Introduction
Inflammation is an important adaptive mechanism of
the host immune response, and typically occurs at the site of
infection. Inflammation is caused by cell injury and toxin exposure
and is associated with a number of pathological conditions
(1,2). Inflammation plays a vital role in the
body's defense system via multiple mediators that allow cells to
recover from damage and to regulate tissue homeostasis (3,4).
Excessive and continuous inflammation can cause severe tissue
damage that can lead to a series of disordered states, including
hepatitis, pneumonia, atherosclerosis, septic shock and rheumatoid
arthritis (5–7). The normal inflammatory response is
self-limiting and is maintained by the downregulation of
pro-inflammatory proteins and the upregulation of anti-inflammatory
proteins (4). Therefore, targeting
dysregulated inflammatory processes and regulating inflammatory
mediators are regarded to be effective therapeutic approaches to
managing inflammatory disorders (8,9).
Lipopolysaccharide (LPS) is one of the most
effective activators of macrophages (10). LPS induces the production of
inflammatory cytokines and mediators such as tumor necrosis factor
alpha (TNF-α), interleukin-1 (IL-1), IL-2 and IL-18, resulting in
the induction of inflammatory genes like TNF-α, CXCL10, IL-2B and
IL-10 via the activation of specific signaling cascades, at the
transcription level (4,11). The imbalance and build-up of
oxidative stress in the cellular environment in disease conditions,
including inflammation, stroke, cancer and diabetes, often
increases the generation of reactive oxygen species (12–14).
LPS-stimulated macrophages induce the production of large amounts
of inflammatory mediators, including nitric oxide (NO) and
prostaglandin E2 (PGE2). NO and
PGE2 are secreted in inducible isoforms, iNOS and
cyclooxygenase-2 (COX2), during the inflammatory response (15,16).
The nuclear transcription factor NF-κB is an
important regulator that initiates inflammation (17). NF-κB is activated by a series of
intracellular signals involving inhibitor of κB kinase (IKK)α/β
proteins and its phosphorylation (18). The binding of LPS to the toll-like
receptors (TLR) of activated macrophages leads to the
phosphorylation and activation of IKK proteins, which allows the
translocation of NF-κB into the cell nucleus, where
pro-inflammatory cytokines are transcribed (19). A reduction in NF-κB signaling pathway
activity and the expression of its related proteins have been shown
to alleviate inflammatory responses (20). Thus, the NF-κB signaling pathway is a
crucial target in the treatment of inflammatory diseases.
Artemisia iwayomogi, commonly known as
Dowijigi (DJ), is a perennial herb that belongs to the composite
asteraceae family, primarily found in Korea (21). A. iwayomogi exerts
various biological effects such as anti-oxidation, anti-inhibition
and anti-inflammation, and has been widely used in the treatment of
various diseases, including hepatitis, inflammation, cholecystitis
and immune-related disorders (22).
Methanolic extracts of A. iwayomogi have been shown to
exhibit scavenging activity in a number of diseases involving
inflammation, and to inhibit NO production by LPS-activated
macrophages (23). However, the
mechanism underlying the anti-inflammatory effect of DJ remains
largely unknown. The anti-inflammatory activities of the
polyphenolic extract of Dowijigi (PDJ) can be assessed by measuring
their potential to inhibit the production of inflammatory
intermediates (24). Since
macrophages play an important role in inflammation and immune
defense responses, RAW264.7 mouse macrophage cells are one of the
most commonly used cell line models to evaluate the
anti-inflammatory effect of drugs in vitro (25). In the present study, the inhibitory
effect of PDJ on inflammatory mediators in LPS-stimulated RAW264.7
cells and the underlying mechanism of action were investigated.
Materials and methods
Plant material
A. iwayomogi plants were collected in May
2018 from Namhae Island. The plant samples were authenticated under
the Korea Animal Bioresource Research Bank (plant registration no.
00754C). The voucher specimens were deposited at the herbarium of
the Research Institute of Life Science. The flowers were separated
from the plants and were washed with water, lyophilized and stored
at −20°C prior to extraction.
Preparation of the PDJ
The lyophilized flowers were weighed (100 g) and
refluxed in 70% methanol (2 liters) at 60°C for 20 h. The extracted
mixture was filtered through a Büchner funnel and concentrated to
~300 ml at 35°C at a variable pressure, using a rotary evaporator.
To remove non-polar impurities from the concentrated filtrate, the
filtrate was washed with n-hexane (300 ml) three times.
Furthermore, the filtrate was extracted using ethyl acetate (100
ml) three times and dried over anhydrous magnesium sulfate. The
solvent was removed from the rotatory evaporator. The resulting
sticky residue was placed on the top of a silica gel sorbent
(40×2.5 cm) and eluted with ethyl acetate to eliminate highly polar
impurities. The solvent was then removed to yield solids of
polyphenol mixture (1.74 g; 1.74% of the lyophilized plants). The
mixture was stored at −20°C until analysis.
High-performance liquid
chromatography-tandem mass spectrometry (HPLC-MS/MS) analysis
HPLC analysis was conducted using a 1260 series HPLC
system (Agilent Technologies, Inc.) with a multiple wavelength
detector set at 254, 280, 320 or 360 nm. A Prontosil C18 column
(length, 250 mm; inner diameter, 4.6 mm; particle size, 5 µm;
Phenomenex Co., Ltd.); Bischoff Chromatography) set at 30°C was
used. The binary mobile phase system consisted of 0.1% formic acid
in water (solvent A) and 0.1% formic acid in acetonitrile (solvent
B). The gradient conditions were 0–10 min at 10% B, 10–60 min at
10–40% B, 60–70 min at 40–50% B, 70–80 min at 50–10% B and 80–90
min at 10% B. The flow rate was maintained at 1 ml/min and a sample
injection volume of 10 µl was used in each experiment. The
electrospray ionization MS/MS analysis was conducted using a 3200
QTrap LC/MS/MS system (Applied Biosystems, Fortser, CA, USA)
operated in negative ion mode (spray voltage set at −4.5 kV) and
nitrogen at a pressure of 45 psi was used as nebulizing agent and
drying gas was supplied. The mass spectra were recorded in the
range of m/z 100–1000. The obtained data were analyzed using
BioAnalystTM software (version 1.4.2; SCIEX).
Cell culture
Mouse RAW264.7 macrophage cells were obtained from
the American Type Culture Collection and cultured in complete DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10%
heat-inactivated fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and supplemented with 100 U/ml penicillin and 100
µg/ml streptomycin (Thermo Fisher Scientific, Inc.). The cells were
incubated at 37°C in a humidified atmosphere containing 5%
CO2.
Cell viability assay
RAW264.7 cells were seeded at a density of
1×104 cells per well in 96 well plate and then cultured
with or without LPS (1 µg/ml; Sigma-Aldrich; Merck KGaA)
pre-treatment at 37°C for 1 h, followed by treatment with various
concentration of PDJ (0.5, 1, 2.5, 5 and 10 µg/ml) at 37°C for 24
h. After incubation, MTT solution (10 µl; 5 mg/ml) was added to the
plate and the cells were incubated at 37°C for ~4 h. Then, the
culture media was washed off completely and the insoluble formazan
crystals formed were dissolved in DMSO. The absorbance was measured
at a wavelength of 590 nm using a PowerWave HT microplate
spectrophotometer (BioTek Instruments, Inc.).
Griess assay for NO detection
NO production in the cell cultures was measured
using the Promega Griess Reagent system (Promega Corporation)
according to the manufacturer's instructions. Briefly, RAW264.7
cells were cultured at a density of 1×104 cells per well
in 96-well plates with or without LPS pre-treatment at 37°C for 1
h. Subsequently, cells were treated with either 2.5 or 5 µg/ml PDJ
and incubated at 37°C for 24 h. After incubation, the media in each
group was collected and mixed with 50 µl sulfanilamide solution and
50 µl Griess reagent for 10 min at room temperature, protected from
light. The nitrate concentration was measured at a wavelength of
520 nm. Sodium nitrite was used to generate the standard curve and
NO production in the culture medium was estimated from the
NO2 concentration.
ELISA
RAW264.7 cells were seeded at a density of
5×104 per well in 48-well plates. The cells were
pre-treated with 1 µg/ml LPS at 37°C for 1 h and then incubated
with 2.5 or 5 µg/ml of PDJ for 24 h at 37°C. Levels of IL-1β, IL-6
and TNFα were quantified using the mouse IL-1β (cat. no.
ADI-900-132A; Enzo Life Sciences, Inc.), IL-6 (cat. no.
ADI-900-045; Enzo Life Sciences, Inc.) and TNFα (cat. no.
ADI-900-047; Enzo Life Sciences, Inc.) ELISA kits, respectively,
according to the manufacturer's instructions.
