Introduction
The tibia is the larger and stronger one of the two
lower leg bones below the knee, and a tibial shaft fracture or
breakage is a common orthopedic injury that may be attributed to
road traffic accidents, falls from height and sports activities,
with an annual morbidity rate of approximately 0.02% (1,2). Tibial
fractures represent 1.66% of the total cases in adults, and nearly
10% of fracture patients have delayed healing or nonunion, which is
the main complication in tibial fracture patients. In recent
decades, as many as several million fracture patients are disabled
due to poor healing (3,4), so that the patients have decreased
productivity and even lose working capacity, thereby increasing
social cost (5,6). Therefore, it is extremely important to
study the physiological mechanism in the repair of fractures for
facilitating fracture healing. The repair of fractures is an
extremely complex physiological process that involves a wide
variety of cells, including bone marrow-derived mesenchymal stem
cells (BMMSCs), osteoprogenitors, osteoblasts and osteoclasts
(2,7), and osteoblasts, the critical functional
cells derived from BMMSCs, are responsible for the metabolism and
fracture healing of adult bones (7).
Mesenchymal stem cells (MSCs) are stem cells with
the multi-directional differentiation potential, first discovered
in bone marrows, and they can differentiate in vitro into
such functional cells as osteoblasts, chondrocytes, lipocytes and
myoblasts (8). Relevant studies have
found that after fractures, BMMSCs can rapidly migrate into the
fracture site and then start to proliferate and osteogenically
differentiate. The osteogenic differentiation of BMMSCs is
modulated by multiple hormones and local factors, so the
stimulation of their osteogenic differentiation has been considered
as an important mechanism by which fracture healing is accelerated
(9,10). The Janus kinase-signal transducer and
activator of transcription (JAK-STAT) signaling pathway plays a
vital role in multiple processes such as cell proliferation and
differentiation. Several studies have demonstrated that the
JAK-STAT pathway can regulate the function of osteoblasts and bone
tissue regeneration and promote angiogenesis (11), while modulating the differentiation
and migration of preosteoclasts (12).
The present study explored the role of the JAK-STAT
signaling pathway in the osteogenic differentiation of BMMSCs and
its influence on the repair of tibial fractures, providing
theoretical bases for related research on fracture repair and
offering potential clinical treatments.
Materials and methods
Main materials
Rabbits aged 15 weeks, xylazine, ketamine and
enrofloxacin, Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), phosphate buffered saline (PBS), and double
antibodies (Gibco; Thermo Fisher Scientific, Inc.), cluster of
differentiation (CD) 45-PE, CD90-PE, CD105-PE, JAK2, phosphorylated
(p)-JAK2, p-STAT3 and β-actin antibodies (Abcam), TG101348 (Selleck
Chemicals), and 0.22 µm pinhole filter (EMD Millipore).
Establishment of rabbit fracture
models
Rabbits underwent osteotomy and external fixation of
the left middle tibia. Anesthesia was performed using xylazine (2.5
mg/kg) and ketamine (22 mg/kg) and maintained with isoflurane. Then
the animals were placed in right lateral position, and the left
pelvis and limb were prepared for operation. A 1 cm-long cranial
lateral incision was made at the tibial shaft, and the skull tibial
muscle and gastrocnemius muscle were bluntly anatomized and
separated to expose the tibia which was then fixed using a mini
fixator. With the lateral tibial periosteum longitudinally cut
open, transverse osteotomy was conducted using a saw, and sterile
saline was used for continuous flushing. Subsequently, four needles
with a diameter of 1.6 mm were led into the lateral middle tibial
shaft, and the fracture site was fixed using two proximal needles
and the distal needles in tibial osteotomy. Finally, the fixator
was fixed into the pin for routine muscular and subcutaneous
closure. A preventive dose of enrofloxacin (5 mg/kg) was
subcutaneously administered before operation and at 3 days after
operation. This study was approved by the Animal Ethics Committee
of Shandong Provincial Third Hospital Animal Center (Jinan,
China).
Isolation and culture of BMMSCs
The tibia of rabbits was removed under sterile
conditions, and cleaned using PBS. Then the bone ends were sawed
off, and the bone cavity was rinsed with the DMEM containing 10%
FBS and 1% double antibodies using a syringe. The cell suspension
was harvested, centrifuged at 4°C, 1,050 × g for 5 min, added with
an appropriate amount of DMEM containing 10% FBS and 1% double
antibodies for re-suspension and sedimentation. Following counting,
the cells were inoculated into a 100 mm culture dish at the density
of 1×105 cells/ml and cultured in an incubator
containing 5% CO2 at 37°C. The medium was replaced every
other day until 90% of the dish bottom was covered. The resulting
cells were washed using PBS twice, digested by 0.25% trypsin and
sub-cultured at the density of 1:3.
Cell injection
BMMSCs were amplified through in vitro
culture, and passage 5 (P5) BMMSCs growing well were collected and
prepared into the cell suspension at the density of
1×107 cells/ml. The fracture rabbits prepared as above
were divided into control group, inhibitor group, BMMSC injection
group and BMMSCs + inhibitor group. The fracture end was locally
injected with 500 µl of the cell suspension in BMMSC injection
group, 500 µl of the cell suspension was locally injected into the
fracture end, and 100 mg/kg TG101348 was orally taken in BMMSCs +
inhibitor group, an equal volume of normal saline was locally
injected into the fracture end, and 100 mg/kg TG101348 was orally
taken in inhibitor group, and the fracture end was locally injected
with 500 µl of normal saline in control group.
