Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, and non-small-cell lung cancer (NSCLC)
accounts for ~85% of all lung cancer cases (1). To date, several management strategies
such as surgery, radiochemotherapy, immunotherapy and other
targeted approaches have been applied for NSCLC (2). However, the mortality rate of patients
with NSCLC remains high (1,3). Therefore, it is imperative to
investigate efficient treatment strategies to incorporate into
NSCLC treatment.
FK506-binding protein 51 (FKBP51) is a member of the
immunophilin family that is involved in multiple signaling
pathways, tumorigenesis and chemoresistance (4,5). As a
genetic factor regulating the hypothalamic-pituitary-adrenal (HPA)
axis, FKBP51 plays an important role in stress regulation (6,7).
However, the functions of FKBP51 in the tumorigenesis of lung
cancer remains to be elucidated. FKBP51 protein contains two
domains: FK506-binding domains (FK1 and FK2) and
tetratricopeptide-repeat (TPR) domains. These unique domains
contribute to the different roles of FKBP51 in different cancerous
tissues (6,8,9). FKBP51
is robustly stimulated by steroid hormones such as progesterone,
glucocorticoid and androgen, but not by estrogen (10). Moreover, the TPR domains can form a
super complex with a steroid receptor (11); therefore, these domains play
important roles in cellular processes. FKBP51 is reportedly
overexpressed in prostate cancer and glioma tissues and also
contributes to the promotion of tumorigenesis and cancer
development (11). Moreover, FKBP51
may promote tumorigenesis by acting on cell metastasis (12). Nevertheless, FK506-binding domains
could interact with AKT to promote AKT dephosphorylation, thereby
hindering cancer development (13–16).
FKBP51 is expressed in almost all normal human tissues, including
lung tissue, and is overexpressed in patients with melanoma,
prostate cancer, lymphoma, head and neck cancer and brain cancer
(5,17). By contrast, it is downregulated in
endometrial adenocarcinoma, pancreatic cancer colon cancer and
testicular cancer (18).
Furthermore, FKBP51 can bind to beclin-1, modifying its
phosphorylation and protein levels, enhancing the levels of
autophagy markers and autophagic flux (19,20).
Up to 50–80% of patients with NSCLC exhibit
inactivation or loss of tumor suppressor p53 (3,21).
Moreover, tumor suppressor p53 is a transcription factor that
responds to carcinogenic stress (22,23). In
addition, mutant p53 cells were found to be sensitive to
chemotherapy and to show decreased AKT expression (24,25). p53
may inhibit AKT activation and promote cellular autophagy (26). However, to the best of our knowledge,
the association between FKBP51 and cellular apoptosis via the p53
signaling pathway in NSCLC, and the extent to which this
association impedes tumorigenesis, remains to be clarified.
In the present study, tissue samples from lung
cancer patients were collected, and the association between lung
cancer and FKBP51 was examined using immunohistochemistry (IHC).
Additionally, the effects of FKBP51 on tumor cell apoptosis were
explored, as evidenced by FKBP51 overexpression in the A549 cell
line. Finally, the association between FKBP51 and the p53 signaling
pathway was studied and the role of FKBP51 in the sensitivity of
A549 cells to cisplatin was examined.
Materials and methods
Human lung carcinoma and healthy lung
tissues
The study was approved by the Institutional Ethics
Committee of Hunan Normal University. A total of 15 paired primary
lung carcinoma tissue specimens and samples of their respective
adjacent normal tissues were collected from patients who underwent
complete surgical resection from January 2018 to December 2018 at
the Hunan Tumor Hospital. All samples were obtained at the time of
operation, immediately snap-frozen in liquid nitrogen, and stored
at −80°C. Patients in this study did not receive chemotherapy or
radiation therapy before surgical resection. An experienced
pathologist established the pathological diagnosis for each
patient. In total, 7 of the 15 patients (age, 48–70 years; 3 women
and 12 men) were diagnosed with tumor-node-metastasis (TNM) stage
I/II tumors, while the remaining 8 were diagnosed with TNM stage
III/IV tumors. Lymph node metastasis was observed in 4 patients.
All patients provided their signed informed consent before study
participation.
Immunohistochemistry
The collected tissue samples were fixed in 10%
neutral formalin at 4°C overnight, embedded in paraffin, and sliced
to obtain 4-µm-thick sections. Immunostaining was performed using
the streptavidin peroxidase method. The sections were incubated
with monoclonal rabbit anti-FKBP51 antibody (1:250; cat. no.
ab126715; Abcam) overnight at 4°C, followed by incubation with
biotinylated goat anti-rabbit immunoglobulin G secondary antibody
(1:500; cat. no. CW0109; Beijing Cwbio Biotech Co., Ltd.) for 30
min at 37°C. After washing with PBS, sections were incubated with
horseradish peroxidase-conjugated streptavidin-biotin (Servicebio
G1211; Wuhan Servicebio Technology Co., Ltd.) and developed at room
temperature for 2 min with 3-diaminobenzidine tetrahydrochloride
(Sinopharm Chemical Reagent Co., Ltd.). Finally, the samples were
lightly counterstained at room temperature for 10 min with
hematoxylin, dehydrated in alcohol, and mounted. The slides were
semiquantitatively scored by evaluating the staining intensity and
the percentage of stained cells in representative areas. The
staining intensity was scored as 0 (no signal), 1 (weak), 2
(moderate), or 3 (high) points. The percentage of cells stained was
scored as 1 (1–25%), 2 (26–50%), 3 (51–75%), or 4 (76–100%) points.
