Introduction
Diabetes mellitus (DM) is a very common metabolic
disorder with a very high morbidity in the world (1). DM is a group of metabolic diseases
characterized by hyperglycemia caused by insulin secretion
deficiency, insulin action, or both. Chronic hyperglycemia of DM is
associated with long-term damage, dysfunction, and failure of
different organs, especially eyes, kidneys, nerves, heart, and
blood vessels (2). DM is the disease
with the highest morbidity in the world at present. With the
progress of society and the improvement of people's quality of
life, the morbidity tend to be increasing (3). It was reported that the global DM
patients will reach 25.6% in 2015 (4). DM causes various complications, such as
cardiovascular and cerebrovascular diseases, nervous system
diseases, and kidney diseases. The disease deteriorates without
timely treatment, malignant tumor diseases will be directly caused.
DM is defined by the elevation of blood glucose markers and is a
major risk factor for cardiovascular diseases, which is the most
common cause of death for DM patients (5).
Reducing cardiovascular disease burden of DM
patients is an important clinical task. Priority should be given to
reducing premature death, improving quality of life, reducing
individual and economic burden of related diseases (6). Glucagon-like peptide-1 (GLP-1) is a
physiological incretin hormone that is released after the intake of
nutrients from the lower digestive tract and stimulates insulin
secretion at elevated blood glucose concentrations (7). GLP-1 can effectively stimulate insulin,
inhibit glucagon secretion, inhibit gastric emptying, and reduce
appetite and food intake (8).
However, whether GLP-1 receptor agonist can affect cardiovascular
complications in DM patients still needs further research.
Therefore, this investigation was carried out to provide reference
for future clinical practice on the concentration of blood sugar
and blood lipid before and after DM treatment, and the predictive
value of blood sugar and blood lipid concentration after DM
treatment on cardiovascular disease complications of DM
patients.
Patients and methods
General data
This study includes 132 DM patients who were treated
in Tengzhou Central People's Hospital (Tengzhou, China) from April
2013 to September 2016. In the research group, basic drugs plus
GLP-1 were used. Patients in the research group included 35 males
and 36 females, aged 33–65 years, with a mean age of 45.3±8.2.
Sixty-one cases with glipizide controlled release tablets were in
the control group, including 36 males and 25 females, aged 35–68
years, with a mean age of 46.7±9.5. This study was approved by the
Medical Ethics Committee of Tengzhou Central People's Hospital.
Inclusion and exclusion criteria
Inclusion criteria: Patients were diagnosed and
treated in Tengzhou Central People's Hospital; with complete
clinical data, aged 30–70 years, able to cooperate with the
investigation, and no allergy to test drugs. There are no other
serious organ diseases affecting the study, and the informed
consent was signed by the patients or family members.
Exclusion criteria: Patients who died in the course
of treatment; combined with other tumors; combined with other
cardiovascular and cerebrovascular diseases, physical disability,
pregnancy; combined with other autoimmune diseases; combined with
other chronic diseases, transfer, mental diseases, and language
dysfunction, as well as diseases affecting the results of this
study.
Treatment plan for patients
The control group was treated with conventional
drugs. The patients took glipizide controlled release tablets
(Beijing Honglin Pharmaceutical Co., Ltd., SFDA approval no.
H20084634) orally, with an initial dose of 5 mg/time and 1
time/day, and then the dose was adjusted according to the results
of blood glucose monitoring, but ≤20 mg/day, for 4 consecutive
months. The research group was given exenatide injection treatment
(Guangzhou UWA Technology Co., Ltd., Art. no. 22-197, at 10 µg.
