Introduction
Diabetes is a worldwide health problem with a
prevalence of ~6% in adults (1).
Type 2 diabetes mellitus (T2DM) accounts for 90-95% of all diabetic
cases and is characterized by insulin resistance and impaired
glucose and lipid metabolism (2).
Treatment of diabetes and its complications are primarily dependent
on chemical and biological agents, which are associated with
certain side effects, including gastrointestinal problems and
hypoglycemia. Natural medicines have exhibited anti-diabetic
activity (3,4). Icariin
(C30H40O15; molecular weight,
676.67), the molecular structure of which is shown in Fig.
1(5), is a flavonoid isolated from
the traditional oriental herbal medicine, Epimedium koreanum
Nakai. Icariin exhibits a variety of beneficial
biological activities, including immunological functions (6), sexual function (7), cardiovascular diseases (8), and anti-cancer (9) and anti-Alzheimer's disease effects
(10). In rats, Icariin was
also found to alleviate renal damage (11), enhance neurite growth in retinal
ganglion cells (12), ameliorate
signs of impotence (13) and lower
lipid levels (14). However, there
is no direct evidence demonstrating how Icariin regulates
glucose homeostasis.
Adiponectin is a biologically active polypeptide
produced by adipocytes (15).
Adiponectin shows anti-diabetic potential by improving insulin
sensitivity (16,17). AMP-mediated protein kinase (AMPK) is
a key molecule involved in regulation of energy metabolism, by
increasing the ratio of intracellular AMP/ATP (18-20).
Additionally, LKB1, an upstream kinase of the AMPK pathway,
activates AMPK, promoting the phosphorylation of Thr172.
Accordingly, LKB1, regulates glucose absorption during contractions
of muscles (21). Drugs which
regulate adiponectin levels or the AMPK-mediated pathway exhibit
hyperglycemic actions which may be used for the treatment of
diabetes (22,23).
Defects in skeletal muscle function have been
associated with insulin resistance in diabetes (24). Glucose transporter isoform 4 (GLUT-4)
expression is upregulated in skeletal muscle and adipose tissues
(25). Insulin promotes
intracellular GLUT-4 translocation to the cytoplasmic membrane,
increasing glucose uptake in skeletal muscle (26). Exercise increases GLUT-4 expression
and AMPK activation in skeletal muscles (27,28).
Overexpression of GLUT-4 improves glucose homeostasis (29). Flavonoids function as an
antidiabetic, primarily by increasing the expression of and
promoting translocation of GLUT-4 via the AMPK signaling pathway
(4). The results of the present
study suggest that regulation of the AMPK/GLUT-4 pathway in
skeletal muscles may be an effective potential therapy for
treatment of hyperglycemia.
The primary aim of the present study was to
investigate the effects of Icariin on the levels of glucose
in a rat model of diabetes. Additionally, the role of AMPK/GLUT-4
signaling pathway in the antidiabetic effects of Icariin
were examined.
Materials and methods
Animal models
Animal experiments were performed in accordance with
the Guide for the Care and Use of Laboratory Animals published by
the US National Institutes of Health (publication no. 85-23,
revised 1996). The present study was approved by the Animal Ethics
Committee of Qingdao University. Sixty five-week-old male
Sprague-Dawley rats, (100-120 g) provided by the Institute of
Qingdao Platford Breeding Co., were maintained in a pathogen-free
environment with a 12 h light/dark cycle with free access to food
and water. The diabetic group (n=50) was fed with high-sugar and
high-fat diet (kcal%: 45% fat, 20% protein, and 35% 100
carbohydrate; 4.73 kcal/gm, Research Diet, New Brunswick, NJ, USA)
for 4 weeks (30), whereas the
control group was fed with a normal diet for 4 weeks. Diabetes was
induced by intraperitoneal injection of 40 mg/kg streptozotocin
(STZ; S0130, Sigma).
Three days after STZ injection, T2DM was confirmed,
as blood glucose levels were increased. A total of 50 rats with
diabetes were randomly divided into five groups (n=10 per group):
Diabetic control; metformin (400 mg/kg dissolved in water,
administered by gavage) (31); and
rats treated with either 5, 10 or 20 mg/kg Icariin (32)(489-32-7, Sigma) dissolved in
carboxymethylcellulose sodium administered by intraperitoneal
injection, once a day. 10 normal rats served as the control group.
After a total of 3 weeks of drugs treatment, the body weight and
fasting blood glucose levels were recorded. All the experimental
animals survived.
Blood sample collection and tissue
extraction
First of all, rats were anesthetized with 30 mg/kg
sodium pentobarbital. Then, blood samples were collected from tail
veins. An oral glucose tolerance test, in which 20% glucose was fed
with a syringe at a dose of 2 g/kg, was performed after the rats
were fasted for 10 h (33). Blood
samples were collected from the caudal vein by means of a small
incision at the end of the tail at 0, 15, 30, 60 and 120 min after
the glucose administration. Subsequently, the level of blood
glucose was measured.
After OGTT test, rats were euthanized using 150
mg/kg sodium pentobarbital. Pancreatic tissues were dissected,
processed as paraffin blocks, then stained with hematoxylin eosin.
Pancreatic tissues were rehydrated, incubated, washed, rapidly
dehydrated and subsequently mounted on cover slips. Tissues were
imaged using a microscope (DM750M, Leica) at x200
magnification.
Serum adiponectin measurement
Serum adiponectin concentrations were determined
using a specific ELISA kit (ab108786, Abcam).
RNA extraction and gene microarray
hybridization
Total RNA was extracted from bisected soleus muscle
tissue using an RNA isolation kit (AM1912, Invitrogen, America).
