Introduction
Osteoarthritis (OA) is the most common form of
rheumatic disease with the highest rate of incidence, which
ultimately leads to limited joint mobility, chronic pain and
disability (1). At the cellular
level, OA is characterized by decreased tissue cellularity and
damage to the extracellular matrix (1). It was previously demonstrated that
apoptosis in chondrocytes was enhanced in the articular cartilage
of patients with OA. Apoptotic cells in the cartilage of patients
with OA were identified by the detection of DNA strand breaks using
TUNEL assay, which identified high levels of apoptotic cells in the
zones of cartilage known as the superficial and middle zones
(2).
Resveratrol (Res) can regulate the expression of
several intracellular signaling proteins and it is known to be
associated with specific anti-inflammatory properties (3,4). In
addition, Res can regulate cell proliferation while preventing
inflammation and apoptosis in both the chronic and acute phases of
OA (5). Res reduces the
morphological changes of chondrocytes and inhibits the induction of
the pro-inflammatory cytokine interleukin (IL)-1β (4). Furthermore, Res suppresses nuclear
factor kappa B subunit 1 (NF-κB1)-dependent pro-inflammatory
signaling and inhibits membrane-bound and mature IL-1β production
in chondrocytes (3). By contrast,
in vitro studies demonstrated that IL-1β inhibits the
chondrocyte proliferation (3–5).
Until recently, non-coding RNAs (ncRNAs) were
considered to have generic intracellular roles (6). Ribosomal RNAs (rRNAs) and transfer RNAs
(tRNAs) are involved in the translation of mRNA, whereas small
nuclear RNAs (snRNAs) participate in RNA splicing and small
nucleolar RNAs (snoRNAs) mediate rRNA modification (6). Previous studies demonstrated that long
non-coding RNAs (lncRNAs), RNAs >200 nucleotides in length with
no or limited protein-coding ability (6,7), can
serve crucial roles in several types of human cancer (8–10). In
addition, lncRNAs may function to regulate gene expression at both
the transcriptional and post-transcriptional levels based on
genetic and epigenetic mechanisms (11,12).
Furthermore, associations between lncRNAs and OA were previously
investigated. Xing et al (13) identified 121 lncRNAs that were up- or
downregulated in OA. MicroRNAs (miR) are small non-coding RNA
molecules derived from the introns and exons of both protein-coding
and non-coding transcripts transcribed by RNA polymerase II
(13–15). In addition, processed pseudogenes can
activate certain miRs (16).
A previous study demonstrated that treatment with
Res downregulated the expression of MALAT1, and as an lncRNA,
MALAT1 can function as a molecular sponge of miR-9 (17). In addition, miR-9 can directly target
NF-κB, and as an inflammatory cytokine NF-κB can induce apoptosis
in chondrocytes contributing to the development of OA (18). In the current study, to explore the
role of Res in OA and its underlying mechanism, the in vivo
model of OA was established and the effect of Res was examined
in vitro and in the in vivo model of OA. PCR
techniques and western blot analysis, immunohistochemical analysis,
dual-luciferase reporter assays were performed to study underlying
mechanisms, while MTT assay was used to study the effect of Res on
cell proliferation in vitro. Res treatment was indicated to
inhibit MALAT1 and modulate MALAT1/miR-9/NF-κB signaling
pathway.
Materials and methods
Animals and experimental design
A total of 30 male C57BL/6 mice (age, 10 weeks;
weight, 20–30 g) were purchased from the Shanghai Laboratory Animal
Centre, Chinese Academy of Sciences (Shanghai, China). The mice
were divided into three groups: Sham surgery (sham group, n=10), OA
with vehicle injection (OA group, n=10) and OA with Res treatment
(OA + Res group, n=10). Following 7 days acclimatization, OA was
induced using the destabilizing medial meniscus (DMM) model
(18). Briefly, mice were
anesthetized with pentobarbital (50 mg/kg IP) and an incision was
made in the right knee. The joint capsule immediately medial to the
patellar tendon was incised and the joint capsule was opened using
microsurgical scissors. DMM was achieved by sectioning the medial
meniscotibial ligament with microsurgical scissors. In the sham
group, an incision was made in the right knee joint and ligaments
were visualized only and not transected. All experimental protocols
were approved by the Institutional Animal Care and Use Committee at
Nanjing University of Chinese Medicine (Nanjing, China). The
current study was approved by the Institutional Ethics Committee on
Animal Research at Nanjing University of Chinese Medicine.
Res treatment
Res (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck
KGaA) to give a final concentration of 100 mg/ml (stock solution).
The stock solution was diluted with PBS to give a final
concentration of 12.5 mg/ml. At five weeks post-surgery, mice in
the OA + Res group received 10 ml Res, while mice in the sham group
received 10 ml PBS via intra-articular injection through the
patellar tendon with the use of a U-100 insulin syringe (BD
Biosciences, San Jose, CA, USA). All injections were performed
twice a week for 8 weeks. At 13 weeks post-surgery, the mice were
sacrificed and knee joints were harvested.
Cell culture and transfection
Mouse chondrocytes (ATCC® CRL-12424™)
were purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). Chondrocytes were cultured in Dulbecco's
modified Eagle medium (DMEM)/F12 (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS, 0.1 mg/ml streptomycin and 1,000
U/ml penicillin (Thermo Fisher Scientific, Inc.) and maintained in
a 5% CO2-humidified incubator at room temperature.
Chondrocytes were grown to 70% confluence and treated with 15 or 30
µM Res prior to transfection with miR-9 mimic or scramble control
which were manufactured by Shanghai GenePharma Co., Ltd. using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Each experiment was performed in triplicate.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue samples and
chondrocytes using the mirVana™ miRNA Isolation kit (Ambion; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Total RNA was reverse transcribed into cDNA using the
PrimeScript® RT reagent kit (Takara Biotechnology Co.,
Ltd., Dalian, China), followed by qPCR. For miR-9 and MALAT1
expression, qPCR was performed using the TaqMan microRNA assay
(Applied Biosystems; Thermo Fisher Scientific, Inc.). For NF-κB1
expression, qPCR was performed using the SYBR® Premix Ex
Taq™ kit (Takara Biotechnology Co., Ltd.). All qPCR were performed
using an ABI-7500 Sequence Detection System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The relative miR-9, MALAT1 and
NF-κB1 expression levels were quantified using the
2−ΔΔCq method (19). U6
and GAPDH mRNA were used as endogenous controls for miR-9, MALAT1
and NF-κB1, respectively. The primer pairs used were as follows:
miR-9 forward, 5′-GGTCTTTGGTTATCTAGCTGTATGA-3′ and reverse,
5′-3CAGTGCGTGTCGTGGAGT-3); MALAT1 forward,
5′-CAGACCACCACAGGTTTACAG-3′ and reverse,
5′-AGACCATCCCAAAATGCTTCA-3′); NF-κB1 forward,
5′-CAAGCGAGGAGGGGACGTG-3′ and reverse, 5′-CCCCCAGAGCCTCCACCC-3′);
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′; GAPDH mRNA forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The thermocycling conditions were as
follows: 94°C for 5 min, 30 cycles of 94°C for 30 sec and 60°C for
30 sec and 72°C for 30 sec, 72°C for 10 min. Each experiment was
performed in triplicate.