Dosage scale injection pen: 0.25 mg/ml, 2.4 ml/branch, a single
injection dose of 10 µg, including 60 injections), subcutaneous
injection 2 times/day. The first 3 weeks were the lead-in period,
on the basis of the above treatment, the patient was injected
subcutaneously with 1 ml of normal saline. From the 4th to the 7th
week, patients were treated with GLP-1 receptor agonist (H20090382,
Hangzhou Haoxin Biotechnology Co., Ltd., Art. no. HYP0014-1, at 10
µg dose scale injection pen: 0.25 mg/ml, 2.4 ml/branch, 10 µg for
single injection, 60 injections), 10 µg/time, 2 times/day. Patients
were injected with GLP-1 receptor agonist from the 8th to 15th
weeks, 10 µg/time, 2 times/day for continuous treatment for 4
months.
Efficacy assessment
The criteria for clinical efficacy: markedly
effective: clinical signs, symptoms completely disappeared or
significantly relieved; effective: comprehensive symptoms have some
relief; invalid: no change in clinical symptoms and signs. The
criteria for cardiac autonomic function: markedly effective:
cardiac autonomic function returned to normal or significantly
improved; effective: cardiac autonomic function improved; invalid:
no change in cardiac autonomic function.
Detection methods
Venous blood (5 ml) was drawn from patients in the
two groups before and after treatment, respectively. The venous
blood was quiescent for 30 min and centrifuged at 1,500 × g at 24°C
for 10 min. The blood glucose function (fasting blood glucose FPG,
glycosylated hemoglobin HbAlc) and blood lipid function (serum
total cholesterol TC, low density lipoprotein cholesterol LDL-C,
high density lipoprotein cholesterol HDL-C) were detected by a full
automatic biochemical analyzer (Jiaozuo Lufeifan Biotechnology Co.,
Ltd., Art. no. LFF-LC-1781).
Observation indicators
Indicators to be observed: The improvement of
clinical efficacy of patients in the two groups after treatment
were observed; the concentrations of FPG, HbAlc, TC, LDL-C, and
HDL-C in serum of patients in the two groups before and after
treatment were compared; and the incidence rate of cardiovascular
disease complications of diabetic were recorded.
Secondary observation indicators: The predictive
value of concentrations of FPG, HbAlc, TC, LDL-C, and HDL-C on
cardiovascular disease was observed.
Statistical methods
In this study, SPSS 20.0 software was used to carry
out statistical analysis on the collected data, GraphPad 7 software
was used to draw the required illustrations and K-S test was used
to analyze the distribution of dose data. Normal distribution data
were expressed by mean ± standard deviation (mean ± SD).
Inter-group comparison was conducted by independent-samples t-test,
and intra-group comparison was conducted by paired t-test. Counting
data, utilization (%), were expressed by Chi-square (χ2)
test. The predictive value of FPG, HbAlc, TC, LDL-C, and HDL-C on
cardiovascular diseases of DM patients after treatment was plotted
by ROC (receiver operating characteristic); P<0.05 was
considered to indicate a statistically significant difference.
Results
Basic clinical data of patients
Age, sex, BMI, marital status, ethnicity, place of
residence, smoking and drinking history, and movement condition in
the clinical data of the research group and the control group were
not significantly different (P>0.05), as shown in Table I.
 | Table I.Clinical data of patients [n (%)]. |
Table I.
Clinical data of patients [n (%)].
