Introduction
For patients with hepatic malignancy, liver
resection may often be the only treatment option (1). Excessive blood loss during surgery is
associated with poor postoperative outcomes (2); to avoid this, vascular inflow occlusion
(VIO) is often performed during liver transaction (3). Although VIO effectively reduces blood
loss, hepatic oxygen supply is interrupted, which results in
metabolic disruption that subjects the liver to hepatic
ischemia/reperfusion (I/R) injury once oxygen is reintroduced
(4).
Apoptosis is a process of programmed cell death that
serves an important role in the progression of I/R injury (5). Intrinsic apoptosis, also termed the
mitochondrial apoptosis pathway, is induced by intracellular
stress, such as oxidative stress, and subsequent activation of the
caspase family-mediated apoptotic cascade (6). Apoptotic hepatocytes are observed in
liver I/R injury (7).
Pharmacological inhibition of hepatocyte apoptosis has been
demonstrated to improve I/R injury (8,9).
Therefore, targeting apoptosis may be a promising preventive and
therapeutic strategy for hepatic I/R injury.
CD5-like (CD5L) protein is a soluble glycoprotein,
also known as apoptosis inhibitor of macrophage (AIM) (10). CD5L serves diverse roles in the
relationship between lipid homeostasis and the immune response
(11). CD5L localizes to cell
surface receptor cluster of differentiation 36 (CD36) to promote
the transcription of genes involved in the regulation of
mitochondrial biogenesis to maintain energy and metabolic
homeostasis; thus, CD5L may promote antiapoptotic effects in
hepatocellular carcinoma (12,13).
Therefore, exogenous CD5L may have beneficial effects in protecting
liver from I/R induced injury.
The autophagy salvage pathway is an additional means
of energy generation in cells and can be activated by various
cellular stressors, including I/R (14). Autophagy is a process of degradation
and recycling of large molecules and dysfunctional organelles,
protecting cells from apoptosis (15). As an inducer of autophagy, CD5L may
serve a functional role in cytoprotection processes in macrophages
and hepatocytes (12,16).
Hepatic I/R injury is a sterile inflammatory
response that follows hepatic ischemia and is characterized by
overproduction of reactive oxygen species (ROS) followed by
hepatocyte apoptosis (17,18). During ischemia, the absence of oxygen
leads to an accumulation of ROS and a reduction in antioxidative
enzymes (19). A previous study has
suggested that CD5L may promote an anti-inflammatory cytokine
profile through the modulation of autophagy, leading to an
inhibition of ROS generation (20).
The present study investigated whether CD5L was able to modulate
cellular oxidative stress to alleviate the I/R injury.
The aim of the present study was to determine
whether exogenous CD5L would enhance autophagy through the CD36
receptor and decrease I/R-induced oxidative stress, leading to
inhibition of hepatocyte apoptosis, thereby attenuating hepatic I/R
injury.
Materials and methods
Isolation, culture and treatment of
hepatocytes
All animals received humane care according to
protocols approved by the institutional care and use committee at
the Wenzhou Medical University (Ethics Committee of Wenzhou Medical
University, Wenzhou, China; approval no. WMU18825). A total of 60
C57BL/6 mice were used for the present study. The housing
conditions for the mice were as follows: Light/dark cycle, 12-h;
temperature, 21±2˚C; relative humidity, 30-70%; food and water,
freely available to each animal throughout. Mouse hepatocytes were
isolated from 3-month-old male C57BL/6 mice (n=12/group) using the
collagenase perfusion method as previously described (21). Briefly, mice were anesthetized with
3.5% chloral hydrate (10 µl/g body weight; 350 mg/kg). Following a
laparotomy, the vena cava was catheterized. The liver was perfused
with pre-warmed EGTA buffer followed by collagenase buffer. The
liver was immediately dissected and placed in a 10-cm cell culture
dish for mincing. The separated hepatocytes were filtered through a
70-µm cell strainer to remove tissue debris. Hepatocytes were
washed twice with cold low-glucose (5.5 mM) Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum and centrifuged at 50 x g for 5
min at 4˚C.