ELISA for measuring PGE2
levels
PGE2 levels in the cells were analyzed
using a PGE2 assay kit (cat. no. ADI-900-001; Enzo Life
Sciences, Inc.), according to manufacturer's protocol. RAW264.7
cells were cultured at a density of 1×104 cells per well
in 96-well plates with or without LPS pre-treatment at 37°C for 1
h. Cells were subsequently treated with either 2.5 or 5 µg/ml PDJ
and incubated at 37°C for 24 h. After PDJ treatment, 100 µl
standard diluent was added to the ELISA plate, followed by 100 µl
sample and ~50 µl assay buffer. Then, 50 µl PGE2
conjugate was added to the plate, followed by 50 µl PGE2
antibody. No antibody was added to the blank wells. The plates were
incubated for 2 h on a shaker at room temperature. After 2 h, the
contents of the wells were emptied and the wells were washed three
times with 400 µl of 1X wash solution from the PGE2
assay kit. After the final wash, media were aspirated from the
wells and 5 µl conjugate solution was added. Subsequently, 200 µl
p-nitrophenyl phosphate substrate solution was added to the wells
followed by incubation at room temperature for ~45 min without
shaking. The reaction was stopped by the addition of 50 µl stop
solution to the wells and the absorbance was measured at a
wavelength of 405 nm.
Determination of protein expression by
western blot analysis
For western blot analysis, RAW264.7 cells were
seeded into 6-well plates at a density of 6×105
cells/well and treated with 2.5 or 5 µg/ml PDJ for 24 h at 37°C.
Then, the cells were lysed in ice-cold RIPA buffer [50 mM Tris-HCl
(pH 8.0), 0.5% sodium deoxycholate, 1 mM EDTA, 150 mM NaCl, 0.1 SDS
and 1% NP-40]. Protein concentrations were determined using the
Pierce™ bicinchoninic acid protein assay kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Equal amounts of protein (~10 µg) were separated on via SDS-PAGE on
10% gels and transferred onto PVDF membranes using the TE 77
Semi-Dry Transfer Unit (GE Healthcare Life Sciences). The blots
were then blocked with 5% skimmed milk and 5% bovine serum albumin
(BSA; Thermo Fisher Scientific, Inc.) for 1 h at room temperature.
Membranes were further incubated with 1:1,000 dilutions of primary
antibodies overnight at 4°C. Primary antibodies of COX-2 (cat. no.
12282S; 1:1,000), iNOS (cat. no. 13120S; 1:1,000), p65 (cat. no.
8242S; 1:1,000), p-p65 (cat. no. Ser536; 3033S; 1:1,000), IκBα
(cat. no. 4812S; 1:1,000), p-IκBα (Ser32; cat. no. 2859S; 1:1,000),
JNK (cat. no. 9258S, 1:1,000), p-JNK1/2 (Thr183/Tyr185; cat. no.
4671S, 1:1,000), p38 (cat. no. 8690S; 1:1,000), p-p38
(Thr180/Tyr182; cat. no. 9216S, 1:1,000), ERK1/2 (cat. no. 4695S;
1:1,000), p-ERK1/2 (Thr202/Tyr204; cat. no. 4970S, 1:1,000), and
β-actin (cat. no. 3700S, 1:10,000) were purchased from Cell
Signaling Technology, Inc. The membranes were washed three times
for 10 min with TBST and then probed with the appropriate
horseradish peroxidase-conjugated secondary antibody (anti-rabbit
and anti-mouse, A120-101P and A90-116P, respectively, Bethyl
Laboratory, Inc.) for 3 h at room temperature. The blots were
visualized using Clarity™ ECL substrate reagent (Bio-Rad
Laboratories, Inc.) and quantified by densitometry using ImageJ
software (National Institutes of Health) with β-actin as the
loading control. The experiment was performed in triplicate.
Determination of mRNA expression by
reverse transcription-quantitative PCR (RT-qPCR)
To determine the mRNA expression levels of related
proteins, RAW264.7 cells were seeded into 6-well plates at a
density of 5×105 cells/well and treated with LPS at 37°C
for 1 h, followed by co-treatment with 2.5 or 5 µg/ml PDJ for 24 h
at 37°C. The total RNA content was isolated using
Trizol® reagent (Thermo Fisher Scientific, Inc.) and the
concentration of RNA was measured using a spectrophotometer. Total
RNA (1 µg) was converted to cDNA using the iScript™ cDNA synthesis
kit (Bio-Rad Laboratories, Inc.) and qPCR was performed with
AccuPower® 2X Greenstar™ qPCR Mastermix (Bioneer
Corporation) and a CFX384 Real Time PCR Detection system (Bio-Rad
Laboratories, Inc.) according to each manufacturer's protocol,
respectively. The qPCR primers used were as follows: TNFα sense,
5′-TGGAGTCATTGCTCTGTGAAGGGA-3′ and antisense,
5′-AGTCCTTGATGGTGGTGCATGAGA-3′; IL-6 sense,
5′-GAGGATACCACTCCCAACAGACC-3′ and antisense,
5′-AAGTGCATCATCGTTGTTCATACA-3′; IL-1β sense,
5′-TGCAGAGTTCCCCAACTGGTACATC-3′ and antisense,
5′-GTGCTGCCTAATGTCCCCTTGAATC-3′; iNOS sense,
5′-TCCTACACCACACCAAAC-3′ and antisense, 5′-CTCCAATCTCTGCCTATC-3′;
COX2 sense, 5′-CCTCTGCGATGCTCTTCC-3′ and antisense,
5′-TCACACTTATACTGGTCAAATCC-3′; and β-actin sense,
5′-TACTGCCCTGGCTCCTAGCA-3′ and antisense,
5′-TGGACAGTGAGGCCAGGATAG-3′. The thermocycling conditions were as
follows: Pre-denaturation for 2 min at 95°C, followed by 40 cycles
at 95°C for 5 sec, 58°C for 30 sec and 95°C for 5 sec. All the data
were analyzed using Bio-Rad CFX Manager Version 3.1 software.
Relative quantification was measured using the 2−ΔΔCq
method (26). The mRNA expression
levels were normalized against β-actin.
Statistical analysis
The data are expressed as the mean ± SEM. Data were
analyzed using GraphPad Prism software (version 5.02; GraphPad
Software, Inc.). Statistically significant differences were
calculated using one-way ANOVA followed by Bonferroni's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Separation and quantification of
PDJ
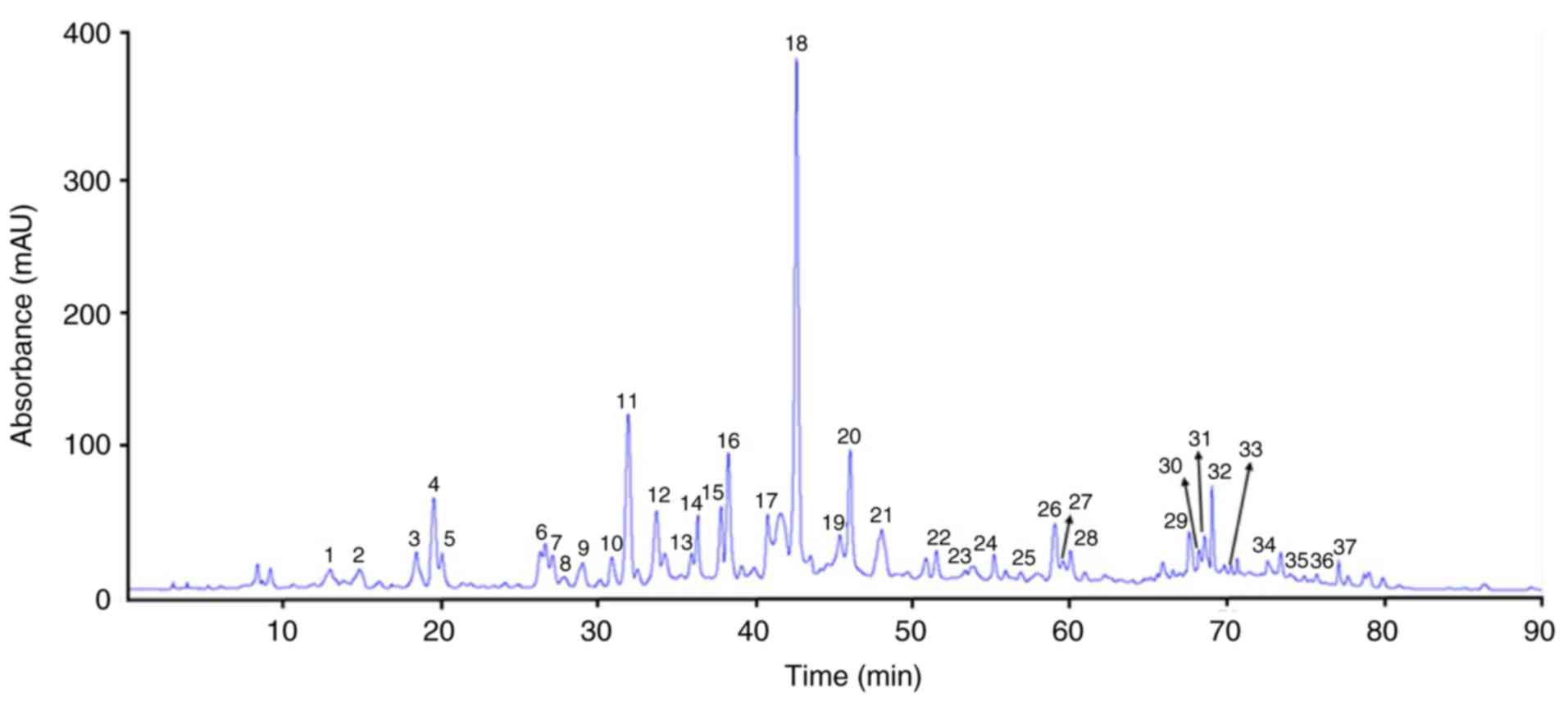
PDJ was separated and quantified using HPLC-MS/MS. A
total of 37 peaks were identified based on the HPLC retention times
and the UV-vis spectra (Fig. 1). The
phenolic compounds and flavonoids were identified according to the
peaks obtained in the HPLC chromatogram and the description of
their mass spectrometry quantification data based on the reference
compounds from published sources are provided in Table I.