MTT assay
Cell proliferation was examined in chondrocytes by
MTT assay following treatment with Res. Following a 48 h
incubation, 0.5 mg/ml MTT (Sigma-Aldrich; Merck KGaA) was added to
chondrocytes and incubated at 37°C for 4 h. Following incubation,
culture medium was removed and 150 µl DMSO was added into each well
(48-well plate at a density of 1×105 cells/well). The
absorbance was measured at a wavelength of 490 nm using a
multi-mode microplate reader (CHAMELEON™ V; Hidex, Turku, Finland)
to analyze cell survival. All experiments were performed in
triplicate.
Dual-luciferase reporter assay
The mutant 3′UTR of MALAT1/NF-κB1 was generated by
mutating the miR-9 binding site sequence in the wild-type 3′UTR of
MALAT1/NF-κB1. The wild-type or mutant 3′UTR of MALAT1/NF-κB1 were
PCR amplified and cloned into the pRL-TK reporter vector (Promega
Corporation, Madison, WI, USA). Chondrocytes were seeded into
48-well plates at a density of 1×105 cells/ml and
co-transfected with 300 ng luciferase reporter vector containing
the wild-type or mutant 3′UTR of MALAT1/NF-κB1 and 20 pmol miR-9
mimic or scramble control using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Following 48-h transfection, chondrocytes
were lysed and cell lysates were collected. Relative luciferase
activities were detected using a Dual-Luciferase Reporter Assay
system (Promega Corporation). Firefly luciferase activity was
normalized to Renilla luciferase activity. Each test was
performed in triplicate.
MALAT1 luciferase assay
The promoter region of wild-type MALAT1 was PCR
amplified and cloned into the pRL-TK reporter vector (Promega
Corporation). Chondrocytes were seeded into 48-well plates at a
density of 1×105 cells/ml and transfected with 300 ng
luciferase reporter vector containing the promoter region of MALAT1
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Subsequently, chondrocytes were treated with 15 or 30 µM Res for 48
h. Following 48-h treatment with Res, chondrocytes were lysed and
cell lysates were collected. The relative Renilla luciferase
activity was detected using a Luciferase Reporter Assay system
(Promega Corporation). Each experiment was performed in
triplicate.
Western blot analysis
Chondrocytes were washed three times with ice-cold
PBS and total protein was extracted using 0.2 ml RIPA lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China). Chondrocytes
were incubated in lysis buffer for 30 min on ice followed by
centrifugation at 18,894 × g for 20 min at 4°C. Total protein was
quantified using a bicinchoninic acid assay kit (Thermo Fisher
Scientific, Inc.) and 25 µg protein was separated via SDS-PAGE on a
6 or 10% gel. The separated proteins were transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA)
and blocked for 1 h at 25°C with Tris-buffered saline containing
0.1% Tween™ 20 and 5% bovine serum albumin (Sigma-Aldrich; Merck
KGaA) to prevent non-specific binding. The membranes were incubated
with mouse primary antibodies against NF-κB1 (1:5,000; cat. no.
MA5-15128), MMP-13 (1:5,000; cat. no. MA5-14247), caspase-3
(1:5,000; cat. no. MA1-91637), IL-6 (1:5,000; cat. no. M621B all
Invitrogen; Thermo Fisher Scientific, Inc.) or β-actin (1:10,000;
cat. no. 3700S; Cell Signaling Technology, Inc., Danvers, MA, US)
for 12 h at 4°C. Following primary incubation, membranes were
incubated with anti-mouse horseradish peroxidase (HRP)-labeled
secondary antibodies (1:12,000; cat. no. 7076S; Cell Signaling
Technology, Inc.) at room temperature for 1 h. Protein bands were
visualized using RapidStep™ ECL detection reagent (EMD Millipore)
and Syngene GeneGenius Gel Light Imaging system (Syngene,
Frederick, MD, USA), according to the manufacturer's protocol. Each
experiment was performed in triplicate.
Immunohistochemistry (IHC)
Tissue samples were fixed with 10% formalin at 4°C
for 12 h and embedded in paraffin and paraffin-embedded samples
were cut into 5-mm sections, which was later put to blocking stage
with 3% hydrogen peroxide for 60 min at room temperature. For
antigen retrieval, tissue sections were incubated with 0.01 M
sodium citrate (pH 6) in a microwave oven for 10 min. Following
antigen retrieval, tissue sections were incubated with mouse
primary antibodies against NF-κB1 (cat. no. PA5-17654; 1:500) and
MMP-13 (cat. no. MA5-14238; 1:500; both Invitrogen; Thermo Fisher
Scientific, Inc.) for 12 h at 4°C. Following primary incubation,
tissue sections were incubated with HRP-labeled secondary
antibodies (cat. no. 7074S; 1:1,000; CST, Danvers, MA, US) for 1 h
at room temperature. Subsequently, tissue sections were stained
with hematoxylin (Dako Cytomation, Glostrup, Denmark) at 37°C for 2
h. Dimethyl benzene was used to mount the tissue sections and
images were captured using a light microscope (magnification,
×400). Two independent pathologists scored the staining intensity
of each protein. The staining intensity was scored as follows: no
staining, 0; weak staining, 1; moderate staining, 2; and strong
staining, 3. Each experiment was performed in triplicate.