For experiments involving hypoxia, the medium was
replaced with serum-free DMEM/F12 medium (Gibco; Thermo Fisher
Scientific, Inc.) saturated with 95% N2/5%
CO2 at 37˚C. The cells were placed in an experimental
hypoxia chamber in a saturated atmosphere of 95% N2 and 5%
CO2. To simulate re-oxygenation, hepatocytes were
cultured under normal conditions, as previously described (9). Following I/R injury, hepatocytes
adhered to the culture dish; 0.25% trypsin-EDTA at 37˚C was used to
release them from the plate.
For CD5L treatment, cells (I/R model) were cultured
with DMEM/F12 containing 1 µg/ml recombinant CD5L and incubated at
37˚C for 1 h before cell I/R treatment as previously described
(16).
Flow cytometry
Flow cytometry using Annexin V-PI Apoptosis
Detection kit (BD Biosciences) was performed to assess apoptosis as
described previously (22).
Hepatocytes (I/R model; 1x105 cells/well) were stained
with Annexin V and propidium iodide according to the manufacturer's
protocols. Data were acquired on a FACScan instrument (BD
Biosciences) and analyzed using CellQuest software (version 8.0.1;
BD Biosciences).
Caspase assay
Caspase3/7/8 activity was determined using Cell
Meter™ Caspase 3/7 and Caspase 8 Activity Apoptosis Assay kits (AAT
Bioquest, Inc.) according to the manufacturer's instructions
(23). The results were read at 520
nm using a microplate reader (Bio-Rad Laboratories, Inc.) and
expressed as fold change in caspase 3/7/8 activity from
control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells (I/R model),
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse transcribed to cDNA using Transcriptor First Strand cDNA
Synthesis kit (Roche Molecular Systems, Inc.) according to
manufacturer's protocol. qPCR was performed using the
SYBR® Green PCR Master Mix Reagent kit (Takara Bio,
Inc.), and GAPDH was used for data normalization. The thermocycling
conditions were as follows: 5 min at 95˚C, followed by 35-40 cycles
of 5 sec at 95˚C and 10 sec at 60˚C. The fold-change of expression
of the transcript mRNA was analyzed using the
2-ΔΔCq method (24). The primers used were as follows: CD36
forward, 5'-GAGCCATCTTTGAGCCTTCA-3' and reverse,
5'-TCAGATCCGAACACAGCGTA-3'; GADPH forward,
5'-GGAGCCAAAAGGGTCATCAT-3' and reverse, 5'-GTGATGGCATGGACTGTGGT-3';
autophagy-related 7 (ATG7) forward, 5'-CGGCTGAGATCTGGGACA-3' and
reverse, 5'-AGCCAGATTGAGCGACTGAT-3' (all purchased from Invitrogen;
Thermo Fisher Scientific, Inc.).
Western blot analysis
Hepatocytes (I/R model) were lysed with ice-cold
Cell Lysis Buffer (cat. no. ab152163; Abcam) to obtain total
protein, which was subsequently quantified using bicinchoninic acid
protein assay. Protein samples (20 µg/lane) were then separated by
10% SDS-PAGE and transferred to polyvinylidene difluoride membranes
(EMD Millipore; Merck KGaA). Following transfer, the membranes were
blocked with 5% milk in Tris-buffered saline and incubated with the
appropriate primary antibodies (all 1:1,000 dilution; LC3B, cat.
no. 3868; ATG7, cat. no. 8558 and β-actin, cat. no. 4970; all from
Cell Signaling Technology, Inc.) overnight at 4˚C. The membranes
were subsequently incubated with horseradish peroxidase-conjugated
secondary antibody (1:2,000 dilution; cat. no. 7074, Cell Signaling
Technology, Inc.) and visualized using ECL Substrate Kit (Abcam).
The stained protein bands were visualized using ChemiDoc XRS
equipment (Bio-Rad Laboratories, Inc.), and quantified and analyzed
using Quantity One software (version 4.5.2; Bio-Rad Laboratories,
Inc.).
Small interfering (si)RNA
transfection
Hepatocytes were resuspended (1x106
cells/ml) in serum- and antibiotic-free siRNA Transfection Medium
(cat. no. sc-36868; Santa Cruz Biotechnology, Inc.). CD36, ATG7 and
control siRNAs (CD36, 5'-CTGTCCATCCCGCACCTGCG-3' and ATG7,
5'-CTCGCCGAGCTCGCCCA-3'; scramble control 5'-ACGTCTATACGCCCA-3')
were purchased from Invitrogen (Thermo Fisher Scientific, Inc.).
