Introduction
Rectal cancer represents approximately a third of
colorectal cancer cases in the USA and remains an important
contributor to the global tumor burden (1,2). The
5-year survival rate of patients with rectal cancer undergoing
surgery is 50% because of the high risk of local recurrence (LR),
despite resection being considered as curative (3). The LR of rectal cancer occurs in 30-50%
of patients who have undergone only radical resection (4). Among randomized trials published from
2004 to 2009, 30-40% of patients with rectal cancer developed
metastatic rectal cancer (5). With
decades of research and practice, important advances have been made
in the treatment of rectal cancer with the introduction of total
mesorectal excision (TME), the addition of (chemo)radiotherapy
preoperatively and the use of magnetic resonance imaging for more
accurate clinical staging (6-9).
Improvements in surgical technique have
significantly lowered the incidence of rectal cancer LR. Intact
removal of the entire mesorectum in cancer of the mid or lower
third of the rectum has resulted in LR rates as low as 5-10%
(10). Parallel to improvements in
surgical technique, adjuvant therapy regimens have been tested in
clinical trials in an effort to improve survival and reduce LR
(11). Several randomized controlled
trials have evaluated neoadjuvant radiotherapy in patients
undergoing resection for rectal cancer and collectively found that
it decreased the risk of LR but did not significantly improve
overall survival (OS) or the rate of distant metastases (7,12,13).
However, survival benefit was observed in some trials (14,15).
In order to improve tumor response and long-term
survival, preoperative radiotherapy has been combined with
chemotherapeutic regimens (16).
After long-term exploration and unremitting efforts, preoperative
radiotherapy or chemoradiotherapy followed by TME and postoperative
chemotherapy is the current recommended regimen for patients with
stage II and III rectal cancer (17). Using such multimodality strategies
reduces LR rates to <10% (11).
The addition of neoadjuvant chemotherapy resulted in greater tumor
downsizing and downstaging, improved the pathological complete
response rate (pCR) and local control, but still led to little OS
benefit when compared with preoperative radiotherapy without
chemotherapy (18,19). Considering preoperative radiotherapy
increases the risk of treatment-related toxicities and the
frequency of postoperative complications, some research teams are
investigating neoadjuvant chemotherapy without radiotherapy in
patients with rectal cancer (20-22).
The FOWARC study compared preoperative chemoradiotherapy using
folinic acid, fluorouracil and oxaliplatin (FOLFOX) chemotherapy
plus radiotherapy, with FOLFOX-based chemotherapy alone. The
aforementioned study found that FOLFOX alone seems to have an
identical LR rate, 3-year disease-free survival (DFS) and 3-year OS
compared to standard FOLFOX plus radiotherapy (20). However, there is still a lack of
evidence directly comparing surgery preceded by neoadjuvant
chemoradiotherapy (CRT) with surgery preceded by neoadjuvant
chemotherapy (CT).
Clinical trials and conventional meta-analysis do
not allow comparisons to be made between all regimens, and opinions
concerning a definition for optimum neoadjuvant treatment strategy
for resected rectal cancer differ (23). Therefore, network meta-analysis (NMA)
may be a potential consideration with which to advance the current
understanding of the best regimen for resected rectal cancer and to
help guide clinical decision making. NMA is a statistical method
that aims to combine information from all randomized comparisons
between a set of treatments for a given medical condition (24). By using Bayesian NMA in the present
study, the objective was to find the most effective neoadjuvant
therapy regimen for resected rectal cancer. Regimens were compared
in terms of the primary outcomes OS and LR, and the secondary
outcomes DFS, distant metastases, pCR, organ preservation, 30-day
mortality and anastomotic leak.
Materials and methods
Literature search
MEDLINE (OvidSP; http://ovidsp.ovid.com/), EMBASE (https://www.embase.com/) and Cochrane Central Registry
of Controlled Trials (CENTRAL) (http://cochranelibrary-wiley.com/o/cochrane/clcentral/)
were systematically searched in the range between 1946 up to and
including May 29 2018. Search terms included extensive controlled
vocabulary (medical subject headings and embase subject headings)
in various combinations, supplemented with key words including
rectal cancer, chemotherapy, radiotherapy, chemoradiotherapy and
randomized clinical trials (RCTs). There were no language
restrictions made. Electronic searches were performed and
supplemented with manual searching for all available articles,
including review articles and abstracts from conferences. The
literature search strategy used for the present study is depicted
in Table SI.
Study selection and data
extraction
RCTs that met the following criteria were included
in the present study: i) The study enrolled patients with
resectable rectal cancer; ii) treatments that administered surgery
alone, surgery preceded by neoadjuvant radiotherapy (RT), CT or
CRT; and iii) the study reported on at least one of the following
outcomes: OS, DFS, perioperative deaths (30-day mortality), pCR,
LR, distant metastases, surgery complications and organ
preservation. Exclusion criteria included the following:
Non-resectable or metastatic rectal cancer, any prior intervention
other than diagnostic biopsy and non-randomized trials.
The selection of studies was carried out by two
reviewers (WZ and XJX) and included independently screening titles
and abstracts for inclusion, extracting the data and assessing the
methodological quality of the included studies. Disagreements were
resolved by consensus or with a third adjudicator. Data regarding
study and population characteristics, as well as treatments and
outcomes, were extracted. For OS and DFS, the hazard ratio (HR) and
95% confidence interval (CI) were extracted when available. If HRs
were not reported in the original publications, the HR was
calculated using methods outlined by Tierney et al (25). Odds ratios (ORs) were calculated for
patterns of recurrence, distant metastases, pCR, organ preservation
and surgery complications (for example, perioperative mortality).
For multiple reports of the same trial, reports containing the
longest follow-up data were used.
Risk of bias assessment
For the included studies, assessment of risk of bias
was conducted independently by two reviewers (WZ and XJX) using the
Cochrane risk of bias assessment tool (26,27).
Studies were assessed on the basis of sequence generation,
allocation concealment, blinding, incomplete outcome data,
selective outcome reporting and other sources of bias. Any
discrepancies were resolved through consensus with a third reviewer
(LZD).
Quality of evidence assessment
The quality of evidence for each direct, indirect
and NMA outcome was evaluated according to the Grading of
Recommendations Assessment, Development and Evaluation method
(28,29). The quality of evidence of each direct
comparison outcome was ranked as high, moderate, low and very low,
based on its risk of bias, consistency, directness, precision of
the results and publication bias (28). The quality of evidence of indirect
and network effects estimates were derived from those of
direct-effects estimates by evaluating network geometry,
intransitivity and incoherence (29). For a particular comparison, both
direct and indirect evidence were available, the higher of the two
quality ratings was presented as the quality rating for the NMA
estimate. Detailed information for the quality of evidence of
direct and indirect comparisons is shown in Table SII.
Statistical analysis
Evidence for eight outcomes were synthesized:
Primary outcomes (OS and LR) and secondary outcomes (DFS, distant
metastases, pCR, 30-day mortality, anastomotic leak and organ
preservation). Direct comparison was performed using a
random-effects model to estimate pooled HR or OR and 95% CI
incorporating within- and between-study heterogeneity (30). Statistical heterogeneity of direct
comparison was assessed using the I2 index and Cochrane
Q test. For each outcome, a Bayesian NMA using Markov chain Monte
Carlo simulation with non-informative prior distribution (A prior
distribution which is non-committal about a parameter, for example,
a uniform distribution) was also performed. The analysis used
generalized linear models with a logit link function with 4 chains
and 100,000 iterated simulations, discarding the initial 5,000
iterations as burn-in. Convergence was assessed using the
Brooks-Gelman-Rubin statistic (31).
To test the robustness of this assumption, the node-splitting
method was used to assess whether there was incoherence in the
closed loop (32).
