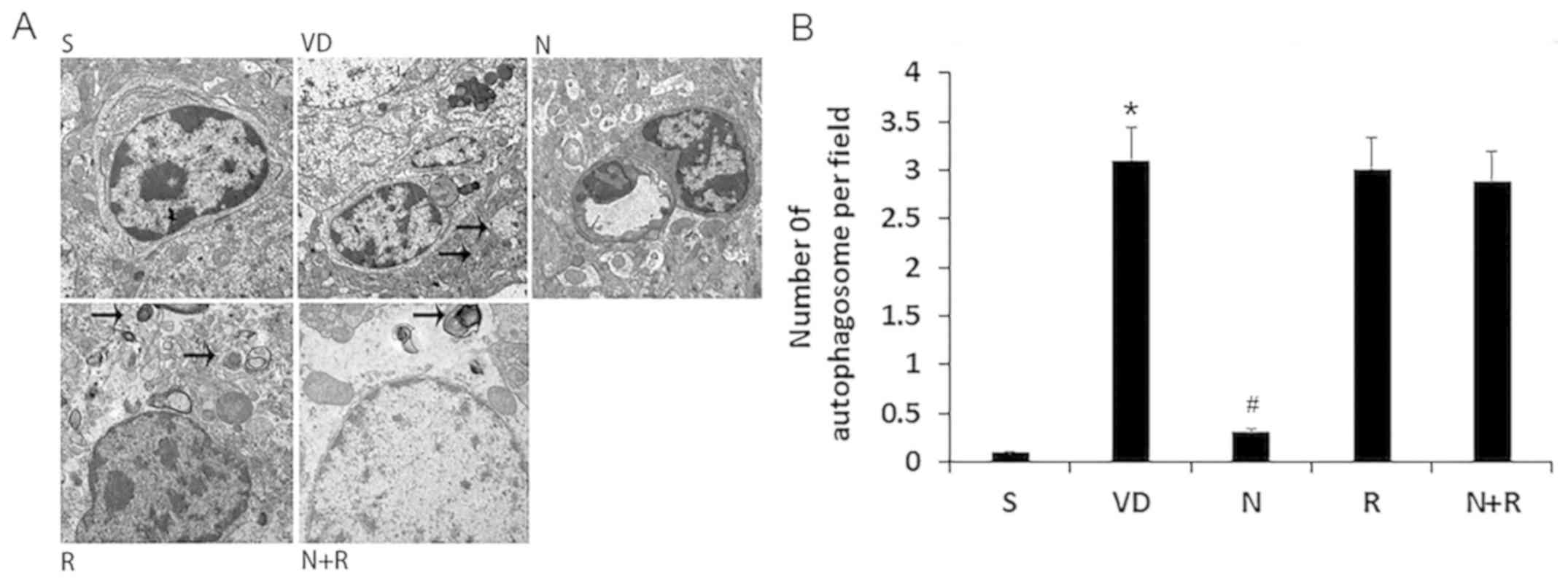 | Figure 4.Effects of NBP on autophagosome
formation in the CA1 area of the hippocampus. (A) Electron
microscopic examination (magnification, ×10,000) of autophagosomes
in the CA1 area. Normal ultrastructure was observed in the S group,
and scattered autophagosomes were observed in the VD, R and N+R
groups. A low number of autophagosomes was observed in the N group.
Arrow marked the autophagosomes. (B) Quantitative analysis of the
number of autophagosomes of per field. Data are expressed as means
± SEM. *P<0.05 vs. S group, #P<0.05 vs. VD group.
