Introduction
Temporary changes in the architecture of the free
gingival margin, as well as the gingival crevicular fluid flow and
the control of bleeding effect into gingival sulcus, are necessary
for the precise treatment procedures in restorative dentistry. Past
several decades have witnessed the use of chemo-mechanical methods
with various retraction/displacement media and chemical
retraction/displacement agents by dentists (1). This technique provides optimal
conditions for imaging and transmission of morphological status of
the prepared tooth and/or design of the implant's structure and the
surrounding periodontal configuration to the dental laboratory
through impression materials or optical/digital scanning in the
patient's mouth in the computer-aided design/computer-aided
manufacturing (CAD/CAM) techniques (2).
In dental practice, two categories of chemical
retraction agents are used: Conventional retraction agents (CRAs)
(e.g., astringents (e.g., coagulants and hemostatics)) and
experimental retraction agents (ERAs) (e.g., vasoconstrictors
(e.g., adrenergics)) (3,4). The astringents contain inorganic
metallic salts such as aluminum chloride and sulfate, ferric
sulfate, and others. From the clinical point of view, CRAs are very
effective agents. However, they have been shown to have numerous
adverse-reversible and irreversible-local effects on the gingival
tissue, which are associated with the low acidity of the
astringents (pH < 3) (5–10). The vasoconstrictors used previously
were based on the organic salts of HCl, as well as on the α- and
β-adrenergics (HCl-epinephrine) and α-adrenergics
(HCl-tetrahydrozoline,-oxymetazoline, and-phenylephrine). Recently,
HCl-xylometazoline was also experimentally verified (11,12). The
most popular HCl-epinephrine has been used at different
concentrations. Few studies have reported that 4% epinephrine can
induce systemic side effects manifested as ‘Epinephrine Syndrome’
(13,14). Fazekas et al (15) studied 0.1% HCl-epinephrine and
Csillag et al (16) studied
0.01% HCl-epinephrine and reported that these agents showed
vasoconstrictor response in gingival tissues with reduced systemic
side effects. Moreover, it has been shown that the exo-and
endogenic effects of epinephrine can be accumulated in human body
during gingival margin retraction procedures. Bowles et al
(17) proposed α-adrenergic
sympathomimetic amines as potential alternative retraction agents
with effective constriction of gingival blood vessels and minimal
systemic action.
Previous studies on the comparative histological
evaluation of the response of gingival tissue in beagle dogs and
rabbits after exposure to the selected CRAs and ERAs revealed a low
damaging potential of 0.05% HCl-tetrahydrozoline (18–20).
Several in vitro studies on various cell lines have shown a
significantly higher cytotoxicity of astringents than that of the
vasoconstrictors (21–26). A previous study compared the
cytotoxic effects on primary human gingival fibroblasts (HGFs) by
using selected chemical retraction agents and demonstrated the
following array: 0.01% HCl-epinephrine <0.1% HCl-epinephrine
<5% aluminum sulfate <20% aluminum sulfate <15.5% ferric
sulfate (24). All these
aforementioned studies evaluated the cytotoxicity of chemical
retraction agents only in the solution form. However, Nowakowska
et al investigated the dynamic response of primary HGFs
after treatment with CRAs and ERAs in solution and gel formulations
(25,26). They isolated HGFs from healthy
gingival tissues by the method described by Saczko et al
(27) and incubated the cells with
retraction agents for 3, 5, and 10 min, according to the clinical
habits of dentists performing gingival retraction, as well as for
24 h. MTT ((3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide) tetrazolium reduction)) assay was performed to test the
cytotoxicity (27,28). After 10 min of exposure to both the
evaluated groups of chemicals, the mitochondria of HGFs showed a
higher activity, which suggests an increase in their antioxidative
defense capabilities. In a subsequent in vitro study, it was
demonstrated that the cytotoxicity of the evaluated vasoconstrictor
retraction agents decreased in the following order: 0.1%
HCl-epinephrine >parallel 0.01 and 0.05% HCl-epinephrine
>α-sympathomimetic amine solutions >0.05%
HCl-tetrahydrozoline gels. The minimal cytotoxic effect on the
mitochondrial oxidoreductive potential was demonstrated by three
self-prepared experimental gels at all evaluated time periods,
including 24 h, and the differences in the cell viability after
treatment with gels were not statistically significant (26).
The biological activity of the chemical retraction
agents in the surrounding periodontal tissues is a crucial factor.
However, their mechanism of action has not been completely
clarified yet. The results presented in the previous studies based
on in vitro experiments have expanded the knowledge on the
action of vasoconstrictive retraction agents in primary HGFs
(1,26,29).
Through the assessment of selected oxidative stress markers, such
as lipid peroxidation (the concentration of malondialdehyde (MDA)),
protein damage (-SH), colony formation, and the expression of
manganese superoxide dismutase (MnSOD), it was concluded that the
experimental gels induced oxidative changes in primary HGFs at the
lowest level. The evaluation of proteins (F-actin and β-tubulin) in
the cellular cytoskeleton of primary HGFs after 24 h of incubation
with gingival retraction agents showed that the studied
vasoconstrictive chemical retraction agents can have a cytotoxic
potential toward gingival tissue under clinical condition. These
observations of the rearrangement of the cytoskeleton also
suggested that the experimental gels caused degradation of the
cellular structure of primary HGFs (29).