| Item | Research group
(71) | Control group
(61) | χ2 or
t | P-value |
|---|
| Age | 45.3±8.2 | 46.7±9.5 | 0.909 | 0.365 |
| Sex |
|
| 1.247 | 0.264 |
| Male | 35 (49.30) | 36 (59.02) |
|
|
|
Female | 36 (50.70) | 25 (40.98) |
|
|
| BMI
(kg/m2) | 25.26±0.37 | 25.21±0.25 | 0.894 | 0.373 |
| Marital status |
|
| 0.356 | 0.551 |
|
Married | 63 (88.73) | 52 (85.25) |
|
|
|
Unmarried | 8
(11.27) | 9
(14.75) |
|
|
| Ethnicity |
|
| 0.382 | 0.536 |
| Han | 61 (85.92) | 50 (81.97) |
|
|
|
Minority | 10 (14.08) | 11 (18.03) |
|
|
| Place of
residence |
|
| 0.115 | 0.735 |
| City | 41 (57.75) | 37 (60.66) |
|
|
|
Countryside | 30 (42.25) | 24 (39.34) |
|
|
| Smoking history |
|
| 0.065 | 0.799 |
| Yes | 38 (53.52) | 34 (55.74) |
|
|
| No | 33 (46.48) | 27 (44.26) |
|
|
| Drinking history |
|
| 1.988 | 0.159 |
| Yes | 32 (45.07) | 35 (57.38) |
|
|
| No | 39 (54.93) | 26 (42.62) |
|
|
| Exercise habits |
|
| 0.131 | 0.717 |
| Yes | 35 (49.30) | 32 (52.46) |
|
|
| No | 36 (50.70) | 29 (47.54) |
|
|
Improvement of clinical efficacy of
patients in the two groups after treatment
Total of 38 cases (53.52%) were markedly effective,
and 24 cases (33.80%) were effective in the research group; 22
cases (36.07%) were markedly effective and 21 cases (34.43%) were
effective in the control group. In terms of marked effect, the
research group was significantly higher than the control group
(P<0.05), while in terms of effectiveness, the two groups had no
statistical significance (P>0.05). The effective treatment rate
in the research group (87.32%) was significantly higher than the
control group (70.49%). P<0.05, indicates a statistically
significant difference (Table
II).
 | Table II.Efficacy of patients in the two