S, sham; VD, vascular dementia; N, NBP; N+R, NBP + rapamycin; NBP,
Dl-3n-butylphthalide. |
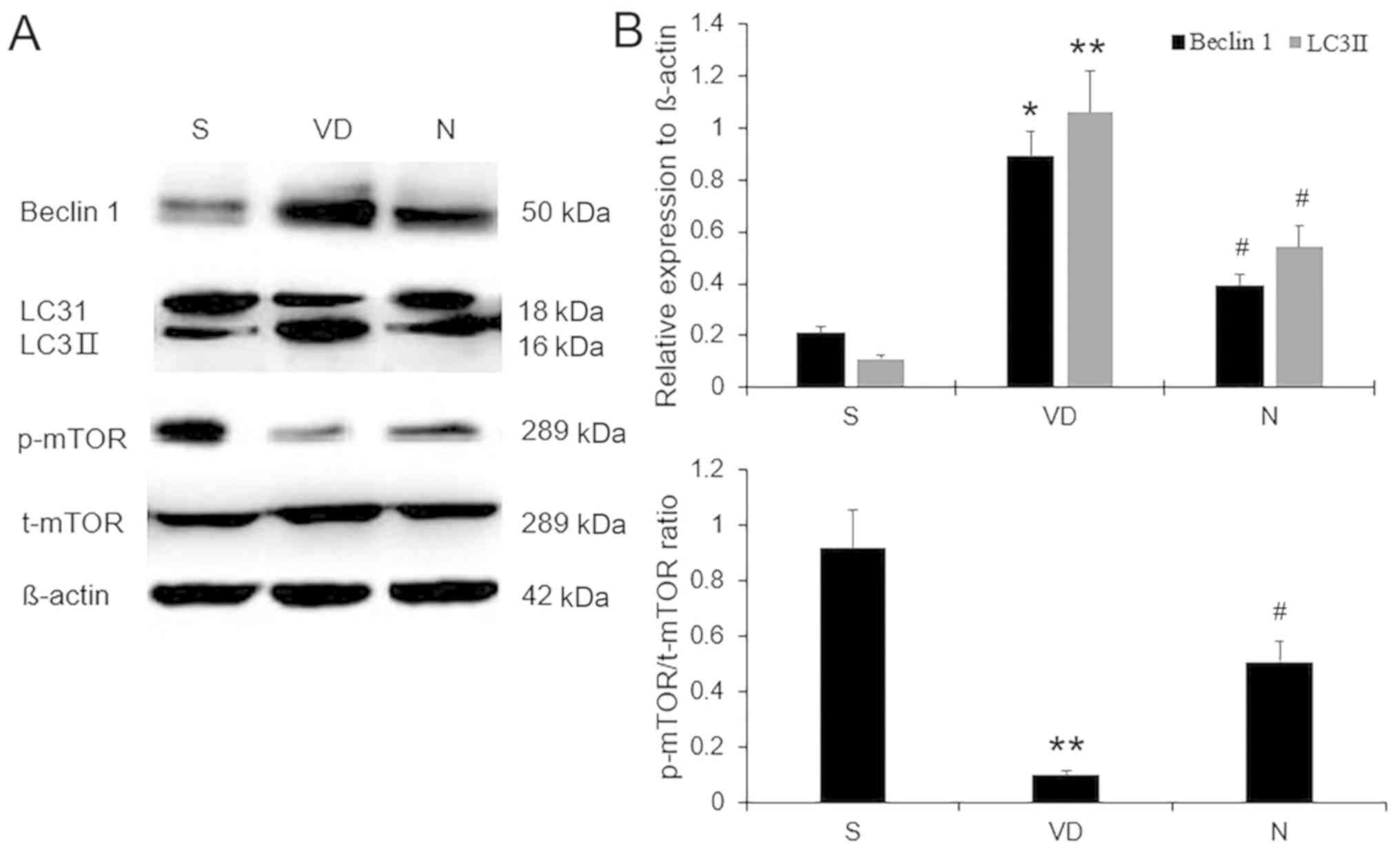
NBP inhibits the expression of
characteristic autophagy proteins and increases mTOR
phosphorylation in rats with VD
The changes in Beclin 1, LC3II and p-mTOR protein
expression levels were determined by western blot analysis
(Fig. 5). Low levels of Beclin 1 and
LC3II were present in the hippocampal CA1 region in rats in the S
group. However, expression levels of Beclin 1 and LC3II were
significantly increased in the VD group (P<0.05 and P<0.01,
respectively) but reduced in the NBP group (both P<0.05). By
contrast, the levels of p-mTOR Ser-2448 in the S group were higher
compared with those in the VD group (P<0.01), and this reduced
phosphorylation of mTOR was partially reversed by NBP
administration in rats with VD (P<0.05). These results
demonstrated that NBP partially inhibited excessive
ischemia-induced autophagy and promoted mTOR phosphorylation in
rats with VD.