Therefore, in the present study, we aimed to
evaluate, in more detail, if the proposed vasoconstrictive
experimental gels are more biocompatible with the gingival margin
tissue and if they can be applied as minimally invasive chemical
retraction agents. In addition, we propose an experimental approach
that can be used as a validation method in the evaluation of
biocompatibility of the newly developed retraction agents. HGFs
were established as a research model for this study to enable a
comparison of the results with the other studies concerning issue
of biocompatibility of gingival retraction agents. Observation of
collagen and fibronectin expressed by HGFs was performed in order
to evaluate the influence of the selected retraction agents on the
organization of cytoskeleton and the extracellular matrix.
Additionally, the level of reactive oxygen species (ROS) was
determined using the DCF assay.
Materials and methods
Chemical retraction agents
Six adrenergic commercial solutions and three
self-prepared experimental gel formulations-EG-1, EG-2, and EG-3
(patent No P. 397505 ‘Dental composition’)-were studied. Table I shows their characteristics. These
chemicals were diluted with Dulbecco's modified Eagle's medium
(DMEM) to 1:20 ratio, which was further studied based on our
previous studies (1,25).
 | Table I.Characteristics of the studied
gingival retraction agents.25 |
Table I.
Characteristics of the studied
gingival retraction agents.25
|
|
|
|
| Level of pH in
dilution |
|
|---|
|
|
|
|
|
|
|
|---|
| Trade name | Manufacturer | Active
ingredients | Chemical group | 1:10 | 1:20 | Clinical form |
|---|
| Afrin | Schering-Plow | 0.05%
HCl-oxymetazoline | α-adrenergics | 4.85 | 5.58 | Solution |
| Visine classic | Pfizer | 0.05%
HCl-tetrahydrozoline | α-adrenergics | 6.85 | 7.15 | Solution |
| Starazolin | Polpharma | 0.05%
HCl-tetrahydrozoline | α-adrenergics | 5.67 | 5.70 | Solution |
| Neosynephrin POS
10% | Ursapharm | 10%
HCL-phenylephrine | α-adrenergics | 4.30 | 5.18 | Solution |
| Injec. Adrenalini
0.05% | Self-prepared
dilution of Injec. Adrenalini 0.1%, Polfa | 0.05%
HCl-epinephrine | α and
β-adrenergics | 3.85 | 5.25 | Solution |
| Injec. Adrenalini
0.01% | Self-prepared
dilution of Injec. Adrenalini 0.1%, Polfa | 0.01%
HCl-epinephrine | α and
β-adrenergics | 3.90 | 5.36 | Solution |
| EG-1 | Self-prepared | 0.05%
HCl-tetrahydrozoline | α-adrenergics | 5.73 | 6.08 | Gel |
| EG-2 | Self-prepared | 0.05%
HCl-tetrahydrozoline | α-adrenergics | 6.16 | 6.64 | Gel |
| EG-3 | Self-prepared | 0.05%
HCl-tetrahydrozoline | α-adrenergics | 5.26 | 5.68 | Gel |
Cell culture
Primary HGFs were mechanically isolated from a
fragment of gingival tissue (1–2 mm) obtained from healthy
patients, according to the procedure described by Dominiak and
Saczko (27). The biopsies were
provided by the Department of Dental Surgery of the Faculty of
Medicine and Dentistry of Wroclaw Medical University in accordance
with the requirements of the Bioethics Commitee of the Wroclaw
Medical University. Patients were two women and one men, who were
subjected to surgery for tooth extraction and agreed as volunteers
for tissue biopsy (Bioethical Committee approval, no: KB-8/2010).
Patients were recruited from January till December 2018, they were
healthy people in the age between 18–50. All patients provided
their informed consent to participate in the present study. No
differences were observed in experimental protocol depending on the
patients' sex. Inclusion criterion was healthy periodontium of the
tissue donors, and exclusion criterion were diseases of gingival
tissues and/or oral cavity tissues. HGFs from several different
donors were used for different repetitions of experiments, however,
the results were not distinguished on the basis of the cells donor.
Before surgery, gingival tissue was rinsed by boric acid, to avoid
mycological infection. The taken biopsy did not exceed 3×2×2 mm.
The tissue was taken by a scalpel and immediately placed in a cell
culture medium (Dulbecco's modified Eagle's medium, DMEM)
containing 10% fetal bovine serum (FBS), and antibiotics
penicillin/streptomycin (Sigma). Primary cells were grown on Petri
dishes (60 mm, Nunc) and during the next passages routinely in 25
cm2 flasks (Equimed). The cells were maintained in a
humidified atmosphere at 37°C and 5% CO2. For
experimental reasons, the cells were detached by trypsinization
(0.25% trypsin-EDTA, Sigma). Successfully establishing of HGFs was
confirmed in immunofluorescent images of cells stained for
fibronectin, one of the markers of HGFs. The obtained primary cell
cultures were frozen in −80°C (up to 3 months) or in −160°C (up to
6 months) using standard protocol with Bambanker freezing medium
(ABO).