Cell Counting Kit (CCK)-8 assay
The P3 BMMSCs with good growth were seeded into a
96-well plate at 1×104 cells/ml and cultured using DMEM
+ 10% FBS or DMEM + 10% FBS + 1 µM of TG101348 for 7 days. The
corresponding wells were added with 10 µl of CCK-8 solution daily
(Dojindo Molecular Technologies, Inc.), and the culture plate was
incubated in an incubator for 1–2 h. Finally, the absorbance at 450
nm was determined using a microplate reader.
Wound healing assay
The P3 BMMSCs growing well were inoculated into a
6-well plate at 2×105 cells/ml and cultured using DMEM +
10% FBS until the cells completely covered the dish bottom, and a
straight scratch was made using a pipette tip. The cells were
washed using PBS 3 times. With the scratched cells removed, the
resulting cells were cultured in DMEM or DMEM + 1 µM of TG101348,
and they were photographed and analyzed at 24 and 48 h.
Osteogenic induction of BMMSCs
The P3 BMMSCs growing well were seeded into a 6-well
plate at 2×105 cells/ml and started to be osteogenically
induced when 90% of the dish bottom was covered by the cells using
the following medium: DMEM + 50 mg/l ascorbic acid + 0.5 mM of
sodium β-glycerophosphate + 1 µM of dexamethasone and 10% FBS. The
BMMSCs cultured in osteogenic induction medium were taken as
induction group, those in osteogenic induction medium + 1 µM of
TG101348 as inhibitor group, and those in DMEM + 10% FBS as control
group, and they were cultured in an incubator containing 5%
CO2 at 37°C. The medium was changed every other day, and
the culture lasted for 3 weeks.
Flow cytometry analysis
Flow cytometry analysis was performed in the P3
BMMSCs growing well as follows. After being washed using PBS twice,
the cells were digested using 0.25% trypsin into single-cell
suspension, and rinsed using PBS 3 times. With the density adjusted
to 3×105 cells/ml, the cells were incubated separately
with CD45-PE, CD90-PE and CD105-PE antibodies for flow cytometry in
the dark at 4°C, and the cell samples incubated with anti-rat
IgG1-PE antibody were taken as homotype controls. The resulting
cells were washed using PBS 3 times, and detected with a flow
cytometer (Beckman Coulter, Inc.). Finally, the data were analyzed
using Kaluza software.
Alizarin red staining and
semi-quantitative analysis
After the induction of osteogenic differentiation
for 3 weeks, the cells were rinsed with PBS twice, fixed in an
appropriate volume of 4% paraformaldehyde at room temperature,
washed with PBS twice again and incubated with 40 mM of alizarin
red staining solution at 37°C for 30 min. With the staining
solution aspirated, PBS was added to wash the cells 3 times, and
the cells were observed and photographed under a microscope. After
PBS was discarded, the calcium nodules were dissolved using 10 mM
of cetylpyridinium chloride. Finally, the absorbance at a
wavelength of 550 nm was measured using the microplate reader for
semi-quantitative analysis.
Western blotting
The cells induced to differentiate and cultured
in vitro were added with cell lysis buffer in moderation.
After the cell lysis suspension was collected using a cell scraper,
the cells were lysed at 4°C overnight, and centrifuged at 4°C,
11,500 × g for 10 min to extract total proteins. Then the
concentration of the proteins was determined via bicinchoninic acid
(BCA) protein assay (Pierce; Thermo Fisher Scientific, Inc.), and
the proteins were separated through 8% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto a polyvinylidene fluoride (PVDF) membranes (EMD
Millipore). Subsequently, the membrane was sealed in Tris buffered
saline using 5% skim milk powder and 0.1% Tween-20 and slightly
shaken and reacted with the JAK2, p-JAK2, p-STAT3 and β-actin
primary antibodies at 4°C overnight. Afterwards, the horseradish
peroxidase (HRP)-labeled secondary antibodies were added for
incubation. Finally, the proteins were treated with
electrochemiluminescence (ECL) reagent, exposed and detected.