groups. |
Table II.
Efficacy of patients in the two
groups.
|
|
| Efficacy [n (%)] |
|
|---|
|
|
|
|
|
|---|
| Group | Number (n) | Markedly
effective | Effective | Ineffective | Effective treatment
rate (%) |
|---|
| Research group | 71 | 38 (53.52) | 24 (33.80) | 9 (12.68) | 87.32 |
| Control group | 61 | 22 (36.07) | 21 (34.43) | 18 (29.51) | 70.49 |
| χ2 |
| 4.032 | 0.006 | 5.713 | 5.713 |
| P-value |
| 0.045 | 0.940 | 0.017 | 0.017 |
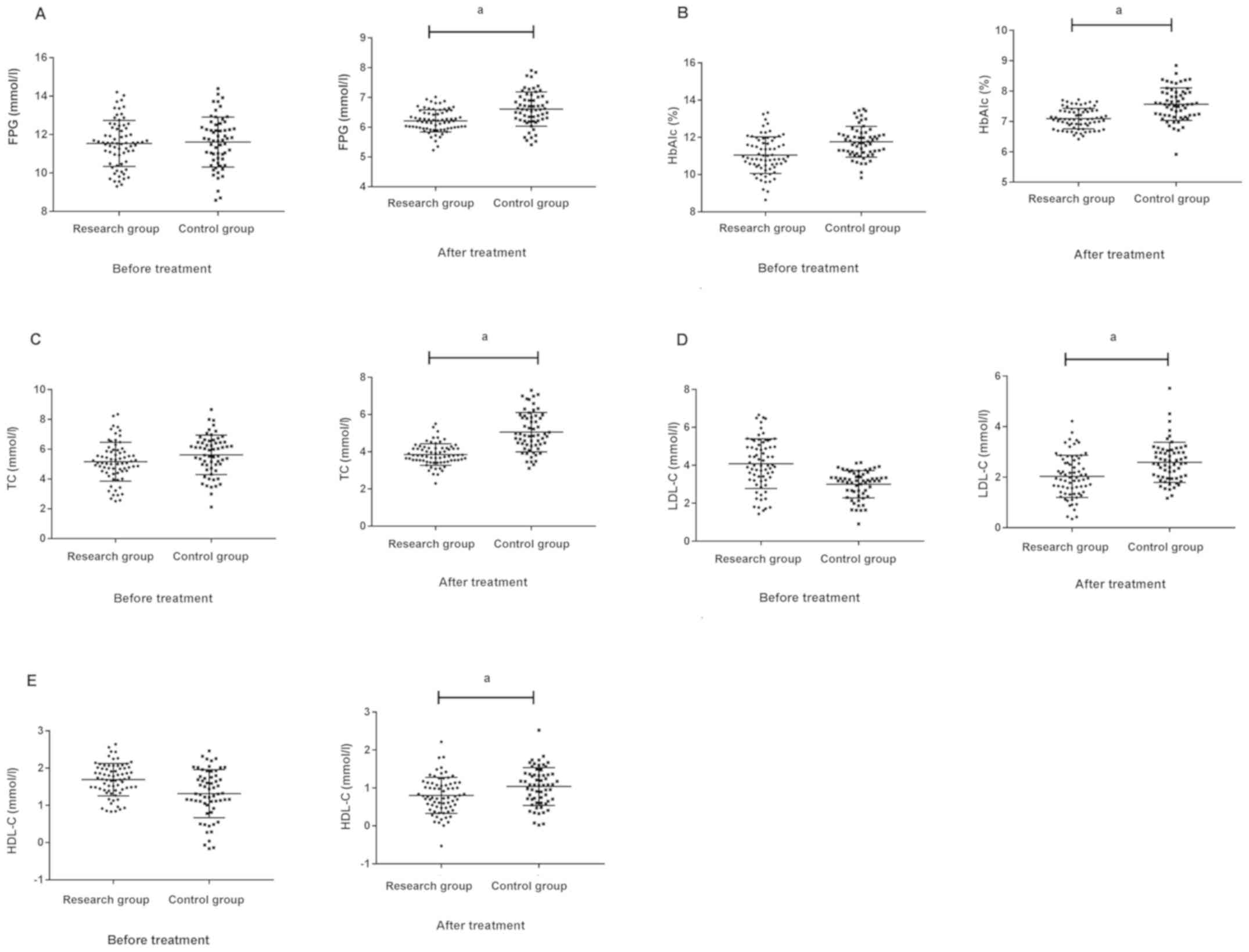
Comparison of concentrations of FPG,
HbAlc, TC, LDL-C, and HDL-C in serum of patients between the two
groups before and after treatment
The concentrations of FPG, HbAlc, TC, LDL-C, and
HDL-C in the research group were, respectively, 11.54±1.28 mmol/l,
11.03±1.18%, 5.48±1.27, 4.18±1.19 and 1.68±0.43 mmol/l before
treatment. After treatment, the concentrations of FPG, HbAlc, TC,
LDL-C, and HDL-C in the research group were, respectively,
6.13±0.35 mmol/l, 7.15±0.34%, 3.93±0.62, 1.95±0.84 and 0.75±0.42
mmol/l. The concentrations of FPG, HbAlc, TC, LDL-C, and HDL-C in
the control group were, respectively, 11.62±1.34, 11.53±0.82%,
5.53±1.31, 3.06±0.82 and 1.30±0.67 mmol/l before treatment. After
treatment, the concentrations of FPG, HbAlc, TC, LDL-C, and HDL-C
in the control group were, respectively, 6.57±0.63 mmol/l,
7.62±0.56%, 4.95±0.92, 2.68±0.73 and 1.08±0.57 mmol/l. There was no
significant difference of patients between the two groups before
treatment, P>0.05. After treatment, the indicators of patients
in the research group were significantly lower than those in the
control group. P<0.05, as shown in Table III and Fig. 1.
 | Table III.Comparison of the two groups of
patients before and after treatment. |
Table III.