Immunocytochemical evaluation of
collagen types I and III
The biological effect of the selected retraction
agents was determined by the evaluation of the expression of
collagen types I and III by immunocytochemical (ABC) method using
DAKO kit (LSAB®2 System-HRP, cat. no. K0673) based on
the labelled streptavidin-biotin (LSAB) method in which a
biotinylated secondary antibody reacts with several
peroxidase-conjugated streptavidin molecules. Primary HGFs (1,000
cells) were seeded onto 8-well slides (Equimed) and then incubated
with retraction agents for 24 h in 37°C. Next, the fibroblasts were
fixed using 4% paraformaldehyde (PFA, Roth) for 10 min at room
temperature. Collagen types I and III were visualized with the
polyclonal goat antibodies (Collagen types I (cat. no. sc-59772)
and III (cat. no. sc-271249); Santa Cruz Biotechnology). The
incubation with primary antibodies was performed overnight in 4°C.
After incubation the staining protocol was applied according to the
manufacturer (DAKO) requirements in RT. DAB (3,3-diaminobenzidine)
was utilized for immunodetection (5 min of incubation in RT) and
hematoxylin (Roth) for nuclei staining (1 min of incubation in RT).
At the end the dehydration (5 min in each of 6 various
concentrations of ethanol) and transparentizing steps (3×5 min)
were performed in RT and finally microscopic slides were mounted
with DPX medium (Sigma-Aldrich) and were examined using an upright
microscope Olympus BX51 using 20× or 40× magnification. The
percentages of the stained cells were obtained by counting 100–200
cells in three randomly selected fields. Each slide was examined by
two independent investigators. Cells were evaluated as positive if
the stained reaction was noted in more than 5% of the cells
(30–32). The intensity of immunocytochemical
staining was rated as follows: (−) negative (no stained reaction),
(+) weak, (++) moderate, (++/+++) higher than moderate, and (+++)
strong. Negative controls with phosphate buffered saline (PBS, Lab
Empire, Poland) were prepared on each slide.
Dichlorofluorescein (DCF) assay
We determined the level of reactive oxygen species
(ROS) using the DCF assay (Life Technologies), which was conducted
with 6-carboxy-2,7-dichlorodihydrofluorescein diacetate
(carboxy-H2DCFDA). Briefly, the stock solution of carboxy-H2DCFDA
(50 µg/ml in sterile DMSO (Sigma)) was prepared at room temperature
in the dark and then diluted using the cell culture medium without
FBS. Cells were cultivated on black 96-well plates with transparent
bottom overnight before the experiment to achieve 70% of
confluency. For experimental protocol retraction agents were
diluted in cell culture medium (DMEM) to 1:20 ratio. Then cells
were incubated with gingival retraction agents and reactive oxygen
species were measured after 5, 20, and 25 min of exposition. After
washing out of the incubation medium from cells using PBS with 6 mM
glucose, the working solution of carboxy-H2DCFDA was added to the
cell culture medium to a final concentration of 10 µM and the cells
were incubated at 37°C in darkness for 30 min. After this, the
excitation and emission were measured at 495 and 530 nm,
respectively. ROS level was detected after 5, 20, and 25 min of
treatment with retraction agents by a multiwell scanning
spectrophotometer (EnSpire Perkin Elmer). All results were compared
to the control untreated cells cultivated in the same conditions.
The results were expressed as a mean values of measured
fluorescence intensity.
Confocal laser scanning microscopy for
the evaluation of fibronectin
For the evaluation of distribution of fibronectin,
the following procedure of immunofluorescence was performed.
Primary HGFs (1,000 cells) were grown on coverslips for 24 h at
37°C, and then incubated with retraction agents diluted in the
culture medium for 24 h at 37°C. Next, the fibroblasts were washed
with PBS (5 min at room temperature), fixed using 4% PFA in PBS (10
min at room temperature), blocked with 1% FBS in PBS (for 1 h at
37°C), and permeabilized with 1% triton X-100 (Sigma) in PBS (v/v)
(3×3 min at room temperature). All washing steps were performed
with PBS. After an overnight incubation of cells with mouse
monoclonal anti-fibronectin antibody [IST-9] (ab6328, diluted
1:200; Abcam) at 4°C, the cells were washed with PBS (2×10 min at
room temperature) and labeled with antibody Alexa Fluor®
488 AffiniPure Goat Anti-Mouse IgG (H+L) (115-545-003, diluted
1:100, Jackson ImmunoResearch) for 1 h at 37°C. Then, the cells
were mounted in a fluorescence mounting medium with
4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma) for DNA
staining. For the imaging of the fibronectin distribution, Olympus
FluoView FV1000 confocal laser scanning microscope (Olympus) with
60× magnification was used.