X-ray examination
The fracture site was examined using an X-ray
machine through the perspective shooting, with the tube projection
distance of 100 cm, and at 100 mA and 50 kV, immediately after the
establishment of rabbit fracture models and at 3 weeks after
operation, so as to observe the fracture models and postoperative
and post-treatment fractures. Finally, X-ray images were analyzed
using medical image analysis software.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
20.0 software (IBM Corp.) was applied to statistically process
data. The data in each group were expressed as mean ± standard
deviation (mean ± SD), and inter-group comparison was made using
independent-samples t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
In vitro isolation and culture of
rabbit BMMSCs
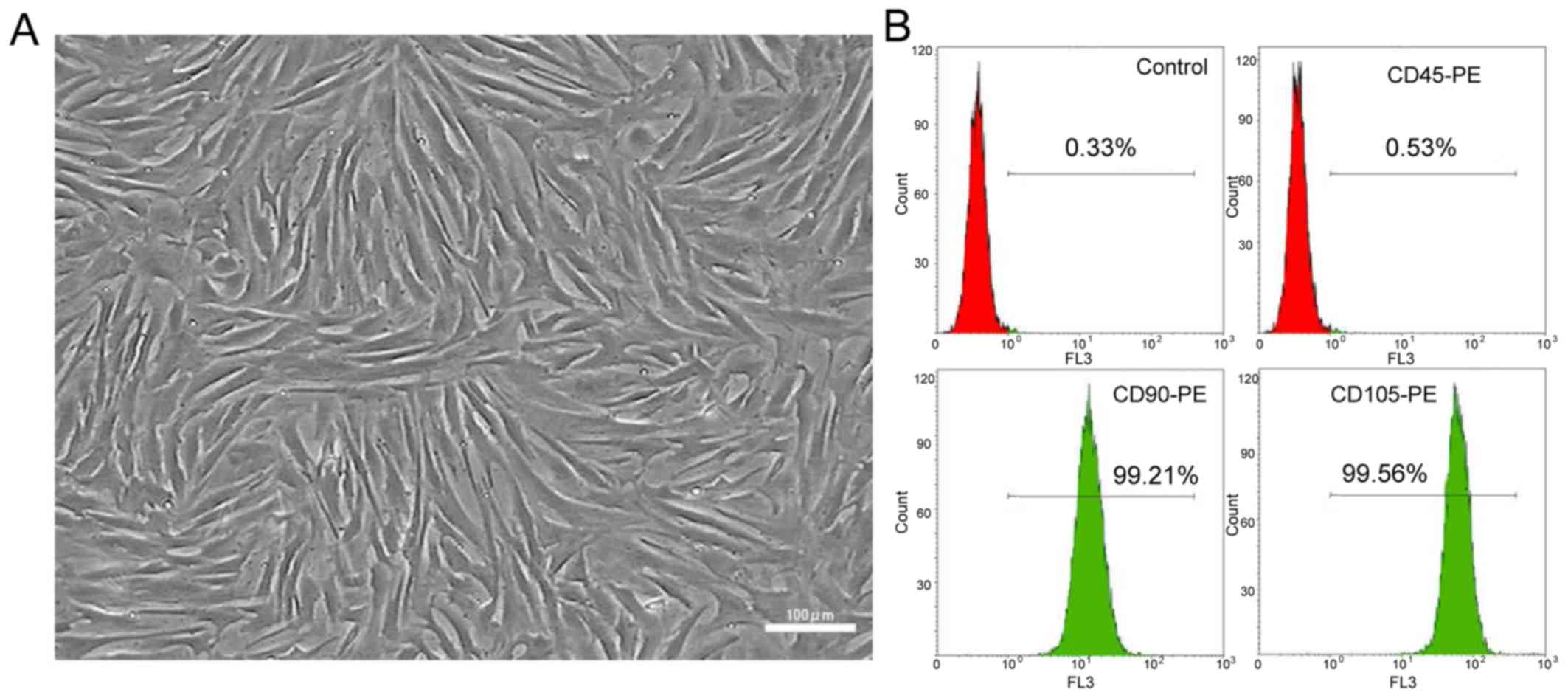
The BMMSCs isolated and cultured in vitro