Statistical analysis
Data were presented as the mean ± standard
deviation. All statistical analyses were performed using SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA). The
difference between two groups was analyzed using a two-tailed
Student's t-test, whilst the difference among three or more groups
was analyzed using one-way analysis of variance and Scheffe's test
was used as a post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
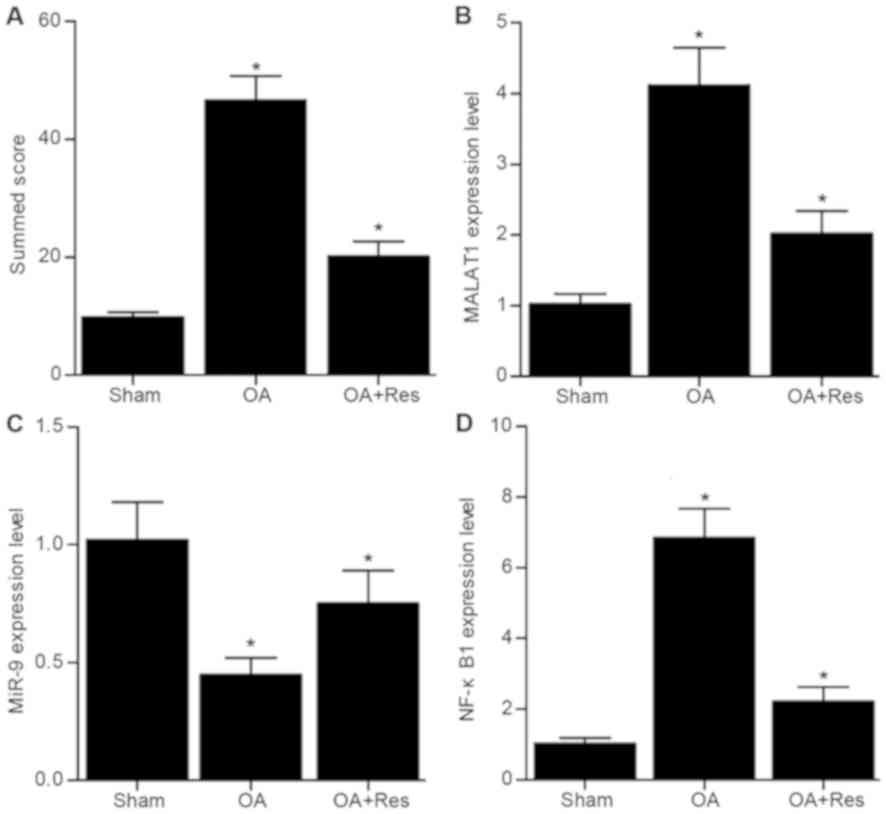
MALAT1, miR-9 and NF-κB1 expression in
the in vivo model of OA
As shown in Fig. 1,
the relative expression levels of MALAT1, miR-9 and NF-κB1 were
analyzed in tissue samples from mice in the sham, OA and OA + Res
groups. The relative mRNA expression levels of MALAT1 and NF-κB1
were significantly increased, while miR-9 expression was
significantly decreased in the OA group compared with the sham
group (Fig. 1B-D). Meanwhile,
compared with OA group, treatment with Res partially reversed the
effects of OA on the mRNA expression levels of MALAT1, miR-9 and
NF-κB1.
NF-κB1, IL-6, MMP-13 and caspase-3
expression in the in vivo model of OA
The relative protein expression levels of NF-κB1,
IL-6, MMP-13 and caspase-3 were determined by western blot analysis
in tissue samples from mice in the sham, OA and OA + Res groups
(Fig. 2). The NF-κB1, IL-6, MMP-13
and caspase-3 protein expression levels were significantly
increased in the OA group compared with the sham group (Fig. 2B-E). However, treatment with Res
partially reversed the effects of OA on the protein expression
levels of NF-κB1, IL-6, MMP-13 and caspase-3.
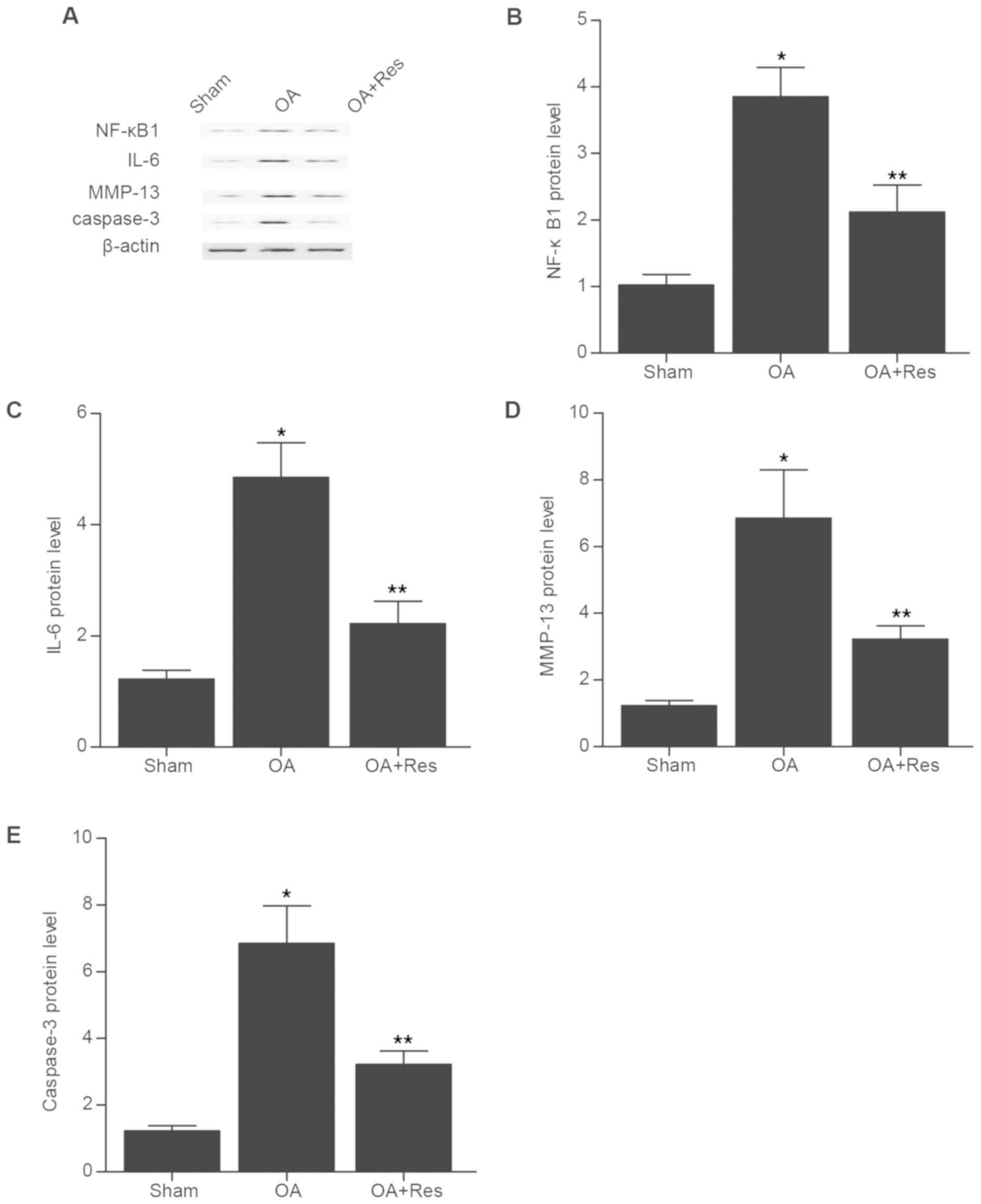 | Figure 2.NF-κB1, IL-6, MMP-13 and caspase-3
expression in the in vivo model of OA. (A) The relative
protein expression levels of NF-κB1, IL-6, MMP-13 and caspase-3
were determined by western blot analysis in tissue samples from
mice in the Sham, OA and OA + Res groups. Quantification of (B)
NF-κB1, (C) IL-6, (D) MMP-13 and (E) caspase-3 protein expression.