Comparison of the two groups of
patients before and after treatment.
|
| Research group
(n=71) | Control group
(n=61) |
|---|
|
|
|
|
|---|
| Indicator | Before treatment | After treatment | Before treatment | After treatment |
|---|
| FPG (mmol/l) | 11.54±1.28 |
6.13±0.35a |
11.62±1.34b |
6.57±0.63a,c |
| HbAlc (%) | 11.03±1.18 |
7.15±0.34a |
11.53±0.82b |
7.62±0.56a,c |
| TC (mmol/l) | 5.48±1.27 |
3.93±0.62a |
5.53±1.31b |
4.95±0.92a,c |
| LDL-C (mmol/l) | 4.18±1.19 |
1.95±0.84a |
3.06±0.82b |
2.68±0.73a,c |
| HDL-C (mmol/l) | 1.68±0.43 |
0.75±0.42a |
1.30±0.67b |
1.08±0.57a,c |
| F | 1,068.000 | 1,724.000 | 1,312.000 | 1,404.000 |
| P-value | 0.001 | 0.001 | 0.001 | 0.001 |
Incidence rate of cardiovascular
diseases in DM patients
Comparing the incidence rate of cardiovascular
diseases and residual vascular risks of patients between the two
groups, the incidence rate of cardiovascular diseases and residual
vascular risks in the research group were significantly lower than
those in the control group. P<0.05 (Table IV).
 | Table IV.Comparison of incidence rate of
cardiovascular disease and residual vascular risks [(n (%)]. |
Table IV.
Comparison of incidence rate of
cardiovascular disease and residual vascular risks [(n (%)].
| Group | Incidence rate of
cardiovascular diseases | Incidence rate of
residual vascular risks |
|---|
| Research group
(n=71) | 4
(5.63) | 1 (1.41) |
| Control group
(n=61) | 10 (16.13) | 7 (11.48) |
| χ2 | 3.871 | 5.840 |
| P-value | 0.049 | 0.016 |
The predictive value of the
concentration of FPG, HbAlc, TC, LDL-C, and HDL-C on cardiovascular
diseases in DM patients after treatment
Patients were divided into the occurrence group and
the non-occurrence group. The expression of FPG, HbAlc, TC, LDL-C,
and HDL-C in the two groups was further detected and found to be
different. The ROC curves were drawn, showing that the AUC of FPG,
HbAlc, TC, LDL-C and HDL-C were 0.742, 0.780, 0.737, 0.726, and
0.721, respectively, which had good prediction value (Table V and Fig.
2).
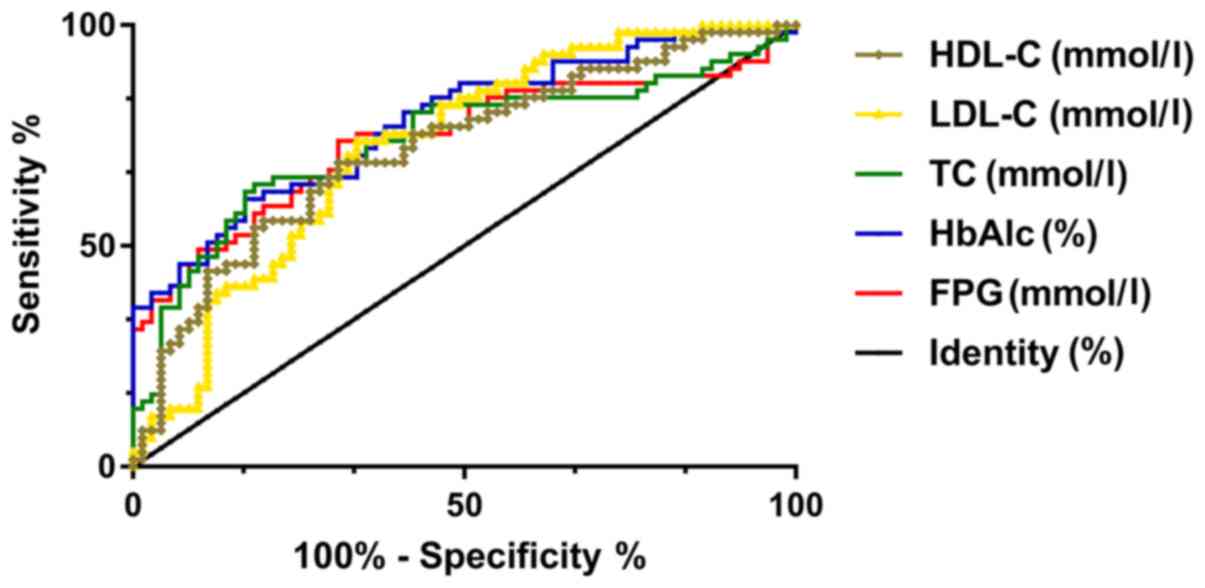 | Figure 2.Predictive value of concentrations of
FPG, HbAlc, TC, LDL-C, and HDL-C on cardiovascular diseases in DM
patients after treatment. ROC curve analysis shows that FPG has a
sensitivity of 73.77% and specificity of 69.01% for predicting
cardiovascular diseases in DM patients when cut-off value is 6.349.