 | Table I.MS/MS data of the polyphenols in
Artemisia iwayonogi (Dowijigi). |
Table I.
MS/MS data of the polyphenols in
Artemisia iwayonogi (Dowijigi).
| Peak no. | Compound | Retention time
(min) | UV max, nm |
[M-H]− | MS/MS | (Refs.) |
|---|
| 1 | Protocatechuic
aldehyde | 12.866 | 278, 310 | 137 | 109 | (40) |
| 2 | Gallocatechin | 14.713 | 272 | 305 | 261, 221, 219,
179 | (41) |
| 3 | Methyl
catechin | 18.337 | 272 | 305 | 137 | (42) |
| 4 | Epicatechin | 19.460 | 272 | 305 | 139 | (42) |
| 5 | Caffeic acid | 19.979 | 323 | 179 | 135 | (43) |
| 6 |
Epigallocatechin | 26.570 | 272 | 305 | 261, 221, 219,
179 | (41) |
| 7 | Catechin | 27.003 | 272 | 289 | 245, 205 | (41) |
| 8 | 5-caffeoylquinic
acid | 27.718 | 284 | 353 | 191 | (41) |
| 9 |
Epigallocatechin-3-gallate | 28.929 | 294 | 457 | 331, 305, 169 | (41) |
| 10 | 5-Feruoylquinic
acid | 30.802 | 272 | 367 | 193, 191, 173 | (44) |
| 11 |
Rhamnose-C-acetyl-hexoside apigenin | 31.828 | 344 | 619 | 499, 457, 413, 341,
311, 293, 315 | (45) |
| 12 |
Liquiritigenin-7-O-sulfate | 33.610 | 342 | 335 | 255, 135, 119 | (46) |
| 13 |
Quercetin-rutinoside | 35.853 | 254, 352 | 609 | 301 | (41) |
| 14 |
Quercetin-3-galactoside | 36.234 | 254, 370 | 463 | 301 | (47) |
| 15 |
Quercetin-3-glucoside | 37.718 | 256, 354 | 463 | 301 | (47) |
| 16 |
Malyidin-3-glucoside | 38.211 | 256, 354 | 493 | 331 | (48) |
| 17 |
3,4-di-O-Caffeoylquinic acid | 40.702 | 326, 300 | 515 | 353, 335, 191, 179,
173 | (49) |
| 18 |
3,5-di-O-Caffeoylquinic acid | 42.525 | 328, 300 | 515 | 353, 354, 191,
179 | (9) |
| 19 | Quinic acid
derivate | 45.298 | 324, 226 | 515 | 353, 335, 191, 179,
173 | (50) |
| 20 |
4,5-di-O-Caffeoylquinic acid | 45.929 | 326, 300 | 515 | 353, 335, 317, 299,
191, 179, 173 | (49) |
| 21 |
Mearnsetin-O-diglucoside | 47.971 | 330 | 655 | 535, 493, 492, 331,
329, 316, 301 | (50) |
| 22 | Quercetin
diglycoside | 51.437 | 330 | 771 | 609, 463, 608, 301,
300 | (49) |
| 23 | Quercetin
dihexoside | 53.260 | 354 | 625 | 463, 301 | (45) |
| 24 | Caffeoyl
dihexoside | 53.816 | 328 | 503 | 342, 341, 179 | (49) |
| 25 |
3-Caffeoyl-4-feruoyl-quinic acid | 56.817 | 322, 286 | 529 | 367, 353, 255, 203,
191, 179, 173 | (50) |
| 26 | Luteolin | 58.962 | 358, 254 | 285 | 133 | (51) |
| 27 | Isorhamnetin | 59.497 | 344, 268 | 315 | 300 | (51) |
| 28 |
5,7,4′,5′-Tetrahydroxy-6,3′-Dimethoxyflavone | 59.991 | 348, 270 | 345 | 330, 315, 287, 259,
136 | (44) |
| 29 | Mearnsetin-glu | 67.527 | 335, 265 | 493 | 478, 331, 330, 316,
315 | (50) |
| 30 | Gliricidin | 68.182 | 330, 272 | 299 | 284, 271, 256, 212,
175 | (52) |
| 31 | Kaempferol
methylether | 68.523 | 348, 266 | 299 | 285, 255, 227 | (45) |
| 32 |
5,7,3′-Trihydroxy-6,4′-dimetoxyflavone | 69.006 | 343, 272 | 329 | 314, 299 | (47) |
| 33 |
5,7,3′-Trihydroxy-6,4′-dimetoxyflavone | 70.197 | 343, 272 | 329 | 314, 299 | (47) |
| 34 |
5,7,3′-Trihydroxy-6,4′-dimetoxyflavone | 72.526 | 343, 272 | 329 | 314, 299 | (47) |
| 35 |
3-Hydroxy-6,7,4′-trimethoxy flavone | 74.865 | 336, 247 | 343 | 328, 327, 313,
261 | (50) |
| 36 |
Quercetagetin-tetramethyl ester | 75.648 | 354, 272 | 373 | 358, 343, 328,
313 | (47) |
| 37 | Genkwanin | 77.049 | 335, 268 | 283 | 268 | (47) |
Cytotoxic effect of PDJ in RAW264.7
cells
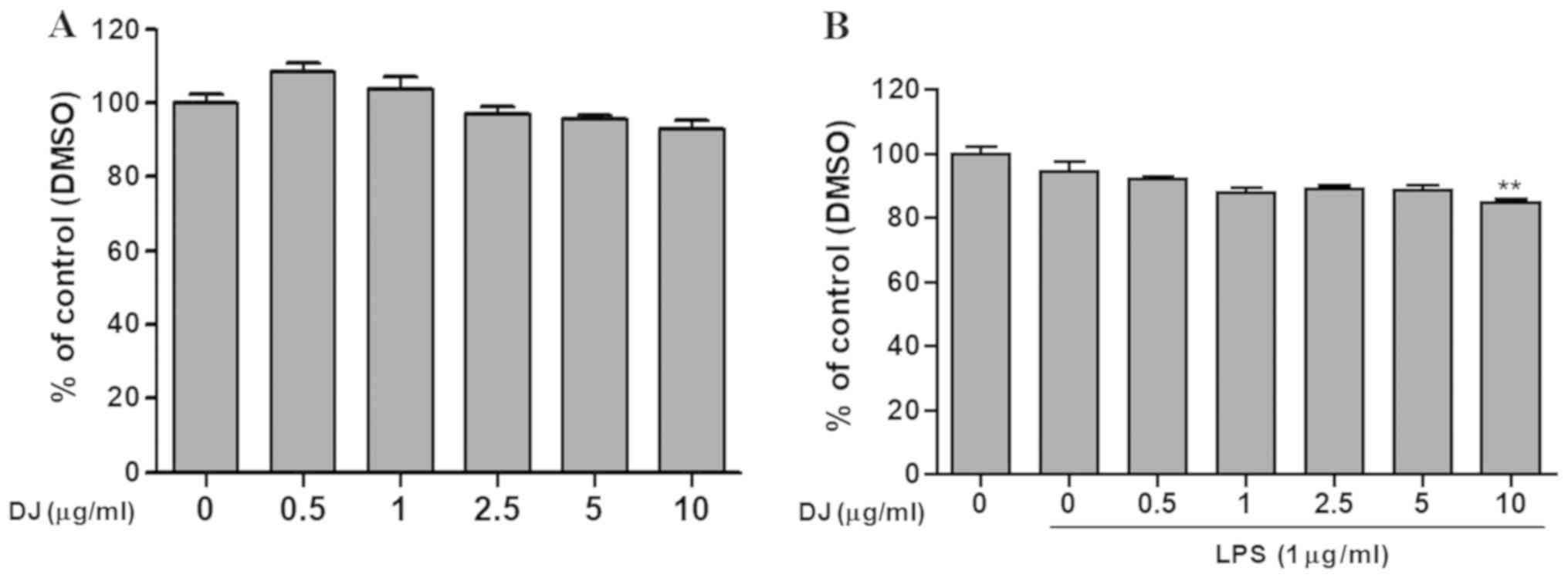
To identify the non-toxic dose of PDJ, cells were
treated with increasing concentrations of PDJ (0, 0.5, 1, 2.5, 5
and 10 µg/ml) following pre-treatment with or without LPS for 1 h.