The Bayesian NMAs also allow for the probabilistic
interpretation of uncertainty and ranking of interventions
(33). Rank probabilities were
calculated from proportions of Markov chain cycles, according to
the pooled effect size of each intervention. Surface under the
cumulative ranking curve (SUCRA) for each treatment was calculated
from a cumulative ranking (34). All
the analyses were conducted using R (version 3.4.1; http://www.R-project.org) with R packages gemtc
(version 0.8-2; http://cran.r-project.org/web/packages/gemtc/index.html),
meta (version 4.9-5; https://cran.r-project.org/web/packages/meta/index.html)
and rjags (vesioin 4-8; https://cran.r-project.org/web/packages/rjags/index.html)
and JAGS (version 4.3.0; http://mcmc-jags.sourceforge.net/).
Results
Study characteristics
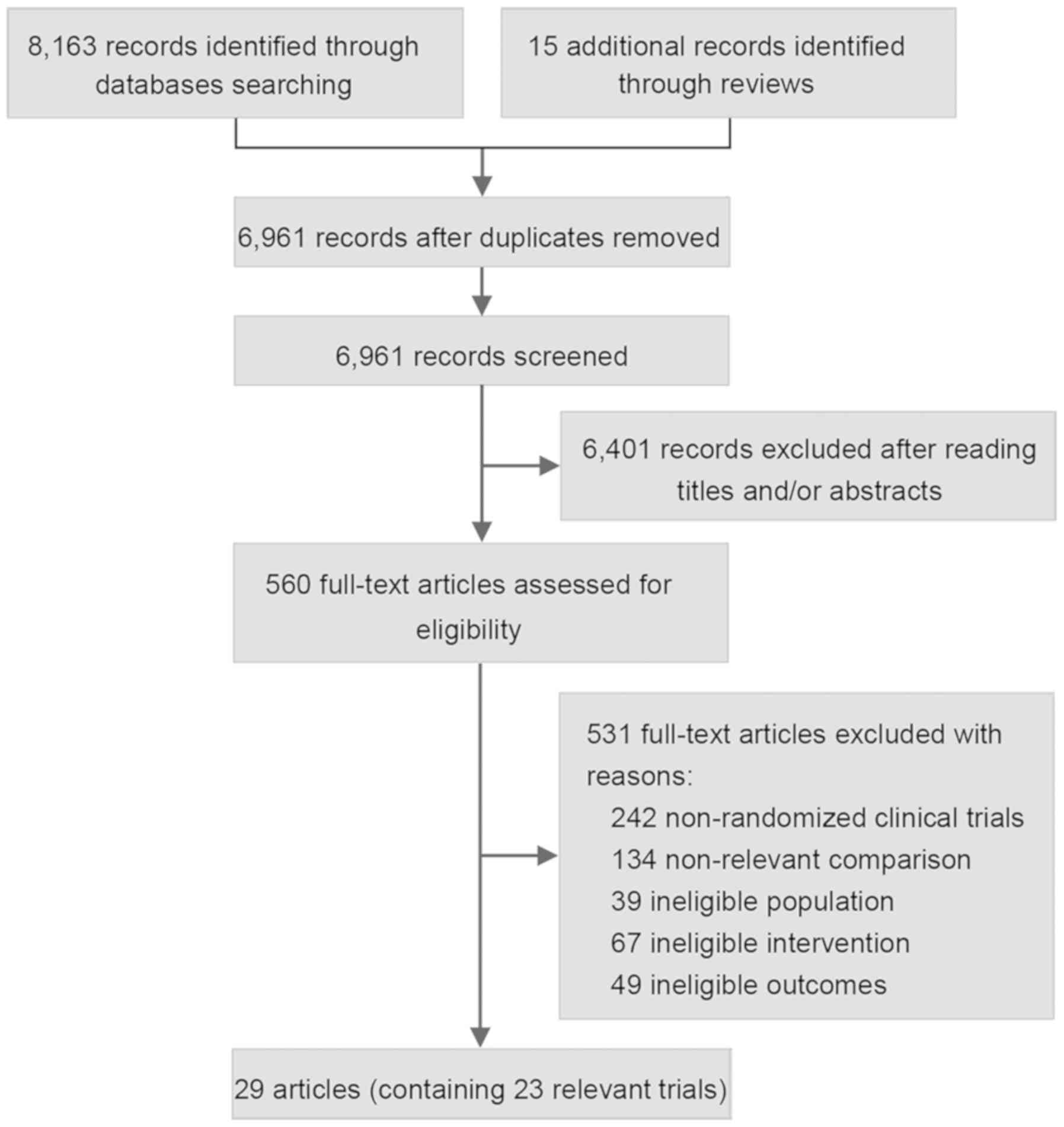
Of the 8,178 citations identified via the literature
search in the present study, 6,961 citations were retained after
removing duplicates and subsequently 6,401 articles were excluded
after title and abstract screening, leaving 560 studies for a
full-text review. Following exclusion of a further 531 articles
which were deemed unsuitable, a total of 23 RCTs with a cumulative
sample size of 10,895 patients were included in the review and NMA
(7,8,11-14,18-20,35-54;
Fig. 1). The number of patients
involved in each study ranged from 68-1,805. Of the 23 studies, 15
included trials that made comparisons between RT and surgery alone
(7,11-13,35-49).
In 6 trials, RT was compared with CRT (8,14,17-18,54).
For the two remaining trials, one compared CRT with surgery alone
(50,51) and the other compared CRT with CT
(30,31). No trial directly comparing CT with RT
or surgery alone was included. Details of the baseline
characteristics of the included trials and the treatment regimens
are presented in Table I.
 | Table IBaseline characteristics of included
trials. |
Table I
Baseline characteristics of included
trials.