*P<0.05 vs. OA + Res group; **P<0.05 vs. sham group. NF-κB1,
nuclear factor kappa B subunit 1; IL-6, interleukin-6; MMP-13,
matrix metallopeptidase 13; OA, osteoarthritis; Res,
Resveratrol. |
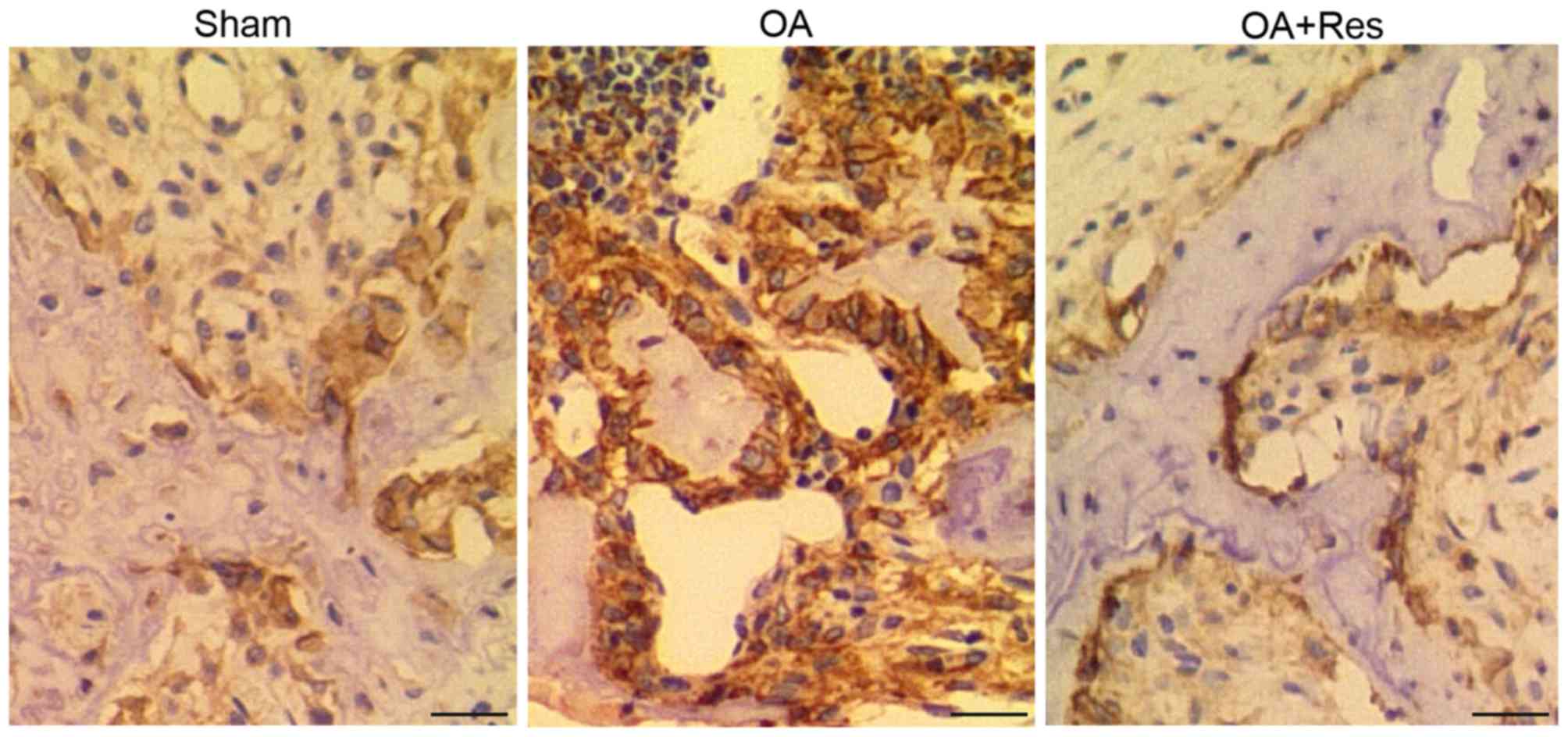
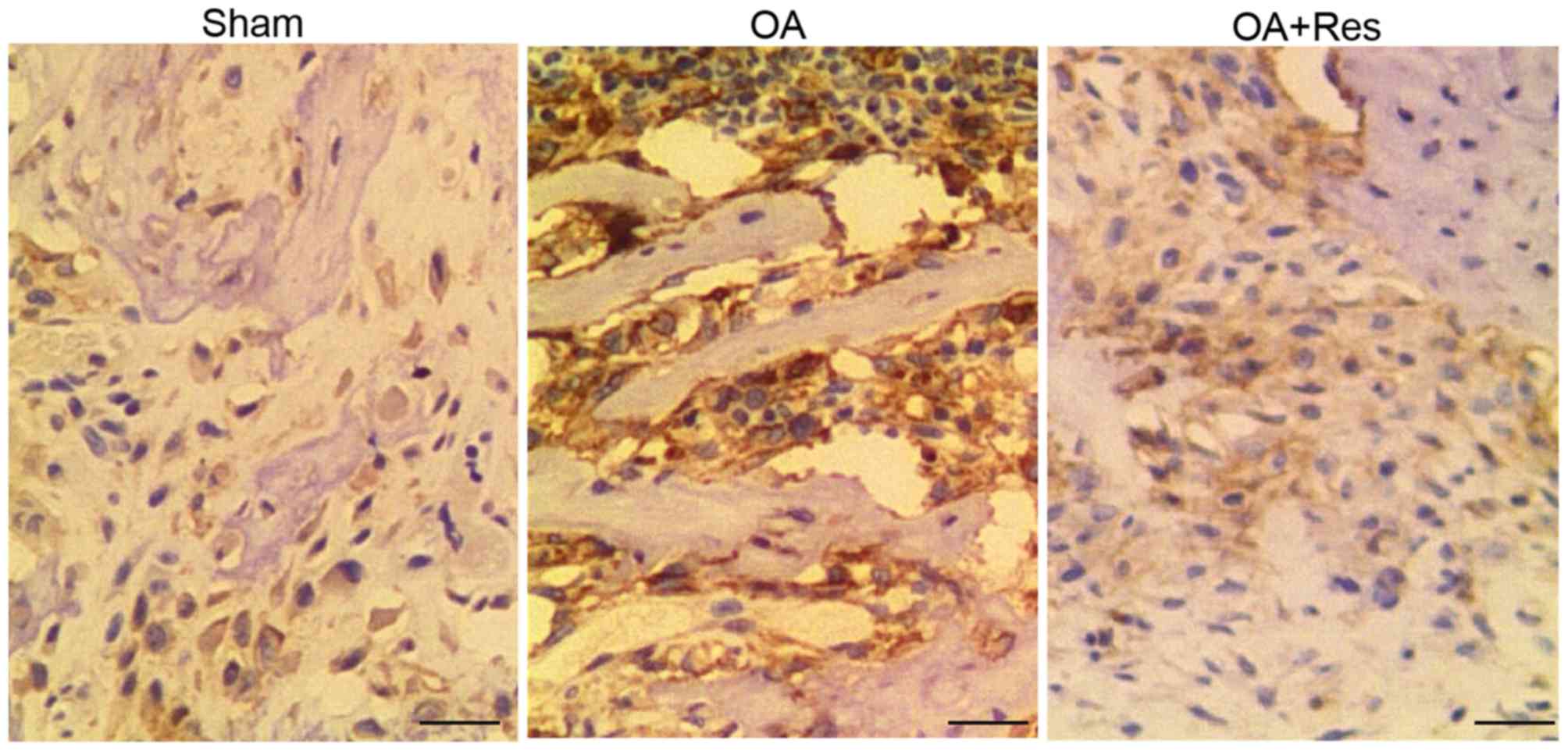
Immunohistochemical analysis of NF-κB1
and MMP-13 in the in vivo model of OA
IHC was performed to examine the protein levels of
NF-κB1 and MMP-13 in tissue samples from mice in the sham, OA and
OA + Res groups. As shown in Fig. 3,
strong NF-κB1 staining was observed in the OA group compared with
the sham group. Similarly, strong MMP-13 staining was observed in
the OA group compared with the sham group (Fig. 4). These results suggest that the
in vivo DMM-induced OA model increased NF-κB1 and MMP-13
expression. However, treatment with Res partially reversed the
effects of OA on NF-κB1 and MMP-13 expression.
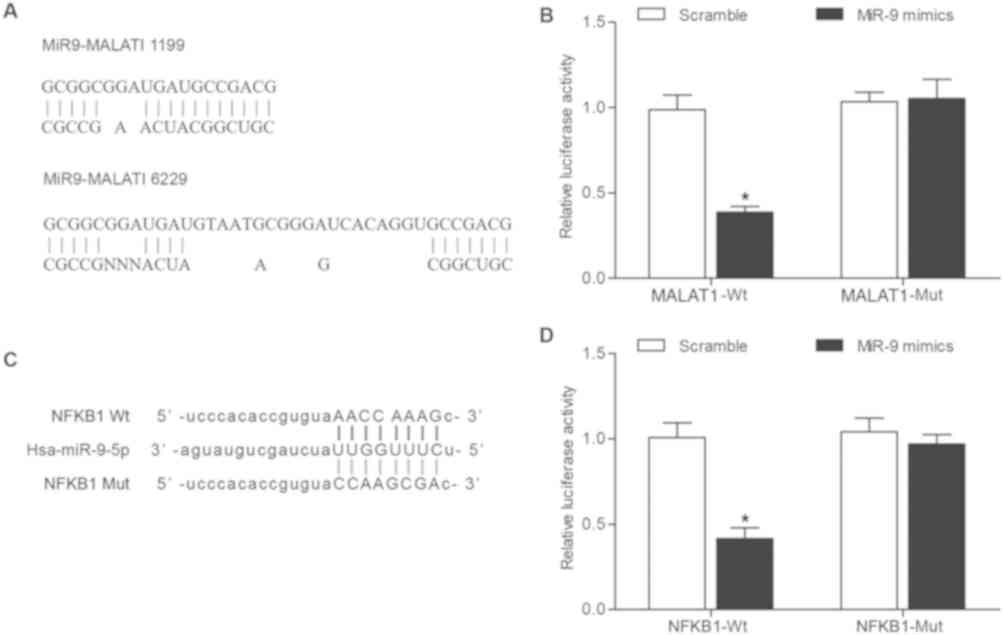
MALAT1 directly regulates miR-9 and
miR-9 directly targets NF-κB1
The 3′UTR of MALAT1 was revealed to contain a
putative binding site for miR-9 (Fig.
5A) via computational analysis using the online microRNA
database (www.mirdb.org). The dual-luciferase
reporter gene assay was performed to confirm the interaction
between MALAT1 and miR-9 in chondrocytes. Following co-transfection
with miR-9 mimic, the dual-luciferase reporter gene assay revealed
that miR-9 significantly decreased the luciferase activity of
wild-type MALAT1 compared with mutant MALAT1 (Fig. 5B). In addition, co-transfection with
scramble control had no effect on the luciferase activity of
wild-type or mutant MALAT1. To further investigate the role of
miR-9 in OA, potential target genes of miR-9 were examined.
Bioinformatics analysis was performed using the online microRNA
database (www.mirdb.org) to identify NF-κB1 as a
putative target gene of miR-9 (Fig.
5C). Following co-transfection with miR-9 mimic, the
dual-luciferase reporter gene assay revealed that miR-9
significantly decreased the luciferase activity of wild-type 3′UTR
NF-κB1 compared with mutant NF-κB1 (Fig.
5D), while co-transfection with scramble control had no effect
on the luciferase activity of wild-type or mutant NF-κB1. Taken
together, these results suggest MALAT1 directly regulated miR-9,
and NF-κB1 was confirmed as a target gene of miR-9.