The results suggested that concentrations of PDJ ≤10 µg/ml were
non-toxic, therefore, doses of 2.5 and 5 µg/ml were used for
subsequent experiments (Fig. 2A and
B).
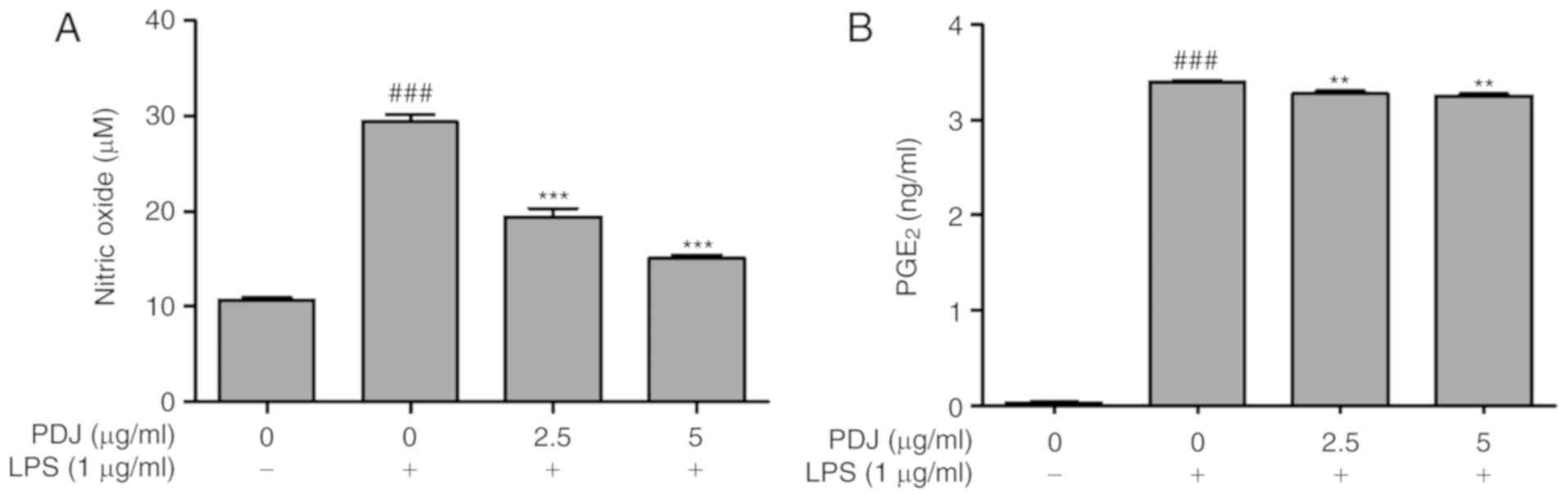
PDJ inhibits LPS-induced NO production
and PGE2
The effect of PDJ on NO production in LPS-induced
RAW264.7 cells was measured by the Griess assay. Cells were
pre-treated with LPS (1 µg/ml) for 1 h followed by treatment with
PDJ (2.5 or 5 µg/ml). Treatment with LPS induced a significant
(P<0.001) increase in NO production, which was suppressed upon
further treatment with PDJ at both concentrations (Fig. 3A). Thus, there was a decrease in the
NO accumulation in the cells co-treated with PDJ and LPS compared
with those treated with LPS alone. The level of PGE2 in
the LPS-induced RAW264.7 cells treated with PDJ was measured using
a PGE2 ELISA kit. The intensity of the bound antibody
was measured to calculate the concentration of PGE2 in
the PDJ-treated cells. The results displayed that PGE2
levels significantly (P<0.001) decreased upon treatment with PDJ
compared with LPS-only treated cells (Fig. 3B).
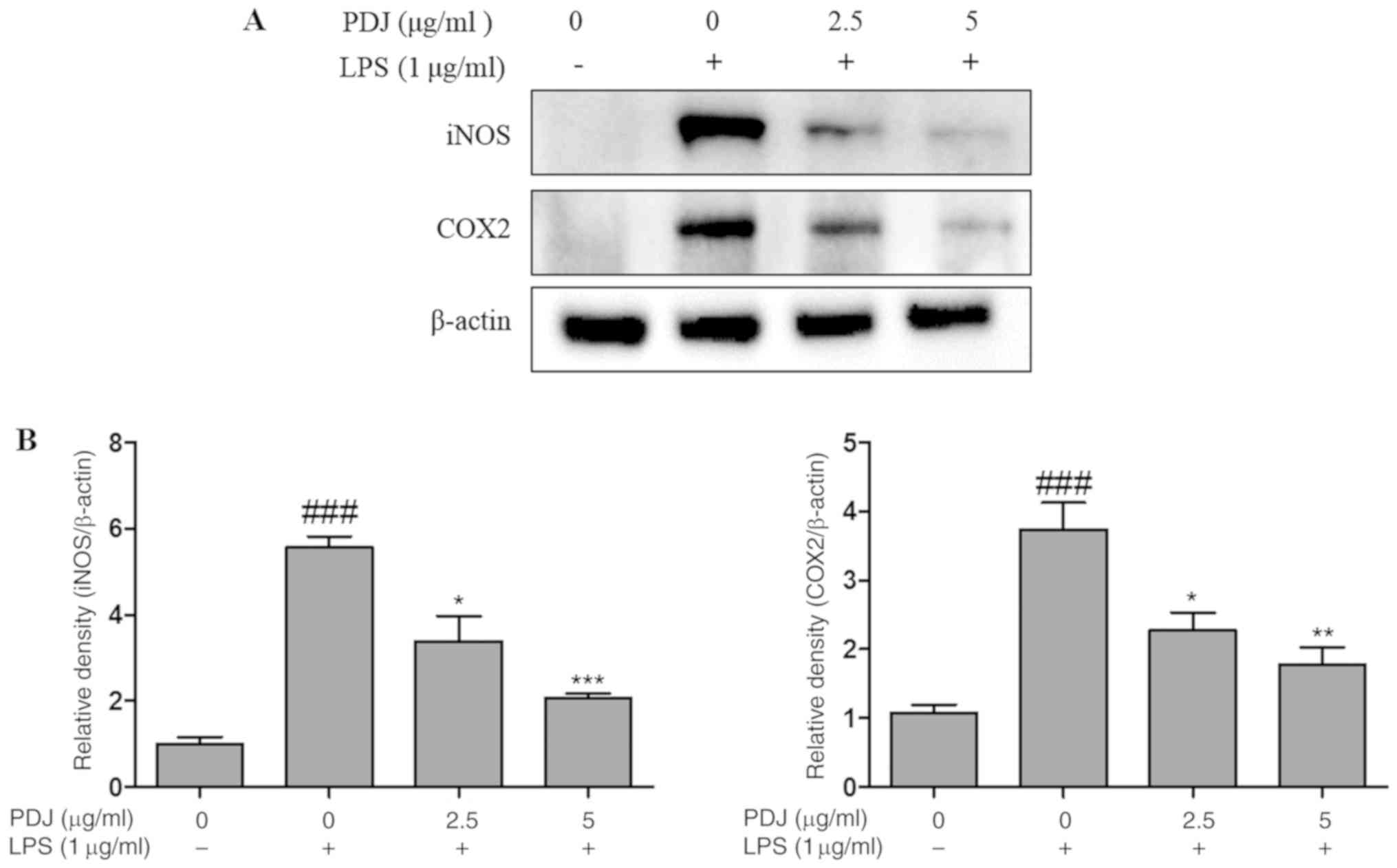
PDJ inhibits LPS-induced mRNA and
protein expression of iNOS and COX2
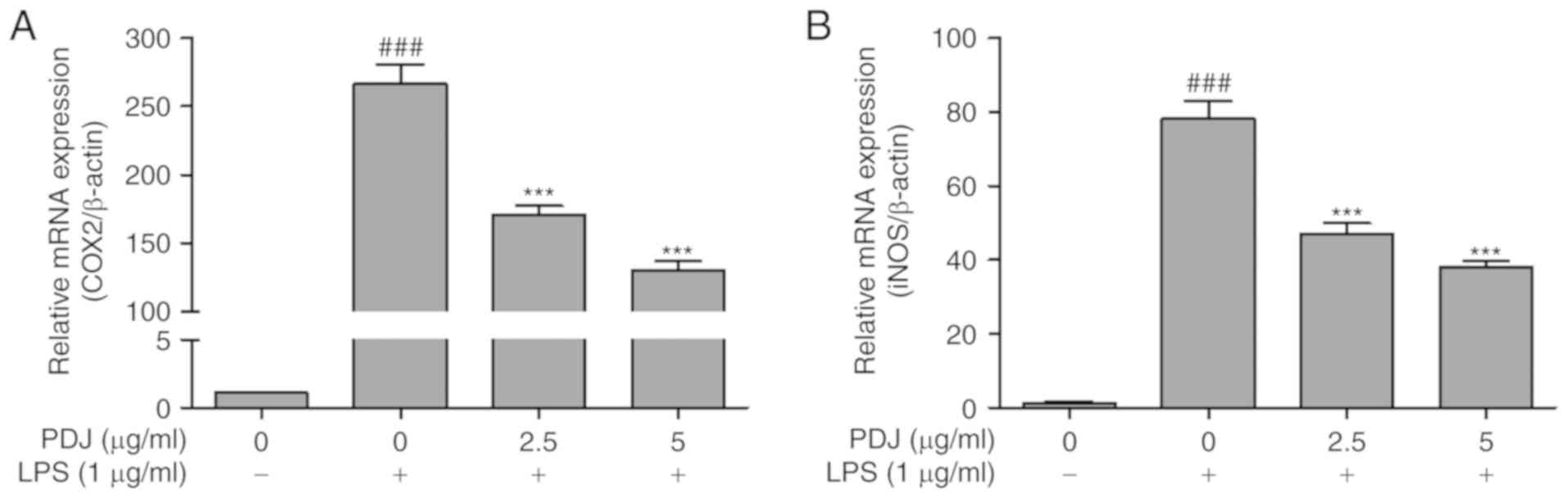
The effect of PDJ on COX2 and iNOS mRNA and protein
expression levels in LPS-induced RAW264.7 cells was evaluated by
RT-qPCR analysis and western blotting, respectively. The expression
of COX2 and iNOS in RAW264.7 cells stimulated with LPS was
significantly (P<0.001) increased compared with the non-LPS
treated control cells, at both the protein (Fig. 4A and B) and mRNA (Fig. 5A and B) level. However, the protein
and mRNA expression levels of COX2 and iNOS in the RAW264.7 cells
were significantly (P<0.001) decreased after treatment with PDJ.