| Author, year | Study period | Treatment | Sample size | Treatment
schedule | Cancer type and
clinical stage | Follow-up
(months) | Outcomes | (Refs.) |
|---|
| Higgins et
al, 1975 | 1964-1966 | RT+SS | 347353 | 20.0-25.0 Gy, 20
fr, 12 d; surgery at any time after RT | Operable rectal or
sigmoid cancer | 60.0 (min) | OS, mortality | (35,36) |
| Rider et al,
1977 | NR | RT+SS | 5650 | 5 Gy, 1 fr | Rectal cancer | NR | OS, mortality | (37) |
| Duncan et
al, 1984 | 1975-1978 | RT+SS | 272275 | 20.0 Gy, 10 fr, 14
d; surgery within 1 week after RT | Rectal cancer | 60.0 | OS, DFS,
mortality | (39) |
| Higgins et
al, 1986 | NR | RT+SS | 180181 | 34.5 Gy, 18 fr, 24
d; surgery immediately after RT | Resectable rectal
or sigmoid Cancer | 45.0 | OS, mortality | (40) |
| Gérard et
al, 1988 | 1976-1981 | RT+SS | 224226 | 34.5 Gy, 15fr, 19
d; surgery after RT | T2, T3, T4 NX or M0
resectable rectal cancer | 75.0 | OS, LR, distant
metastases, mortality | (41) |
| Reis et al,
1989 | 1978-1980 | RT+SS | 3434 | 40.0 Gy, 20 fr, 28
d; surgery 1 week after RT | Resectable rectal
or rectosigmoid Cancer | 96.0 | OS, LR, distant
metastases, mortality | (42) |
| Dahl et al,
1990 | 1976-1985 | RT+SS | 155145 | 31.5 Gy, 18 fr, 24
d; surgery 2-3 weeks after RT | Resectable rectal
cancer | 73.2 | OS, DFS, LR,
distant metastases, mortality | (13) |
| Marsh et al,
1994 | 1982-1986 | RT+SS | 143141 | 20.0 Gy, 4 fr, 4 d;
surgery within 1 week after RT | Locally advanced
rectal cancer | 96.0 (min) | OS, DFS, LR,
distant metastases | (12) |
| Goldberg et
al, 1994 | 1980-1984 | RT+SS | 228239 | 15.0 Gy, 3 fr, 5-7
d; surgery 2 days after RT | Resectable rectal
cancer | 60.0 (min) | OS, LR,
mortality | (43) |
| Cedermark et
al, 1995 | 1980-1987 | RT+SS | 424425 | 25.0 Gy, 5 fr, 5-7
d; surgery within 7 days after RT | Resectable rectal
cancer | 144.0 | OS, LR, distant
metastases, mortality | (44) |
| Oates et al,
1996 | 1981,1989 | RT+SS | 139140 | 40.0 Gy, 20 Fr, 28
d; surgery after a minimum of 4 weeks | Rectal cancer | 120.0 | OS, DFS, LR,
distant metastases, mortality | (45) |
| Cedermark et
al, 1997 | 1987-1990 | RT+SS | 375374 | 25.0 Gy, 5 fr, 7 d;
surgery within 1 week after RT | Resectable rectal
cancer | 156.0 | OS, DFS, LR,
mortality | (14) |
| Petersen et
al, 1998 | 1988-1993 | RT+SS | 4746 | 16.5 Gy, 5 fr, 7 d;
surgery within 2 days after RT | Rectal cancer | 44.1.0 | OS, LR,
mortality | (46) |
| Martling et
al, 2001 | 1987-1993 | RT+SS | 272285 | 25.0 Gy, 5 fr, 7 d;
surgery within 1 week after RT | Resectable rectal
cancer | 105.6 | OS, DFS, LR,
distant metastases, mortality | (47) |
| Marijnen et
al, 2011 | 1996-1999 | RT+SS | 897908 | 25.0 Gy, 5 fr, 5 d;
surgery within 10 days of the start of RT | Resectable rectal
cancer | 139.2 | OS, LR, distant
metastases, mortality | (7) |
| Fan et al,
2015 | 2008-2012 | CRT+SS | 9094 | 46.0-50.0 Gy, 23-25
fr, 28 d; 2 cycles of modified XELOX regimen before surgery, 4
cycles of standard XELOX regimen and 4 cycles of capecitabine after
surgery | T3–T4 or
node-positive resectable rectal cancer | 38.0 | OS, LR, distant
metastases, pCR, mortality | (51) |
| Boulis-Wassif et
al, 1984 | 1972-1976 | CRT+SRT+S | 126121 | CRT: 34.5 Gy, 15
fr, 18 d; 5-Fu (375 mg/m2/d) in first 4 days, 4-6 h
before RT RT: 34.5 Gy, 15 fr, 18 d; surgery within 2 weeks after
RT | T2, T3, or
resectable T4 rectal cancer | 62.4 | OS, LR, distant
metastases, pCR, mortality | (15) |
| Gerard et
al, 2006 | 1993-2003 | CRT+SRT+S | 375367 | CRT: 45.0 Gy, 25
fr, 35 d 5-Fu (350 mg/m2/d), LV (20 mg/m2/d)
in 20 min during d 1-5 and 29-33, 1 h before RT RT: 45.0 Gy, 25 fr,
35 d; surgery 3-10 weeks after RT | Resectable T3-4,
NX, M0 rectal cancer | 81.0 | OS, DFS, LR, pCR,
mortality | (19) |
| Bujko et al,
2006 | 1999-2002 | CRT+SRT+S | 157155 | CRT: 50.4 Gy, 28
fr, 5-Fu (325 mg/m2/d), LV (20 mg/m2/d) as
rapid infusion on 5 days in week 1 and 5 of RT RT: 25.0 Gy, 5 fr, 7
d; surgery after 4-6 week | T3 or T4 resectable
rectal cancer | 48.0 | OS, DFS, LR,
distant metastases, pCR | (8) |
| Bosset et
al, 2006 | 1993-2003 | CRT+SRT+S | 505506 | CRT: 45 Gy, 25 fr,
35 d 5-Fu (350 mg/m2/d), LV (20 mg/m2/d) in
20 min in first and fifth week of RT RT: 45.0 Gy, 25 fr, 35 d;
surgery 3-10 weeks after RT | T3 or T4 resectable
rectal cancer | 64.8 | OS, DFS, LR, pCR,
mortality | (52,53) |
| Ngan et al,
2012 | 2001-2006 | CRT+SRT+S | 161162 | CRT: 50.4 Gy, 28
fr, 5 weeks with continuous infusion of FU (225
mg/m2/d), surgery 4-6 weeks after RT RT: 25.0 Gy, 5 fr,
5 d, surgery 3-7 d after RT | T3 resectable
rectal cancer | 70.8 | OS, DFS, LR,
pCR | (54) |
| Latkauskas et
al, 2016 | 2007-2010 | CRT+SRT+S | 7268 | CRT: 50.0 Gy, 25
fr, 35 d, 5-FU (400 g/m2/d), LV (20 mg/m2/d)
during first and last week of RT RT: 25.0 Gy, 5 fr, 35 d in both
arms; surgery 6 week after RT | Stage II-III
resectable rectal cancer | 39.7 | OS, DFS, LR,
distant metastases | (18) |
| Deng et al,
2015 | 2010-2015 | CRT+SCT+S | 158163 | RT: 46.0-50.4 Gy,
23-28 fr, 5-6 weeks for both arms CT: mFOLFOX regimen | Stage II-III
resectable rectal cancer (T3-4 N0 or T1-4 N1-2) | 45.2 | DFS, LR, pCR,
mortality | (20) |
Risk of bias of included studies
Results of the quality assessment of the trials
according to the Cochrane risk-of-bias tool (55) are shown in Fig. S1. The overall risk bias was low and
was well agreed between reviewers. All the studies included in the
NMA were randomized, meaning that the overall selection and
attrition bias were minimized. None of the included studies were
described as double blind or used blinded outcome assessment. In
addition, there were no imbalances between treatment arms in the
number of patients that did not undergo the complete trial
procedure.