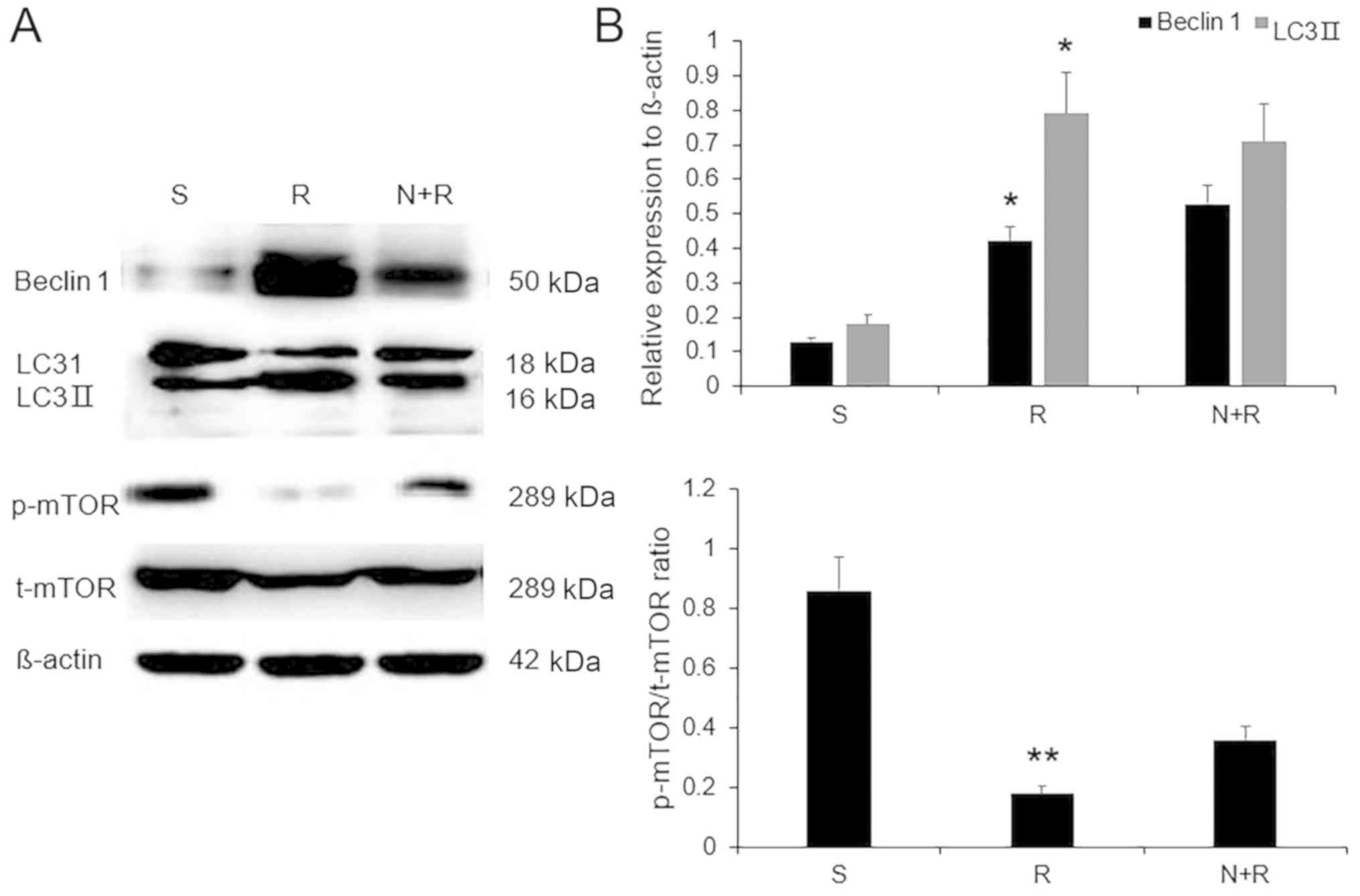
NBP inhibits autophagy via an
mTOR-dependent pathway in rats with VD
The role of mTOR in ischemia-induced autophagy was
assessed by measuring mTOR phosphorylation levels in the five
groups. The administration of the mTOR inhibitor rapamycin reduced
mTOR phosphorylation (P<0.01) and simultaneously increased the
expression levels of Beclin 1 and LC3II in VD rats compared with
the S group (both P<0.05), whereas NBP + rapamycin failed to
reverse these effects compared with rapamycin alone (P>0.05;
Fig. 6). These results indicated
that NBP may suppress autophagy through an mTOR-dependent pathway
in rats with VD.