showed favorable and whirlpool-like growth, and plumpness in
morphology (Fig. 1A). According to
the results of flow cytometry analysis in P3 cells, the positive
expression rates of CD45, CD90 and CD105 in cells were 0.53, 99.21
and 99.56%, respectively (Fig.
1B).
Proliferation and wound healing
results of BMMSCs
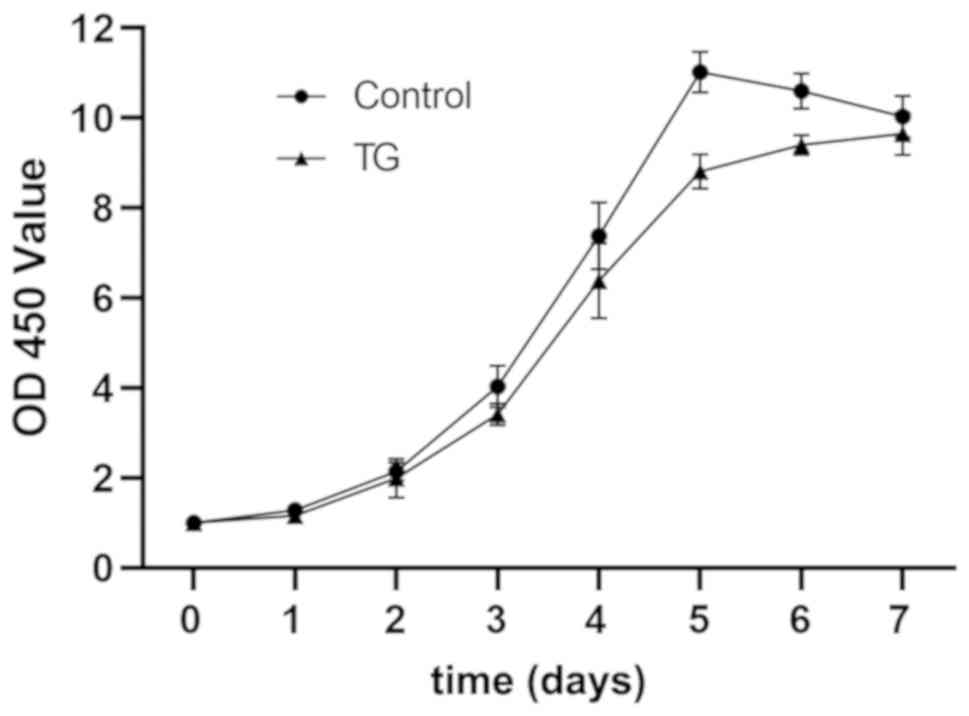
BMMSCs were cultured using JAK2 inhibitor TG101348,
with those normally cultured as controls. At 0, 1, 2, 3, 4, 5, 6
and 7 days, the optical density (OD) was measured using CCK-8
assay, and it was found that the cells proliferated in a sigmoid
curve, while the application of TG101348 lowered the proliferation
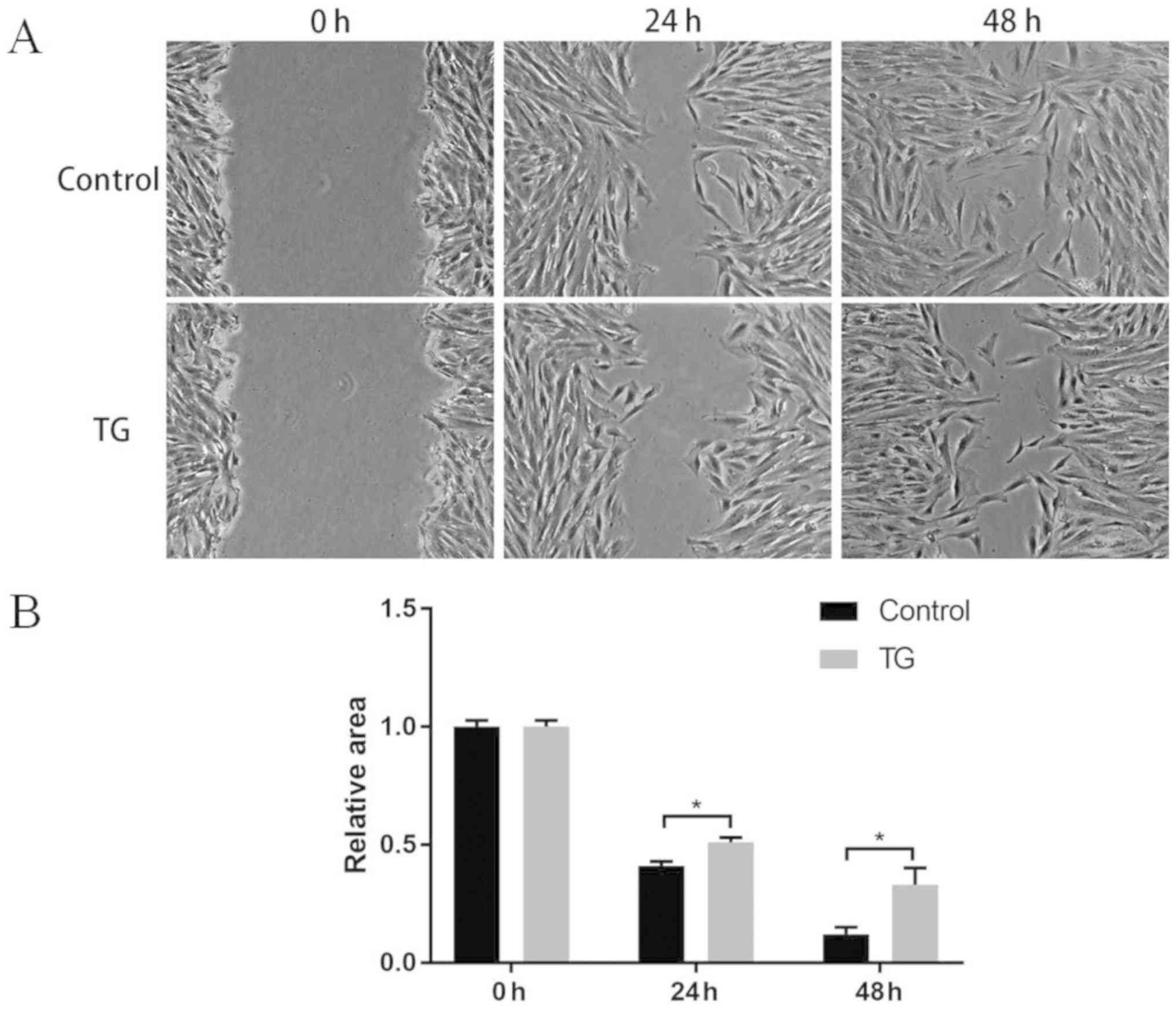
level of BMMSCs (Fig. 2). The wound
healing assay results revealed that compared with those in control
group, the BMMSCs cultured with TG101348 had a notably weakened
migration ability at 24 and 48 h (P<0.05) (Fig. 3).