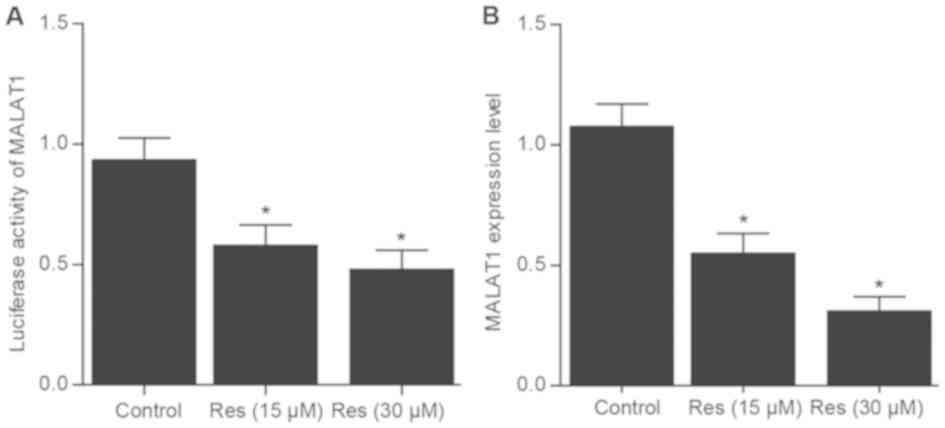
Res influences the transcriptional
activity of the MALAT1 promoter
To further explore the underlying mechanism of
MALAT1 in OA, a dual-luciferase reporter construct driven by MALAT1
promoter was examined in chondrocytes following treatment with Res.
The luciferase reporter gene assay revealed that the
transcriptional activation of the MALAT1 promoter was significantly
decreased following treatment with Res in a dose-dependent manner
compared with control (Fig. 6A). In
addition, the relative mRNA expression level of MALAT1
significantly decreased following treatment with Res in a
dose-dependent manner compared with control (Fig. 6B). Taken together, these results
suggest that treatment with Res suppressed the transcriptional
activity of the MALAT1 promoter thereby inhibiting MALAT1
expression.
Res effects miR-9 and the NF-κB1
signaling pathway in chondrocytes
The expression levels of miR-9, NF-κB1, IL-6,
MMP-13, caspase-3 were detected in cells following treatment with
different doses (15 and 30 µM) of Res. The relative expression
level of miR-9 was significantly increased, whilst the mRNA
expression level of NF-κB1 was significantly decreased following
treatment with Res in a dose-dependent manner compared with control
(Fig. 7A and B). Similarly, the
protein expression levels of NF-κB1, IL-6, MMP-13 and caspase-3
were significantly decreased following treatment with Res compared
with control (Fig. 7C-G).
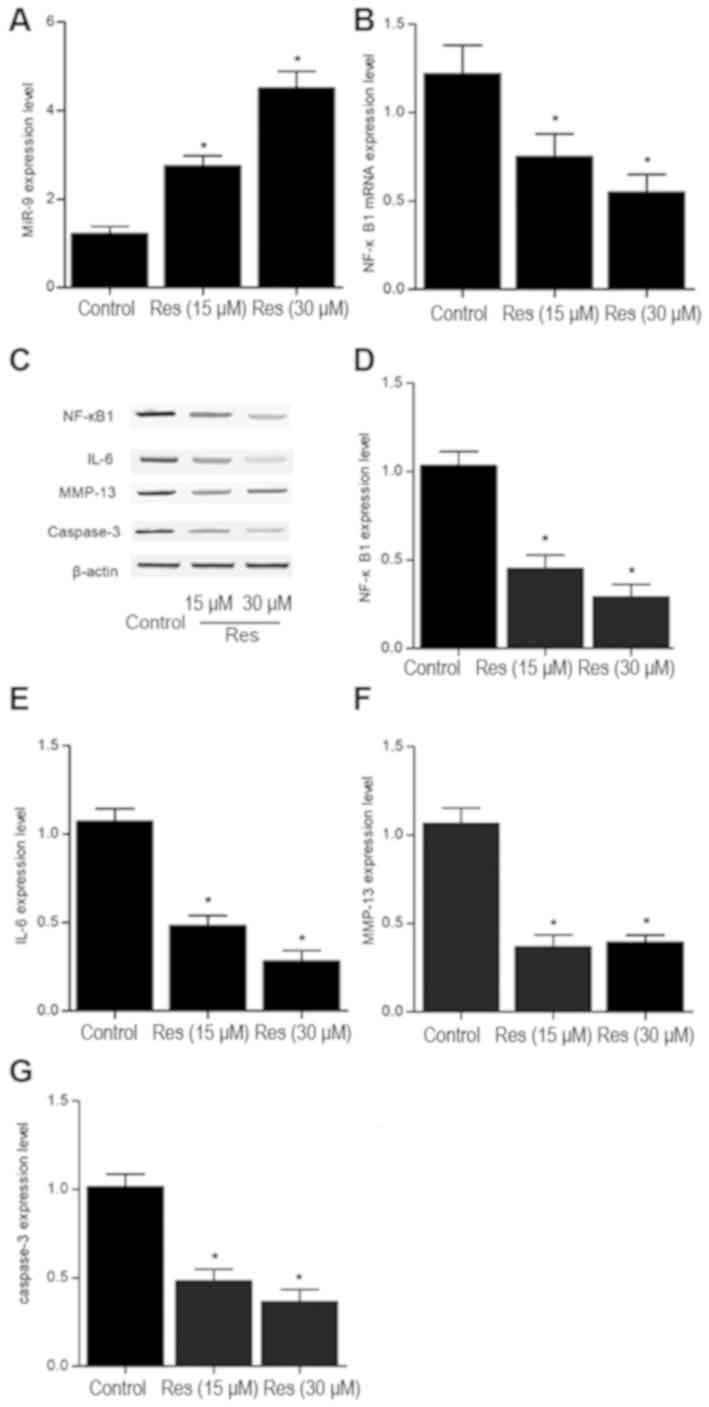 | Figure 7.Effect of Res treatment on miR-9,
NF-κB1, IL-6, MMP-13, caspase-3 expression in mouse chondrocytes.