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) was
used to perform siRNA transfections (100 nM) according to the
manufacturer's protocol. Cells were analyzed at 48 h following
transfection.
ROS production measurement
Intracellular production of ROS was detected by
staining with 2',7'-dichlorofluorescin diacetate (DCFH-DA;
Sigma-Aldrich; Merch KGaA). Following treatments, cells were washed
thrice with sterile PBS and incubated with 10 µM DCFH-DA for 30 min
at 37˚C. The fluorescence intensity of the cells was measured using
a fluorescence spectrophotometer at excitation and emission
wavelengths of 488 nm and 525 nm, respectively.
Superoxide dismutase (SOD)
activity
SOD activity in treated hepatocytes was determined
using a colorimetric Superoxide Dismutase Activity Assay Kit (cat.
no. ab65354; Abcam) according to the manufacturer's protocol.
Briefly, protein was isolated from hepatocytes using the lysis
buffer as part of the kit, and SOD activity was measured in 10 µg
of total protein extract. Absorbance was measured at 450 nm.
Glutathione peroxidase (GSH-Px)
Assay
The level of GSH-Px was assayed using fluorometric
green GSH/GSSG Ratio Detection Assay (cat. no. ab138881; Abcam) and
performed as previously described (25). The absorbance of the samples was
assessed using a spectrophotometer at 340 nm.
Catalase (CAT) activity assay
CAT activity in cells was determined using a
colorimetric Catalase Activity Assay Kit (cat. no. ab83464; Abcam)
according to the manufacturer's instructions. The absorbance of the
samples was assessed using a spectrophotometer at 570 nm.
Statistical analysis
Data are expressed as the mean ± SD. SPSS version
19.0 (IBM Corp.) was used for statistical analysis. Differences
among ≥3 groups were tested by one-way analysis of variance and
Tukey's test for multiple comparisons. Differences between two
groups were evaluated by Student's t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
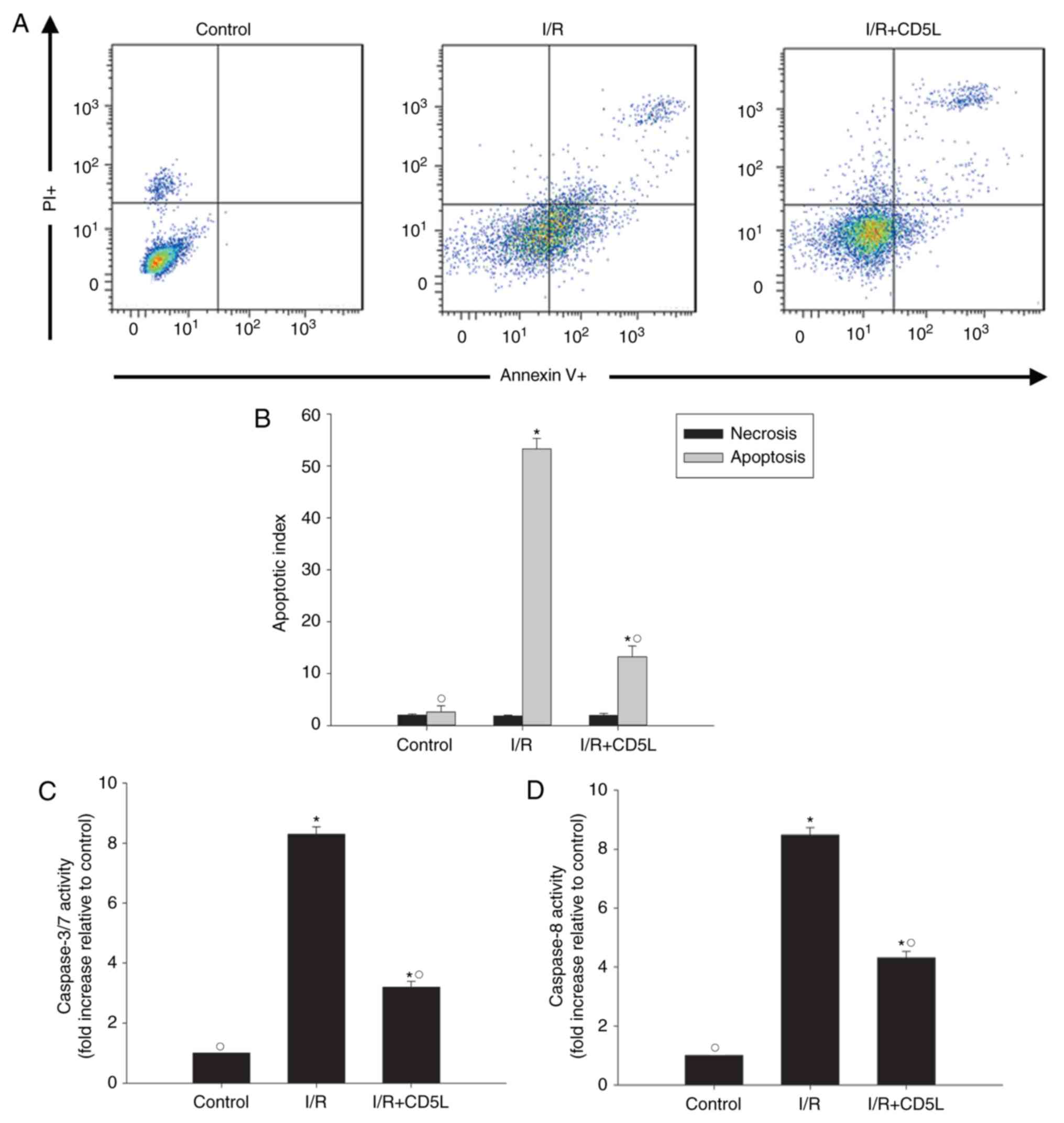
Recombinant CD5L inhibits I/R-induced
hepatocyte apoptosis
As hepatocyte apoptosis has been implicated in I/R
injury (26), the apoptotic rate of
hepatocytes was tested in the I/R model in the present study.
Annexin V-FITC fluorescence-activated cell sorting (FACS) results