The results indicated that PDJ downregulated LPS-induced COX2 and
iNOS expression at both the mRNA and protein levels.
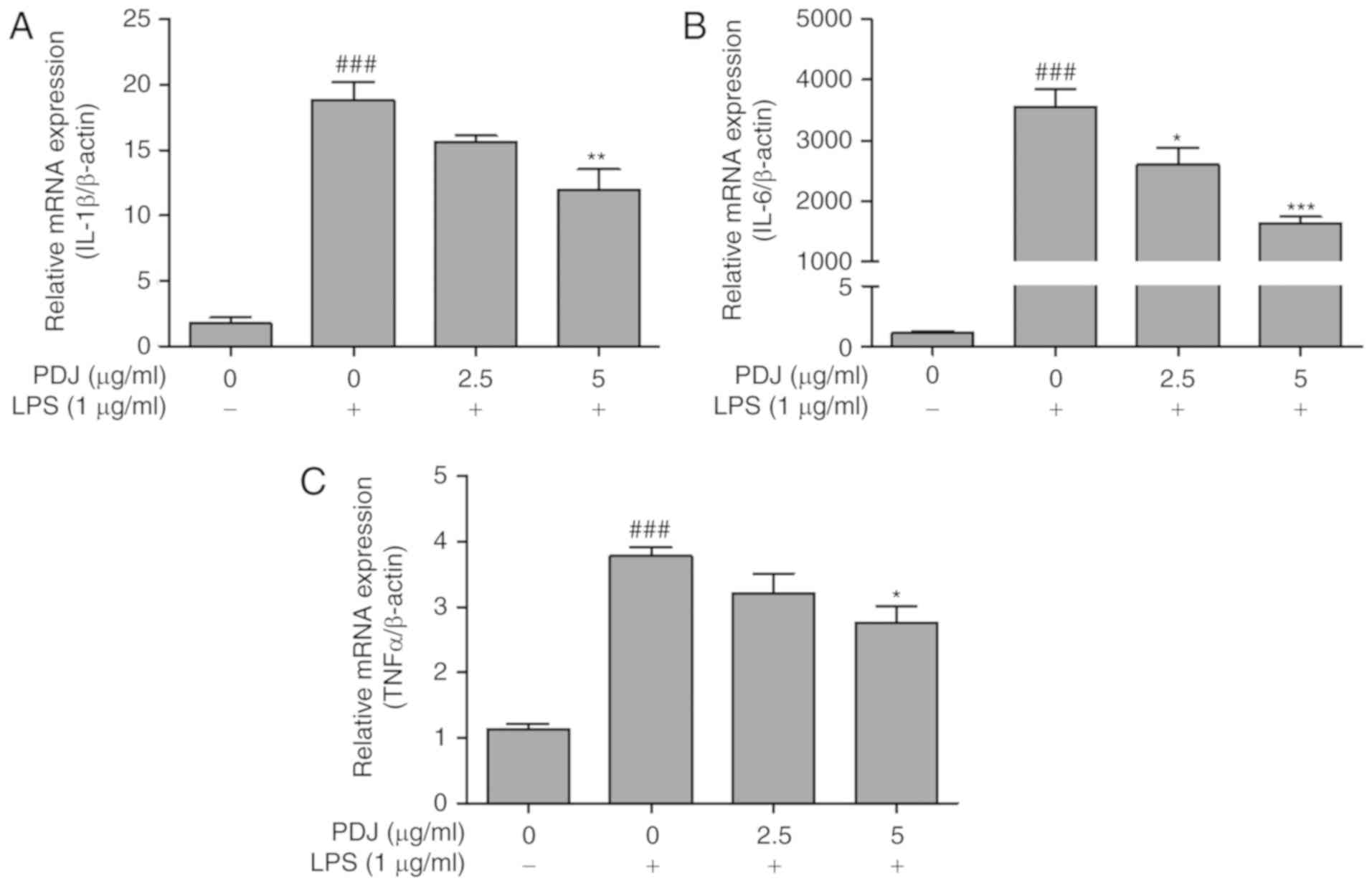
PDJ inhibits LPS-induced mRNA
expression of the inflammatory cytokines TNFα, IL-6 and IL-1β
The effect of PDJ on the mRNA expression levels of
inflammatory cytokines IL-6, IL-1β and TNFα in LPS-induced RAW264.7
cells was evaluated by RT-qPCR. mRNA levels of the inflammatory
cytokines were significantly increased (P<0.001) following LPS
treatment compared with the untreated control group. The levels of
IL-6, IL-1β and TNFα expression decreased in LPS-treated RAW264.7
cells that were also treated with PDJ (Fig. 6A-C). The results indicated that PDJ
suppressed cytokine expression at the transcriptional level in
LPS-induced RAW264.7 cells.