The relative (A) miR-9 and (B) NF-κB1 mRNA expression levels were
determined by RT-qPCR in chondrocytes following treatment with 0,
15 or 30 mM Res for 48 h. (C) The relative NF-κB1, IL-6, MMP-13 and
caspase-3 protein expression levels were determined by western blot
analysis in chondrocytes following treatment with 0, 15 or 30 mM
Res for 48 h. Quantification of (D) NF-κB1, (E) IL-6, (F) MMP-13
and (G) caspase-3 protein expression. n=3. *P<0.05 vs. control
group. miR, microRNA; NF-κB1, nuclear factor kappa B subunit 1;
IL-6, interleukin-6; MMP-13, matrix metallopeptidase 13; Res,
Resveratrol. |
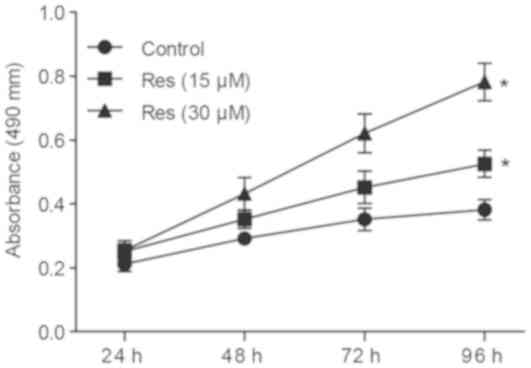
Res promotes cell proliferation in
mouse chondrocytes
Following treatment with various doses (15 and 30
µM) of Res, cell viability was determined by MTT assay. The growth
rate of chondrocytes significantly increased following treatment
with Res in a dose-dependent manner compared with control (Fig. 8). These results suggest that
treatment with Res can significantly increase chondrocyte
proliferation.
Discussion
As an abundant phytoalexin found in grape skins and
red wine, resveratrol (3,4′, 5-trihydroxystilbene) is a potent and
selective inhibitor of NF-κB activation (18–20). In
addition, Res inhibits cyclooxygenase 2 (COX-2) transcription and
activity in human mammary epithelial cells (21,22). As
a polyphenolic phytoestrogen, Res can activate sirtuin 1,
expression of which was demonstrated previously to be inhibited in
OA. In addition, Res can promote the differentiation of OA and
therefore Res may be beneficial in maintaining healthy cartilage
(23). In the current study, the
effect of Res on the transcriptional activity of the MALAT1
promoter was examined. Treatment with Res suppressed the
transcriptional activity of the MALAT1 promoter thereby inhibited
MALAT1 expression. In addition, RT-qPCR and western blot analysis
were used to determine the relative expression levels of MALAT1,
miR-9, NF-κB1, IL-6, MMP-13 and caspase-3 in vitro and in
the in vivo model of OA, as well as examining the effect of
Res. Following treatment with Res, the relative expression level of
miR-9 was significantly increased in a dose-dependent manner,
whereas the protein expression levels of NF-κB1, IL-6, MMP-13 and
caspase-3 were significantly decreased.
A previous study demonstrated that Res reduced the
expression of MALAT1 (16). To
investigate the effect of Res on MALAT1 expression in OA, a dual
luciferase reporter construct driven by MALAT1 promoter was
examined in chondrocytes following treatment with Res. The
luciferase reporter gene assay revealed that treatment with Res
significantly suppressed the transcriptional activity of the MALAT1
promoter. These results suggest that Res may directly influence the
transcription of MALAT1. In the current study, the luciferase
reporter gene assay was also used to investigate the regulatory
relationship between miR-9 and MALAT1 or NF-κB1. MALAT1 directly
regulated miR-9, and NF-κB1 was confirmed as a target gene of
miR-9.
Several studies have demonstrated the effect of Res
on NF-κB (24–26). NF-κB is a transcription factor that
mediates immune and inflammatory responses, and it is hypothesized
that Res exerts its effects by partially inhibiting NF-κB
activation (24). Res reduces IκBα
degradation and prevents the nuclear translocation of p65 NF-κB in
mast cells stimulated by PMA and A23187. It is likely that Res
inhibits the expression of COX-2, TNF-α, IL-6 and IL-8 through
NF-κB activation and IκB degradation (24). NF-κB1 regulates the transcription of
genes which function in cell adhesion, proliferation and
differentiation as well as the immune response, apoptosis and
angiogenesis (25). NF-κB1 encodes
both p50 and p105 proteins (26).
p105 is a cytoplasmic protein that does not bind to DNA directly.
However, p50 can directly bind to DNA and is subunit is derived
from the N-terminus of the precursor protein, p105 (26). In the current study, DMM was used to
establish an in vivo model of OA, and Res was used to treat
OA. The summed score and the relative MALAT1, miR-9 and NF-κB1
expression levels were examined in tissue samples from mice in the
sham, OA and OA + Res groups. The relative mRNA expression levels
of MALAT1 and NF-κB1 were significantly increased, while miR-9
expression was significantly decreased in the OA group compared
with the sham group. However, treatment with Res partially reversed
the effects of OA on the mRNA expression levels of MALAT1, miR-9
and NF-κB1. In addition, western blot analysis demonstrated that
the relative protein expression levels of NF-κB1, IL-6, MMP-13 and
caspase-3 were significantly increased in the OA and OA + Res
groups compared with the sham group although treatment with Res
partially reversed the effects of OA on protein expression.
Furthermore, NF-κB1 and MMP-13 expression was examined by IHC in
samples from mice in the sham, OA, OA + Res groups. NF-κB1 and
MMP-13 were highly expressed in the OA and OA + Res groups compared
with sham group, however treatment with Res partially reversed the
effects of OA on NF-κB1 and MMP-13 expression.
The interactions between miR-9 and NF-κB were
hypothesized to suppress apoptosis during chondrogenesis (27), as cells transfected with miR-9
inhibitors were associated with a higher level of caspase-3
activity and an increased rate of apoptosis, whereas cells
transfected with miR-9 mimics were associated with a lower level of
caspase-3 activity and a decreased rate of apoptosis (28). Different cell types and distinct
intercellular environments may cause differences observed. In
particular, a previous study reported low miR-9 expression in the
knee tissues of patients with OA which may be related with the
enhanced level of apoptosis in chondrocytes (27). In addition, miR-9 can negatively
regulate the expression of NFkB1, thus suggesting that a reduction
in miR-9 expression may increase the expression of NF-κB and
therefore inhibit cell proliferation (29,30–32)
In conclusion, treatment with Res downregulates
MALAT1 expression, and MALAT1 may function as a molecular sponge of
miR-9. In addition, miR-9 can directly target NF-κB, and as an
inflammatory cytokine NF-κB can induce apoptosis in chondrocytes
contributing to the development of OA. In the current study, DMM
was used to establish an in vivo model of OA, which was
treated with Res and the effect of Res was examined in vitro
and in the in vivo model of OA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data that support the findings of the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
GZ and ZG planned the study, HZ and WY collected the
literature, GZ, HZ, WY, and ZG collected the data, XT, XL and ZG
analyzed the data, GZ and ZG prepared the manuscript and all the
other co-authors approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Institutional
Ethics Committee on Animal Research at Nanjing University of
Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sasaki H, Takayama K, Matsushita T, Ishida
K, Kubo S, Matsumoto T, Fujita N, Oka S, Kurosaka M and Kuroda R:
Autophagy modulates osteoarthritis-related gene expression in human
chondrocytes. Arthritis Rheum. 64:1920–1928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goggs R, Carter SD, Schulze-Tanzil G,
Shakibaei M and Mobasheri A: Apoptosis and the loss of chondrocyte
survival signals contribute to articular cartilage degradation in
osteoarthritis. Vet J. 166:140–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Csaki C, Keshishzadeh N, Fischer K and
Shakibaei M: Regulation of inflammation signalling by resveratrol
in human chondrocytes in vitro. Biochem Pharmacol. 75:677–687.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Csaki C, Mobasheri A and Shakibaei M:
Synergistic chondroprotective effects of curcumin and resveratrol
in human articular chondrocytes: Inhibition of IL-1beta-induced
NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther.