RNA concentrations were measured using spectrophotometric analysis
by measuring the A260/280 ratio. The instrument for detecting RNA
concentration is spectrophotometer (E300, Thermo, America). Reverse
transcription-quantitative PCR was performed and analyzed on a
Rotor-Gene 6000 system (Corbett Research). PCR was performed using
a SYBR® Premix Ex Taq™ (Tli RNaseH Plus) kit (RR420A,
Takara). The thermocycling conditions were: Initial denaturation,
9˚C for 30 sec; followed by 40 cycles of denaturation at 60˚C for
30 sec, primer annealing at 9˚C for 5 sec, and extension at 64˚C
for 1 min. Fluorescence was measured at 72˚C in each cycle. To
determine the specificity of PCR reactions, melt curve analysis was
performed following amplification by slowly ramping the heat from
72˚C to 9˚C, with fluorescence acquisition at 1˚C intervals and a
5-sec hold at each increment. The forward and reverse primer
sequences were as follows: GLUT-4 forward,
5'-TCATTCCTGTGAAAGTGATGACGA-3' and reverse,
5'-CTGCCACAGTGTCATATCATCCAA-3'; and β-actin forward,
5'-CCGTAAAGACCTCTATGCCAACA-3' and reverse
5'-GCTAGGAGCCAGGGCAGTAATC-3'. Expression of the target gene was
normalized to β-actin. Primer 5 software was used for the primer
design.
Western blotting
Homogenized skeletal muscle (0.1 g) at 4˚C in 1 ml
of lysis buffer containing 50 mM Tris.HCl, pH 7.4, 150 mM NaCl, 1
mM EDTA, 1% Nonidet P-40, 5 mM Na3VO4, 20 mM
NaF, 10 mM sodium pyrophosphate and 50 µl protease inhibitor
cocktail (B14001, Bimake). Equal quantities (50 µg) of total
protein were resolved on a 12% gel using SDS-PAGE and transferred
to a PVDF membrane (FFP32, Beyotime). After transfer, membranes
were blocked using 5% skimmed milk containing 0.1% Tween-20.
Subsequently the membranes were incubated with anti-GLUT-4 (2213S,
Cell Signaling Technology, America), anti-phospho (p)-AMPK (2535S,
Cell Signaling Technology), total-AMPK (2532S, Cell Signaling
Technology) or anti-β-actin (ab8227, Abcam). Membranes were then
incubated with horse radish peroxidase (HRP)-conjugated secondary
antibodies, including HRP-conjugated goat anti-rabbit
immunoglobulin G (IgG) (AP132P, Merck Millipore), or HRP-conjugated
goat anti-mouse IgG (ab6789, Abcam). Enhanced chemiluminescence
plus kit (PE0010, Solarbio) was used to visualize the signals.
Relative protein expression levels were quantified using
densitometry analysis and normalized to β-actin expression
levels.
Statistical analysis
GraphPad Prism version 4.0 (GraphPad Software, Inc.)
was used to analyze the data. Statistical analysis was performed
using a one-way ANOVA with a Tukey's post hoc test. All data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
The effect of Icariin on body weights,
blood glucose levels and serum fasting blood glucose levels in
diabetic rats
After 3 weeks of treatment with drugs, the body
weight of rats decreased significantly (*P<0.05; Table I) compared with the non-diabetic
control. There was no significant difference in the body weight of
rats treated with 5 mg/kg Icariin compared with the diabetic
control (P>0.05; Table I).
However, the body weights of rats were significantly increased when
treated with 10 or 20 mg/kg Icariin compared with the
diabetic control (^P<0.05, Table
I). There was no significant difference in body weight between
the Icariin (10 and 20 mg/kg) group and the metformin group
(P>0.05, vs. the metformin group, Table I).
 | Table IThe effect of Icariin on body weight
in T2DM rats. |
Table I
The effect of Icariin on body weight
in T2DM rats.
| Groups | Basal body weight
(g) | Body weight (g) 21
days after drug treatment |
|---|
| Control | 201.8±9.8 | 223.8±12.1 |
| Diabetic | 198.8±11.4 |
187.8±11.4a |
| Metformin | 201.4±7.2 |
219.3±9.1b |
| Icariin 5
mg/kg | 203.3±10.9 | 195.3±9.7 |
| Icariin 10
mg/kg | 202.3±11.2 |
208.7±6.9b |
| Icariin 20
mg/kg | 200.9±8.2 |
215.3±12.1b |
The diabetic rats treated with 10 or 20 mg/kg
Icariin exhibited reduced blood glucose levels compared with
the diabetic control rats (^P<0.05; Table II). Meanwhile, there was no
significant difference in blood glucose level between the
Icariin (10 and 20 mg/kg) group and the metformin group
(P>0.05, vs. the metformin group, Table II). However, the change in blood
glucose levels were not considered significant in the rats treated
with 5 mg/kg Icariin compared with the diabetic control rats
(P>0.05, Table II).
 | Table IIThe effect of Icariin on blood
glucose in T2DM rats. |
Table II
The effect of Icariin on blood
glucose in T2DM rats.
| Groups | Blood glucose
(mmol/l) before STZ injection | Blood glucose
(mmol/l) 21 days after drug treatment |
|---|
| Control | 2.21±0.4 | 2.88±0.7 |
| Diabetic | 2.29±0.5 |
5.89±1.1a |
| Metformin | 2.25±0.5 |
3.16±0.9b |
| Icariin 5
mg/kg | 2.25±0.6 | 5.48±1.3 |
| Icariin 10
mg/kg | 2.35±0.4 |
4.02±1.2b |
| Icariin 20
mg/kg | 2.27±0.7 |
3.27±0.7b |
In the oral glucose tolerance test, the blood
glucose levels reached peak levels after 30 min, and returned to
resting levels after ~120 min, except in rats treated with 5 mg/kg
Icariin, where peak blood glucose levels were reached after
60 min. Glucose levels were significantly lower in the rats treated
with 10 or 20 mg/kg Icariin compared with the diabetic
control (^P<0.05; Table III).