Primary outcome
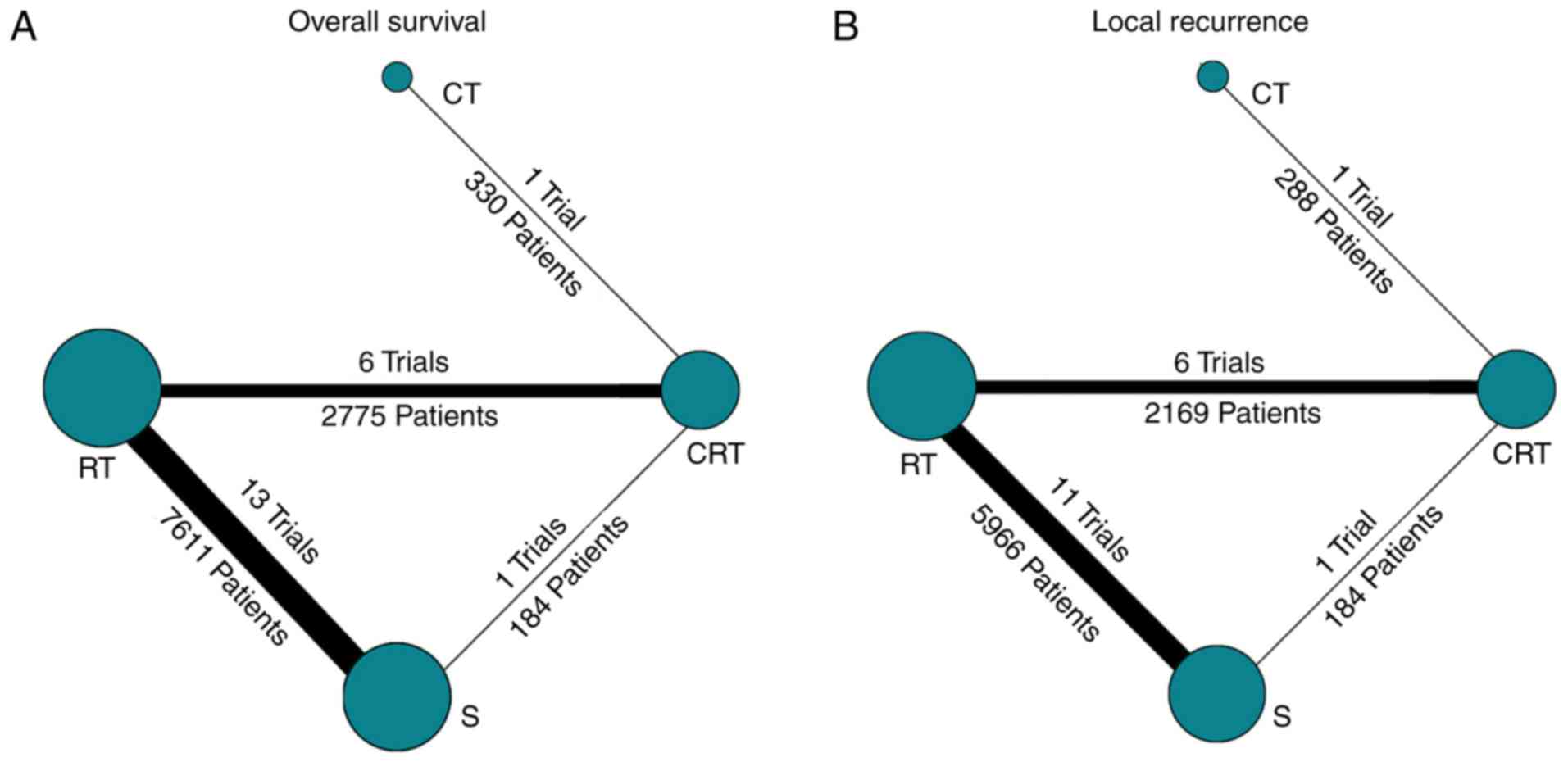
A total of 21 trials including 10,900 patients and
comparing four treatment strategies were included in the 5-year OS
analysis (Fig. 2A). As can be seen
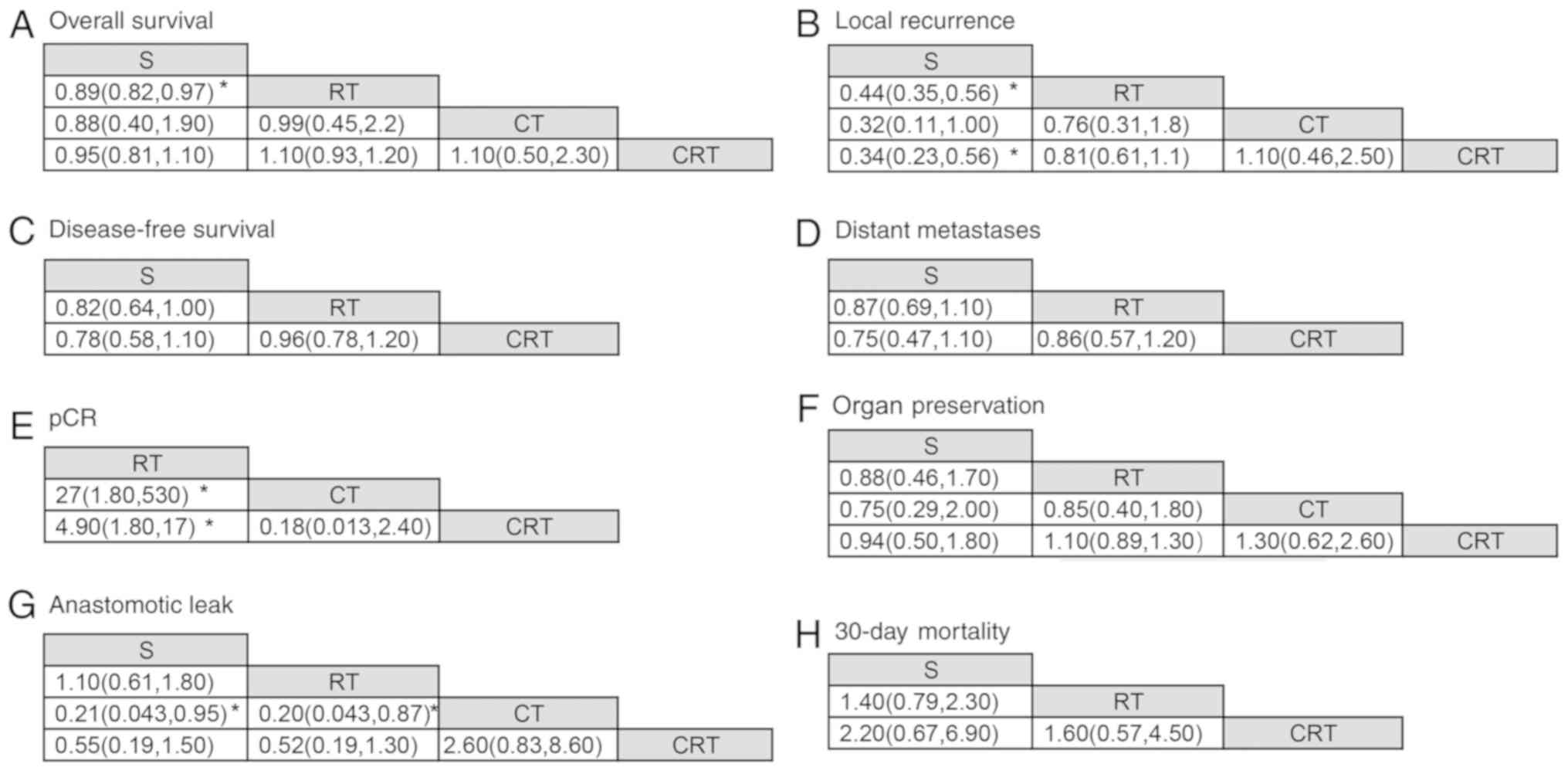
from pairwise comparison (Table
II), RT was associated with improved OS benefit compared with
surgery alone (HR, 0.89; 95% CI, 0.83-0.96; quality of evidence,
high). For NMA, there were no significant differences in OS for all
comparisons except RT vs. surgery alone (HR, 0.89; 95% CI,
0.82-0.97; Table II). The SUCRA
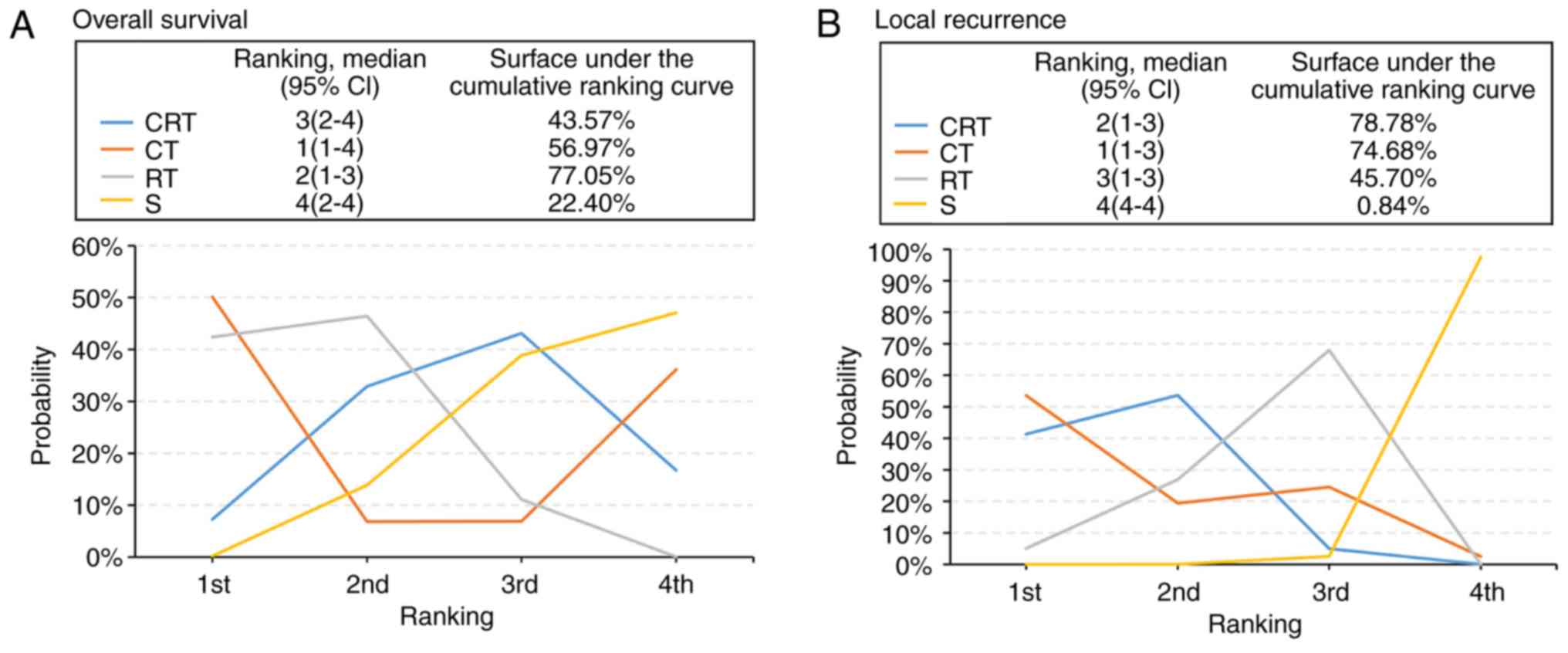
values of 77.05, 56.97 and 43.57% for RT, CT and CRT, respectively
(Fig. 3A), suggested that these were
the three treatments with the highest chance of improving OS in
rectal cancer. Sensitivity analyses were also performed with 16
trials for which the total radiation dose ≥25 Gy. The results were
similar to those for the analysis of all 21 trials.
 | Table IIResults from direct pairwise
comparison and the network meta-analysis. |
Table II
Results from direct pairwise
comparison and the network meta-analysis.
| A, RT vs. S |
|---|
| Outcomes | Pairwise
comparison | Network
meta-analysis |
|---|
| Overall
survival | HR 0.89
(0.83-0.96)a | HR 0.89
(0.82-0.97)a |
| Local
recurrence | OR 0.44
(0.37-0.51)a | OR 0.44
(0.35-0.56)a |
| DFS | HR 0.80
(0.63-1.02) | HR 0.82
(0.64-1.00) |
| Distant
metastases | OR 0.87
(0.73-1.05) | OR 0.87
(0.69-1.10) |
| Mortality | OR 1.38
(0.89-2.12) | OR 1.40
(0.79-2.20) |
| pCR | NA | NA |
| Anastomotic
leak | OR 1.12
(0.75-1.67) | OR 1.10
(0.61-1.80) |
| Organ
preservation | NA | OR 0.88
(0.46-1.70) |
| B, CRT vs. S |
| Outcomes | Pairwise
comparison | Network
meta-analysis |
| Overall
survival | HR 1.34
(0.43-4.21) | HR 0.95
(0.81-1.10) |
| Local
recurrence | OR 1.05
(0.25-4.30) | OR 0.34
(0.23-0.56) |
| DFS | HR 1.48
(0.34-6.52) | HR 0.78
(0.58-1.10) |
| Distant
metastases | OR 0.76
(0.30-1.90) | OR 0.75
(0.47-1.10) |
| Mortality | NA | OR 2.10
(0.68-6.40) |
| pCR | NA | NA |
| Anastomotic
leak | OR 0.24
(0.05-1.18) | OR 0.55
(0.19-1.50) |
| Organ
preservation | OR 0.94
(0.50-1.77) | OR 0.94
(0.50-1.80) |
| C, CRT vs. CT |
| Outcomes | Pairwise
comparison | Network
meta-analysis |
| Overall
survival | HR 1.08
(0.51-2.29) | HR 1.10
(0.50-2.30) |
| Local
recurrence | OR 1.06
(0.46-2.40) | OR 1.10
(0.46-2.50) |
| DFS | NA | NA |
| Distant
metastases | NA | NA |
| Mortality | NA | NA |
| pCR | OR 0.18