11:R1652009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shakibaei M, Csaki C, Nebrich S and
Mobasheri A: Resveratrol suppresses interleukin-1beta-induced
inflammatory signaling and apoptosis in human articular
chondrocytes: Potential for use as a novel nutraceutical for the
treatment of osteoarthritis. Biochem Pharmacol. 76:1426–1439. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
St Laurent G, Wahlestedt C and Kapranov P:
The Landscape of long noncoding RNA classification. Trends Genet.
31:239–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ji P, Diederichs S, Wang W, Boing S,
Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, et
al: MALAT-1, a novel noncoding RNA, and thymosin beta4 predict
metastasis and survival in early-stage non-small cell lung cancer.
Oncogene. 22:8031–8041. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holoch D and Moazed D: RNA-mediated
epigenetic regulation of gene expression. Nat Rev Genet. 16:71–84.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoon JH, Abdelmohsen K and Gorospe M:
Posttranscriptional gene regulation by long noncoding RNA. J Mol
Biol. 425:3723–3730. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xing D, Liang JQ, Li Y, Lu J, Jia HB, Xu
LY and Ma XL: Identification of long noncoding RNA associated with
osteoarthritis in humans. Orthop Surg. 6:288–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mattick JS and Makunin IV: Small
regulatory RNAs in mammals. Hum Mol Genet. 1:R121–R132. 2005.
View Article : Google Scholar
|
|
15
|
Rodriguez A, Griffiths-Jones S, Ashurst JL
and Bradley A: Identification of mammalian microRNA host genes and
transcription units. Genome Res. 14:1902–1910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Devor EJ: Primate microRNAs miR-220 and
miR-492 lie within processed pseudogenes. J Hered. 97:186–190.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L,
Sun J, Cai J, Qin J, Ren J and Li Q: Resveratrol inhibits invasion
and metastasis of colorectal cancer cells via MALAT1 mediated
Wnt/β-catenin signal pathway. PLoS One. 8:e787002013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu R, Liu N, Luo S, Huang W, Zha Z and
Yang J: MicroRNA-9 regulates the development of knee osteoarthritis
through the NF-kappaB1 pathway in chondrocytes. Medicine
(Baltimore). 95:e43152016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu L, Polur I, Servais JM, Hsieh S, Lee
PL, Goldring MB and Li Y: Intact pericellular matrix of articular
cartilage is required for unactivated discoidin domain receptor 2
in the mouse model. Am J Pathol. 179:1338–1346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Manna SK, Mukhopadhyay A and Aggarwal BB:
Resveratrol suppresses TNF-induced activation of nuclear
transcription factors NF-kappa B, activator protein-1, and
apoptosis: Potential role of reactive oxygen intermediates and
lipid peroxidation. J Immunol. 164:6509–6519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Estrov Z, Shishodia S, Faderl S, Harris D,
Van Q, Kantarjian HM, Talpaz M and Aggarwal BB: Resveratrol blocks
interleukin-1beta-induced activation of the nuclear transcription
factor NF-kappaB, inhibits proliferation, causes S-phase arrest,
and induces apoptosis of acute myeloid leukemia cells. Blood.
102:987–995. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holmes-McNary M and Baldwin AS Jr:
Chemopreventive properties of trans-resveratrol are associated with
inhibition of activation of the IkappaB kinase. Cancer Res.
60:3477–3483. 2000.PubMed/NCBI
|
|
24
|
Subbaramaiah K, Chung WJ, Michaluart P,
Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM and Dannenberg AJ:
Resveratrol inhibits cyclooxygenase-2 transcription and activity in
phorbol ester-treated human mammary epithelial cells. J Biol Chem.
273:21875–21882. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Surh YJ, Chun KS, Cha HH, Han SS, Keum YS,
Park KK and Lee SS: Molecular mechanisms underlying chemopreventive
activities of anti-inflammatory phytochemicals: Down-regulation of
COX-2 and iNOS through suppression of NF-kappa B activation. Mutat
Res. 480-481:243–268. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shan T, Wang Y, Wu T, Liu C, Guo J, Zhang
Y, Liu J and Xu Z: Porcine sirtuin 1 gene clone, expression
pattern, and regulation by resveratrol. J Anim Sci. 87:895–904.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Salemi M, Barone C, Romano C, Scillato F,
Ragalmuto A, Caniglia S, Salluzzo MG, Sciuto G, Ridolfo F and Bosco
P: NF-κB1 gene expression in Down syndrome patients. Neurol Sci.
36:1065–1066. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song J, Kim D, Chun CH and Jin EJ:
MicroRNA-9 regulates survival of chondroblasts and cartilage
integrity by targeting protogenin. Cell Commun Signal. 11:662013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tomek M, Akiyama T and Dass CR: Role of
Bcl-2 in tumour cell survival and implications for pharmacotherapy.
J Pharm Pharmacol. 64:1695–1702. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu N, Sun Q, Chen J, Li J, Zeng Y, Zhai
S, Li P, Wang B and Wang X: MicroRNA-9 suppresses uveal melanoma
cell migration and invasion through the NF-κB1 pathway. Oncol Rep.
28:961–968. 2012.PubMed/NCBI
|
|
31
|
Guo LM, Pu Y, Han Z, Liu T, Li YX, Liu M,
Li X and Tang H: MicroRNA-9 inhibits ovarian cancer cell growth
through regulation of NF-kappaB1. FEBS J. 276:5537–5546. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baltaci SB, Mogulkoc R and Baltaci AK:
Resveratrol and exercise. Biomed Rep. 5:525–530. 2016. View Article : Google Scholar : PubMed/NCBI
|