There was no significant difference in oral glucose tolerance
between the Icariin (10 and 20 mg/kg) group and the
metformin group (P>0.05, vs. the metformin group, Table III).
 | Table IIIThe effect of Icariin on blood
glucose level during the oral glucose tolerance test in T2DM
rats. |
Table III
The effect of Icariin on blood
glucose level during the oral glucose tolerance test in T2DM
rats.
| | Blood glucose
(mmol/l) |
|---|
| Groups | 0 min | 15 min | 30 min | 60 min | 120 min |
|---|
| Control | 2.88±0.7 | 6.45±1.3 | 7.09±1.2 | 6.24±1.0 | 3.87±0.7 |
| Diabetic | 5.89±1.1 | 12.3±1.8 | 19.3±1.0 | 20.51±1.1 |
3.83±1.8a |
| Metformin | 3.16±0.9 | 8.23±1.7 | 9.63±1.4 | 7.98±1.3 |
3.82±1.1b |
| Icariin 5
mg/kg | 5.48±1.3 | 8.70±1.3 | 12.88±1.8 | 13.86±2.1 | 9.83±2.3 |
| Icariin 10
mg/kg | 4.02±1.2 | 10.31±1.9 | 11.41±0.9 | 10.87±1.5 |
5.31±1.3b |
| Icariin 20
mg/kg | 3.27±0.7 | 8.29±1.7 | 9.81±1.6 | 8.68±1.8 |
4.03±1.8b |
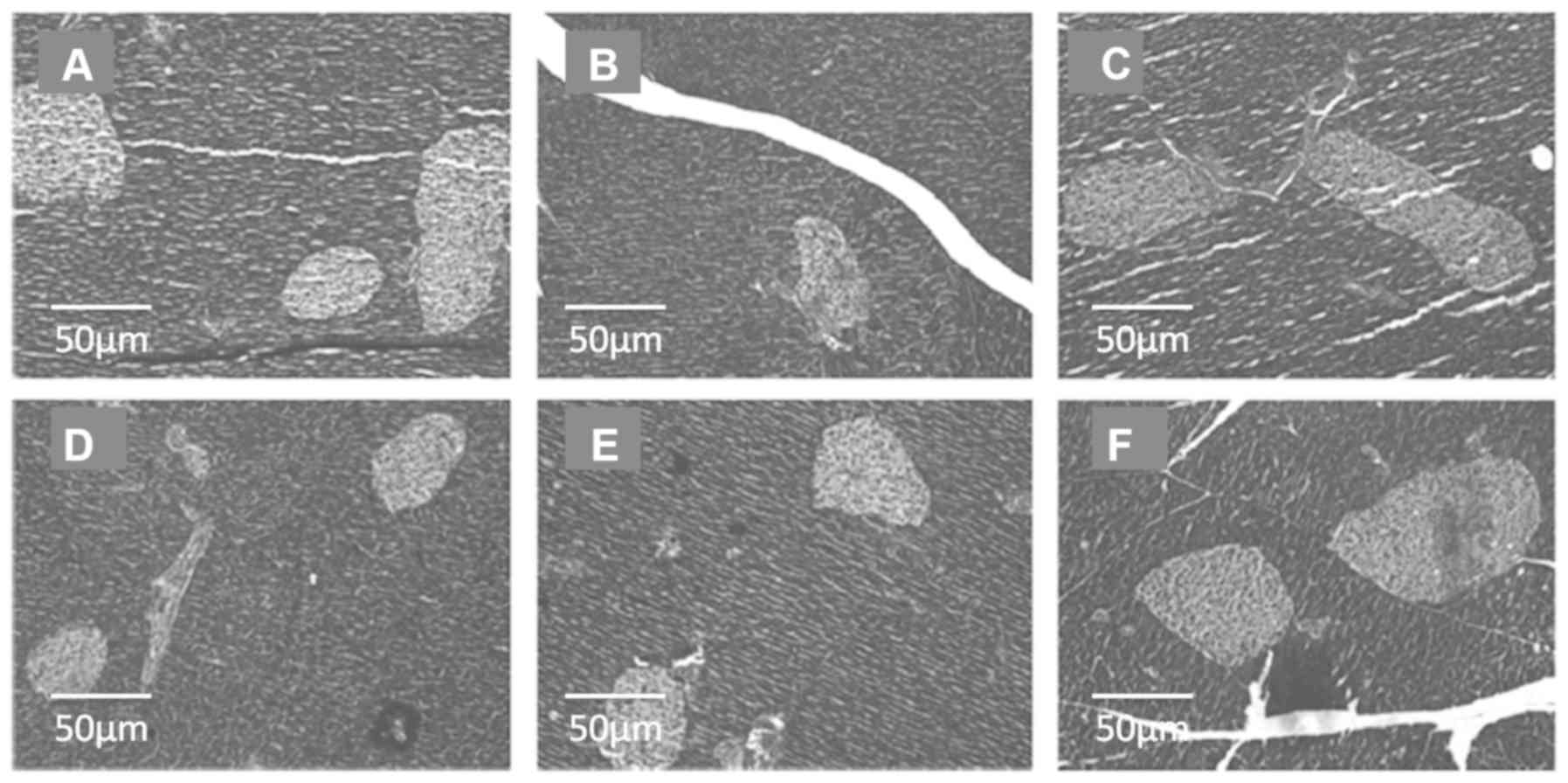
The effect of Icariin on pancreatic
tissues in diabetic rats
The morphology of islets in the pancreatic tissues
from the different groups are shown in Fig. 2. STZ treatment resulted in impaired
pancreatic tissues, with fewer islets compared with the normal
control (Fig. 2A and B, respectively). Icariin treatment
reduced the loss in the number of islets, compared with the
diabetic control rats, irrespective of the dose used, thus serving
a protective role during the diabetic process (Fig. 2D-F). Metformin treatment also reduced
the loss in the number of islets, compared with the diabetic
control rats (Fig. 2C).