revealed that I/R-induced apoptosis compared with that in control
cells, but this effect could be partially reversed by the
application of recombinant CD5L (Fig.
1A and B). To further explore
the antiapoptotic effects of CD5L, the changes in apoptosis marker
caspases 3/7 and caspase 8 were measured following I/R induction
with or without CD5L pretreatment. The results demonstrated that
CD5L significantly reduced the caspase activity compared with
untreated I/R model cells (Fig. 1C
and D).
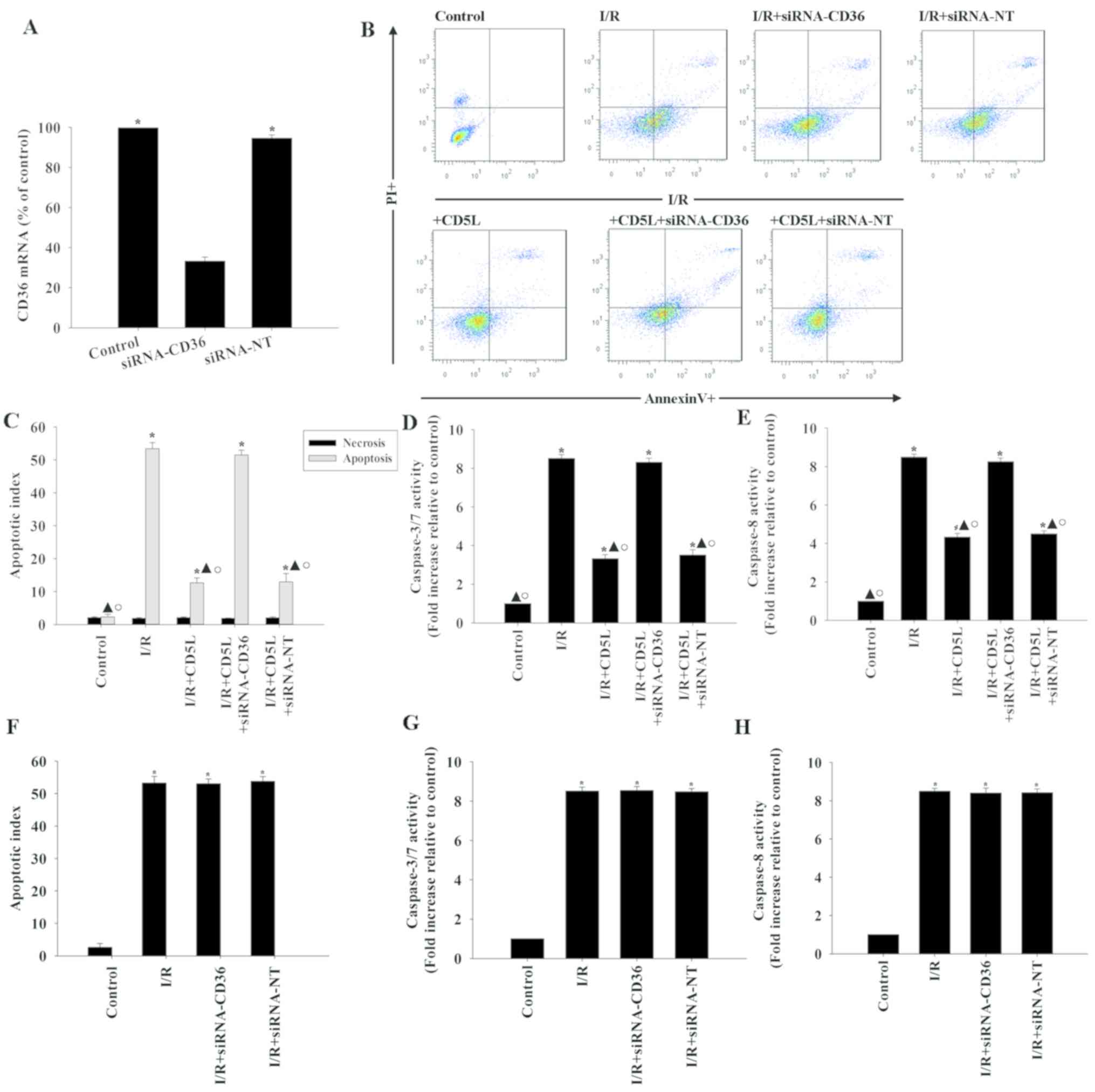
CD5L inhibits hepatocyte apoptosis via
the CD36 receptor
CD5L activates the scavenger receptor CD36 on the
cell surface to mediate the cellular protection process (16). Therefore, the role of CD36 in
CD5L-induced hepatocyte protection was investigated. siRNAs
targeting CD36 silenced its expression in hepatocytes by >80%
compared with the non-targeting siRNA (siRNA-NT) negative control
(Fig. 2A). FACS analysis of
CD36-silenced hepatocytes treated with CD5L compared with
siRNA-NT-transfected cells treated with CD5L demonstrated an
increase in apoptosis (Fig. 2B and