Statistical analysis
The data are presented as mean ± error. The minimum
number of repeats performed was n=9. The evaluation of statistical
significance was performed by two-way ANOVA with Tukey post-hoc
test using the control group represented by the untreated
HGFs-cells incubated with a culture medium (DMEM) without any
retraction agent. The evaluation involved separate controls for
each time point in DCF assay. P<0.05 was considered to indicate
a statistically significant difference. GraphPad Prism 7.0 software
was implemented for the analysis.
Results
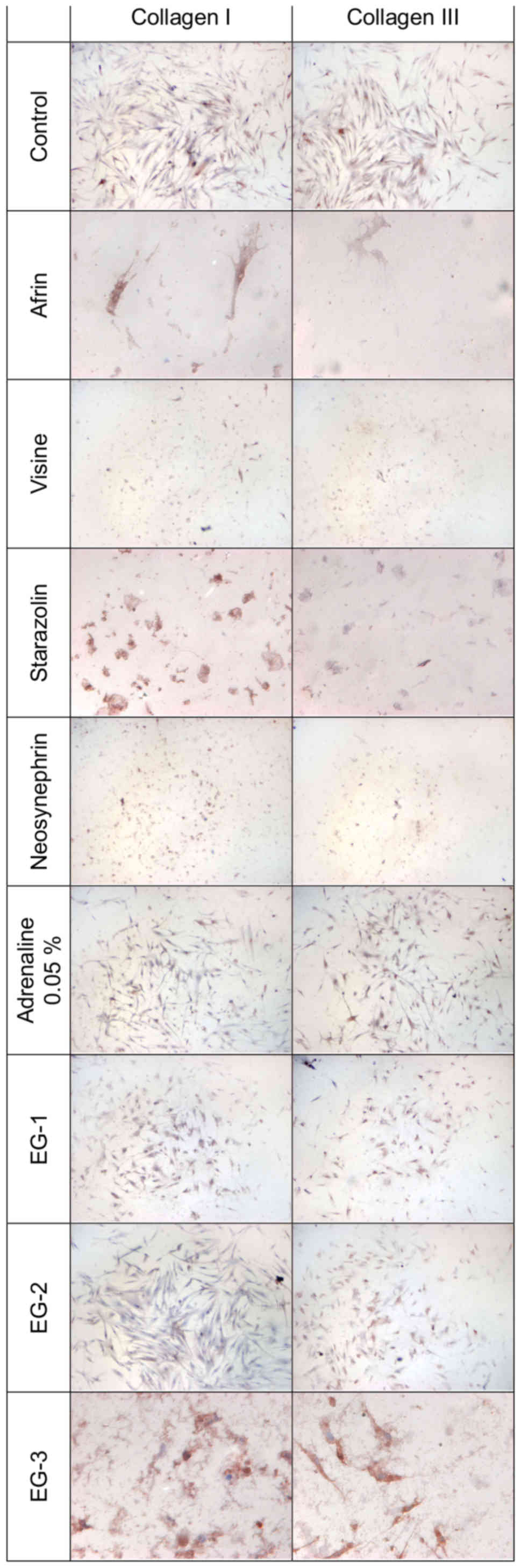
Expression of collagen types I and
III
Fig. 1 presents the
results of experimental collagen types I and III evaluation in
primary HGFs. Table II presents
semi-quantitative results. The results of the expression of
collagen types I and III in primary HGFs were determined after 24 h
of incubation with gingival retraction agents using 1:20 dilution.
A slight increase in collagen types I and III quantity was observed
in HGFs after 24 h of incubation with all the investigated
retraction agents. The highest intensity of immunocytochemical
reaction was observed after incubation with the experimental gels
(EG-1, EG-2, and EG-3).
 | Table II.Evaluation of the expression of
collagen types I and III in primary human gingival fibroblasts
after 24 h of incubation with gingival retraction agents diluted
with the cell culture medium (DMEM) to 1:20 concentration. |
Table II.
Evaluation of the expression of
collagen types I and III in primary human gingival fibroblasts
after 24 h of incubation with gingival retraction agents diluted
with the cell culture medium (DMEM) to 1:20 concentration.