A final score of 0–12 points was obtained by multiplying the
staining intensity by the percentage of cells stained. Adjacent
nontumorous tissue samples were designated as having positive
FKBP51 expression when a score of ≥5 points was noted. Conversely,
samples presenting weak expression (1–4 points) or no expression (0
points) were defined as displaying a lack of FKBP51 expression. All
samples were observed using a light microscope (×200 and ×400
magnification; Olympus Corporation).
Cell culture
The NSCLC cell line A549 was purchased from the
American Type Culture Collection and stored in liquid nitrogen.
A549 cells are human alveolar basal epithelial adenocarcinoma cells
and are commonly used in lung cancer research (27). A549 cells were maintained at 37°C
under a humidified atmosphere containing 5% CO2 and
cultured in RPMI 1640 medium supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), with 2.0 mM glutamine, 100 µg/ml
ampicillin and 100 U/ml streptomycin sulfate.
Plasmid construction and
transfection
Total RNA from cells was extracted using an
EZNA® Total RNA kit I (Omega Bio-Tek, Inc.) according to
the manufacturer's instructions. RNA was reverse transcribed to
cDNA using the PrimeScript RT Reagent kit (Takara Bio, Inc.).
FKBP51 was amplified from cDNA by PCR and subcloned into the
pCMV-tag2B (Agilent Technologies, Inc.) plasmid to obtain a FKBP51
expression vector using the ClonExpress II One-Step Cloning kit
(Vazyme Biotech Co., Ltd.). PCR was performed using the following
primer pair: Forward 5′-ACGACGATAAGAGCCCGGGCATGACTACTGATGAAGGTGC-3′
and reverse 5′-ATTAAGGTACCGGGCCCCCCTCATACGTGGCCCTCAGGTT-3′. The
following cycling conditions were used: Initial denaturation at
95°C for 3 min, followed by 30 cycles of 95°C for 30 sec, 60°C for
30 sec and 72°C for 90 sec, and a final extension at 72°C for 5
min. PCR products were obtained using a DNA Gel Recovery kit
(Beyotime Institute of Biotechnology). For transfection, A549 cells
were seeded in 24-well plates at a density of 2×104
cells/well and incubated for 14 h. Then, transfection was performed
using the vectors (0.5 µg) mixed with Lipofectamine®
2000 (Thermo Fisher Scientific, Inc.) at a ratio of 1:2
(volume/volume), according to the manufacturer's
specifications.
Dual-luciferase reporter assay
A549 cells in 24-well plates were co-transfected
with pCMV-tag2B, pCMV-tag2B-FKBP51 (Agilent Technologies, Inc.),
pRL-TK and pGL4.1-p53-LUC (Promega Corporation). The total amount
of plasmid was balanced with the empty vector for each transfection
using the vectors (0.5 µg) mixed with Lipofectamine®
2000 (Thermo Fisher Scientific, Inc.) at a ratio of 1:2
(volume/volume). The cells were harvested and lysed with the
Dual-Luciferase® Reporter Assay System (Promega
Corporation) on ice at 24 h after transfection. Relative luciferase
activity against Renilla luciferase activity was used for
normalization. The data above were analyzed using
GloMax® 20/20 Luminometer (Promega, Inc.). The plasmids
pCMV-tag2B were used as negative controls.
Western blotting
To prepare total protein extracts, cells were
collected at 48 h post-transfection and lysed using RIPA buffer
(Beijing Cwbio Biotech Co., Ltd.) at 4°C for 10 min. Subsequently,
the mixture was centrifuged at 12,000 × g at 4°C for 10 min, and
the supernatant was transferred to a fresh tube. Total protein
concentration was detected using a BCA assay (Beyotime Institute of
Biotechnology). Proteins (50 µg) were separated by 10% SDS-PAGE and
transferred onto PVDF membranes (EMD Millipore). After blocking in
5% non-fat milk for 1 h at room temperature, the membranes were
incubated with the appropriate primary antibody overnight at 4°C,
followed by a secondary antibody incubation for 2 h at room
temperature. Bands were visualized with the Tanon 5500 Multi
Automatic Chemiluminescence-Fluorescence Image Analysis System
(Tanon Science & Technology Co., Ltd.).
Anti-FLAG primary antibodies were purchased from
Abcam (1:1,000; cat. no. ab205606). Anti-β-actin, -p53, -Bcl-2 and
-cleaved caspase-3 were purchased from Cell Signaling Technology,
Inc. (1:1,000; cat. nos. 4970, 2527, 4223, 9664, respectively).
Goat anti-rabbit IgG-HRP was purchased from Cwbio (1:5,000; cat.
no. CW0103S).
Flow cytometry
Apoptosis levels were measured using the
FITC-Annexin V Apoptosis Detection kit (Becton, Dickinson and
Company). The FKBP51 expression plasmid was transfected into the
cells in a culture flask for 48 h. Before testing, the cells were
trypsinized, resuspended in 500 µl binding buffer [10 mM HEPES (pH
7.4), 140 mM NaCl, 1 mM MgCl2, 5 mM KCl and 2.5 mM
CaCl2] containing 5 µl FITC-conjugated Annexin V and 5
µl propidium iodide (PI), and incubated at room temperature in the
dark for 10 min. A total of 1×105 cells were harvested
and analyzed using the BD FACSCalibur™ flow cytometer (Becton,
Dickinson and Company). Following PI excitation with an argon ion
laser at a wavelength of 488 nm and acceptance through a filter at
a wavelength of 630 nm, 1×104 cells were collected using
the forward scatter/side scatter scatterplot method to exclude
mutually adherent cells and cell debris. The percentage of cells in
each phase of cell cycle was presented on the PI fluorescence
histogram.