When cut-off value is 7.440, HbAlc has a sensitivity of 60.66% and
specificity of 83.10% for predicting cardiovascular disease in DM
patients. When the cut-off value is 4.924, the sensitivity of TC to
the predictive value of cardiovascular diseases in DM patients is
63.93%, the specificity is 81.69%, and the AUC is 0.773. When
cut-off value is 2.238, LDL-C has a sensitivity of 73.77%,
specificity of 66.20%, and AUC of 0.726 for predicting
cardiovascular disease in DM patients. When cut-off value is 1.108,
the sensitivity and specificity of HDL-C for predicting
cardiovascular diseases in DM patients are 68.58 and 69.01%,
respectively. DM, diabetes mellitus. |
 | Table V.ROC diagnosis. |
Table V.
ROC diagnosis.
| Indicators | AUC | 95% CI | Sensitivity | Specificity | Standard error | Cut-off |
|---|
| FPG | 0.742 | 0.654–0.831 | 73.77% | 69.01% | 0.045 | 6.349 |
| HbAlc | 0.780 | 0.701–0.859 | 60.66% | 83.10% | 0.040 | 7.440 |
| TC | 0.737 | 0.647–0.826 | 63.93% | 81.69% | 0.046 | 4.924 |
| LDL-C | 0.726 | 0.640–0.812 | 73.77% | 66.20% | 0.044 | 2.238 |
| HDL-C | 0.721 | 0.633–0.808 | 68.58% | 69.01% | 0.045 | 1.018 |
Discussion
DM is defined as a group of metabolic diseases,
characterized by hyperglycemia caused by insulin secretion
deficiency, insulin action, or both (2). In 2014, the global prevalence of DM was
~9% (9), and nearly 1.3 million
people died of DM (10) in 2010. DM
is also associated with high morbidity due to a wide range of
complications, such as retinopathy, nephropathy, neuropathy, and
cardiovascular diseases (11,12).
Prevention and management of these complications have become the
main aspect of modern DM nursing.
Epidemiological studies show that overweight and
obesity are important risk factors for DM, cardiovascular diseases,
cancer, and premature death (13).
The main risk factor for complications of DM is poor blood glucose
control (14). Some studies have
shown that chronic hyperglycemia is related to microvascular
complications (15,16). Although it has been proved that
improving blood sugar control can reduce microvascular
complications in DM patients (17),
its relationship with macrovascular complications and all-cause
mortality is still uncertain. In the study of Nadkarni et al
(18), GLP-1's hypoglycemic effect
was shown to be dependent on glucose. GLP-1 can reduce blood
glucose level only when blood glucose concentration is higher than
fasting level. The postprandial blood glucose level decreases with
the decrease of GLP-1, and the hypoglycemic effect of GLP-1 is
self-terminated. This remarkable glucose-dependent characteristic
of GLP-1 leads to a situation that intravenous injection of GLP-1
cannot reduce blood glucose below fasting level. These drugs can
protect the heart muscle, improve myocardial infarction and heart
failure by regulating blood sugar, blood lipids, blood pressure,
and through anti-inflammatory and anti-oxidative stress mechanisms.