|
| Collagen type
I | Collagen type
III |
|---|
|
|
|
|
|---|
| Gingival retraction
agent | Positive cells,
% | Reaction
intensity | Positive cells,
% | Reaction
intensity |
|---|
| Control | 98 | ++/+++ | 97 | +++ |
| Afrin | 100 | ++ | 100 | + |
| Visine | 100 | + | 100 | + |
| Starazolin | 100 | + | 100 | −/+ |
| Neosynephrin | 100 | ++ | 100 | + |
| Adrenaline
0.05% | 100 | + | 100 | + |
| EG-1 | 100 | ++/+++ | 100 | +++ |
| EG-2 | 100 | ++ | 100 | ++/+++ |
| EG-3 | 100 | ++ | 100 | ++/+++ |
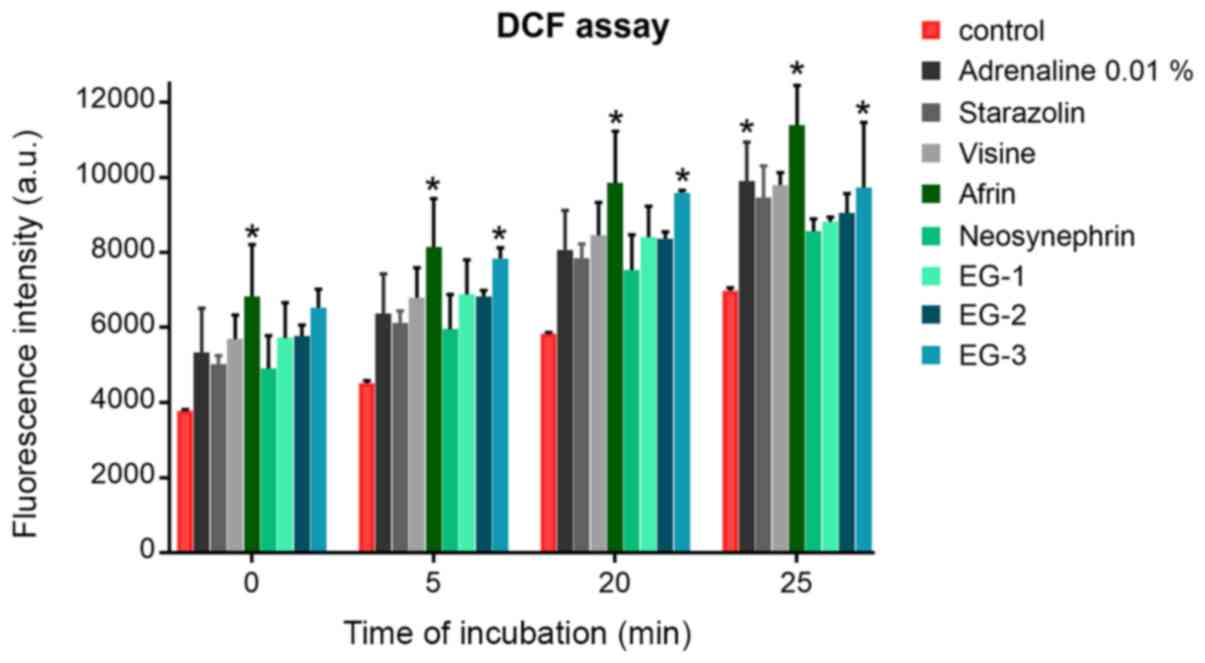
Level of ROS
The effect of retraction agents on the generation of
free radicals and promotion of oxidative stress was examined.
Oxidative stress was evaluated by DCF assay after different times
of incubation (5, 20, and 25 min) with various retraction agents
using 1:20 concentration. The increase in the level of free
radicals was proportional to the time of incubation in HGFs. In
most cases, the level of increase of ROS after incubation with
retraction agents did not differ from that of the control cells
(Fig. 2). The newly developed
experimental gels stimulated the production of ROS similar to the
commercial retraction agents (e.g., Neosynephrin or Visine).
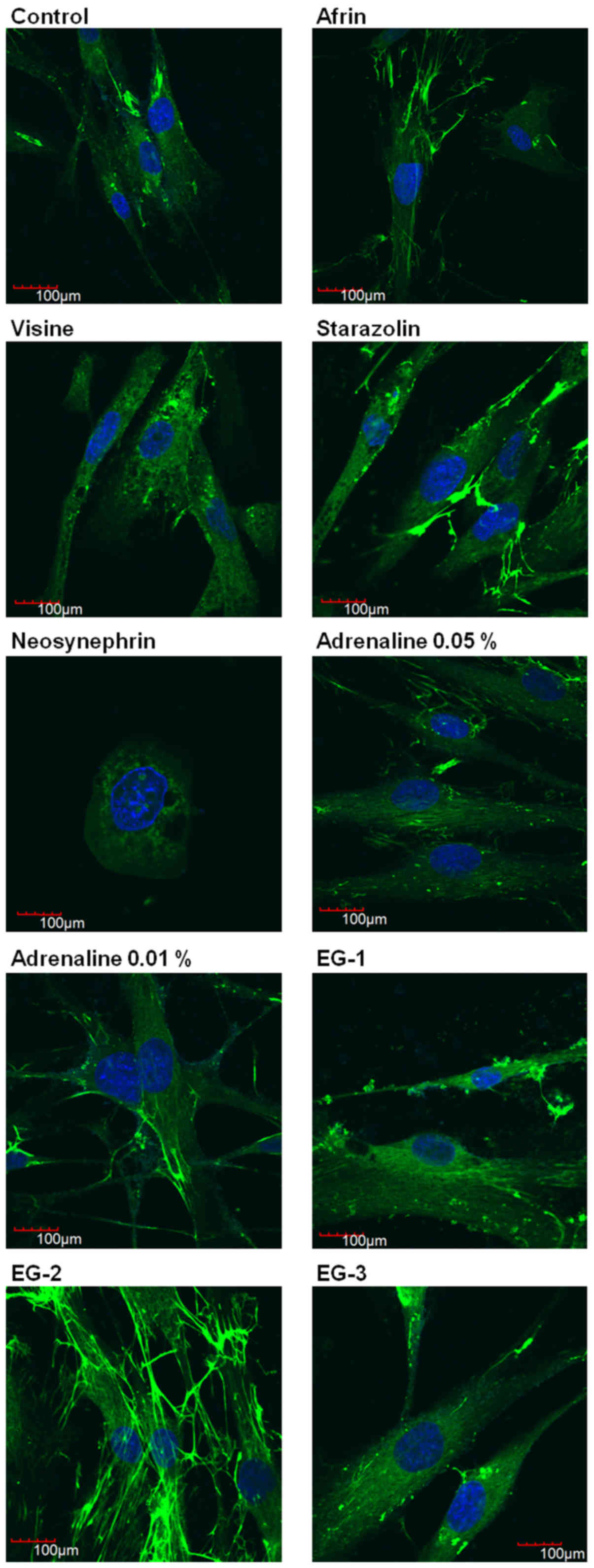
Evaluation of fibronectin
distribution
We performed a semi-quantitative determination of
fibronectin as a marker of cell attachment and proliferation.