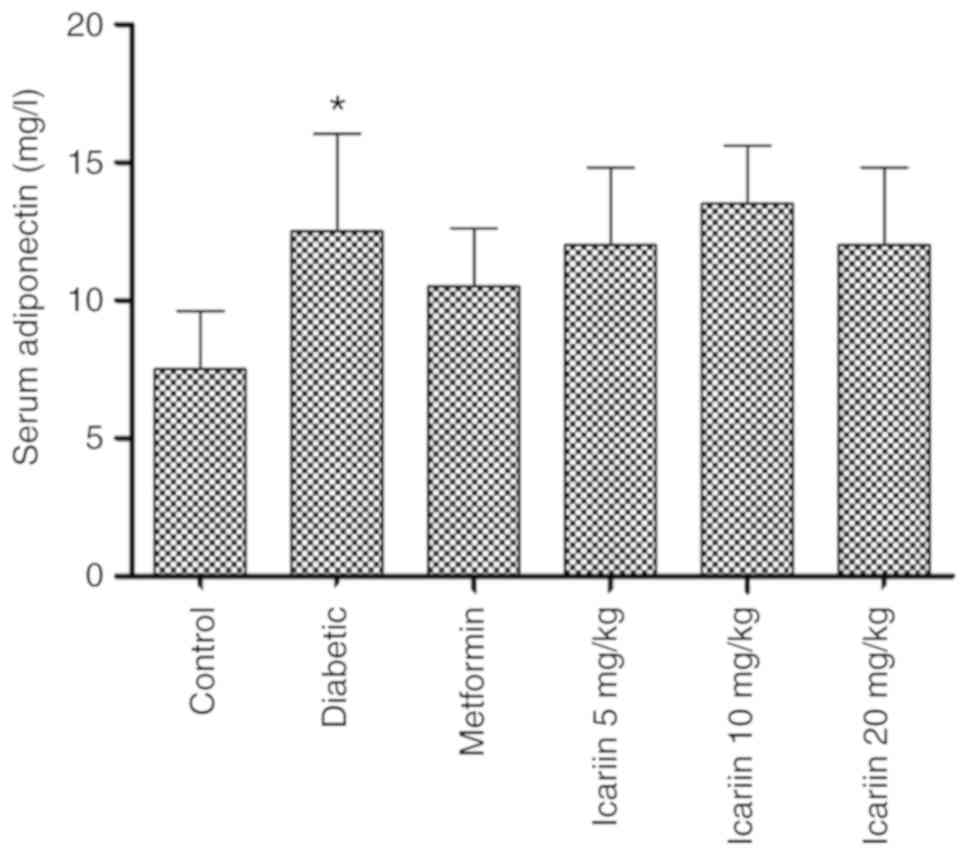
The effect of Icariin on serum
adiponectin levels in diabetic rats
STZ treatment increased the serum adiponectin levels
compared with the non-diabetic control (Fig. 3; *P<0.05); However, no significant
changes were observed in the serum adiponectin levels in the rats
treated with Icariin (P>0.05, vs. the diabetic
group).
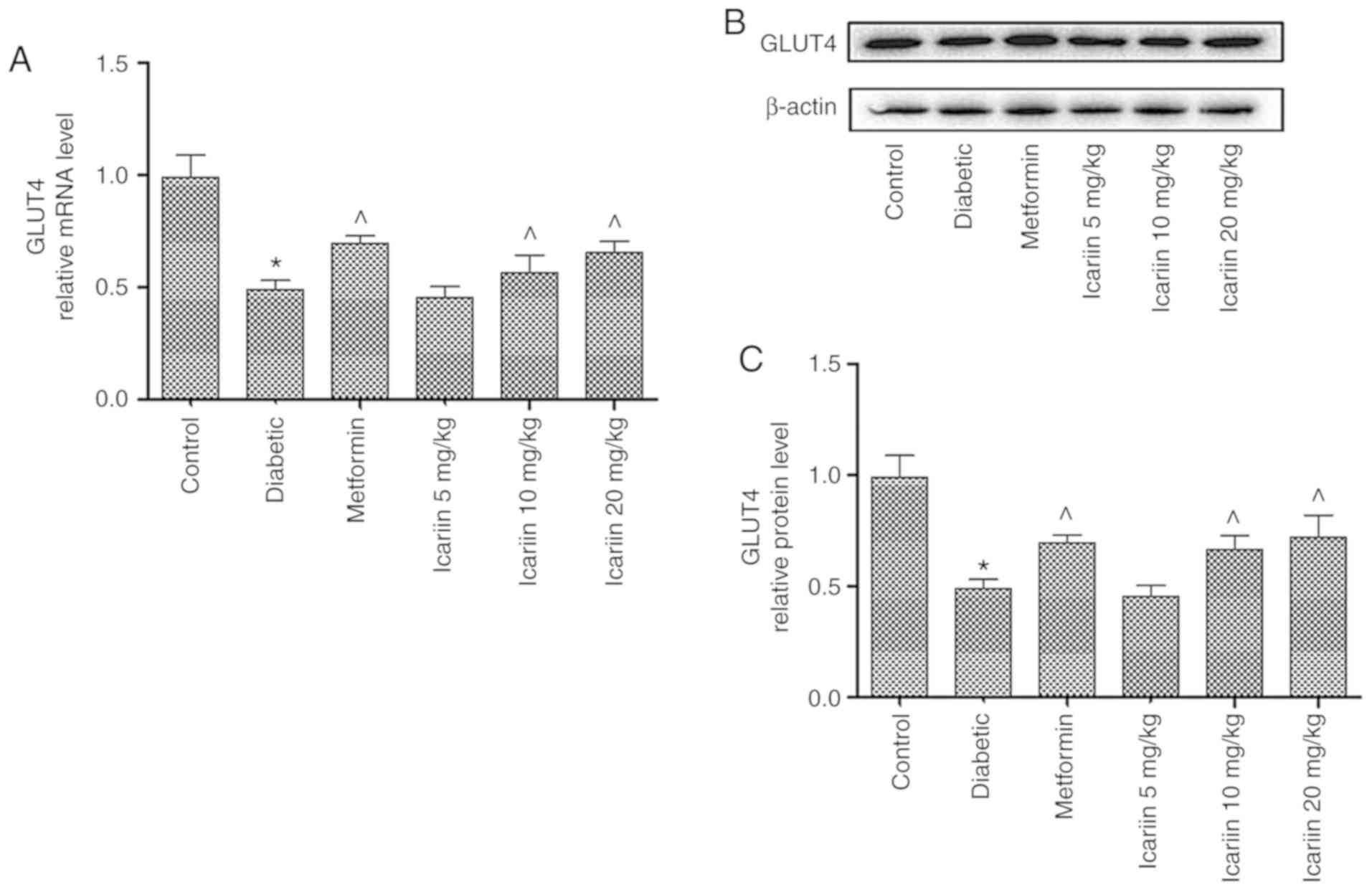
The effect of Icariin on the mRNA and
protein expression levels of GLUT-4 in diabetic rats
As shown in Fig. 4,
the GLUT-4 mRNA expression levels in skeletal muscles were
significantly lower in the diabetic rats compared with the control
(P<0.05). In the rats treated with 10 and 20 mg/kg
Icariin, GLUT-4 mRNA expression levels were significantly
increased compared with the diabetic control (Fig. 4A; ^P<0.05). Similarly, GLUT-4