Cell Counting Kit-8 (CCK8) assay
A549 cells were seeded into a 96-well plate at a
density of 5×103/well and cultured in 5% CO2
atmosphere at 37°C. Cisplatin (Selleck Chemicals, Inc.) was
dissolved in dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA). The
cells were cultured for 16 h before transfection with
pCMV-tag2B-FKBP51, followed by treatment with cisplatin (20 µM) for
24 h. Finally, CCK8 (MedChemExpress) was added to the plate in the
vehicle group. The CCK8 assay was performed according to the
manufacturer's guidelines after 4 h of incubation.
Statistical analysis
SPSS 19.0 software (IBM Corp.) was used for
statistical analysis. Results of IHC and clinical correlations were
evaluated using the χ2 test. Data are expressed as the
mean ± SD. Student's t-test was used to evaluate the differences
between the two groups, with P<0.05 considered to indicate a
statistically significant difference.
Results
FKBP51 and p53 expression are
downregulated in human lung carcinoma
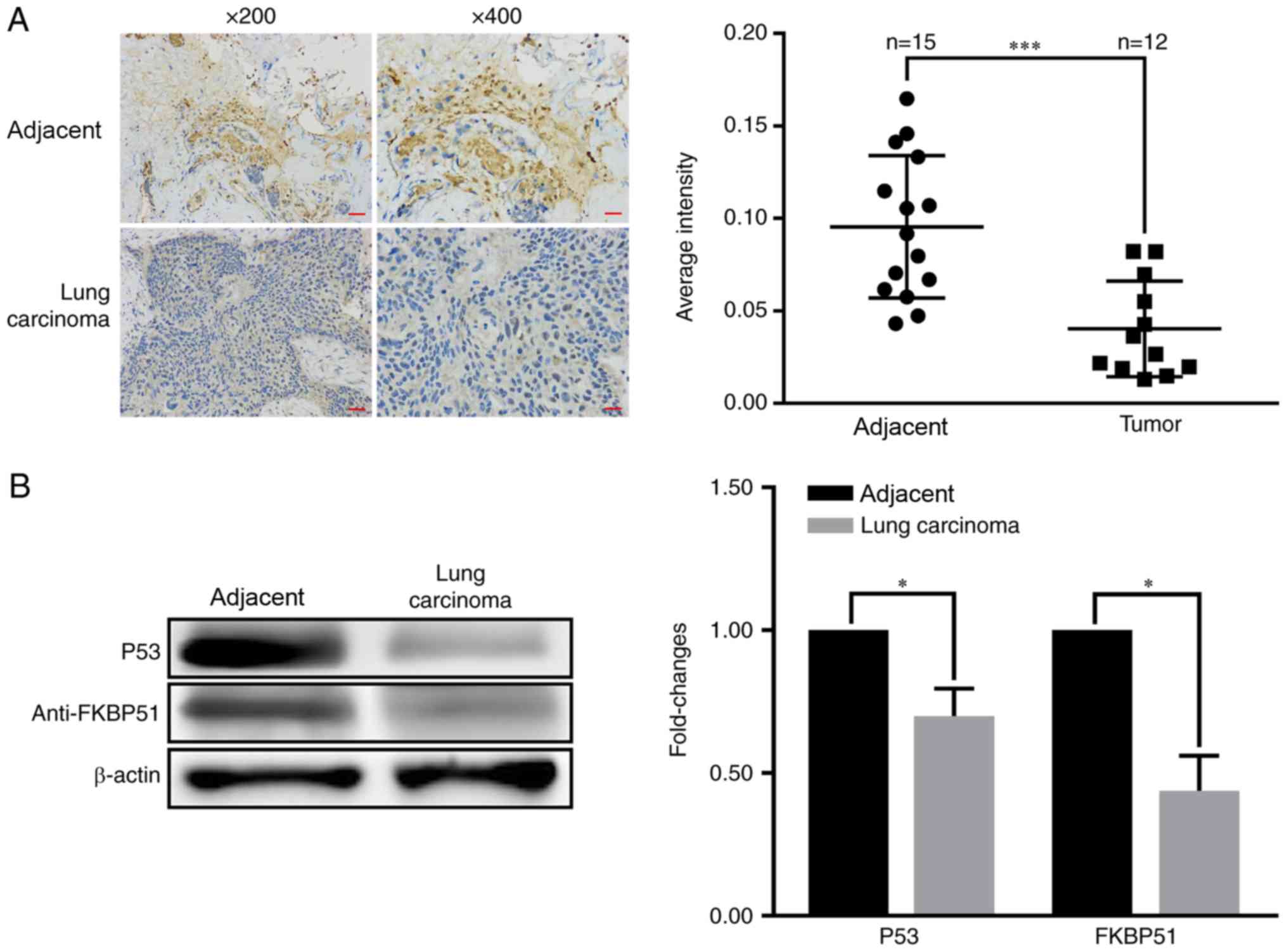
A total of 15 paired primary lung carcinoma tissue
samples were collected to investigate the role of FKBP51 in lung
carcinoma development and IHC was performed to examine the
association between FKBP51 expression and lung cancer development.