(0.09-0.39)a | OR 0.18
(0.01-2.40) |
| Anastomotic
leak | OR 2.58
(1.25-5.31)a | OR 2.6
(0.83-8.60) |
| Organ
preservation | OR 1.24
(0.61-2.51) | OR 1.3
(0.62-2.60) |
| D, CRT vs. RT |
| Outcomes | Pairwise
comparison | Network
meta-analysis |
| Overall
survival | HR 1.06
(0.92-1.21) | HR 1.10
(0.93-1.20) |
| Local
recurrence | OR 0.78
(0.48-1.25) | OR 0.81
(0.61-1.10) |
| DFS | HR 0.92
(0.82-1.05) | HR 0.96
(0.78-1.20) |
| Distant
metastases | OR 0.82
(0.51-1.30) | OR 0.86
(0.57-1.20) |
| Mortality | OR 1.55
(0.86-2.79) | OR 1.60
(0.58-4.30) |
| pCR | OR 4.01
(2.24-7.18)a | OR 4.9
(1.80-17.0)a |
| Anastomotic
leak | OR 0.75
(0.30-1.85) | OR 0.52
(0.19-1.30) |
| Organ
preservation | OR 1.06
(0.90-1.26) | OR 1.10
(0.89-1.30) |
A total of 19 trials (8,607 patients) comparing
three neoadjuvant treatments and surgery were included in the LR
analysis (Fig. 2B). The results of
NMA suggested a significant advantage of RT or CRT compared with
surgery alone (Table II; RT vs.
surgery alone: OR, 0.44; 95% CI, 0.35-0.56; quality of evidence,
high and CRT vs. surgery alone: OR, 0.34; 95% CI, 0.23-0.56;
quality of evidence, moderate). There were no significant
differences for both direct pairwise analysis and NMA between CRT
and RT or CT. Sensitivity analyses were also performed with 15
studies for which the total dose of radiation was ≥25 Gy. The
results were similar to those for the analysis of all 19 studies.
Ranking probabilities analysis further supported the conclusion
that CRT and CT were probably the best and second-best strategies,
respectively, for local tumor control (Fig. 3B). The SUCRA values of CRT and CT
were 78.78 and 74.68%, respectively (Fig. 3B).
Secondary outcome
The NMAs for the 6 secondary outcomes (5-year DFS,
distant metastases, pCR, organ preservation, 30-day mortality and
anastomotic leak) included 6 to 16 trials involving 2,767 to 7,410
patients with clinically resectable rectal cancer (Fig. S2). The incidence of distant
metastases was 28.2% (1,558 of 5,516), pCR,9.0% (267 of 2,971),
organ preservation 54.0% (1,496 of 2,767), 30-day mortality 4.1%
(316 of 7,410) and anastomotic leak 5.8% (175 of 3,042). In DFS
analysis, 10 trials comparing the three preoperative treatments
were included. The HRs are shown in Table II and Fig. 4. RT was associated with improved DFS
than surgery alone (HR, 0.82; 95% CI, 0.64-1.00; quality of
evidence, low). CRT displayed no significant improvement in DFS
compared with RT (HR, 0.96; 95% CI, 0.78-1.20).