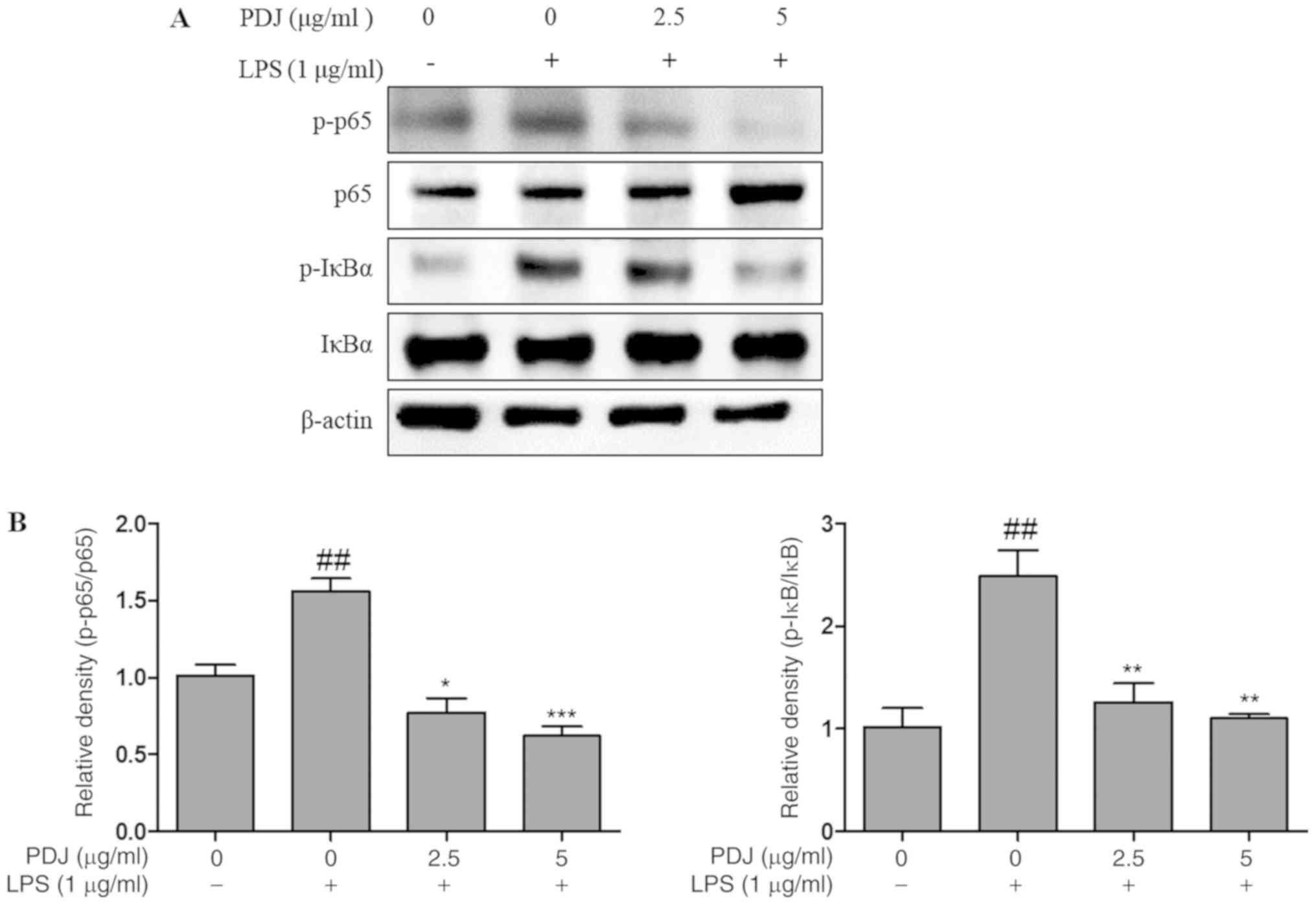
PDJ induces anti-inflammatory effects
in LPS-stimulated RAW264.7 cells via regulation of the NF-κB
signaling pathway
The effect of PDJ on the LPS-induced degradation and
phosphorylation of NF-κB proteins and the expression levels of
inhibitor of κBα (IκBα) and p65 were analyzed by western blotting.
The results indicated that PDJ treatment following LPS-stimulation
decreased p-IκBα and p-p65 protein expression, whereas the
expression of p65 and IκBα remained unchanged (Fig. 7). The results suggested that PDJ
increased IκBα and p65 protein levels by dephosphorylating IκBα and
p65 in LPS-induced RAW264.7 cells.
Discussion
Plant extracts are attracting greater interest in
anti-inflammatory drug discovery due to their low side effect
profile and effective mode of action (27). Numerous varieties of
phytoconstituents present in plants, including phenols, flavonoids
and alkaloids, are responsible for the effective biological
activities of plants, including antioxidant, anti-inflammatory,
anti-atherosclerotic, anti-tumor, anti-bacterial and anti-viral
effects (28). Compounds isolated
from DJ exert pharmacological effects in a number of diseases and
processes, including obesity, diabetes, fatty liver, inflammation
and aging (23,29,30).
However, to the best of our knowledge, no previous studies have
reported the inhibitory activities of PDJ on macrophage cells for
the treatment of inflammation. The present study examined the
anti-inflammatory effect of PDJ on LPS-stimulated RAW264.7
cells.
The polyphenolic content in the DJ flower extract
was analyzed and identified by HPLC and MS/MS. The results of the
MTT assay suggested that PDJ was not cytotoxic to RAW264.7 cells at
concentrations ≤10 µg/ml. Therefore, 2.5 and 5 µg/ml PDJ were used
for further experiments to examine the anti-inflammatory effects of
PDJ.
Oxidative stress leads to an excessive accumulation
of reactive oxygen species, such as NO and PGE2, in
activated macrophages that has been observed in both acute and
chronic inflammation in a number of disease conditions, including
atherosclerosis, obesity and arthritis (31–33).
Inhibition of NO production and PGE2 accumulation
modulates the inflammatory response in macrophages, leading to the
reduction of swelling and redness at the infection site (34,35).
Therefore, the present study investigated NO and PGE2
production to determine whether PDJ could mediate the inflammatory
response in RAW264.7 cells stimulated by LPS and co-treated with
PDJ. The results suggested that PDJ effectively inhibited the
production of NO and PGE2.
NO and PGE2 are produced from L-arginine
and arachidonic acid metabolites of the proteins iNOS and COX2
(36). Thus, the mRNA and protein
expression levels in LPS-stimulated RAW264.7 cells were analyzed
using RT-qPCR and western blotting, respectively. Pretreatment with
LPS increased the mRNA and protein expression levels of iNOS and
COX2, whereas the expression levels were significantly decreased in
a concentration-dependent manner in cells co-treated with PDJ.
Collectively, these data suggested that the suppressive effect of
PDJ on NO and PGE2 production was a result of the
inhibition of iNOS and COX2 expression, respectively.
Through synergetic interplay with the
pro-inflammatory cytokines TNFα, IL-1β and IL-6, iNOS and COX2
aggravate inflammation and the inflammatory response in
pathological conditions (37, 38). The release of pro-inflammatory
cytokines, such as TNFα, IL-1β and IL-6, by activated macrophages
at the site of infection is an important target for
anti-inflammatory therapeutic strategies (15,19).
Consistent with the RT-qPCR results, PDJ significantly
downregulated the mRNA expression levels of TNFα, IL-1β and IL-6,
suggesting that PDJ exerted anti-inflammatory effects via
inhibition of the pro-inflammatory cytokines.
The NF-κB signaling pathway is activated by the
phosphorylation, ubiquitination and subsequent proteolytic
degradation of NF-κB-bound IκB kinase proteins such as IκBα and
p65, which in turn triggers the response of cytokine genes such as
TNFα, IL-1β and IL-6 in the nucleus. Moreover, the majority of
anti-inflammatory drugs interrupt the inflammatory genes including
TNFα, IL-1β and IL-6 via activation of the NF-κB signaling pathway
(35,39). The expression of the NF-κB signaling
pathway-related proteins IκBα and p65 in the present study
suggested that treatment with PDJ inhibited the phosphorylated
forms of p65 and IκBα, while the expression levels of the whole
form of the proteins remained unchanged. The ratio of p-p65/p65 and
p-IκBα/IκBα were elevated in the LPS-only treated group but were
not elevated in the LPS group co-treated with PDJ, indicating that
PDJ promoted phosphorylation of the p65 and IκBα proteins via the
NF-κB signaling pathway to increase immune function in the RAW264.7
cells.
In conclusion, the results of the present study
suggested that PDJ exerted anti-inflammatory effects in RAW264.7
cells in vitro by suppressing NO and PGE2
production, and further displayed the inhibitory effects of PDJ on
the inflammatory mediators iNOS and COX2 and the pro-inflammatory
cytokines TNFα, IL-1β and IL-6. Furthermore, the anti-inflammatory
activity of PDJ might have occurred by direct inhibition of
upstream kinases in the activation of the NF-κB signaling pathway,
as summarized in Fig. 8.