FKBP51 expression in lung cancer tissue samples was significantly
lower compared with that in adjacent tissues (Fig. 1A). Western blotting revealed that
FKBP51 and p53 levels were decreased in lung carcinoma tissues
compared with adjacent tissues (Fig.
1B).
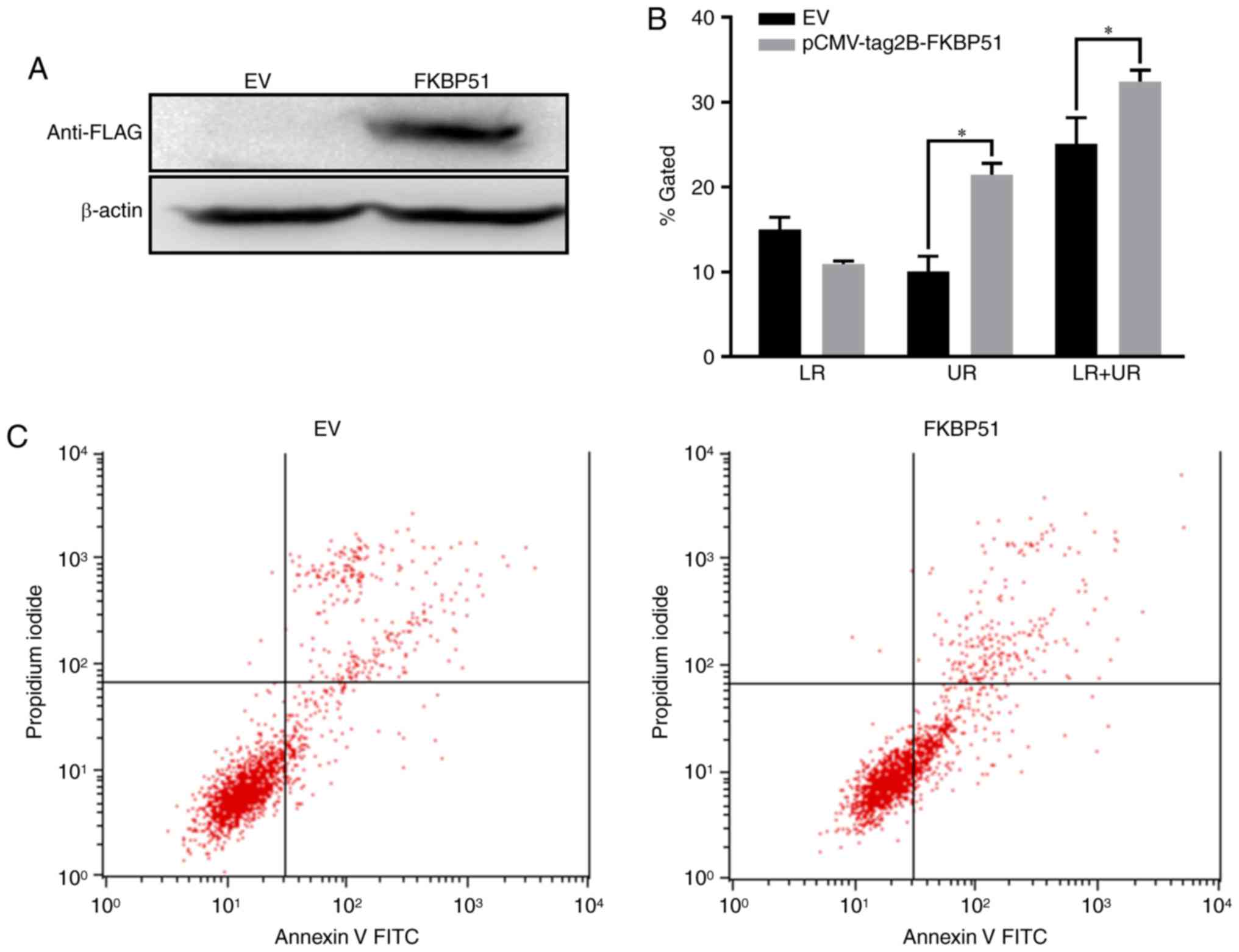
FKBP51 overexpression promotes A549
cell apoptosis
Since FKBP51 expression was downregulated in lung
carcinoma tissue, it was hypothesized that this protein may play a
role in lung cancer development. To test this hypothesis, FKBP51
was overexpressed in A549 cells and cellular apoptosis was detected
using flow cytometry. FLAG expression significantly increased after
transfection compared with empty vector controls, indicating that
FKBP51 was successfully overexpressed in A549 cells (Fig. 2A). Flow cytometry revealed that
FKBP51 overexpression in A549 cells significantly promoted cellular
apoptosis compared with empty vector controls (Fig. 2B and C).