Fig. 3 presents the
results of evaluation of the intracellular distribution of
fibronectin in HGFs after 24 h of incubation with selected gingival
retraction preparations using immunofluorescence technique. The
results show that Neosynephrin was the most cytotoxic agent, which
caused disorders of the cellular morphology and significant
antiproliferative effect. Incubation of HGFs with Visine slightly
disturbed the expression of fibronectin. Other studied retraction
agents had no significant influence on the distribution of
fibronectin. However, we were able to observe that EG-2 stimulated
the expression of fibronectin. Our results confirm the safety of
using the studied experimental gels. Moreover, our results suggest
the beneficial effect of EG-2.
Discussion
Vasoconstrictive gingival retraction agents should
have a sufficient clinical efficiency without local and minimal
systemic side effects (18–20). A systemic reaction is infrequent in
some vasoconstrictor materials of the alpha agonists-including
tetrahydrozoline, oxymetazoline, and phenylephrine-that are
commonly used as eye and nose decongestant drops. Therefore, a dose
that is lower than the maximum allowed can be used in the gingival
retraction. Bowles et al (17) reported that tetrahydrozoline is a
powerful retraction agent. Similar investigations have shown that
tetrahydrozoline is better than epinephrine for gingival retraction
protocols (15,16). However, in clinical practice, the
vasoconstrictive gingival retraction agents are available only in
solution form.
Previously, we demonstrated that cytotoxic effect of
the product is dependent on the purity of the active substance and
the pharmacological form of chemical agents (26). In this study, we focused on the
biological properties of the experimental gels EG-1, EG-2, and EG-3
in comparison with the commonly used vasoconstrictive retraction
agents-epinephrine and experimental α-adrenergic sympathomimetic
amines solutions. The experimental gels are based on the pure form
of the active substance-tetrahydrozoline, contrary to other
retraction agents used in prosthodontics, such as Visine classic
and Starazolin. All these compounds belong to sympathomimetic
amines, which contain other chemical substances in addition to
tetrahydrozoline. We hypothesized that these chemical differences
can influence and modify the biological effects of retraction
agents in gingival fibroblasts, which have been shown in our
previous studies (26,29). In our previous in vitro study,
the examination revealed minor cytotoxic effect of the experimental
gels in contrast to the commercial preparations (Visine,
Starazolin, Afrin, and Neosynephrin) (26). Based on these results, three gels
containing 0.05% HCl-tetrahydrozoline were prepared. In this study,
we performed additional experiments to evaluate the cytotoxicity
and biocompatibility of our experimental gels in more detail. To
investigate whether cells exposed to retraction agents show
increased level of oxidative stress, we measured the level of ROS
with DCF assay. In most cases, the level of ROS after incubation
with retraction agents was on similar to the control cells. Several
in vitro tests can be used to determine the cytotoxicity of
the biomaterial. Regarding oral tissues and practical motives, cell
culture model seems to be more appropriate than that of in
vivo studies as it enables the performance of multiple tests
for the estimation of precise biological response (26).
Furthermore, in this study, we focused on the
evaluation of the influence of the selected retraction agents on
the organization of cytoskeleton and the extracellular matrix.