The estimated OR of pairwise analysis and NMA for
distant metastases, pCR, organ preservation and anastomotic leak
are also shown in Table II and
Fig. 4. There were no significant
differences between interventions for the likelihood of the distant
metastases. For trials comparing RT with surgery alone, pCR was not
reported and could not be estimated on the basis of the information
provided. CRT and CT were associated with improved pCR compared
with RT (CRT vs. RT: OR, 4.90; 95% CI, 1.80-17.00; quality of
evidence, low and CT vs. RT: OR, 27.0; 95% CI, 1.80-530.00; quality
of evidence, very low; Fig. 4).
For the analysis of complications, 30-day mortality
and anastomotic leak data were extracted. A total of 16 trials
(7726 patients) comparing three treatments were included in the
30-day mortality analysis. One trial compared CRT with CT (20), reporting no treatment-related
mortality and being the only trial including results of CT, this
trial was excluded from the NMA. Neoadjuvant strategies (RT and
CRT) tended to have more treatment-related mortality than surgery
alone, but there were no significant differences (Table II and Fig. 4). The surgical complication of
anastomotic leak was reported in nine trials. CT was associated
with a lower likelihood of anastomotic leak compared to all other
treatments (CT vs. surgery alone: OR, 0.21; 95% CI, 0.04-0.95;
quality of evidence, low; CT vs. RT: OR, 0.20; 95% CI, 0.04-0.87;
quality of evidence, low and CRT vs. CT: OR, 2.60; 95% CI,
0.83-8.60; quality of evidence, low). The SUCRA analysis suggested
that CT had the lowest risk of anastomotic leak with a SUCRA value
of 97.26% (Fig. S3E).
Ranking probability
The ranking probability and the results of SUCRA
analysis are shown in Figs. 3 and
S3. For the primary outcomes, CT
had the highest overall probability of being the best strategy for
the neoadjuvant treatment of resectable rectal cancer. Considering
all the outcomes, CRT was the best strategy based on SUCRA.
Discussion
The NMA in the present study included 23 trials with
10,895 patients with resectable rectal cancer and simultaneously
estimated relative effects of four currently used treatment
strategies. The present study revealed that preoperative
radiotherapy displayed significant survival benefit over surgery
alone. No statistically significant differences were observed
between CRT and RT or CT. In addition, primary analysis of LR
suggested that RT and CRT played a role in local control of rectal
cancer when compared with surgery alone, but there were no
noticeable differences in survival benefit between the comparisons
of three neoadjuvant treatments. With the ranking analysis, CT
seemed to be the best strategy among all the included strategies
for primary outcomes assessed (CT ranked the first and had the
biggest total SUCRA value considering the primary outcomes), whilst
CRT was the best strategy when considering all the included
outcomes. The evidence for the secondary outcomes was of low
quality overall. Out of the outcomes assessed, only anastomotic
leak estimated the relative effects of four strategies and CT
remained to be the best strategy. The analysis for DFS, distant
metastases, pCR and 30-day mortality compared three different
strategies, all of them with no significant difference.
Several systemic reviews have evaluated various
strategies using conventional pairwise comparison (3,16,56,57).
Three meta-analyses confirmed that preoperative radiotherapy
improved OS and significantly reduced the LR compared with surgery
alone (3,56,57). The
present study analyzed the majority of these trials and the results
for the primary outcomes were consistent with the results from the
previous aforementioned studies. Previously, three systematic
reviews have reported that CRT provides no superior OS compared
with RT and the result of local control rate was different between
these analyses (16,57,58). A
previous meta-analysis identified five trials that reported that
chemoradiotherapy improved local tumor control as opposed to
radiotherapy, with no impact on perioperative outcome or long-term
survival (57). The pairwise
analysis and NMA carried out in the present study displayed no
improvement in both local control and long-term survival for
patients treated with CRT compared to those treated with RT.
However, significant heterogeneity remained when the data for LR
were analyzed using the random effects assumption
(I2=61%, P=0.03). In this present study, it is unclear
whether the heterogeneity is attributable to the addition of
chemotherapy to a different RT schedule and a different waiting
period until surgery.
To the best of our knowledge, the present study is
the first NMA to provide estimates of the outcomes and effects of
pairwise comparisons of potential neoadjuvant therapy regimens for
resectable rectal cancer. The efficacy of each potential regimen
could be ranked using these polled outcomes. However, there was
only one eligible trial for CT compared with other strategies
(20), which decreased the quality
of the whole analysis. Therefore, future studies that directly
evaluate survival outcomes and side effects in patients receiving
neoadjuvant CT are warranted.
The present study had some further limitations.
Firstly, there were some differences in baseline characteristics of
the included trials that could lead to biased results. For example,
three of the trials included patients with cancer of the
rectosigmoid (40,41,43). The
majority of the early trials using Dukes classification included
patients from Dukes A-C (15,32,35),
whereas previous studies using TNM classification for clinical
staging only included patients with stage II/III rectal cancer
(18,20). In the MRC I trial, 28% of patients
with metastatic cancer were found to have a Dukes' stage A lesion
for which adjuvant therapy is not currently advised (38). These included patients may have led
to biased results. Secondly, some regimens with different doses or
duration times were grouped together, which may further increase
the bias. For example, for the CRT treatment strategy, the total
dose of radiation ranged from 34.5-50.4 Gy and three types of
chemotherapy regimen (fluorouracil/leucovorin, modified FOLFOX and
capecitabine plus oxaliplatin) were used. Thirdly, only one trial
compared the neoadjuvant CT regime with CRT therapy strategy,
limiting the assessment (20).
Fourthly, considering there was no significant survival benefit
between the three neoadjuvant treatment strategies, the analysis of
the side effects, such as toxicity or quality of life, could be
important to evaluate the relative effects of each regime. However,
a number of the included trials did not report these outcomes, but
future trials should describe them.
Based on the primary and secondary results, the NMA
performed in the present study found that neoadjuvant radiotherapy
decreases the LR and improves OS compared with surgery alone for
resected rectal cancer. Neoadjuvant chemoradiotherapy displayed no
significant OS benefit or local control compared with RT. CRT was
the best neoadjuvant therapy and CT was likely the second best for
all outcomes based on the ranking probability and SUCRA. These
findings were limited by overall low quality of evidence.
Supplementary Material
Risk of bias of included studies. Risk
of bias across studies assessed using the Cochrane risk of bias
tool (1,2). Studies are assessed on the basis of sequence
generation, allocation concealment, blinding, incomplete outcome
data, selective reporting and other sources of bias. (A) Risk of
bias graph, each risk of bias item presented as percentages across
all included studies. (B) Risk of bias summary for each included
study.
Network geometry for secondary
outcomes in the network meta-analysis. Each node indicates a
strategy and the node size is proportional to the number of
patients in the treatment group. Lines represent direct comparisons
between two treatments and line thickness represents the number of
randomized controlled trials included in each comparison, also
represented by the numbers. (A) DFS (10 trials), (B) distant
metastases (13 trials), (C) pCR (7 trials), (D) organ preservation
(6 trials), (E) anastomotic leak (9 trials) and (F) 30-day
mortality (16 trials).DFS, disease-free survival; pCR, pathological
complete response rate; S, surgery alone; RT, surgery preceded by
neoadjuvant radiotherapy; CRT, surgery preceded by neoadjuvant
chemoradiotherapy; CT, surgery preceded by neoadjuvant
chemotherapy.