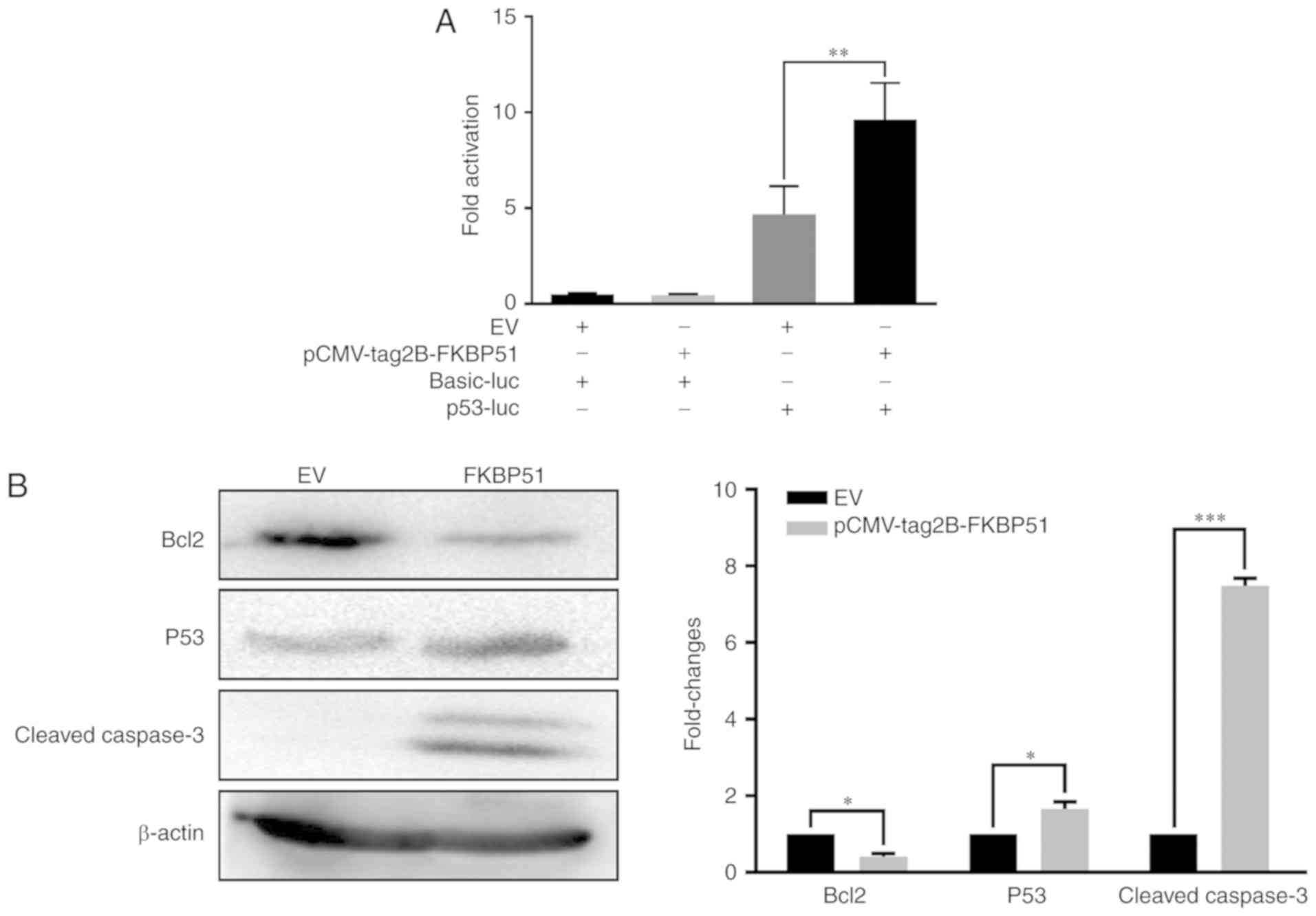
FKBP51 promotes A549 cell apoptosis
through activation of the p53 signaling pathway
Since FKBP51 was shown to promote A549 cell
apoptosis, the expression of proteins known to be involved in
tumorigenesis pathways was investigated to further elucidate the
molecular mechanisms underlying this process. The p53 signaling
pathway was found to be involved in apoptosis regulation by FKBP51.
A dual-luciferase reporter assay was performed to examine the
association between FKBP51 and p53. p53 levels were significantly
increased in FKBP51-overexpressing A549 cells compared with control
cells (Fig. 3A). To further confirm
whether the p53 signaling pathway was involved in FKBP51-mediated
promotion of apoptosis, p53 levels were detected using western
blotting. p53 and caspase-3 levels were significantly increased
while Bcl-2 levels were significantly decreased in
FKBP51-overexpressing A549 cells compared with control cells
(Fig. 3B). These results suggested
that FKBP51 functions by activating the p53 signaling pathway in
A549 cells.