Cytoskeleton proteins play a very important role in the proper
functioning of cells as these proteins provide the structural
framework for the cell and are responsible for the movement of cell
and internal transport. In this study, we aimed to evaluate the
expression of fibronectin and two types of collagen (types I and
III). Our evaluation showed that some of the studied retraction
agents (Visine and Neosynephrin) influenced the expression of
fibronectin, which can lead to disorders in the construction of the
cytoskeleton. However, none of the experimental preparations (EG-1,
EG-2, and EG-3) altered the intracellular arrangement and
fluorescent signal of fibronectin. We observed numerous cells with
normal cytoplasm and regular distribution of fibronectin after
incubation with new experimental gels. We also observed that EG-2
stimulated the expression of fibronectin. These results confirm
that the studied experimental gels are biologically safe. Moreover,
our results suggest a beneficial effect of EG-2 on cellular matrix
proteins. Fibronectin, binds with the components of the
extracellular matrix such as collagen and fibrin and is very
important for wound healing. It also plays a key role in cell
adhesion, growth, and migration (33). The results of this study demonstrated
a slight increase in the amount of collagen types I and III in HGFs
after 24 h of incubation with the newly investigated retraction
agents (EG-1, EG-2, and EG-3). This indicates that tetrahydrozoline
contained in the gel formulation plays a double role: it not only
acts as the retraction agent but also plays a role in wound healing
by increasing the expression of collagen and fibronectin.
Our study revealed that the oxidative changes
induced by the experimental gel formulations were on the
physiological level. Moreover, the observations of the cytoskeleton
of HGFs clearly indicated that the experimental gels did not affect
their cellular structure and they even stimulated the expression of
collagen and fibronectin.
For the proper understanding of the mechanism of
action of retraction agents, it is necessary to recognize the
limitations of in vivo and in vitro study. In
clinical conditions, sulcular epithelium and subepithelial
connective tissue protect the gingival fibroblasts. In addition,
the concentration of the chemical retraction agents might get
decreased due to the constant humidity and temperature maintained
in the oral cavity. Moreover, the applied agents can get washed
away by the crevicular fluid flow, due to bleeding, or via saliva.
Probably, the systemic blood circulation parameters can also affect
the successful application of the retraction procedure. Therefore,
the same vasoconstrictor retraction agents may act in an unusual
way under clinical conditions. Further clinical studies are
required to extend the results presented in this article and to
find their clinical relevance.
To conclude, regardless of the limitations of this
in vitro study, the results suggest that the proposed
experimental gels are biocompatible with periodontal tissues, and
they can be considered as the new vasoconstrictive chemical
retraction agents.
Acknowledgements
Not applicable.
Funding
The present study was supported by Funds of
Department of Molecular and Cellular Biology, Wroclaw Medical
University (grant no. 846-183) and Funds of Department of
Prosthodontics, Wroclaw Medical University (grant no. ST-365).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
DN, JS and JK contributed to the conception and
design of the study, analysis and interpretation of data, drafting
the article and final approval of the submitted version. AS and OM
contributed to the acquisition of data, revising the article
critically for important intellectual content and final approval of
the submitted version. MZ and WW contributed to the conception and
design of the study, revising the article critically for important
intellectual content and final approval of the submitted version.
JW contributed to the acquisition, analysis and interpretation of
data, drafting the article, and final approval of the submitted
version.
Ethics approval and consent to
participate
All healthy donor patients provided their informed
consent to participate in this study in accordance with The
Declaration of Helsinki. The present study was planned with no risk
and in agreement with the Wroclaw Medical University Bioethical
Committee. All patients signed agreement before the examination.
All patients provided their informed consent to participate in the
present study. Wroclaw Medical University Bioethical Committee
approved the present study (approval no. KB-8/2010).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nowakowska D, Saczko J, Kulbacka J and
Więckiewicz W: Chemical Retraction Agents–in vivo and in vitro
studies into their physico-chemical properties, biocompatibility
with gingival margin tissues and compatibility with elastomer
impression materials. Mini Rev Med Chem. 17:435–444. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bennani V, Schwass D and Chandler N:
Gingival retraction techniques for implants versus teeth: Current
status. J Am Dent Assoc. 139:1354–1363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Felpel LP: A review of
pharmacotherapeutics for prosthetic dentistry: Part I. J Prosthet
Dent. 77:293–305. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nowakowska D: Classification of chemical
retraction agents. Protet Stomatol. 58:202–208. 2008.(In
Polish).
|
|
5
|
Phatale S, Marawar PP, Byakod G, Lagdive
SB and Kalburge JV: Effect of retraction materials on gingival
health: A histopathological study. J Indian Soc Periodontol.
14:35–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chaudari J, Prajapati P, Patel J,
Sethuraman R and Naveen YG: Comparative evaluation of the amunt of
gingival displacement produced by three different retraction
systems: An in vivo study. Contemp Clin Dent. 6:189–195. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akca EA, Yildirim E, Dalkiz M, Yavuzyilmaz
H and Beydemir B: Effects of different retraction medicaments on
gingival tissue. Quintessence Int. 53–59. 2016.
|
|
8
|
Al Hamad KQ, Azar WZ, Alwaeli HA and Said
KN: A clinical study on the effects of cordless and conventional
retraction techniques on the gingival and periodontal health. J
Clin Periodontol. 35:1053–1058. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldberg PV, Higginbottom FL and Wilson
TG: Periodontal considerations in restorative and implant therapy.
Periodontol 2000. 25:100–109. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nowakowska D and Raszewski Z: The pH value
of conventional and experimental gingival margin retraction
medicaments. Dent Med Probl. 47:76–80. 2010.
|
|
11
|
Katareva I, Georgieva K, Simeonov S,
Doychinova M and Tonchev T: Application of xylometazoline for
chemo-mechanical retraction of the free gingiva. J of IMAB.