Discussion
VD is a common cause of dementia, primarily induced
by cerebrovascular disease or risk factors including age,
hypertension, diabetes, and smoking (20). An increasing number of studies
recently focused on the pathological mechanisms and possible
therapies for VD; however, VD remains difficult to treat, and
further studies are therefore urgently needed.
Autophagy, which is an adaptive reaction of cells to
stress produced by alterations in the internal and external
environments, is a dynamic catabolic process involved in multiple
cellular processes (21). However,
excessive autophagy may trigger cell death and contribute to
ischemia-induced neuronal damage (22,23).
Inhibition of autophagy has thus demonstrated beneficial effects in
modulating neurological deficits following cerebral ischemia
(24,25).
The kinase mTOR is a common regulator of autophagy
induction; mTOR activation suppresses, whereas inhibiting mTOR
promotes autophagy (26). Rapamycin
is a direct inhibitor of mTOR and is the most commonly used and
specific inducer of autophagy (27).
The mTOR pathway activation has been demonstrated to protect
hippocampal neurons against hypoxia-induced injury and promote
neuronal recovery (28).
In the present study, a rat model of VD was
established (29), and learning and
memory in the rats were evaluated by conducting MWM and T-maze
tests. The results demonstrated that NBP significantly improved the
cognitive performance of rats with VD, but could not attenuate
cognitive decline in rats with VD following treatment with the
autophagy agonist rapamycin. These results, along with the changes
in the relative numbers of Nissl-positive cells in the CA1 region
of the hippocampus in the NBP-treated or the NBP combined with
rapamycin treated group, indicated that NBP could ameliorate
cognitive impairment and neuronal loss in rats with VD, and that
these effects of NBP could be prevented by the autophagy agonist
rapamycin.
Numerous studies have reported that autophagy is
activated in neurons subjected to ischemia or hypoxia, and
excessive autophagy may lead to autophagic neuronal cell death
(30–33). The results of the transmission
electron microscopy analysis in the present study revealed that
brain ischemia induced autophagosome formation in VD rats, which
was inhibited by NBP. This NBP-induced suppression of autophagy was
confirmed by western blot analysis, which revealed that the
expression levels of the autophagy markers LC3II and Beclin 1 were
downregulated in NBP-treated rats with VD, indicating that
inhibition of autophagy by NBP may prevent the learning and memory
impairments observed in rats with VD.
The upstream signaling pathway of ischemia-mediated
autophagy activation and the role of this pathway in the inhibitory
effect of NBP on autophagy were further elucidated by detecting
mTOR phosphorylation levels in rats with VD. mTOR a negative
regulator of autophagy, and mTOR phosphorylation levels in rats
with VD were notably enhanced by NBP treatment, accompanied by a
significant downregulation of autophagy protein expression. In
addition, mTOR phosphorylation decreased, whereas the expression
levels of autophagy-related proteins increased in rats with VD
following injection of the mTOR inhibitor rapamycin; however, NBP
co-treatment with rapamycin did not enhance mTOR phosphorylation or
inhibit autophagy-related protein expression in rats with VD. These
results indicated that excessive autophagy may occur in rats with
VD and may trigger neuronal damage, which was consistent with
previous studies (21,22). This process may be reversed by NBP
through upregulating mTOR phosphorylation; blocking mTOR signaling
using the specific inhibitor rapamycin prevented the reversal of
autophagy by NBP in rats with VD, suggesting that the
neuroprotective effect of NBP may be mTOR-dependent.