In vitro induction of osteogenic
differentiation of BMMSCs
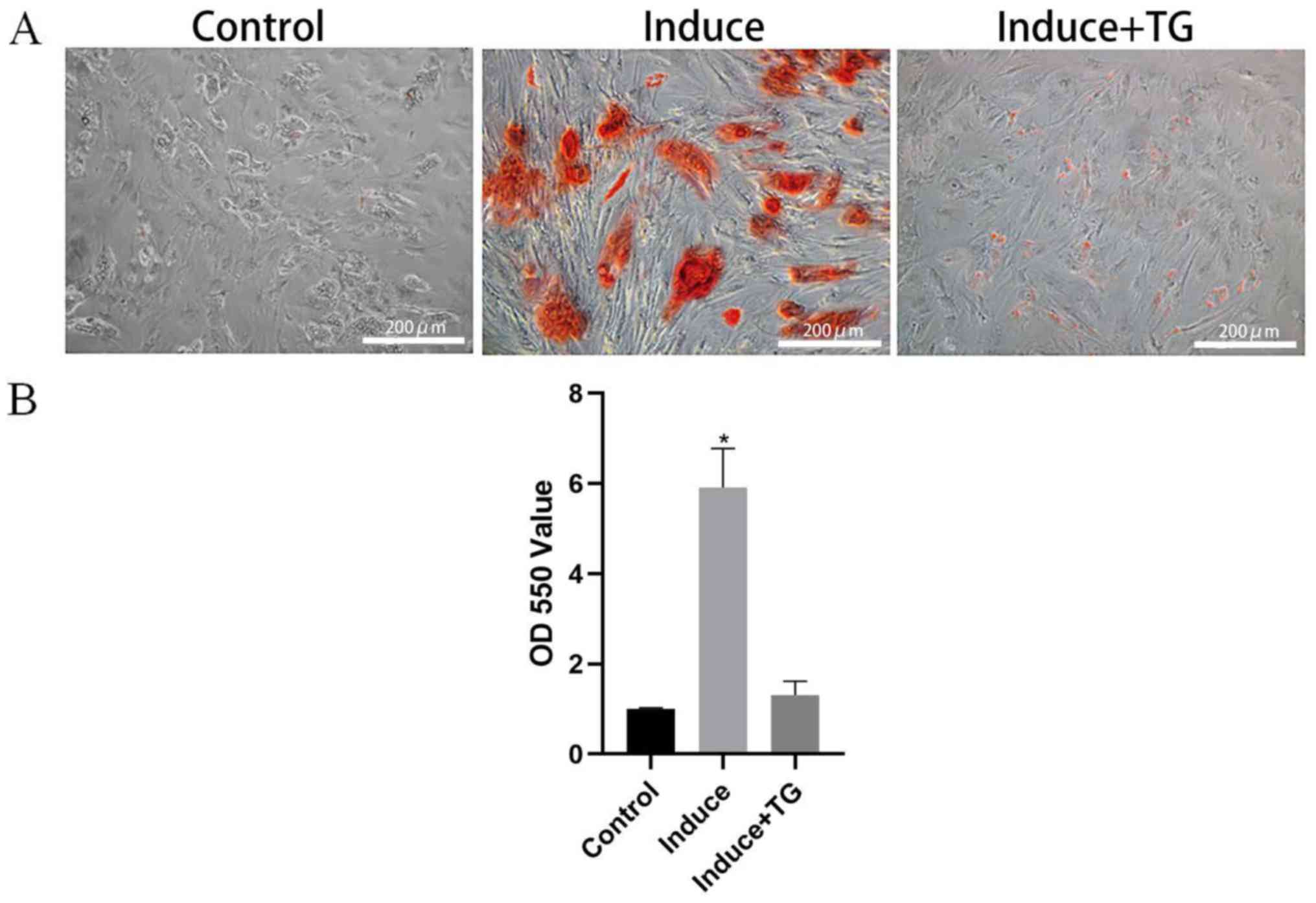
BMMSCs were induced in vitro to differentiate
into osteoblasts for 3 weeks, and the alizarin red staining results
showed that the osteogenically induced BMMSCs had large numbers of
calcium nodules, while only few of those co-induced with JAK2
inhibitor TG101348 were stained red, but the BMMSCs normally
cultured for 3 weeks were not stained red (Fig. 4A). It was found through the
semi-quantitative analysis of the alizarin red staining results
that the OD of osteogenically induced BMMSCs was notably higher
than that of the BMMSCs co-induced with TG101348 (P<0.05)
(Fig. 4B).
Analysis of JAK/STAT signaling pathway
in osteogenic induction
BMMSCs were induced to osteogenically differentiate
for 1 or 3 weeks, and then the JAK-STAT signaling pathway-related
proteins JAK2, p-JAK2 and p-STAT3 were detected using WB. The
results showed that compared with those in control group, the
protein expression levels of p-JAK2 and p-STAT3 increased notably
in induction group at 1 and 3 weeks (P<0.05), whereas the
protein expressions of JAK2, p-JAK2 and p-STAT3 in the osteogenic
induction of cells were considerably repressed by JAK2 inhibitor
TG101348 (P<0.05). As the time of induction was extended, the
expression levels of p-JAK2 and p-STAT3 in the 3rd week of
induction were higher than those in the 1st week (Fig. 5).