21:849–852. 2015. View Article : Google Scholar
|
|
12
|
Gerhard-Szép S: Cytotoxic potential of
HCl-xylometazoline-based gingival retraction solutions. DZZ 2ß16.
38–50. 2016.
|
|
13
|
Buchanan WT, Thayer KE and Yoder JL:
Systemic effects of epinephrine-impregnated retraction cord in
fixed partial denture prosthodontics. J Am Dent Assoc. 104:482–484.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bader JD, Bonito AJ and Shugars DA: A
systematic review of cardiovascular effects of epinephrine on
hypertensive dental patients. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 93:647–653. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fazekas A, Csempesz F, Csabai Z and Vág J:
Effects of pre-soaked retraction cords on the microcirculation of
the human gingival margin. Oper Dent. 27:343–348. 2002.PubMed/NCBI
|
|
16
|
Csillag M, Nyiri G, Vag J and Fazekas A:
Dose-related effects of epinephrine on human gingival blood flow
and crevicular fluid production used as a soaking solution for
chemo-mechanical tissue retraction. J Prosthet Dent. 97:6–11. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bowles WH, Tardy SJ and Vahadi A:
Evaluation of new gingival retraction agents. J Dent Res.
70:1447–1449. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kopač I, Cvetko E, Pavlica Z and Marion L:
Gingival tissue inflammatory response following treatment with
chemical retraction agents in Beagle dogs. Pflugers Arch. 442 (6
Suppl 1):R145–R146. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kopač I, Cvetko E and Marion L: Gingival
inflammatory response induced by chemical retraction agents in
beagle dogs. Int J Prosthodont. 15:14–19. 2002.PubMed/NCBI
|
|
20
|
Kostić I, Mihailović D, Najman S,
Stojanović S and Kostić M: The rabbit gingival tissue response to
retraction liquids and tetrahydrozoline. Vojnosanit Pregl.
71:46–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kopač I, Batista U, Cvetko E and Marion L:
Viability of fibroblasts in cell culture after treatment with
different chemical retraction agents. J Oral Rehabil. 29:98–104.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kopač I, Sterle M and Marion L: Electron
microscopic analysis of the effects of chemical retraction agents
on cultured rat keratinocytes. J Prosthet Dent. 87:51–56. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu CM, Huang FM, Yang LC, Chou LS, Chou
MY and Chang YC: Cytotoxic effects of gingival retraction cords on
human gingival fibroblasts in vitro. J Oral Rehabil. 31:368–372.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu J, Zhang XM, Hao PJ, Hui M and Yu HY:
Comparison of cytotoxicity between chemical retraction agents on
human gingival fibroblasts in vitro. Hua Xi Kou Qiang Yi Xue Za
Zhi. 27:202–205. 2009.(In Chinese). PubMed/NCBI
|
|
25
|
Nowakowska D, Saczko J, Kulbacka J and
Choromanska A: Dynamic oxidoreductive potential of astringent
retraction agents. Folia Biol (Praha). 56:263–268. 2010.PubMed/NCBI
|
|
26
|
Nowakowska D, Saczko J, Kulbacka J,
Choromanska A and Raszewski Z: Cytotoxic potential of
vasoconstrictor experimental gingival retraction agents: In vitro
study on primary human gingival fibroblasts. Folia Biol (Praha).
58:37–43. 2012.PubMed/NCBI
|
|
27
|
Dominiak M and Saczko J: Method of primary
culture of human fibroblasts for autologous augmentation. PL patent
209784B1. Filed December 4, 2006, issued October 31. 2011.
|
|
28
|
Nowakowska D, Panek H, Nowakowska M and
Nowakowska A: Gingival retraction-survey results of Polish
dentists. Part 2. Clinical habits related to retraction procedures.
Protet Stomatol. 56:361–366. 2006.
|
|
29
|
Nowakowska D, Saczko J, Bieżuńska-Kusiak
K, Choromańska A, Dubińska-Magiera M, Ziętek M and Kulbacka J: The
influence of retraction agents on cytoskeleton reorganization and
oxidative stress in primary human gingival fibroblasts (HGFs). Arch
Oral Biol. 59:341–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fedchenko N and Reifenrath J: Different
approaches for interpretation and reporting of immunohistochemistry
analysis results in the bone tissue-a review. Diagn Pathol.
9:2212014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walker RA: Quantification of
immunohistochemistry-issues concerning methods, utility and
semiquantitative assessment I. Histopathology. 49:406–410. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zabel M: Immunocytochemia. 1st. PWN;
Warszawa: 1999
|
|
33
|
Kim MC, Neal DM, Kamm RD and Asada HH:
Dynamic modeling of cell migration and spreading behaviors on
fibronectin coated planar substrates and micropatterned geometries.
PLoS Comput Biol. 9:e10029262013. View Article : Google Scholar : PubMed/NCBI
|