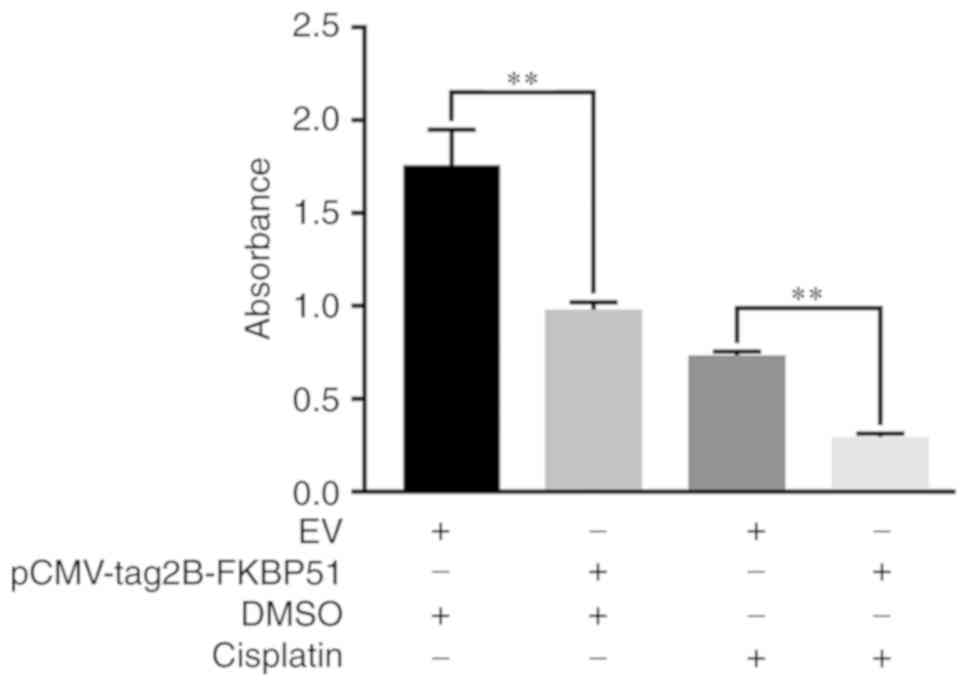
FKBP51 contributes to the sensitivity
of A549 cells to cisplatin
The resistance of tumor cells to drugs has been
known to hinder cancer treatment to a certain extent (18). The cell viability of A549 cells was
tested using a CCK8 assay. FKBP51-overexpressing A549 cells showed
a significantly lower survival rate following cisplatin exposure
compared with empty vector controls (Fig. 4). This finding suggested that FKBP51
improves the sensitivity of A549 cells to cisplatin.
Discussion
Although the diagnosis and treatment of NSCLC has
been well explored, this malignant condition only has a 5-year
survival rate of <15% (28). A
combination of cisplatin-based chemotherapy and EGFR tyrosine
kinase inhibitors (TKIs) represents a relatively common approach to
NSCLC treatment; however, drug resistance is an inevitable factor
(29). Moreover, substantial
evidence indicates that FKBP51 plays an important role in stress
biology, metabolism, pain signaling and the ability to upregulate
gene expression via stress and glucocorticoid hormones (4,7,30). Meanwhile, studies have shown that
FKBP51 may be a potential target for prostate cancer treatment
(10,11,31). In
the present study, FKBP51 expression was detected in human lung
cancer and healthy tissues using IHC. FKBP51 expression in NSCLC
was significantly lower compared with healthy lung tissues. This
effect revealed that lower FKBP51 expression was associated with
lung cancer development. Subsequently, FKBP51 expression was
examined in lung cancer tissues using western blotting. It was
found that FKBP51 and tumor suppressor p53 were poorly expressed in
lung carcinoma tissues compared with adjacent tissues, suggesting
that FKBP51 plays a role in inhibiting lung cancer development.
However, the potential molecular mechanisms of FKBP51 in NSCLC
remain unclear.
To investigate whether FKBP51 was involved in
apoptosis in lung cancer, FKBP51 was overexpressed in A549 cells.
The results showed that FKBP51 plays a key role in the regulation
of apoptosis in irradiated melanoma cells and FKBP51 was regarded
as a candidate marker for the identification of suitable melanoma
tumors (19,32). As previously mentioned, FKBP51 may be
associated with apoptosis in melanomas. However, to our knowledge,
no study on FKBP51 inducing apoptosis in lung cancer has been
reported. In this present study, the fusion protein FLAG-FKBP51 was
overexpressed in A549 cells. FKBP51 overexpression resulted in a
significant increase in the number of apoptotic A549 cells,
suggesting that FKBP51 inhibits lung cancer development by
promoting cellular apoptosis.
FKBP51 inhibits AKT activation, leading to the
inhibition of cell proliferation in endometrial adenocarcinomas
(15). Furthermore, FKBP51 binds to
beclin-1, thereby promoting cell apoptosis in melanoma cells
(19). p53 inactivation promotes
cell apoptosis by suppressing Bcl-2 activity and promoting
caspase-3 expression (30,33–35).
Bcl-2 binds to p53 to inhibit its activity and thus inhibit
apoptosis (36,37). Caspase-3 stimulates cell death via
apoptosis (25,38,39).
These studies revealed that the FKBP51 was involved in the P53
signaling pathway and induces and apoptotic phenotype in cancer
cells. Thus, the flow cytometry results demonstrated that FKBP51
may promote apoptosis in lung cancer cells and inhibits NSCLC
development. Moreover, FKBP51 was found to promote p53 expression.
Among the downstream effectors, Bcl-2 was downregulated and
caspase-3 was upregulated. These results suggested that FKBP51
blocks NSCLC development by promoting A549 cell apoptosis via the
p53 signaling pathway.
The resistance of tumor cells to drugs often reduces
the efficacy of chemotherapy. Thus, improving the sensitivity of
tumor cells to drugs is of great significance in the treatment of
cancer (40,41). Previous studies have shown that
FKBP51 increases the sensitivity of pancreatic cancer to
chemotherapy drugs (13,16). Chemotherapy is one of the key options
available for the treatment of NSCLC (42). Thus, improving the drug sensitivity
of NSCLC is crucial. Our study showed that the FKBP51 acts as an
transducer of the chemotherapy drug cisplatin. When FKBP51 is
overexpressed, the sensitivity of NSCLC cells to cisplatin was
increased, which is of benefit for clinical treatment of this
cancer type.
In conclusion, FKBP51 is downregulated in human lung
cancer tissues. FKBP51 overexpression in A549 cells promoted
cellular apoptosis. Additionally, FKBP51 increased enhanced the
activity of the p53 signaling pathway, suggesting that FKBP51 can
block lung cancer progression via this pathway. Finally,
FKBP51-overexpressing A549 cells showed increased sensitivity to
cisplatin. Collectively, our results suggested that FKBP51 can be a
therapeutic target for NSCLC.