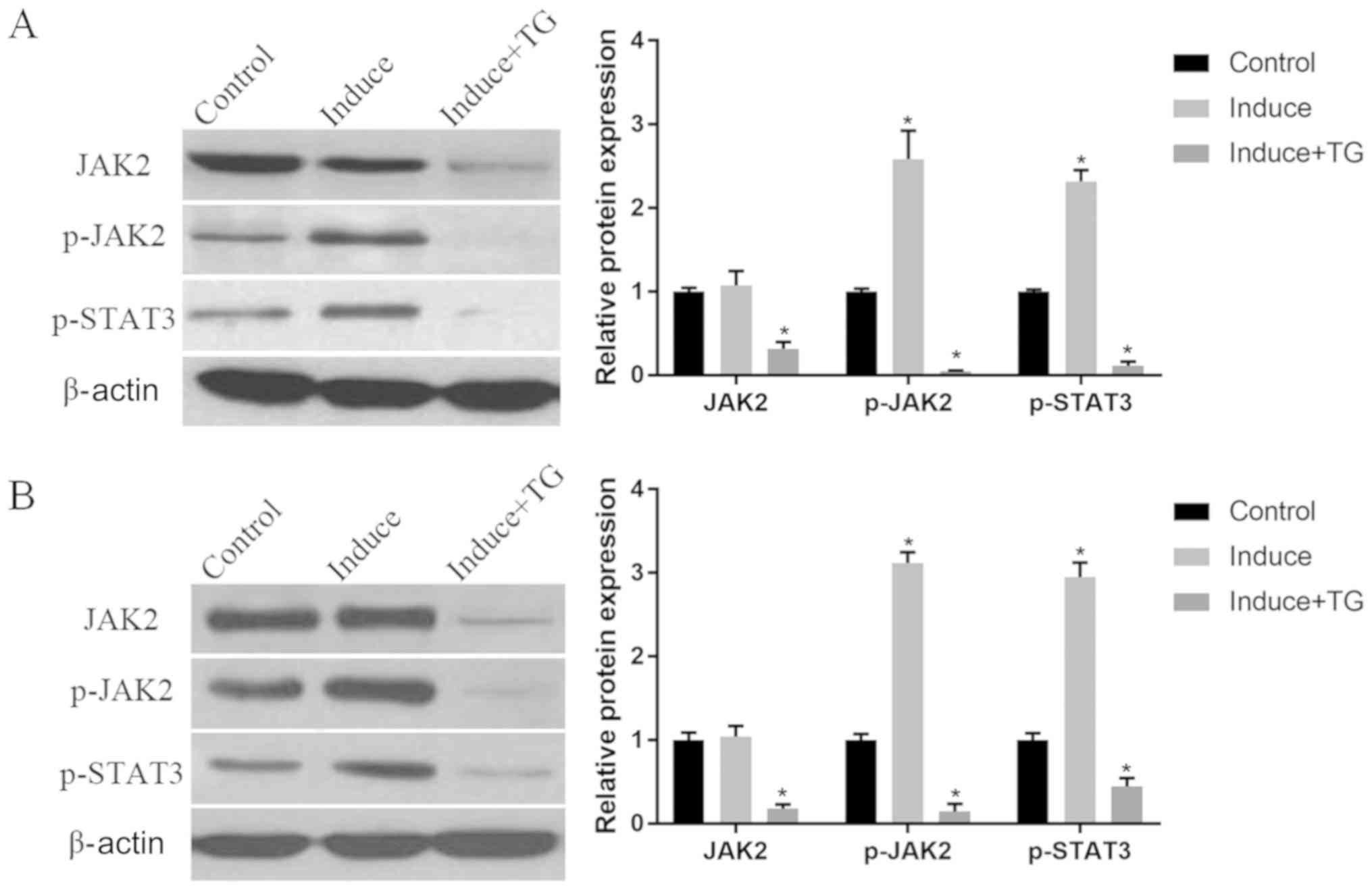 | Figure 5.The JAK-STAT signaling pathway in the
induction of osteogenic differentiation of BMMSCs detected via WB.
(A) Protein expression of JAK2, p-JAK2 and p-STAT3 in the 1st week
of the induction: Compared with those in control group, the protein
expression levels of p-JAK2 and p-STAT3 are substantially elevated
in induction group (P<0.05), while the expressions of JAK2,
p-JAK2 and p-STAT3 are suppressed by TG101348 (P<0.05). (B)
Protein expression of JAK2, p-JAK2 and p-STAT3 in the 3rd week of
the induction: The protein expression levels of p-JAK2 and p-STAT3
in osteogenic induction group are notably higher than those in
control group (P<0.05), whereas the protein expression levels of
JAK2, p-JAK2 and p-STAT3 are lowered by TG101348 compared with
those in control group (P<0.05). *P<0.05, the differences are
statistically significant compared with the other two groups.
Control, BMMSCs normally cultured; Induce, BMMSCs normally
cultured; Induce + TG, BMMSCs osteogenically induced with JAK
inhibitor TG101348. JAK-STAT, Janus kinase-signal transducer and
activator of transcription; BMMSCs, bone marrow-derived mesenchymal
stem cells; WB, western blotting; p-, phosphorylated. |
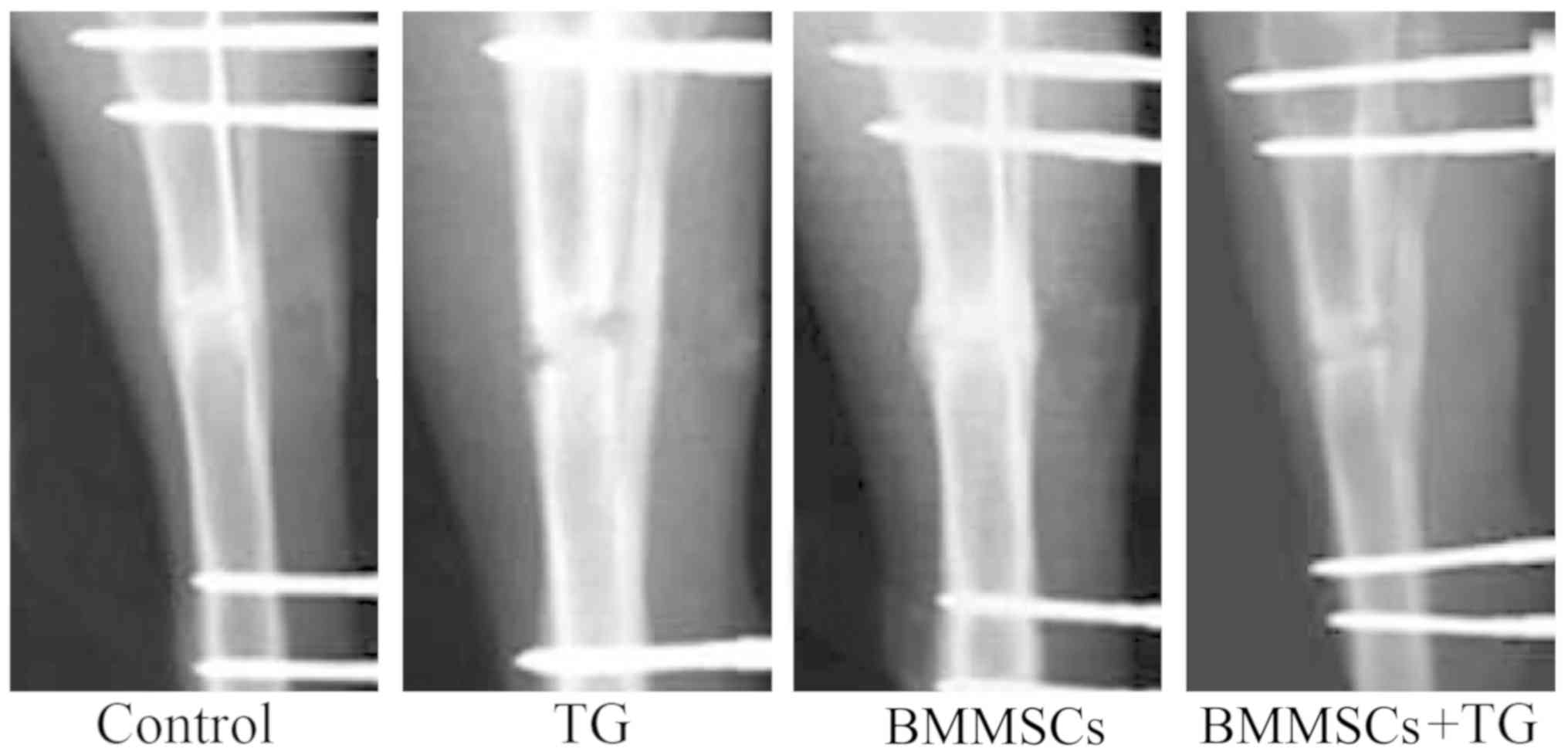
Role of BMMSC transplantation in the
healing of rabbit fractures
After tibial fractures, the rabbits were injected
with BMMSCs, JAK2 inhibitor TG101348 or BMMSCs + TG101348, and 3
weeks later, the recovery of fractures was evaluated via X-ray
examination. Based on the results, the rabbits in control group and
those injected with BMMSCs recovered well. Moreover, the external
calluses at the fracture ends of the rabbits injected with BMMSCs
were larger than those in control group, while the fracture ends
were not completely healed in the rabbits injected with JAK2
inhibitor TG101348 or BMMSCs + TG101348 (Fig. 6).
Discussion
Fracture healing is a complex physiological process
in which the bone is locally repaired. This process involves such
cells as osteoblasts and osteoclasts that are directly implicated
in bone remodeling and bone resorption, and massive osteoblasts
produced in the early stage of healing is vital for the repair of
fractures (13). Relevant studies
have corroborated that after fractures, MSCs migrate to the
fracture site to proliferate and differentiate into osteoblasts.
The mature osteoblasts secrete a matrix material called osteoid,
and then they are embedded in the osteoid and differentiate into