protein expression levels were also increased in Icariin
treated mice compared with the diabetic control (Fig. 4B and C; P<0.05). There was no significant
difference in expression of GLUT-4 between the Icariin (10
and 20 mg/kg) group and the metformin group (P>0.05, vs. the
metformin group).
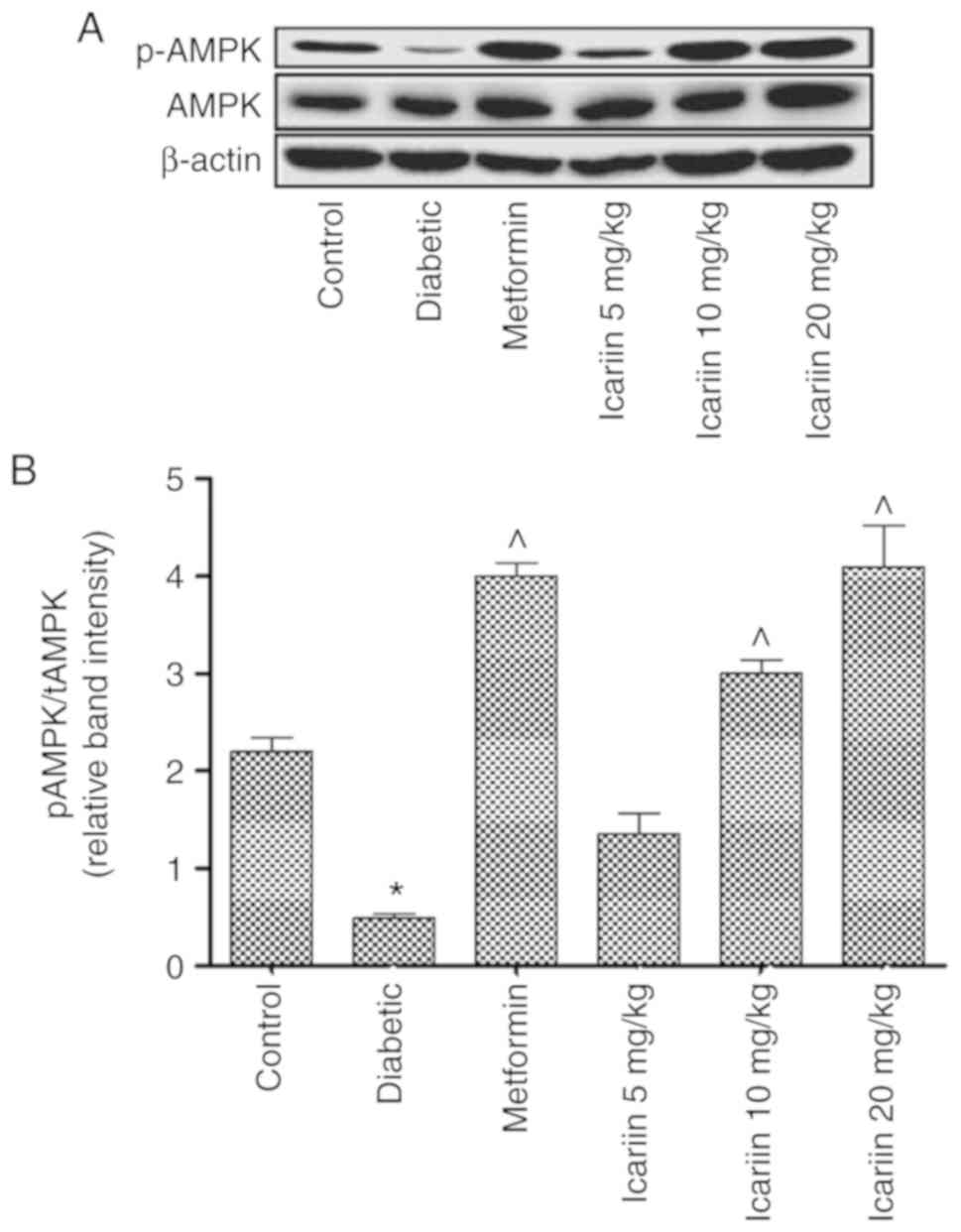
The effect of Icariin on
phosphorylation of AMPK in diabetic rats
The phosphorylation of AMPK was decreased in the
diabetic rats compared with the control (*P<0.05; Fig. 5). Phosphorylation of AMPK in the rats
treated with 10 or 20 mg/kg Icariin was significantly
increased compared with the diabetic control (P<0.05; Fig. 5). There was no significant difference
in phosphorylation of AMPK between the Icariin (10 and 20
mg/kg) group and the metformin group (P>0.05, vs. the metformin
group, Fig. 5).