Because GLP-1 does not produce hypoglycemia, these clinical
findings lead to the use of GLP-1 receptor agonist as a new
hypoglycemic agent, which can be used to treat DM. Therefore, in
this study, DM patients were treated through GLP-1 receptor agonist
scheme, and the improvement of clinical efficacy of DM patients was
observed, to provide reference for clinical treatment.
In this study, the clinical efficacy of patients in
the two groups after treatment was compared. The results showed
that the research group was significantly better than the control
group in terms of markedly effective treatment rate, while there
was no significant difference in effective treatment rate between
the two groups. In terms of overall effective rate, the research
group was higher than the control group, which shows that GLP-1
receptor agonist scheme can improve the effective treatment rate of
patients. At present, the indicators for clinical detection of DM
were generally blood glucose function (FPG, HbAlc) and blood lipid
function (TC, LDL-C, and HDL-C). Although the cellular regulation
of insulin secretion is quite clear, little is known about the
control of glucagon secretion (19,20).
Whether FPG, HbAlc, TC, LDL-C, and HDL-C can be used as predictors
of cardiovascular complications in DM patients has not been
reported. In this study, the concentrations of FPG, HbAlc, TC,
LDL-C, and HDL-C in serum of the research group and the control
group before and after treatment were compared, and it was found
that there was no difference in the concentrations of FPG, HbAlc,
TC, LDL-C, and HDL-C in serum of patients in the two groups before
treatment but in serum of the research group after treatment they
were significantly lower than those in the control group, which
indicated that FPG, HbAlc, TC, LDL-C, and HDL-C were better
inhibited by adding GLP-1 receptor agonist. After treatment, the
incidence rate of cardiovascular diseases in DM patients was
observed, and it was found that the research group was
significantly better than the control group in both the incidence
rate of cardiovascular diseases and the incidence rate of residual
vascular risks, suggesting that GLP-1 receptor agonist therapy can
reduce the incidence rate of complications of cardiovascular
diseases in DM patients. In a study by Than and Newsome (21), it is indicated that liraglutide and
GLP-1 have extremely high sequence homologs, and their activities
interact with GLP-1 receptor to greatly increase the synthesis and
metabolism of cyclic adenosine monophosphate. Glucose stimulates
insulin to accelerate secretion when blood sugar increases,
inhibits glucagon secretion, reduces insulin secretion when blood
sugar decreases, and maintains normal metabolism of glucagon.
Finally, ROC curve analysis was performed, and it was found that
the AUC of FPG, HbAlc, TC, LDL-C, and HDL-C in serum after
treatment for predicting cardiovascular disease complications in DM
patients was respectively 0.742, 0.780, 0.737, 0.726, and 0.721,
respectively, which has high clinical value.
In the above studies, the efficacy of GLP-1 receptor
agonist on DM patients and the predictive value of FPG, HbAlc, TC,
LDL-C, and HDL-C in cardiovascular disease complications of DM
patients were preliminarily proven. However, further studies need
to be carried out for confirmation.