Ranking probability and SUCRA of
strategies for secondary outcomes in the network meta-analysis of
neoadjuvant treatments for resectable rectal cancer. Each line
represents a treatment strategy. The x-axis indicates the ranking
of strategies, with ‘1st’ representing the best. The y-axis
represents the probability of each ranking. (A) DFS, (B) distant
metastases, (C) pCR, (D) organ preservation, (E) anastomotic leak
and (F) 30-day mortality. SUCRA, surface under the cumulative
ranking curve; DFS, disease-free survival; pCR, pathological
complete response rate; S, surgery alone; RT, surgery preceded by
neoadjuvant radiotherapy; CRT, surgery preceded by neoadjuvant
chemoradiotherapy; CT, surgery preceded by neoadjuvant
chemotherapy.
Literature search strategy.
GRADE evidence profile table for
direct comparisons and network meta-analysis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural and
Scientific grants from Zhangzhou (grant no. ZZ2018J11) and the
nursery grants from the Affiliated Southeast Hospital of Xiamen
University (grant nos. 16Y007 and 17Y006).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ and SZ contributed to the study design/planning,
data collection/entry, data analysis/statistics, data
interpretation and funds collection. XJX and LZD contributed to the
preparation of the manuscript and literature analysis/search. RRL
and KN contributed to the data collection/entry. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Stintzing S: Management of colorectal
cancer. F1000Prime Rep. 6(108)2014.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Cammà C, Giunta M, Fiorica F, Pagliaro L,
Craxì A and Cottone M: Preoperative radiotherapy for resectable
rectal cancer: A meta-analysis. JAMA. 284:1008–1015.
2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Young PE, Womeldorph CM, Johnson EK,
Maykel JA, Brucher B, Stojadinovic A, Avital I, Nissan A and Steele
SR: Early detection of colorectal cancer recurrence in patients
undergoing surgery with curative intent: Current status and
challenges. J Cancer. 5:262–271. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Glynne-Jones R and Kronfli M: Locally
advanced rectal cancer: A comparison of management strategies.
Drugs. 71:1153–1177. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Heald RJ and Ryall RD: Recurrence and
survival after total mesorectal excision for rectal cancer. Lancet.
1:1479–1482. 1986.PubMed/NCBI View Article : Google Scholar
|
|
7
|
van Gijn W, Marijnen CA, Nagtegaal ID,
Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius
B and Van de Velde CJ: Dutch Colorectal Cancer Group: Preoperative
radiotherapy combined with total mesorectal excision for resectable
rectal cancer: 12-year follow-up of the multicentre, randomised
controlled TME trial. Lancet Oncol. 12:575–582. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bujko K, Nowacki MP, Nasierowska-Guttmejer
A, Michalski W, Bebenek M and Kryj M: Long-term results of a
randomized trial comparing preoperative short-course radiotherapy
with preoperative conventionally fractionated chemoradiation for
rectal cancer. Br J Surg. 93:1215–1223. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
MERCURY Study Group: Diagnostic accuracy
of preoperative magnetic resonance imaging in predicting curative
resection of rectal cancer: Prospective observational study. BMJ.
333(779)2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Heald RJ, Moran BJ, Ryall RD, Sexton R and
MacFarlane JK: Rectal cancer: The Basingstoke experience of total
mesorectal excision, 1978-1997. Arch Surg. 133:894–899.
1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ceelen WP, Van Nieuwenhove Y and Fierens
K: Preoperative chemoradiation versus radiation alone for stage II
and III resectable rectal cancer. Cochrane Database Syst Rev.
CD006041:2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Marsh PJ, James RD and Schofield PF:
Adjuvant preoperative radiotherapy for locally advanced rectal
carcinoma. Results of a prospective, randomized trial. Dis Colon
Rectum. 37:1205–1214. 1994.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dahl O, Horn A, Morild I, Halvorsen JF,
Odland G, Reinertsen S, Reisaeter A, Kavli H and Thunold J:
Low-dose preoperative radiation postpones recurrences in operable
rectal cancer. Results of a randomized multicenter trial in western
Norway. Cancer. 66:2286–2294. 1990.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Swedish Rectal Cancer Trial, Cedermark B,
Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE and Wilking N:
Improved survival with preoperative radiotherapy in resectable
rectal cancer. N Engl J Med. 336:980–987. 1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Boulis-Wassif S, Gerard A, Loygue J,
Camelot D, Buyse M and Duez N: Final results of a randomized trial
on the treatment of rectal cancer with preoperative radiotherapy
alone or in combination with 5-fluorouracil, followed by radical
surgery. Trial of the European organization on research and
treatment of cancer gastrointestinal tract cancer cooperative
group. Cancer. 53:1811–1818. 1984.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Onaitis MW, Noone RB, Hartwig M, Hurwitz
H, Morse M, Jowell P, McGrath K, Lee C, Anscher MS, Clary B, et al:
Neoadjuvant chemoradiation for rectal cancer: Analysis of clinical
outcomes from a 13-year institutional experience. Ann Surg.
233:778–785. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
National Comprehensive Cancer Network.
Rectal cancer (version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf.
Accessed July, 2018.
|
|
18
|
Latkauskas T, Pauzas H, Kairevice L,
Petrauskas A, Saladzinskas Z, Janciauskiene R, Gudaityte J,
Lizdenis P, Svagzdys S, Tamelis A and Pavalkis D: Preoperative
conventional chemoradiotherapy versus short-course radiotherapy
with delayed surgery for rectal cancer: Results of a randomized
controlled trial. BMC Cancer. 16(927)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gérard JP, Conroy T, Bonnetain F, Bouché
O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E,
Maurel J, et al: Preoperative radiotherapy with or without
concurrent fluorouracil and leucovorin in T3-4 rectal cancers:
Results of FFCD 9203. J Clin Oncol. 24:4620–4625. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Deng Y, Chi P, Lan P, Wang L, Cui L, Chen
D, Cao J, Wei H, Peng X, Cai G, et al: A multicenter randomized
controlled trial of mFOLFOX6 with or without radiation in
neoadjuvant treatment of local advanced rectal cancer (FOWARC
study): Preliminary results. J Clin Oncol. 33:2015.(In Chinese).
View Article : Google Scholar
|
|
21
|
Schrag D, Weiser MR, Goodman KA, Gonen M,
Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem
JG, et al: Neoadjuvant chemotherapy without routine use of
radiation therapy for patients with locally advanced rectal cancer:
A pilot trial. J Clin Oncol. 32:513–518. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang J, Huang M, Cai Y, Wang L, Xiao J,
Lan P, Hu H, Wu X, Ling J, Peng J, et al: Neoadjuvant chemotherapy
with mFOLFOXIRI without routine use of radiotherapy for locally
advanced rectal cancer. Clin Colorectal Cancer. 18:238–244.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lumley T: Network meta-analysis for
indirect treatment comparisons. Stat Med. 21:2313–2324.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Rücker G: Network meta-analysis,
electrical networks and graph theory. Res Synth Methods. 3:312–324.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8(16)2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L and Sterne JA:
Cochrane Bias Methods Group; Cochrane Statistical Methods Group:
The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Higgins JP and Altman DG: Assessing Risk
of Bias in Included Studies. Cochrane Handbook for Systematic
Reviews of Interventions: Cochrane Book Series: 187-241, 2008.
|
|
28
|
Guyatt GH, Oxman AD, Vist GE, Kunz R,
Falck-Ytter Y, Alonso-Coello P and Schünemann HJ: GRADE Working
Group: GRADE: An emerging consensus on rating quality of evidence
and strength of recommendations. BMJ. 336:924–926. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Puhan MA, Schünemann HJ, Murad MH, Li T,
Brignardello-Petersen R, Singh JA, Kessels AG and Guyatt GH: GRADE
Working Group: A GRADE working group approach for rating the
quality of treatment effect estimates from network meta-analysis.