Discussion
The primary findings of the present study were that
treatment with 10 or 20 mg/kg Icariin for 3 weeks reduced
the blood glucose levels in diabetic rats. This treatment also
reduced the peak glucose levels in an oral glucose tolerance test.
Furthermore, treatment with Icariin resulted in reducing the
loss in the number of islets in the pancreatic tissues and
treatment with Icariin was associated with upregulated mRNA
expression of GLUT-4 and increased phosphorylation of AMPK in the
skeletal muscles. These results suggest that the beneficial effects
of Icariin on T2DM may be associated with an AMPK/GLUT-4
signaling pathway.
Recent studies have suggested that polyphenolic
compounds prevent the development of long-term diabetes and its
complications, including cardiovascular disease, neuropathy,
nephropathy and retinopathy (34,35). The
therapeutic properties of Epimedium koreanum have been
attributed to the flavonoid component of Icariin, which has
been reported to exhibit a broad range of pharmacological effects,
including anti-diabetic, anti-Alzheimer's disease, anti-tumor and
hepatoprotective properties (36).
To the best of our knowledge, the present study is the first to
demonstrate the dose-dependent antidiabetic effect of
Icariin, with hypoglycemic effects observed with 10 and 20
mg/kg. Treatment with Icariin for 3 weeks reduced the blood
glucose levels as well as the peak glucose levels following a bolus
dose of glucose.
Metformin is medically considered as the only
biguanide which is used and recommended as oral anti-diabetic
agent, which is crucial for decreasing the levels of plasma
glucose. As known, metformin has been found to exert an increasing
effect on inhibiting hepatic gluconeogenesis, decreasing
hyperinsulinemia, reducing protein synthesis, improving insulin
sensitivity and enhancing glucose use in the muscle. In clinical
practice, previous evidence has reported that metformin is widely
accepted as an effective treatment for DM, and notably to T2DM by
serving as the first-line therapy. Therefore, metformin was chosen
as a positive control drug in this study.
Adipose tissue has been demonstrated to serve an
endocrine role in recent years. Adiponectin, secreted by
adipocytes, is an insulin-sensitizing hormone (15), improving insulin resistance in mice
(37). And in studies on humans,
adiponectin has the potential to be a biomarker for predicting
metabolic diseases such as diabetes mellitus (38). In clinical trials, adiponectin was
demonstrated to exhibit anti-diabetic (39), anti-atherosclerotic (40) and anti-cancer potential (41). Adiponectin improves insulin
sensitivity by increasing insulin receptor expression and signal
transduction, thereby alleviating insulin resistance (16,17,42). In
the present study, there was no statistically significant
difference in the adiponectin levels between the Icariin
treated and diabetes control group, suggesting that the
anti-diabetes effect of Icariin was likely not associated
with the expression of adiponectin in the rat model diabetes
used.
AMPK, a crucial component of cellular metabolism,
has been demonstrated to inhibit many metabolic diseases including
T2DM. Metformin lowers blood glucose levels by inhibiting hepatic
glucose production, which is mediated by an AMPK-dependent
mechanism (43). Increased glucose
uptake following AMPK activation by AICA-riboside in perfused rat
hindlimb muscles is attributed to an increase in translocation of
GLUT-4 to the cell membrane (44).
These results suggest that increasing GLUT-4 expression in skeletal
muscles may be an effective therapy for treating diabetes. In the
present study, Icariin treatment resulted in increased
expression of AMPK and GLUT-4, suggesting that the
anti-hyperglycemic effects of Icariin may be associated with
the AMPK/GLUT-4 signaling pathway.
The limitation of this study is that there is no
measurement of insulin levels under Icariin intervention.
Thus, it is impossible to accurately assess the islet function.
This study does not investigate whether Icariin has side
effects in the treatment of T2DM. In this study, we did not observe
the effect of Icariin on healthy rats. The side effects of Icariin
were not elucidated by literature search. However, Icariin belongs
to flavonoids which have common side effects (45), such as allergic reaction and pyrogen
reaction. Studies have shown that the toxic side effects may be
caused by the charge complexes formed by a class of lipoproteins
and flavonoids. To clarify the common impurities and
physicochemical properties of these flavonoids, effective
separation methods should be adopted to provide safe and effective
drugs for clinical use. Finally, there was no data on diabetic
patients. This is also what needs to be done in future
research.
In summary, the present study demonstrated that
Icariin is an effective therapy for treating diabetes in a rat
model of T2DM. The pharmacological effects of Icariin is related to
preserve pancreatic islet number or function and increased
expression levels of AMPK and GLUT-4 in the skeletal muscles.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81601103)
and the China Postdoctoral Science Foundation (grant no.