In conclusion, GLP-1 receptor agonist can improve
the clinical efficacy of patients. Through ROC analysis, FPG,
HbAlc, TC, LDL-C, and HDL-C can be used as predictors of
cardiovascular disease complications in DM patients, which has high
clinical value.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL conceived and designed the study, and drafted the
manuscript. FL and YK collected, analyzed and interpreted the
experimental data. YK revised the manuscript for important
intellectual content. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Tengzhou Central People's Hospital (Tengzhou, China). Signed
informed consents were obtained from the patients and/or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association: Standards
of medical care in diabetes - 2016 abridged for primary care
providers. Clin Diabetes. 34:3–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Diabetes Association: Diagnosis
and classification of diabetes mellitus. Diabetes Care. 33 (Suppl
1):S62–S69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
American Diabetes Association: (2)
Classification and diagnosis of diabetes. Diabetes Care. 38
(Suppl):S8–S16. 2015. View Article : Google Scholar
|
|
4
|
Zinman B, Wanner C, Lachin JM, Fitchett D,
Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ,
et al EMPA-REG OUTCOME Investigators, : Empagliflozin,
cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J
Med. 373:2117–2128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al
American Heart Association Statistics Committee and Stroke
Statistics Subcommittee, : Executive summary: heart disease and
stroke statistics - 2013 update: a report from the American Heart
Association. Circulation. 127:143–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Low Wang CC, Hess CN, Hiatt WR and
Goldfine AB: Clinical update: Cardiovascular disease in diabetes
mellitus: Atherosclerotic cardiovascular disease and heart failure
in type 2 diabetes mellitus - mechanisms, management, and clinical
considerations. Circulation. 133:2459–2502. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nauck MA, Kleine N, Orskov C, Holst JJ,
Willms B and Creutzfeldt W: Normalization of fasting hyperglycaemia
by exogenous glucagon-like peptide 1 (7–36 amide) in type 2
(non-insulin-dependent) diabetic patients. Diabetologia.
36:741–744. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Drucker DJ and Nauck MA: The incretin
system: Glucagon-like peptide-1 receptor agonists and dipeptidyl
peptidase-4 inhibitors in type 2 diabetes. Lancet. 368:1696–1705.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Basu S, Yoffe P, Hills N and Lustig RH:
The relationship of sugar to population-level diabetes prevalence:
an econometric analysis of repeated cross-sectional data. PLoS One.
8:e578732013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ha KH and Kim DJ: Trends in the Diabetes
Epidemic in Korea. Endocrinol Metab (Seoul). 30:142–146. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Monami M, Adalsteinsson JE, Desideri CM,
Ragghianti B, Dicembrini I and Mannucci E: Fasting and
post-prandial glucose and diabetic complication. A meta-analysis.
Nutr Metab Cardiovasc Dis. 23:591–598. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fowler MJ: Microvascular and macrovascular
complications of diabetes. Clin Diabetes. 26:77–82. 2008.
View Article : Google Scholar
|
|
13
|
Malik VS, Popkin BM, Bray GA, Després JP
and Hu FB: Sugar-sweetened beverages, obesity, type 2 diabetes
mellitus, and cardiovascular disease risk. Circulation.
121:1356–1364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Hu G, Yuan Z and Chen L:
Glycosylated hemoglobin in relationship to cardiovascular outcomes
and death in patients with type 2 diabetes: A systematic review and
meta-analysis. PLoS One. 7:e425512012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaster B and Hirsch IB: The effects of
improved glycemic control on complications in type 2 diabetes. Arch
Intern Med. 158:134–140. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
UK Prospective Diabetes Study (UKPDS)
Group, : Intensive blood-glucose control with sulphonylureas or
insulin compared with conventional treatment and risk of
complications in patients with type 2 diabetes (UKPDS 33). Lancet.
352:837–853. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shichiri M, Kishikawa H, Ohkubo Y and Wake
N: Long-term results of the Kumamoto Study on optimal diabetes
control in type 2 diabetic patients. Diabetes Care. 23 (Suppl
2):B21–B29. 2000.PubMed/NCBI
|
|
18
|
Nadkarni P, Chepurny OG and Holz GG:
Regulation of glucose homeostasis by GLP-1. Prog Mol Biol Transl
Sci. 121:23–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seino S, Shibasaki T and Minami K:
Dynamics of insulin secretion and the clinical implications for
obesity and diabetes. J Clin Invest. 121:2118–2125. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gaisano HY, Macdonald PE and Vranic M:
Glucagon secretion and signaling in the development of diabetes.
Front Physiol. 3:3492012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Than NN and Newsome PN: A concise review
of non-alcoholic fatty liver disease. Atherosclerosis. 239:192–202.
2015. View Article : Google Scholar : PubMed/NCBI
|