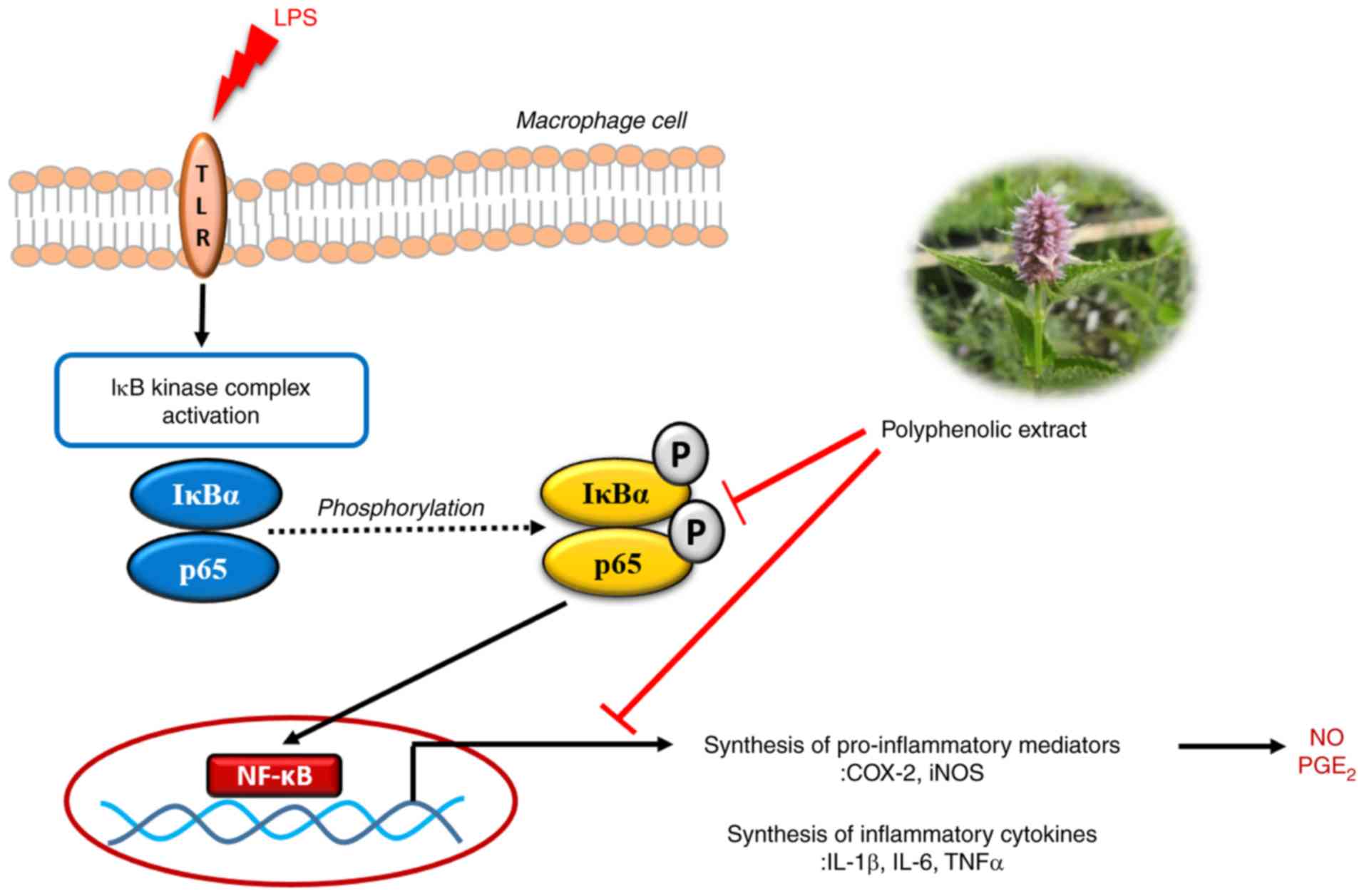 | Figure 8.Schematic representation of the
NF-κB-mediated inhibition of inflammatory responses by the
polyphenolic extract of Dowijigi flower. LPS, lipopolysaccharide;
TLR, Toll-like receptor; IκB, inhibitor of κB; IκBα, inhibitor of
κBα; COX2, cyclooxygenase-2; iNOS, inducible nitric oxide synthase;
IL, interleukin; TNF, tumor necrosis factor; NO, nitric oxide;
PGE2, prostaglandin E2; p,
phosphorylated. |
Acknowledgements
Not applicable.
Funding
The present study was supported partly by The
National Research Foundation of Korea funded by The Ministry of
Science, ICT and Future Planning (grant nos. 2012M3A9B8019303 and
2017R1A2B4003974).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SMK and PV designed the study, performed the
experiments, organized focus group discussions, and collected and
analyzed the data. HHK performed the extraction of polyphenols and
performed the HPLC analysis. SEH and VVGS performed experiments and
prepared the final manuscript. GSK participated in the focus group
discussion and revised the study design, the results and the final
manuscript for publication. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu C, Zhao W, Zhang X and Chen X:
Neocryptotanshinone inhibits lipopolysaccharide-induced
inflammation in RAW264.7 macrophages by suppression of NF-κB and
iNOS signaling pathways. Acta Pharm Sin B. 5:323–329. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jeong SG, Kim S, Kim HG, Kim E, Jeong D,
Kim JH, Yang WS, Oh J, Sung GH, Hossain MA, et al: Mycetia
cauliflora methanol extract exerts anti-inflammatory activity by
directly targeting PDK1 in the NF-κB pathway. J Ethnopharmacol.
231:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Araújo ERD, Félix-Silva J,
Xavier-Santos JB, Fernandes JM, Guerra GCB, de Araújo AA, Araújo
DFS, de Santis Ferreira L, da Silva Júnior AA, Fernandes-Pedrosa MF
and Zucolotto SM: Local anti-inflammatory activity: Topical
formulation containing Kalanchoe brasiliensis and Kalanchoe pinnata
leaf aqueous extract. Biomed Pharmacother. 113:1087212019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang G, Lee K, Lee M, Ham I and Choi HY:
Inhibition of lipopolysaccharide-induced nitric oxide and
prostaglandin E2 production by chloroform fraction of Cudrania
tricuspidata in RAW 264.7 macrophages. BMC Complement Altern Med.
12:2502012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Demoruelle MK, Deane KD and Holers VM:
When and where does inflammation begin in rheumatoid arthritis?
Curr Opin Rheumatol. 26:64–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hotchkiss RS, Moldawer LL, Opal SM,
Reinhart K, Turnbull IR and Vincent JL: Sepsis and septic shock.
Nat Rev Dis Primers. 2:160452016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo G, Cheng BC, Zhao H, Fu XQ, Xie R,
Zhang SF, Pan SY and Zhang Y: Schisandra Chinensis Lignans
suppresses the production of inflammatory mediators regulated by
NF-κB, AP-1, and IRF3 in lipopolysaccharide-stimulated RAW264.7
Cells. Molecules. 23:2018. View Article : Google Scholar
|
|
8
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2017.PubMed/NCBI
|
|
9
|
Abdulkhaleq LA, Assi MA, Abdullah R,
Zamri-Saad M, Taufiq-Yap YH and Hezmee MNM: The crucial roles of
inflammatory mediators in inflammation: A review. Vet World.
11:627–635. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng F and Lowell CA: Lipopolysaccharide
(LPS)-induced macrophage activation and signal transduction in the
absence of Src-family kinases Hck, Fgr, and Lyn. J Exp Med.
185:1661–1670. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hutchins AP, Takahashi Y and
Miranda-Saavedra D: Genomic analysis of LPS-stimulated myeloid
cells identifies a common pro-inflammatory response but divergent
IL-10 anti-inflammatory responses. Sci Rep. 5:91002015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cecilia OM, José Alberto CG, José NP,
Ernesto Germán CM, Ana Karen LC, Luis Miguel RP, Ricardo Raúl RR
and Adolfo Daniel RC: Oxidative stress as the main target in
diabetic retinopathy pathophysiology. J Diabetes Res.
2019:85624082019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zuo L, Prather ER, Stetskiv M, Garrison
DE, Meade JR, Peace TI and Zhou T: Inflammaging and oxidative
stress in human diseases: From molecular mechanisms to novel
treatments. Int J Mol Sci. 20:2019. View Article : Google Scholar
|
|
14
|
Mayouf N, Charef N, Saoudi S, Baghiani A,
Khennouf S and Arrar L: Antioxidant and anti-inflammatory effect of
Asphodelus microcarpus methanolic extracts. J Ethnopharmacol.
239:1119142019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han JM, Lee EK, Gong SY, Sohng JK, Kang YJ
and Jung HJ: Sparassis crispa exerts anti-inflammatory activity via
suppression of TLR-mediated NF-κB and MAPK signaling pathways in
LPS-induced RAW264.7 macrophage cells. J Ethnopharmacol. 231:10–18.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shah M, Ullah MA, Drouet S, Younas M,
Tungmunnithum D, Giglioli-Guivarc'h N, Hano C and Abbasi BH:
Interactive effects of light and melatonin on biosynthesis of
silymarin and anti-inflammatory potential in callus cultures of
Silybum marianum (L.) Gaertn. Molecules. 24:12072019.
View Article : Google Scholar
|
|
17
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song C, Hong YH, Park JG, Kim HG, Jeong D,
Oh J, Sung GH, Hossain MA, Taamalli A, Kim JH, et al: Suppression
of Src and Syk in the NF-κB signaling pathway by Olea
europaea methanol extract is leading to its anti-inflammatory
effects. J Ethnopharmacol. 235:38–46. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han SY, Yi YS, Jeong SG, Hong YH, Choi KJ,
Hossain MA, Hwang H, Rho HS, Lee J, Kim JH and Cho JY: Ethanol
extract of Lilium bulbs plays an anti-inflammatory role by
targeting the IKK[Formula: see text]/[Formula: see text]-mediated
NF-[Formula: see text]B pathway in macrophages. Am J Chin Med.