2016M602100).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and LW made substantial contributions to
conception, design of the experiments, as well as the writing and
revision of this manuscript. XL was responsible for acquisition and
interpretation of data. XL, YW, PS, YL, TL and SL performed the
experiments and participated in the writing of the manuscript. CW
analyzed the data. The manuscript has been read and approved by
each author, and all agree to this submission.
Ethics approval and consent to
participate
The current study was approved by the Medical Ethics
Committee of Affiliated Hospital of Qingdao University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ogurtsova K, da Rocha Fernandes JD, Huang
Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE and
Makaroff LE: IDF Diabetes Atlas: Global estimates for the
prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract.
128:40–50. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Constantin RP, Constantin RP, Bracht A,
Yamamoto NS, Ishii-Iwamoto EL and Constantin J: Molecular
mechanisms of citrus flavanones on hepatic gluconeogenesis.
Fitoterapia. 92:148–162. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li WL, Zheng HC, Bukuru J and De Kimpe N:
Natural medicines used in the traditional Chinese medical system
for therapy of diabetes mellitus. J Ethnopharmacol. 92:1–21.
2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hajiaghaalipour F, Khalilpourfarshbafi M
and Arya A: Modulation of glucose transporter protein by dietary
flavonoids in type 2 diabetes mellitus. Int J Biol Sci. 11:508–524.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xiong W, Ma X, Wu Y, Chen Y, Zeng L, Liu
J, Sun W, Wang D and Hu Y: Determine the structure of
phosphorylated modification of icariin and its antiviral activity
against duck hepatitis virus A. BMC Vet Res. 11(205)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
He W, Sun H, Yang B, Zhang D and Kabelitz
D: Immunoregulatory effects of the herba Epimediia glycoside
icariin. Arzneimittelforschung. 45:910–913. 1995.PubMed/NCBI
|
|
7
|
Makarova MN, Pozharitskaya ON, Shikov AN,
Tesakova SV, Makarov VG and Tikhonov VP: Effect of lipid-based
suspension of Epimedium koreanum Nakai extract on sexual
behavior in rats. J Ethnopharmacol. 114:412–416. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu HB and Huang ZQ: Vasorelaxant effects
of icariin on isolated canine coronary artery. J Cardiovasc
Pharmacol. 49:207–213. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhou J, Wu J, Chen X, Fortenbery N,
Eksioglu E, Kodumudi KN, Pk EB, Dong J, Djeu JY and Wei S: Icariin
and its derivative, ICT, exert anti-inflammatory, anti-tumor
effects, and modulate myeloid derived suppressive cells (MDSCs)
functions. Int Immunopharmacol. 11:890–898. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen YJ, Zheng HY, Huang XX, Han SX, Zhang
DS, Ni JZ and He XY: Neuroprotective effects of icariin on brain
metabolism, mitochondrial functions, and cognition in
triple-transgenic alzheimer's disease mice. CNS Neurosci Ther.
22:63–73. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qi MY, Kai-Chen Liu HR, Su YH and Yu SQ:
Protective effect of Icariin on the early stage of experimental
diabetic nephropathy induced by streptozotocin via modulating
transforming growth factor beta1 and type IV collagen expression in
rats. J Ethnopharmacol. 138:731–736. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xin H, Zhou F, Liu T, Li GY, Liu J, Gao
ZZ, Bai GY, Lu H and Xin ZC: Icariin ameliorates
streptozotocin-induced diabetic retinopathy in vitro and in vivo.
Int J Mol Sci. 13:866–878. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang ZB and Yang QT: The testosterone
mimetic properties of icariin. Asian J Androl. 8:601–605.
2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lu YF, Xu YY, Jin F, Wu Q, Shi JS and Liu
J: Icariin is a PPARα activator inducing lipid metabolic gene
expression in mice. Molecules. 19:18179–18191. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hossain MM, Mukheem A and Kamarul T: The
prevention and treatment of hypoadiponectinemia-associated human
diseases by up-regulation of plasma adiponectin. Life Sci.
135:55–67. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
El Husseny MW, Mamdouh M, Shaban S,
Ibrahim Abushouk A, Zaki MM, Ahmed OM and Abdel-Daim MM:
Adipokines: Potential therapeutic targets for vascular dysfunction
in type ii diabetes mellitus and obesity. J Diabetes Res.
2017(8095926)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gao Q, Yao X and Zheng J: MiR-323 inhibits
prostate cancer vascularization through adiponectin receptor. Cell
Physiol Biochem. 36:1491–1498. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ye JM, Dzamko N, Hoy AJ, Iglesias MA, Kemp
B and Kraegen E: Rosiglitazone treatment enhances acute
AMP-activated protein kinase-mediated muscle and adipose tissue
glucose uptake in high-fat-fed rats. Diabetes. 55:2797–2804.
2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fujii N, Ho RC, Manabe Y, Jessen N, Toyoda
T, Holland WL, Summers SA, Hirshman MF and Goodyear LJ: Ablation of
AMP-activated protein kinase alpha2 activity exacerbates insulin
resistance induced by high-fat feeding of mice. Diabetes.
57:2958–2966. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Richter EA and Ruderman NB: AMPK and the
biochemistry of exercise: Implications for human health and
disease. Biochem J. 418:261–275. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sakamoto K, McCarthy A, Smith D, Green KA,
Grahame Hardie D, Ashworth A and Alessi DR: Deficiency of LKB1 in
skeletal muscle prevents AMPK activation and glucose uptake during
contraction. EMBO J. 24:1810–1820. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sharma AX, Quittner-Strom EB, Lee Y,
Johnson JA, Martin SA, Yu X, Li J, Lu J, Cai Z, Chen S, et al:
Glucagon receptor antagonism improves glucose metabolism and
cardiac function by promoting AMP-mediated protein kinase in
diabetic mice. Cell Rep. 22:1760–1773. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Na RS, Ma C, Liu QR, Wu LM, Zheng XL and
Liu ZW: Itraconazole attenuates hepatic gluconeogenesis and
promotes glucose uptake by regulating AMPK pathway. Exp Ther Med.