In conclusion, the results of the present study
demonstrated that NBP attenuated cognitive decline and neuronal
loss in the CA1 region of the hippocampus in rats exposed to
cerebral ischemia. In addition, the beneficial effects of NBP were
associated with the activation of the mTOR signaling pathway and
suppression of autophagy-related protein expression. However, this
study had certain limitations. In vivo, the influence of
other factors could not be completely ruled out; it is not clear
whether the accumulated autophagic vacuoles in rats with VD were
the result of autophagy induction or autophagic flux impairment,
and whether NBP exerted its effects on autophagy induction or
autophagic flux. In our future research, rat hippocampal neuron
cells will be cultured to measure the influence of NBP on autophagy
induction and the state of autophagic flux in vitro, which
may elucidate the detailed mechanisms by which NBP exerts a
neuroprotective effect in rats with VD.
Acknowledgements
Not applicable.
Funding
This study was supported by National Key R&D
Program of China (grant no. 2018YFC1311600), Liaoning Province
Nature Science Foundation of China (grant no. 2015020471) and
Science and Technology Program of Shenyang (no. 17-230-930)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AT wrote the manuscript and performed statistical
analysis. RZ and AT designed the study. XM established the rat
models. XM and HL collected and analyzed all experimental data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The experiments performed in the present study were
approved by The Animal Research Committee of the First Affiliated
Hospital of China Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
O'Brien JT and Thomas A: Vascular
dementia. Lancet. 386:1698–1706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith EE: Clinical presentations and
epidemiology of vascular dementia. Clin Sci (Lond). 131:1059–1068.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jayant S and Sharma B: Selective modulator
of cannabinoid receptor type 2 reduces memory impairment and
infarct size during cerebral hypoperfusion and vascular dementia.
Curr Neurovasc Res. 13:289–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Voigt O and Pöggeler S: Self-eating to
grow and kill: Autophagy in filamentous ascomycetes. Appl Microbiol
Biotechnol. 97:9277–9290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bharadwaj PR, Verdile G, Barr RK, Gupta V,
Steele JW, Lachenmayer ML, Yue Z, Ehrlich ME, Petsko G, Ju S, et
al: Latrepirdine (dimebon) enhances autophagy and reduces
intracellular GFP-Aβ42 levels in yeast. J Alzheimers Dis.
32:949–967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu J, Qian HY, Liu LJ, Zhou BC, Xiao Y,
Mao JN, An GY, Rui MZ, Wang T and Zhu CL: Mild hypothermia
alleviates excessive autophagy and mitophagy in a rat model of
asphyxial cardiac arrest. Neurol Sci. 35:1691–1699. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujita S, Sakurai M, Baba H, Abe K and
Tominaga R: Autophagy-mediated stress response in motor neurons
after hypothermic spinal cord ischemia in rabbits. J Vasc Surg.
62:1312–1319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abdoulaye IA and Yi JG: A review of recent
advances in neuroprotective potential of 3-N-butylphthalide and its
derivatives. Biomed Res Int. 2016:50123412016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng X, Peng Y, Liu M and Cui L:
DL-3-n-butylphthalide extends survival by attenuating glial
activation in a mouse model of amyotrophic lateral sclerosis.
Neuropharmacology. 62:1004–1010. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong N, Huang J, Chen C, Zhao Y, Zhang Z,
Jia M, Zhang Z, Hou L, Yang H, Cao X, et al: Dl-3-n-butylphthalide,
a natural antioxidant, protects dopamine neurons in rotenone models
for Parkinson's disease. Neurobiol Aging. 1777–1791. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu J, Wang Y, Li J, Deng J and Zhou H:
Intracranial artery stenosis and progression from mild cognitive
impairment to Alzheimer disease. Neurology. 82:842–849. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng Y, Sun J, Hon S, Nylander AN, Xia W,
Feng Y, Wang X and Lemere CA: L-3-n-butylphthalide improves
cognitive impairment and reduces amyloid-beta in a transgenic model
of Alzheimer's disease. J Neurosci. 30:8180–8189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He Z, Zhou Y, Lin L, Wang Q, Khor S, Mao
Y, Li J, Zhen Z, Chen J, Gao Z, et al: Dl-3-n-butylphthalide
attenuates acute inflammatory activation in rats with spinal cord
injury by inhibiting microglial TLR4/NF-κB signalling. J Cell Mol
Med. 21:3010–3022. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheng X, Hua K, Yang C, Wang X, Ji H, Xu
J, Huang Z and Zhang Y: Novel hybrids of 3-n-butylphthalide and
edaravone: Design, synthesis and evaluations as potential
anti-ischemic stroke agents. Bioorg Med Chem Lett. 25:3535–3540.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang F, Ma J, Han F, Guo X, Meng L, Sun Y,
Jin C, Duan H, Li H and Peng Y: DL-3-n-butylphthalide delays the
onset and progression of diabetic cataract by inhibiting oxidative
stress in rat diabetic model. Sci Rep. 6:193962016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lei H, Zhao CY, Liu DM, Zhang Y, Li L,
Wang XL and Peng Y: l-3-n-Butylphthalide attenuates
β-amyloid-induced toxicity in neuroblastoma SH-SY5Y cells through
regulating mitochondrion-mediated apoptosis and MAPK signaling. J
Asian Nat Prod Res. 16:854–864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xing M, Sun Q, Wang Y, Cheng Y and Zhang
N: Hydroxysafflor yellow A increases BDNF and NMDARs in the
hippocampus in A vascular dementia rat model. Brain Res.