osteocytes, followed by the formation of bone matrices via
calcification. Ultimately, the healing of fractures end up with the
formation of bone tissues (13,14).
BMMSCs play a crucial role in the whole repair process of fractures
(9). Stem cells have been applied to
the treatment of fractures, such as nonunion (15), and the transplantation of
culture-amplified BMMSCs can enhance local repair (7). In this study, the fracture site was
locally injected with BMMSCs, and it was discovered that they
promoted the formation of external calluses and repair of
fractures.
The osteoblasts and osteoclasts in bone development
and repair can be modulated by various local factors, including
cytokines in bone microenvironment and multiple cytokine signaling
pathways, such as the JAK-STAT pathway. The JAK-STAT signaling
pathway was originally identified as a pathway that mainly responds
to the activation of receptors of interferon-γ and interleukin-6
family members (16). The JAK family
members comprise JAK1, JAK2, JAK3 and Tyk2, and STAT protein was
initially discovered as the potential cytoplasmic transcription
factor (17). The phosphorylation of
STATs is triggered by the binding of cytokines to their receptors
on the cell surface via the JAK-STAT signaling pathway (18). The JAK-STAT signaling pathway plays a
key role in the growth and differentiation of various types of
cells, and many cytokines that activate this pathway have been
found through studies to affect the proliferation and
differentiation of osteoblasts and osteoclasts. The in vitro
activation of the JAK-STAT signaling pathway can enhance alkaline
phosphatase activity and increase osteocalcin expression,
suggesting that the differentiation of osteoblasts can be promoted
(19). In this study, the expression
of JAK in BMMSCs was inhibited by the JAK inhibitor, and it was
discovered that the proliferation and migration abilities of cells
were weakened, implying that the JAK-STAT signaling pathway has a
certain effect on BMMSCs. A previous study has demonstrated that
STAT3 can promote proliferation and enhance anti-inflammatory
activity, and that the JAK inhibitor inhibits the expression of
JAK2 and weakens the activity of STAT3, thereby leading to the
decline in the proliferative ability of cells (19). The osteogenic differentiation of
BMMSCs was further induced, and the results revealed that JAK
inhibitor substantially inhibited the generation of osteoblasts,
illustrating that the JAK2-STAT3 signaling pathway is important in
the differentiation of stem cells into osteoblasts.