C). In addition, silencing CD36
reversed the inhibition of caspase activity induced by CD5L
(Fig. 2D and E). By contrast, siRNA-CD36 or siRNA-NT did
not affect the apoptotic rates and caspase activity under I/R
conditions (Fig. 2F-H).
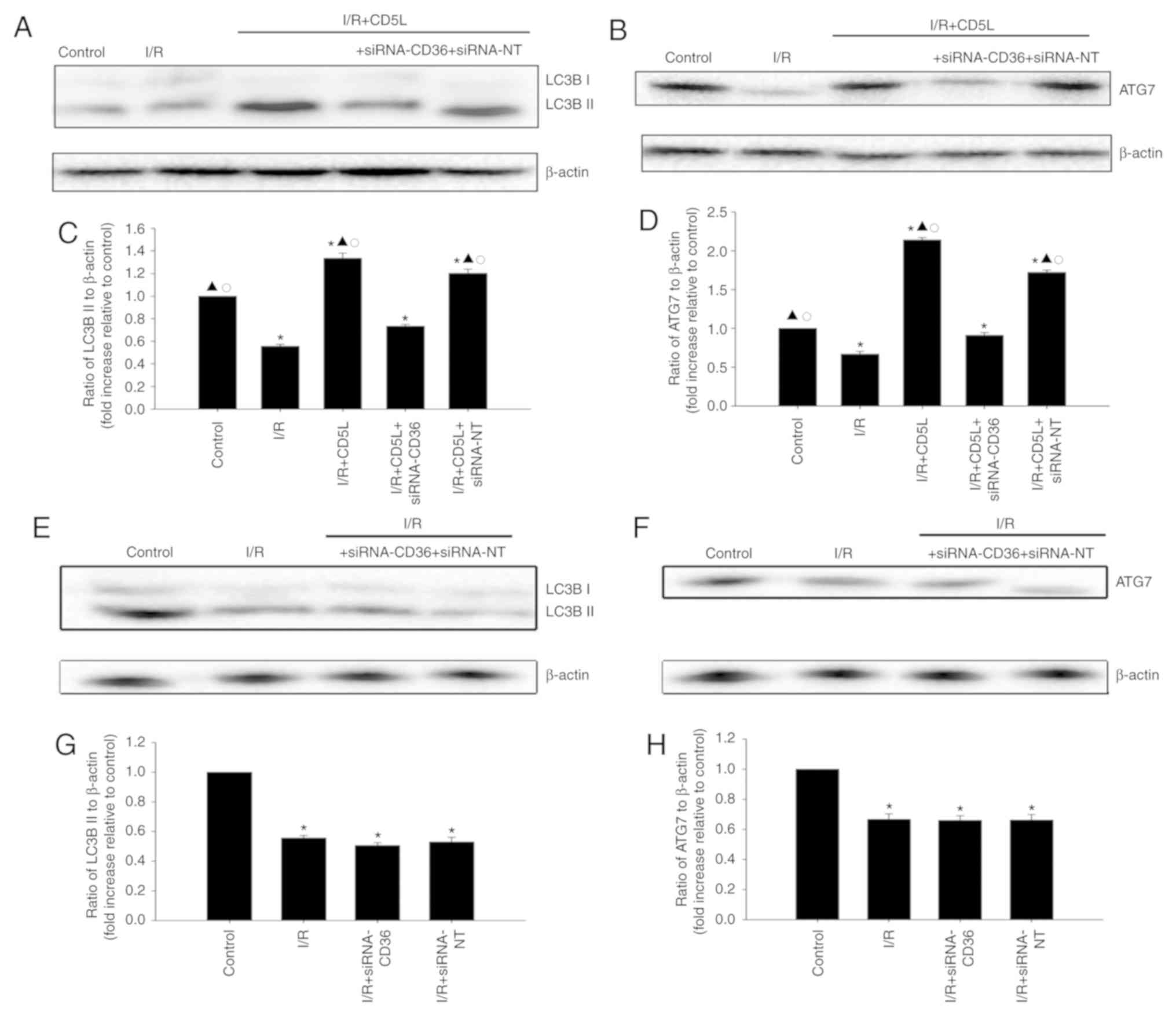
CD5L has a cytoprotective effect by
modulating autophagy
As the induction of autophagy is associated with an
antiapoptotic effect during I/R (15), the autophagic flux was examined in
I/R hepatocytes by assessing the expression of LC3B-II, a marker
for autophagy, by western blotting of total cell lysates. The
present study also identified that ATG7 upregulation was also
associated with protection against I/R liver injury. I/R induction
decreased LC3B-II and ATG7 levels in hepatocytes compared with the
control group, whereas CD5L treatment increased LC3B-II (Fig. 3A and C) and ATG7 levels (Fig. 3B and D) compared with the I/R group; these
results indicated that the induction of autophagy may occur upon
CD5L activation. CD36 silencing reversed the expression of
autophagy-related proteins induced by CD5L (Fig. 3E-H).