1642:419–425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deacon RM and Rawlins JN: T-maze
alternation in the rodent. Nat Protoc. 1:7–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pulsinelli WA, Brierley JB and Plum F:
Temporal profile of neuronal damage in a model of transient
forebrain ischemia. Ann Neurol. 11:491–498. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levine B, Mizushima N and Virgin HW:
Autophagy inimmunity and inflammation. Nature; 469. pp. 323–335.
2011, PubMed/NCBI
|
|
22
|
Zhao Y, Huang G, Chen S, Gou Y, Dong Z and
Zhang X: Homocysteine aggravates cortical neural cell injury
through neuronal autophagy overactivation following rat cerebral
ischemia-reperfusion. Int J Mol Sci. 17:E11962016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zou W, Song Y, Li Y, Du Y, Zhang X and Fu
J: The role of autophagy in the correlation between neuron damage
andcognitive impairmentin ratchronic cerebral hypoperfusion. Mol
Neurobiol. 55:776–791. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li L, Chen J, Sun S, Zhao J, Dong X and
Wang J: Effects of estradiol on autophagy and Nrf-2/ARE signals
after cerebral ischemia. Cell Physiol Biochem. 41:2027–2036. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim YC and Guan KL: mTOR: A pharmacologic
target for autophagy regulation. J Clin Invest. 125:25–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang D, Lin Q, Su S, Liu K, Wu Y and Hai
J: URB597 improves cognitive impairment induced by chronic cerebral
hypoperfusion by inhibiting mTOR-dependent autophagy. Neuroscience.
344:293–304. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy in higher
eukaryotes. Autophagy. 8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Meng F, Cottrell JE, Sacktor TC
and Kass IS: Metabotropic actions of the volatile anaesthetic
sevoflurane increase protein kinase M synthesis and induce
immediate preconditioning protection of rat hippocampal slices. J
Physiol. 590:4093–4107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hölscher C: Time space and hippocampal
functions. Rev Neurosci. 14:253–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kiriyama Y and Nochi H: The function of
autophagy in neurodegenerative diseases. Int J Mol Sci.
16:26797–26812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Che H, Yan Y, Kang XH, Guo F, Yan ML, Liu
HL, Hou X, Liu T, Zong DK, Sun LL, et al: MicroRNA-27a promotes
inefficient lysosomal clearance in the hippocampi of rats following
chronic brain hypoperfusion. Mol Neurobiol. 54:2595–2610. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jia Y, Jin W, Xiao Y, Dong Y, Wang T, Fan
M, Xu J, Meng N, Li L and Lv P: Lipoxin A4 methyl ester alleviates
vascular cognition impairment by regulating the expression of
proteins related to autophagy and ER stress in the rat hippocampus.
Cell Mol Biol Lett. 20:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang X, He C, Liu P, Song X, Thomas T,
Tshimanga S, Wang F, Niu J, Sun T and Li PA: Inhibition of mTOR
pathway by rapamycin reduces brain damage in rats subjected to
transient forebrain ischemia. Int J Biol Sci. 11:1424–1435. 2015.
View Article : Google Scholar : PubMed/NCBI
|