46:1281–1296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han B, Dai Y, Wu H, Zhang Y, Wan L, Zhao
J, Liu Y, Xu S and Zhou L: Cimifugin inhibits inflammatory
responses of RAW264.7 cells induced by lipopolysaccharide. Med Sci
Monit. 25:409–417. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abad MJ, Bedoya LM, Apaza L and Bermejo P:
The Artemisia L. genus: A review of bioactive essential
oils. Molecules. 17:2542–2566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee J, Narayan VP, Hong EY, Whang WK and
Park T: Artemisia iwayomogi extract attenuates high-fat
diet-induced hypertriglyceridemia in mice: Potential involvement of
the adiponectin-AMPK pathway and very low density lipoprotein
assembly in the liver. Int J Mol Sci. 18:2017. View Article : Google Scholar
|
|
23
|
Lee YK, Hong EY and Whang WK: Inhibitory
effect of chemical constituents isolated from Artemisia
iwayomogi on polyol pathway and simultaneous quantification of
major bioactive compounds. Biomed Res Int.
2017:73756152017.PubMed/NCBI
|
|
24
|
Sandhiutami NM, Moordiani M, Laksmitawati
DR, Fauziah N, Maesaroh M and Widowati W: In vitro assesment of
anti-inflammatory activities of coumarin and Indonesian cassia
extract in RAW264.7 murine macrophage cell line. Iran J Basic Med
Sci. 20:99–106. 2017.PubMed/NCBI
|
|
25
|
Erbel C, Rupp G, Helmes CM, Tyka M, Linden
F, Doesch AO, Katus HA and Gleissner CA: An in vitro model to study
heterogeneity of human macrophage differentiation and polarization.
J Vis Exp. e503322013.PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan SY, Zhou SF, Gao SH, Yu ZL, Zhang SF,
Tang MK, Sun JN, Ma DL, Han YF, Fong WF and Ko KM: New perspectives
on how to discover drugs from herbal medicines: CAM's outstanding
contribution to modern therapeutics. Evid Based Complement Alternat
Med. 2013:6273752013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maione F, Russo R, Khan H and Mascolo N:
Medicinal plants with anti-inflammatory activities. Nat Prod Res.
30:1343–1352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu HH, Kim YH, Kil BS, Kim KJ, Jeong SI
and You YO: Chemical composition and antibacterial activity of
essential oil of Artemisia iwayomogi. Planta Med.
69:1159–1162. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park WS, Son YK, Ko EA, Choi SW, Kim N,
Choi TH, Youn HJ, Jo SH, Hong DH and Han J: A carbohydrate
fraction, AIP1, from Artemisia iwayomogi reduces the action
potential duration by activation of rapidly activating delayed
rectifier K channels in rabbit ventricular myocytes. Korean J
Physiol Pharmacol. 14:119–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abramson SB: Nitric oxide in inflammation
and pain associated with osteoarthritis. Arthritis Res Ther. 10
(Suppl 2):S22008. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li T, Liu B, Guan H, Mao W, Wang L, Zhang
C, Hai L, Liu K and Cao J: PGE2 increases inflammatory damage in
Escherichia coli-infected bovine endometrial tissue in vitro
via the EP4-PKA signaling pathway. Biol Reprod. 100:175–186. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ezzat SM, Raslan M, Salama MM, Menze ET
and El Hawary SS: In vivo anti-inflammatory activity and UPLC-MS/MS
profiling of the peels and pulps of Cucumis melo var.
cantalupensis and Cucumis melo var.
reticulatus. J Ethnopharmacol. 237:245–254. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park SB, Park GH, Kim HN, Son HJ, Song HM,
Kim HS, Jeong HJ and Jeong JB: Anti-inflammatory effect of the
extracts from the branch of Taxillus yadoriki being
parasitic in Neolitsea sericea in LPS-stimulated RAW264.7
cells. Biomed Pharmacother. 104:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong GE, Kim JA, Nagappan A, Yumnam S, Lee
HJ, Kim EH, Lee WS, Shin SC, Park HS and Kim GS: Flavonoids
identified from Korean Scutellaria baicalensis georgi
inhibit inflammatory signaling by suppressing activation of NF-κB
and MAPK in RAW 264.7 cells. Evid Based Complement Alternat Med.
2013:9120312013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herencia F, Ferrándiz ML, Ubeda A, Guillén
I, Dominguez JN, Charris JE, Lobo GM and Alcaraz MJ: Novel
anti-inflammatory chalcone derivatives inhibit the induction of
nitric oxide synthase and cyclooxygenase-2 in mouse peritoneal
macrophages. FEBS Lett. 453:129–134. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Muniandy K, Gothai S, Badran KMH, Suresh
Kumar S, Esa NM and Arulselvan P: Suppression of proinflammatory
cytokines and mediators in LPS-induced RAW 264.7 macrophages by
stem extract of Alternanthera sessilis via the inhibition of
the NF-κB pathway. J Immunol Res. 2018:34306842018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014:5614592014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tzeng HE, Tsai CH, Ho TY, Hsieh CT, Chou
SC, Lee YJ, Tsay GJ, Huang PH and Wu YY: Radix Paeoniae Rubra
stimulates osteoclast differentiation by activation of the NF-κB
and mitogen-activated protein kinase pathways. BMC Complement
Altern Med. 18:1322018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fernandes A, Sousa A, Mateus N, Cabral M
and de Freitas V: Analysis of phenolic compounds in cork from
Quercus suber L. by HPLC-DAD/ESI-MS. Food Chem.
125:1398–1405. 2011. View Article : Google Scholar
|
|
41
|
Del Rio D, Stewart AJ, Mullen W, Burns J,
Lean ME, Brighenti F and Crozier A: HPLC-MSn analysis of phenolic
compounds and purine alkaloids in green and black tea. J Agric Food
Chem. 52:2807–2815. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Prasain JK, Peng N, Dai Y, Moore R,
Arabshahi A, Wilson L, Barnes S, Michael Wyss J, Kim H and Watts
RL: Liquid chromatography tandem mass spectrometry identification
of proanthocyanidins in rat plasma after oral administration of
grape seed extract. Phytomedicine. 16:233–243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gardana C, Scaglianti M, Pietta P and
Simonetti P: Analysis of the polyphenolic fraction of propolis from
different sources by liquid chromatography-tandem mass
spectrometry. J Pharm Biomed Anal. 45:390–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Han B, Xin Z, Ma S, Liu W, Zhang B, Ran L,
Yi L and Ren D: Comprehensive characterization and identification
of antioxidants in Folium Artemisiae Argyi using
high-resolution tandem mass spectrometry. J Chromatogr B Analyt
Technol Biomed Life Sci. 1063:84–92. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Barros L, Dueñas M, Carvalho AM, Ferreira
IC and Santos-Buelga C: Characterization of phenolic compounds in
flowers of wild medicinal plants from Northeastern Portugal. Food
Chem Toxicol. 50:1576–1582. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li F, Zhang YB, Wei X, Song CH, Qiao MQ
and Zhang HY: Metabolic profiling of Shu-Yu capsule in rat serum
based on metabolic fingerprinting analysis using HPLC-ESI-MSn. Mol
Med Rep. 13:4191–4204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Olennikov DN, Chirikova NK, Kashchenko NI,
Nikolaev VM, Kim SW and Vennos C: Bioactive phenolics of the genus
Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the
Siberian species and their inhibitory potential against α-amylase
and α-glucosidase. Front Pharmacol. 9:7562018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pati S, Losito I, Gambacorta G, La Notte
E, Palmisano F and Zambonin PG: Simultaneous separation and
identification of oligomeric procyanidins and anthocyanin-derived
pigments in raw red wine by HPLC-UV-ESI-MSn. J Mass Spectrom.
41:861–871. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schütz K, Kammerer DR, Carle R and
Schieber A: Characterization of phenolic acids and flavonoids in
dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb
by high-performance liquid chromatography/electrospray ionization
mass spectrometry. Rapid Commun Mass Spectrom. 19:179–186. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Han J, Ye M, Qiao X, Xu M, Wang BR and Guo
DA: Characterization of phenolic compounds in the Chinese herbal
drug Artemisia annua by liquid chromatography coupled to
electrospray ionization mass spectrometry. J Pharm Biomed Anal.
47:516–525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Orčić D, Francišković M, Bekvalac K,
Svirčev E, Beara I, Lesjak M and Mimica-Dukić N: Quantitative
determination of plant phenolics in Urtica dioica extracts
by high-performance liquid chromatography coupled with tandem mass
spectrometric detection. Food Chem. 143:48–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ye M, Yang WZ, Liu KD, Qiao X, Li BJ,
Cheng J, Feng J, Guo DA and Zhao YY: Characterization of flavonoids
in Millettia nitida var. hirsutissima by HPLC/DAD/ESI-MS
n. J Pharm Anal. 2:35–42. 2012. View Article : Google Scholar : PubMed/NCBI
|