15:2165–2171. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lowell BB and Shulman GI: Mitochondrial
dysfunction and type 2 diabetes. Science. 307:384–387.
2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
James DE, Strube M and Mueckler M:
Molecular cloning and characterization of an insulin-regulatable
glucose transporter. Nature. 338:83–87. 1989.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Goodyear LJ and Kahn BB: Exercise, glucose
transport, and insulin sensitivity. Annu Rev Med. 49:235–261.
1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kraniou Y, Cameron-Smith D, Misso M,
Collier G and Hargreaves M: Effects of exercise on GLUT-4 and
glycogenin gene expression in human skeletal muscle. J Appl Physiol
(1985). 88:794–796. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cao S, Li B, Yi X, Chang B, Zhu B, Lian Z,
Zhang Z, Zhao G, Liu H and Zhang H: Effects of exercise on AMPK
signaling and downstream components to PI3K in rat with type 2
diabetes. PLoS One. 7(e51709)2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Leturque A, Loizeau M, Vaulont S, Salminen
M and Girard J: Improvement of insulin action in diabetic
transgenic mice selectively overexpressing GLUT4 in skeletal
muscle. Diabetes. 45:23–27. 1996.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang Q, Xiao X, Zheng J, Li M, Yu M, Ping
F, Wang T and Wang X: Liraglutide protects cardiac function in
diabetic rats through the PPAα pathway. Biosci Rep, 2018 (Epub
ahead of print).
|
|
31
|
Ismail TA, Soliman MM and Nassan MA:
Molecular and immunohistochemical effects of metformin in a rat
model of type 2 diabetes mellitus. Exp Ther Med. 9:1921–1930.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang X, Liu C, Xu Y, Chen P, Shen Y, Xu Y,
Zhao Y, Chen W, Zhang X, Ouyang Y, et al: Combination of
mesenchymal stem cell injection with icariin for the treatment of
diabetes-associated erectile dysfunction. PLoS One.
12(e0174145)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Du XX, Tao X, Liang S, Che JY, Yang S, Li
H, Chen JG and Wang CM: Hypoglycemic effect of acidic
polysaccharide from schisandra chinensis on T2D rats induced by
high-fat diet combined with STZ. Biol Pharm Bull. 42:1275–1281.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bahadoran Z, Mirmiran P and Azizi F:
Dietary polyphenols as potential nutraceuticals in management of
diabetes: A review. J Diabetes Metab Disord. 12(43)2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang Y, Zhen W, Maechler P and Liu D:
Small molecule kaempferol modulates PDX-1 protein expression and
subsequently promotes pancreatic β-cell survival and function via
CREB. J Nutr Biochem. 24:638–646. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim DH, Jung HA, Sohn HS, Kim JW and Choi
JS: Potential of Icariin metabolites from Epimedium koreanum
Nakai as antidiabetic therapeutic agents. Molecules.
22:2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yamauchi T, Kamon J, Waki H, Terauchi Y,
Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N,
et al: The fat-derived hormone adiponectin reverses insulin
resistance associated with both lipoatrophy and obesity. Nat Med.
7:941–946. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
Nie JM and Li HF: Metformin in combination
with rosiglitazone contribute to the increased serum adiponectin
levels in people with type 2 diabetes mellitus. Exp Ther Med.
14:2521–2526. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Adibian M, Hodaei H, Nikpayam O, Sohrab G,
Hekmatdoost A and Hedayati M: The effects of curcumin
supplementation on high-sensitivity C-reactive protein, serum
adiponectin, and lipid profile in patients with type 2 diabetes: A
randomized, double-blind, placebo-controlled trial. Phytother Res.
33:1374–1383. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nakajima T, Yokota T, Shingu Y, Yamada A,
Iba Y, Ujihira K, Wakasa S, Ooka T, Takada S, Shirakawa R, et al:
Impaired mitochondrial oxidative phosphorylation capacity in
epicardial adipose tissue is associated with decreased
concentration of adiponectin and severity of coronary
atherosclerosis. Sci Rep. 9(3535)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tumminia A, Vinciguerra F, Parisi M,
Graziano M, Sciacca L, Baratta R and Frittitta L: Adipose tissue,
obesity and adiponect in: Role in endocrine cancer risk. Int J Mol
Sci. 20(pii: E2863)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zeng F, Shi J, Long Y, Tian H, Li X, Zhao
AZ, Li RF and Chen T: Adiponectin and endometrial cancer: A
systematic review and meta-analysis. Cell Physiol Biochem.
36:1670–1678. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Duca FA, Côté CD, Rasmussen BA,
Zadeh-Tahmasebi M, Rutter GA, Filippi BM and Lam TK: Metformin
activates a duodenal Ampk-dependent pathway to lower hepatic
glucose production in rats. Nat Med. 21:506–511. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ
and Winder WW: 5' AMP-activated protein kinase activation causes
GLUT4 translocation in skeletal muscle. Diabetes. 48:1667–1671.
1999.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Farzaei MH, Singh AK, Kumar R, Croley CR,
Pandey AK, Coy-Barrera E, Kumar Patra J, Das G, Kerry RG,
Annunziata G, et al: Targeting inflammation by flavonoids: Novel
therapeutic strategy for metabolic disorders. Int J Mol Sci.
20:2019.PubMed/NCBI View Article : Google Scholar
|