Acknowledgements
Not applicable.
Funding
This study was supported in part by grants from the
National Natural Science Foundation of China (grant nos. 81470449,
81670290, 81470377, 31872315, 31572349, 81670288, 81700338 and
81570279), the Cooperative Innovation Center of Engineering and New
Products for Developmental Biology of Hunan Province (grant no.
2013-448-6), National Key Research and Development Program of China
(grant nos. 2018YFA0108700 and 2017YFA0105602), NSFC Projects of
International Cooperation and Exchanges (grant no. 81720102004),
The Research Team Project of Natural Science Foundation of
Guangdong Province of China (grant no. 2017A030312007), Science and
Technology Planning Projects of Guangdong Province of China (grant
nos. 2017A070701013, 2017B090904034, 2017030314109,
2019B020230003), the Special Project of Dengfeng Program of
Guangdong Provincial People's Hospital (grant no. DFJH201802), the
Key Program of Guangzhou Science Research Plan (grant no.
805212639211). Hunan Provincial Natural Science Foundation of China
(grant nos. 2015JJ3087 and 2018JJ2666) and the Scientific Research
Fund of Hunan Provincial Education Department (grant no.
18A028).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC contributed to acquisition of data, revision of
the manuscript, ZL, YQW and JZ contributed to the acquisition of
data and writing the manuscript, YP, XM, JC, YS, MY, WC, YHL, XZ,
WY, YQL, FL, ZZ, GD, XY and YW contributed to the acquisition of
data, ZJ and PZ contributed to the analysis and interpretation of
data, XF contributed to the acquisition of data and revision of the
manuscript, XW contributed to the conception, design, revision of
the manuscript and the acquisition of data.
Ethics approval and consent to
participate
All patients provided their written informed consent
and agreed to the usage of their samples in scientific research.
All human procedures were approved by the Ethics Committee of Hunan
Normal University (Changsha, China). All animal procedures were
performed in accordance with the Guidelines for Care and Use of
Laboratory Animals of Hunan Normal University and the experiments
were approved by the Animal Ethics Committee of Hunan Normal
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis CE, Siegel RL, Sauer AG, Miller
KD, Fedewa SA, Alcaraz KI and Jemal A: Cancer statistics for
African Americans, 2016: Progress and opportunities in reducing
racial disparities. CA Cancer J Clin. 66:290–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsch FR, Suda K, Wiens J and Bunn PA Jr:
New and emerging targeted treatments in advanced non-small-cell
lung cancer. Lancet. 388:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hähle A, Merz S, Meyners C and Hausch F:
The many faces of FKBP51. Biomolecules. 9:E352019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Staibano S, Mascolo M, Ilardi G, Siano M
and De Rosa G: Immunohistochemical analysis of FKBP51 in human
cancers. Curr Opin Pharmacol. 11:338–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Romano S, Di Pace A, Sorrentino A, Bisogni
R, Sivero L and Romano MF: FK506 binding proteins as targets in
anticancer therapy. Anticancer Agents Med Chem. 10:651–656. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stechschulte LA and Sanchez ER: FKBP51-a
selective modulator of glucocorticoid and androgen sensitivity.
Curr Opin Pharmacol. 11:332–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cioffi DL, Hubler TR and Scammell JG:
Organization and function of the FKBP52 and FKBP51 genes. Curr Opin
Pharmacol. 11:308–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L: FKBP51 regulation of AKT/protein
kinase B phosphorylation. Curr Opin Pharmacol. 11:360–364. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jääskeläinen T, Makkonen H and Palvimo JJ:
Steroid up-regulation of FKBP51 and its role in hormone signaling.
Curr Opin Pharmacol. 11:326–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ni L, Yang CS, Gioeli D, Frierson H, Toft
DO and Paschal BM: FKBP51 promotes assembly of the Hsp90 chaperone
complex and regulates androgen receptor signaling in prostate
cancer cells. Mol Cell Biol. 30:1243–1253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takaoka M, Ito S, Miki Y and Nakanishi A:
FKBP51 regulates cell motility and invasion via RhoA signaling.
Cancer Sci. 108:380–389. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou J and Wang L: FKBP5 as a selection
biomarker for gemcitabine and Akt inhibitors in treatment of
pancreatic cancer. PLoS One. 7:e362522012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pei H, Li L, Fridley BL, Jenkins GD,
Kalari KR, Lingle W, Petersen G, Lou Z and Wang L: FKBP51 affects
cancer cell response to chemotherapy by negatively regulating Akt.
Cancer Cell. 16:259–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong J, Jiao Y, Mu W, Lu B, Wei M, Sun L,
Hu S, Cui B, Liu X, Chen Z and Zhao Y: FKBP51 decreases cell
proliferation and increases progestin sensitivity of human
endometrial adenocarcinomas by inhibiting Akt. Oncotarget.
8:80405–80415. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo K, Li Y, Yin Y, Li L, Wu C, Chen Y,
Nowsheen S, Hu Q, Zhang L, Lou Z and Yuan J: USP49 negatively
regulates tumorigenesis and chemoresistance through FKBP51-AKT
signaling. EMBO J. 36:1434–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fagerberg L, Hallström BM, Oksvold P,
Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S,
Danielsson A, Edlund K, et al: Analysis of the human
tissue-specific expression by genome-wide integration of
transcriptomics and antibody-based proteomics. Mol Cell Proteomics.