According to a study (20), JAK2 deficiency causes the decoupling
between growth hormone-receptor signals and the downstream mediator
STAT, thus affecting the normal development of bones. Since STAT3
mediates the anabolism signals in osteoblasts and regulates bone
formation, selectively inactivating STAT3 in osteoblasts can
inhibit bone formation, thereby decreasing the bone mass and
raising the incidence rate of traumatic fractures in individuals
(21,22). Additionally, prior to bone formation,
the mice with JAK2 knocked out die of anemia at E12.5 (20). The fracture sites were injected with
BMMSCs in this study, and it was found that the degree of the
repair of fractures was increased, whereas the healing capacity of
fractures declined in the presence of JAK inhibitor, probably
because the JAK inhibitor suppresses the expression of JAK2,
causing the decoupling of its downstream mediator STAT3, inhibiting
the differentiation of MSCs into osteoblasts and the expression of
anabolism signals in osteoblasts (17), and ultimately weakening the healing
capacity of fractures.
Based on the results of this study, BMMSCs can
differentiate into osteoblasts in vitro and promote the
repair of fractures and the mineralization of bone tissues in the
fracture healing probably through the JAK-STAT signaling
pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PW and ZZ designed the study and performed the
experiments, collected the data, analyzed the data and prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of Shandong Provincial Third Hospital Animal Center
(Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Court-Brown CM and Caesar B: Epidemiology
of adult fractures: A review. Injury. 37:691–697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lillo M, El Ezzo O, Cauteruccio M, Ziranu
A, De Santis V and Maccauro G: Infections in primary intramedullary
nailing of open tibial fractures: A review article. Eur Rev Med
Pharmacol Sci. 23 (Suppl):195–200. 2019.PubMed/NCBI
|
|
3
|
Gamal O and Shams A: Surgical technique
for biological fixation of closed segmental tibial fractures by the
Less Invasive Stabilization System (LISS). SICOT J. 4:482018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kadar A, Sherman H, Glazer Y, Katz E and
Steinberg EL: Predictors for nonunion, reoperation and infection
after surgical fixation of patellar fracture. J Orthop Sci.
20:168–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Antonova E, Le TK, Burge R and Mershon J:
Tibia shaft fractures: Costly burden of nonunions. BMC
Musculoskelet Disord. 14:422013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goh EL, Chidambaram S, Eigenmann D, Ma S
and Jones GG: Minimally invasive percutaneous plate osteosynthesis
versus intramedullary nail fixation for closed distal tibial
fractures: A meta-analysis of the clinical outcomes. SICOT J.
4:582018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bajada S, Harrison PE, Ashton BA,
Cassar-Pullicino VN, Ashammakhi N and Richardson JB: Successful
treatment of refractory tibial nonunion using calcium sulphate and
bone marrow stromal cell implantation. J Bone Joint Surg Br.
89:1382–1386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji M, Bai C, Li L, Fan Y, Ma C, Li X and
Guan W: Biological characterization of sheep kidney-derived
mesenchymal stem cells. Exp Ther Med. 12:3963–3971. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taguchi K, Ogawa R, Migita M, Hanawa H,
Ito H and Orimo H: The role of bone marrow-derived cells in bone
fracture repair in a green fluorescent protein chimeric mouse
model. Biochem Biophys Res Commun. 331:31–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khosla S, Westendorf JJ and Oursler MJ:
Building bone to reverse osteoporosis and repair fractures. J Clin
Invest. 118:421–428. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kamran MZ, Patil P and Gude RP: Role of
STAT3 in cancer metastasis and translational advances. BioMed Res
Int. 2013:4218212013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li DQ, Wan QL, Pathak JL and Li ZB:
Platelet-derived growth factor BB enhances osteoclast formation and
osteoclast precursor cell chemotaxis. J Bone Miner Metab.
35:355–365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu Y, Zhou J, Wang Q, Fan W and Yin G:
Ginsenoside Rg1 promotes osteogenic differentiation of rBMSCs and
healing of rat tibial fractures through regulation of GR-dependent
BMP-2/SMAD signaling. Sci Rep. 6:252822016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geris L, Gerisch A, Sloten JV, Weiner R
and Oosterwyck HV: Angiogenesis in bone fracture healing: A
bioregulatory model. J Theor Biol. 251:137–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee EH and Hui JH: The potential of stem
cells in orthopaedic surgery. J Bone Joint Surg Br. 88:841–851.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J: JAK-STAT and bone metabolism.
JAK-STAT. 2:e239302013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heinrich PC, Behrmann I, Müller-Newen G,
Schaper F and Graeve L: Interleukin-6-type cytokine signalling
through the gp130/Jak/STAT pathway. Biochem J. 334:297–314. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bellido T, Borba VZ, Roberson P and
Manolagas SC: Activation of the Janus kinase/STAT (signal
transducer and activator of transcription) signal transduction
pathway by interleukin-6-type cytokines promotes osteoblast
differentiation. Endocrinology. 138:3666–3676. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Parganas E, Wang D, Stravopodis D, Topham
DJ, Marine JC, Teglund S, Vanin EF, Bodner S, Colamonici OR, van
Deursen JM, et al: Jak2 is essential for signaling through a
variety of cytokine receptors. Cell. 93:385–395. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bromberg J: Stat proteins and oncogenesis.
J Clin Invest. 109:1139–1142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grimbacher B, Holland SM, Gallin JI,
Greenberg F, Hill SC, Malech HL, Miller JA, O'Connell AC and Puck
JM: Hyper-IgE syndrome with recurrent infections - an autosomal
dominant multisystem disorder. N Engl J Med. 340:692–702. 1999.
View Article : Google Scholar : PubMed/NCBI
|