BMJ. 349(g5630)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
|
|
31
|
Brooks SP and Gelman A: General methods
for monitoring convergence of iterative simulations. J Comput
Graphical Statistics. 7:434–455. 1998. View Article : Google Scholar
|
|
32
|
Dias S, Welton NJ, Caldwell DM and Ades
AE: Checking consistency in mixed treatment comparison
meta-analysis. Stat Med. 29:932–944. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jansen JP, Fleurence R, Devine B, Itzler
R, Barrett A, Hawkins N, Lee K, Boersma C, Annemans L and
Cappelleri JC: Interpreting indirect treatment comparisons and
network meta-analysis for health-care decision making: Report of
the ISPOR task force on indirect treatment comparisons good
research practices: Part 1. Value Health. 14:417–428.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Salanti G, Ades AE and Ioannidis JP:
Graphical methods and numerical summaries for presenting results
from multiple-treatment meta-analysis: An overview and tutorial. J
Clin Epidemiol. 64:163–171. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Higgins GA Jr, Conn JH, Jordan PH Jr,
Humphrey EW, Roswit B and Keehn RJ: Preoperative radiotherapy for
colorectal cancer. Ann Surg. 181:624–631. 1975.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Roswit B, Higgins GA and Keehn RJ:
Preoperative irradiation for carcinoma of the rectum and
rectosigmoid colon: Report of a National Veterans Administration
randomized study. Cancer. 35:1597–1602. 1975.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rider WD, Palmer JA, Mahoney LJ and
Robertson CT: Preoperative irradiation in operable cancer of the
rectum: Report of the Toronto trial. Can J Surg. 20:335–338.
1977.PubMed/NCBI
|
|
38
|
Duncan W, Smith AN, Freedman LS, Alderson
MR, Arnott SJ, Bleehen N M, Bond WH, Crowther D, Deeley TJ, Duthie
HL, et al: A trial of preoperative radiotherapy in the management
of operable rectal cancer. Br J Surg. 69:513–519. 1982.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Duncan W, Smith AN, Freedman LS, Alderson
MR, Arnott SJ, Bleehen N M, Bond WH, Crowther D, Deeley TJ, Duthie
HL, et al: The evaluation of low dose pre-operative X-ray therapy
in the management of operable rectal cancer; results of a randomly
controlled trial. Br J Surg. 71:21–25. 1984.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Higgins GA, Humphrey EW, Dwight RW, Roswit
B, Lee LE Jr and Keehn RJ: Preoperative radiation and surgery for
cancer of the rectum. Veterans administration surgical oncology
group trial II. Cancer. 58:352–359. 1986.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gérard A, Buyse M, Nordlinger B, Loygue J,
Pène F, Kempf P, Bosset JF, Gignoux M, Arnaud JP and Desaive C:
Preoperative radiotherapy as adjuvant treatment in rectal cancer.
Final results of a randomized study of the European organization
for research and treatment of cancer (EORTC). Ann Surg.
208:606–614. 1988.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Reis Neto JA, Quilici FA and Reis JA Jr: A
comparison of nonoperative vs. preoperative radiotherapy in rectal
carcinoma. A 10-year randomized trial. Dis Colon Rectum.
32:702–710. 1989.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Goldberg PA, Nicholls RJ, Porter NH, Love
S and Grimsey JE: Long-term results of a randomised trial of
short-course low-dose adjuvant pre-operative radiotherapy for
rectal cancer: Reduction in local treatment failure. Eur J Cancer.
30A:1602–1606. 1994.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cedermark B, Johansson H, Rutqvist LE and
Wilking N: The Stockholm I trial of preoperative short term
radiotherapy in operable rectal carcinoma. A prospective randomized
trial. Stockholm colorectal cancer study group. Cancer.
75:2269–2275. 1995.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Oates G, Stenning S and Hardcastle J:
Randomised trial of surgery alone versus radiotherapy followed by
surgery for potentially operable locally advanced rectal cancer.
Medical research council rectal cancer working party. Lancet.
348:1605–1610. 1996.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Petersen S, Hellmich G, Baumann M,
Herrmann T, Henke G and Ludwig K: Brief preoperative radiotherapy
in surgical therapy of rectal carcinoma. Long-term results of a
prospective randomized study. Chirurg. 69:759–765. 1998.(In
German). PubMed/NCBI View Article : Google Scholar
|
|
47
|
Martling A, Holm T, Johansson H, Rutqvist
LE and Cedermark B: Stockholm Colorectal Cancer Study Group: The
Stockholm II trial on preoperative radiotherapy in rectal
carcinoma: Long-term follow-up of a population-based study. Cancer.
92:896–902. 2001.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Randomized study on preoperative
radiotherapy in rectal carcinoma. Stockholm colorectal cancer study
group. Ann Surg Oncol. 3:423–430. 1996.
|
|
49
|
Kapiteijn E, Marijnen CA, Nagtegaal ID,
Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B,
van Krieken JH, et al: Preoperative radiotherapy combined with
total mesorectal excision for resectable rectal cancer. N Engl J
Med. 345:638–646. 2001.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wang F, Lu ZH, Pan Z, Gao YH, Chen G, Wu
X, Ding PR, Zeng ZF and Wan D: Preoperative radiotherapy combined
with simultaneous chemotherapy with capecitabine plus oxaliplatin
versus surgery alone: A single-centered, phase II study in patients
with mid/low rectal cancer. J Clin Oncol. 32:2014.(In Chinese).
View Article : Google Scholar
|
|
51
|
Fan WH, Wang FL, Lu ZH, Pan ZZ, Li LR, Gao
YH, Chen G, Wu XJ, Ding PR, Zeng ZF and Wan DS: Surgery with versus
without preoperative concurrent chemoradiotherapy for mid/low
rectal cancer: An interim analysis of a prospective, randomized
trial. Chin J Cancer. 34:394–403. 2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bosset JF, Collette L, Calais G, Mineur L,
Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A and Ollier
JC: EORTC Radiotherapy Group Trial 22921: Chemotherapy with
preoperative radiotherapy in rectal cancer. N Engl J Med.
355:1114–1123. 2006.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bosset JF, Calais G, Mineur L, Maingon P,
Radosevic-Jelic L, Daban A, Bardet E, Beny A, Briffaux A and
Collette L: Enhanced tumorocidal effect of chemotherapy with
preoperative radiotherapy for rectal cancer: Preliminary
results--EORTC 22921. J Clin Oncol. 23:5620–5627. 2005.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ngan SY, Burmeister B, Fisher RJ, Solomon
M, Goldstein D, Joseph D, Ackland SP, Schache D, McClure B,
McLachlan SA, et al: Randomized trial of short-course radiotherapy
versus long-course chemoradiation comparing rates of local
recurrence in patients with T3 rectal cancer: Trans-tasman
radiation oncology group trial 01.04. J Clin Oncol. 30:3827–3833.
2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Higgins JPT and Green S (eds): Cochrane
handbook for systematic reviews of interventions, version 5.1.0
(updated Marh 2011). The Cochrane Collaboration 2011.
|
|
56
|
Wong RK, Tandan V, De Silva S and
Figueredo A: Pre-operative radiotherapy and curative surgery for
the management of localized rectal carcinoma. Cochrane Database
Syst Rev. CD002102:2007.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rahbari NN, Elbers H, Askoxylakis V,
Motschall E, Bork U, Buchler MW, Weitz J and Koch M: Neoadjuvant
radiotherapy for rectal cancer: Meta-analysis of randomized
controlled trials. Ann Surg Oncol. 20:4169–4182. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Latkauskas T, Paskauskas S, Dambrauskas Z,
Gudaityte J, Saladzinskas S, Tamelis A and Pavalkis D: Preoperative
chemoradiation vs radiation alone for stage II and III resectable
rectal cancer: A meta-analysis. Colorectal Dis. 12:1075–1083.
2010.PubMed/NCBI View Article : Google Scholar
|