13:397–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L, Lou Z and Wang L: The role of FKBP5
in cancer aetiology and chemoresistance. Br J Cancer. 104:19–23.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Romano S, D'Angelillo A, Pacelli R,
Staibano S, De Luna E, Bisogni R, Eskelinen EL, Mascolo M, Calì G,
Arra C and Romano MF: Role of FK506-binding protein 51 in the
control of apoptosis of irradiated melanoma cells. Cell Death
Differ. 17:145–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gassen NC, Hartmann J, Schmidt MV and Rein
T: FKBP5/FKBP51 enhances autophagy to synergize with antidepressant
action. Autophagy. 11:578–580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cancer Genome Atlas Research Network, .
Comprehensive genomic characterization of squamous cell lung
cancers. Nature. 489:519–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vogelstein B, Lane D and Levine AJ:
Surfing the p53 network. Nature. 408:307–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Donzelli S, Strano S and Blandino G: YAP
and p73: A Matter of mutual specificity in tumor suppression. The
Hippo Signaling Pathway Cancer. 147–172. 2013. View Article : Google Scholar
|
|
24
|
Makkonen H, Kauhanen M, Paakinaho V,
Jääskeläinen T and Palvimo JJ: Long-range activation of FKBP51
transcription by the androgen receptor via distal intronic
enhancers. Nucleic Acids Res. 37:4135–4148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim YY, Jee HJ, Um JH, Kim YM, Bae SS and
Yun J: Cooperation between p21 and Akt is required for
p53-dependent cellular senescence. Aging Cell. 16:1094–1103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cordani M, Butera G, Pacchiana R and
Donadelli M: Molecular interplay between mutant p53 proteins and
autophagy in cancer cells. Biochim Biophys Acta Rev Cancer.
1867:19–28. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lieber M, Smith B, Szakal A, Nelson-Rees W
and Todaro G: A continuous tumor-cell line from a human lung
carcinoma with properties of type II alveolar epithelial cells. Int
J Cancer. 17:62–70. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer Statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang L, Ma L, Xu F, Zhai W, Dong S, Yin L,
Liu J and Yu Z: Role of long non-coding RNA in drug resistance in
non-small cell lung cancer. Thorac Cancer. 9:761–768. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Balsevich G, Häusl AS, Meyer CW,
Karamihalev S, Feng X, Pöhlmann ML, Dournes C, Uribemarino A,
Santarelli S and Labermaier C: Stress-responsive FKBP51 regulates
AKT2-AS160 signaling and metabolic function. Nature Communications.
8((1)): 17252017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Velasco AM, Gillis KA, Li Y, Brown EL,
Sadler TM, Achilleos M, Greenberger LM, Frost P, Bai W and Zhang Y:
Identification and validation of novel androgen-regulated genes in
prostate cancer. Endocrinology. 145:3913–3924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Romano S, D'Arrigo P, Tufano M, Staibano
S, Rea A, Merolla F, Ilardi G, Petrella A and Romano MF: TRAF2 and
FKBP51 as possible markers for identification of suitable melanoma
tumors for tumor necrosis factor-α inhibition. Melanoma Res.
29:145–150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen J, Xie F, Zhang L and Jiang WG: iASPP
is over-expressed in human non-small cell lung cancer and regulates
the proliferation of lung cancer cells through a p53 associated
pathway. BMC Cancer. 10:6942010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fu X, Xu L, Qi L, Tian H, Yi D, Yu Y, Liu
S, Li S, Xu Y and Wang C: BMH-21 inhibits viability and induces
apoptosis by p53-dependent nucleolar stress responses in SKOV3
ovarian cancer cells. Oncol Rep. 38:859–865. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Walsh JG, Cullen SP, Sheridan C, Lüthi AU,
Gerner C and Martin SJ: Executioner caspase-3 and caspase-7 are
functionally distinct proteases. Proc Natl Acad Sci USA.
105:12815–12819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons
MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chee JL, Saidin S, Lane DP, Leong SM, Noll
JE, Neilsen PM, Phua YT, Gabra H and Lim TM: Wild-type and mutant
p53 mediate cisplatin resistance through interaction and inhibition
of active caspase-9. Cell Cycle. 12:278–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kato S, Han SY, Liu W, Otsuka K, Shibata
H, Kanamaru R and Ishioka C: Understanding the function-structure
and function-mutation relationships of p53 tumor suppressor protein
by high-resolution missense mutation analysis. Proc Natl Acad Sci
USA. 100:8424–8429. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng X, Liu H, Zhang Z, Gu Y, Qiu H and He
Z: Annexin A2 contributes to cisplatin resistance by activation of
JNK-p53 pathway in non-small cell lung cancer cells. J Exp Clin
Cancer Res. 36:1232017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sarin N, Engel F, Kalayda GV, Mannewitz M,
Cinatl J Jr, Rothweiler F, Michaelis M, Saafan H, Ritter CA, Jaehde
U and Frötschl R: Cisplatin resistance in non-small cell lung
cancer cells is associated with an abrogation of cisplatin-induced
G2/M cell cycle arrest. PLoS One. 12:e01810812017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang G, Reed E and Li QQ: Molecular basis
of cellular response to cisplatin chemotherapy in non-small cell
lung cancer (Review). Oncol Rep. 12:955–965. 2004.PubMed/NCBI
|
|
42
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|