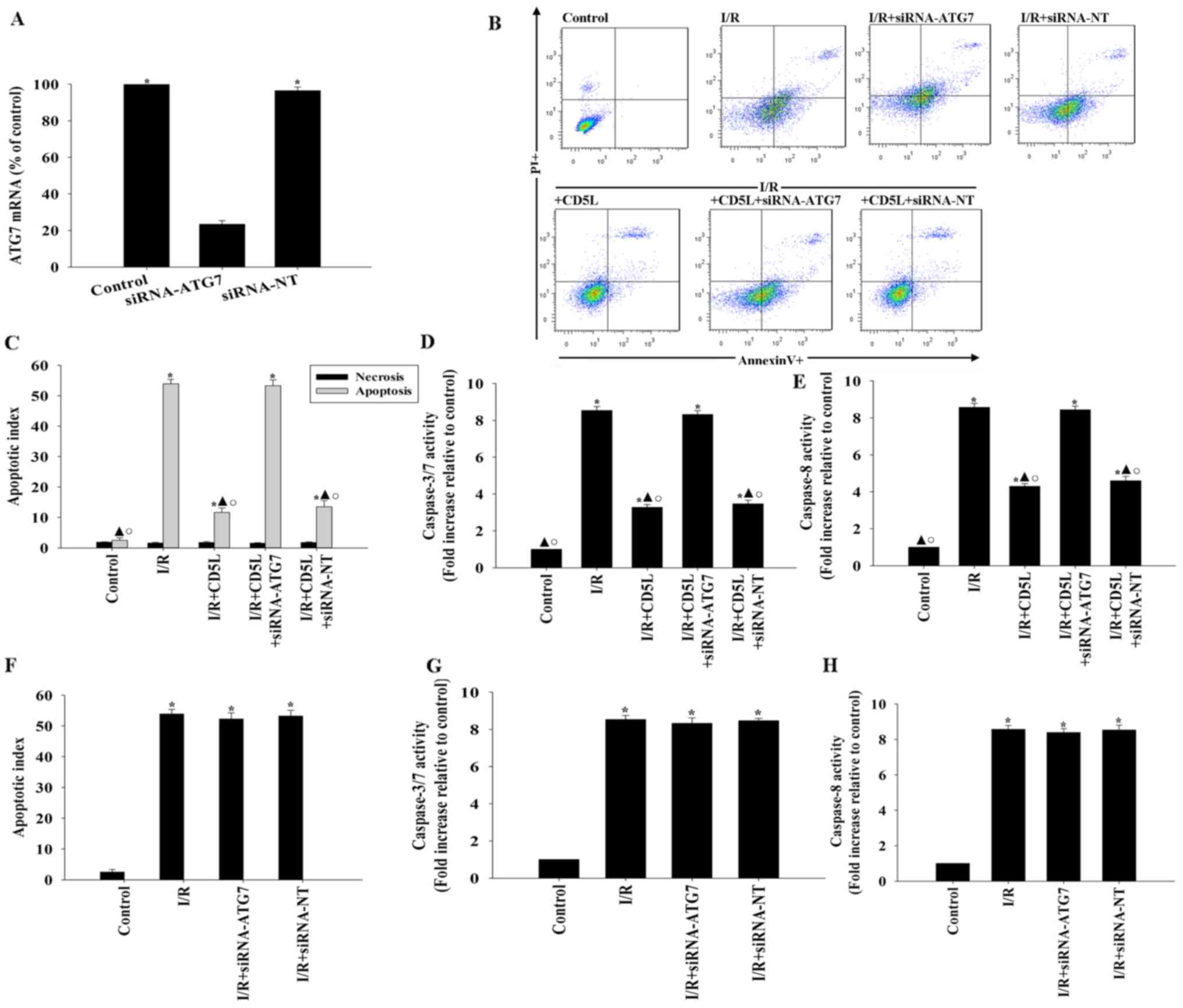
To confirm these results, experiments aimed at
silencing the protein ATG7, an integral component of the autophagy
process (27), were performed. siRNA
transfection targeting ATG7 lowered the expression levels of ATG7
mRNA in hepatocytes (Fig. 4A). ATG7
silencing reversed the antiapoptotic effect of CD5L, and was
associated with an increase in apoptosis compared with the control
groups (Fig. 4B and C). In addition, following CD5L treatment,
ATG7 silencing resulted in increased caspase activity compared with
siRNA-NT-transfected cells (Fig. 4D
and E). By contrast, siRNA-ATG7 and
siRNA-NT had no effects on apoptosis and caspase activity under I/R
condition without CD5L treatment (Fig.
4F-H).
CD36 and ATG7 reverse the antioxidant
effect of CD5L in hepatocytes
Since the oxidative stress is associated with
I/R-related cellular damage (28),
the effects of CD5L on ROS generation and antioxidant enzyme
activity were examined. As demonstrated in Fig. 5A, I/R exposure significantly
increased ROS generation in hepatocytes compared with that in the
control group. In addition, antioxidant enzyme activity analysis
revealed that the levels of SOD, GSH-Px and CAT in the I/R groups
were significantly reduced compared with those in the control
groups (Fig. 5B-D). Following
treatment with CD5L, ROS generation was decreased, but antioxidant
enzyme activity levels were increased compared with the I/R group;
silencing either CD36 or ATG7 reversed the antioxidant effect of
CD5L on hepatocytes (Fig. 5).
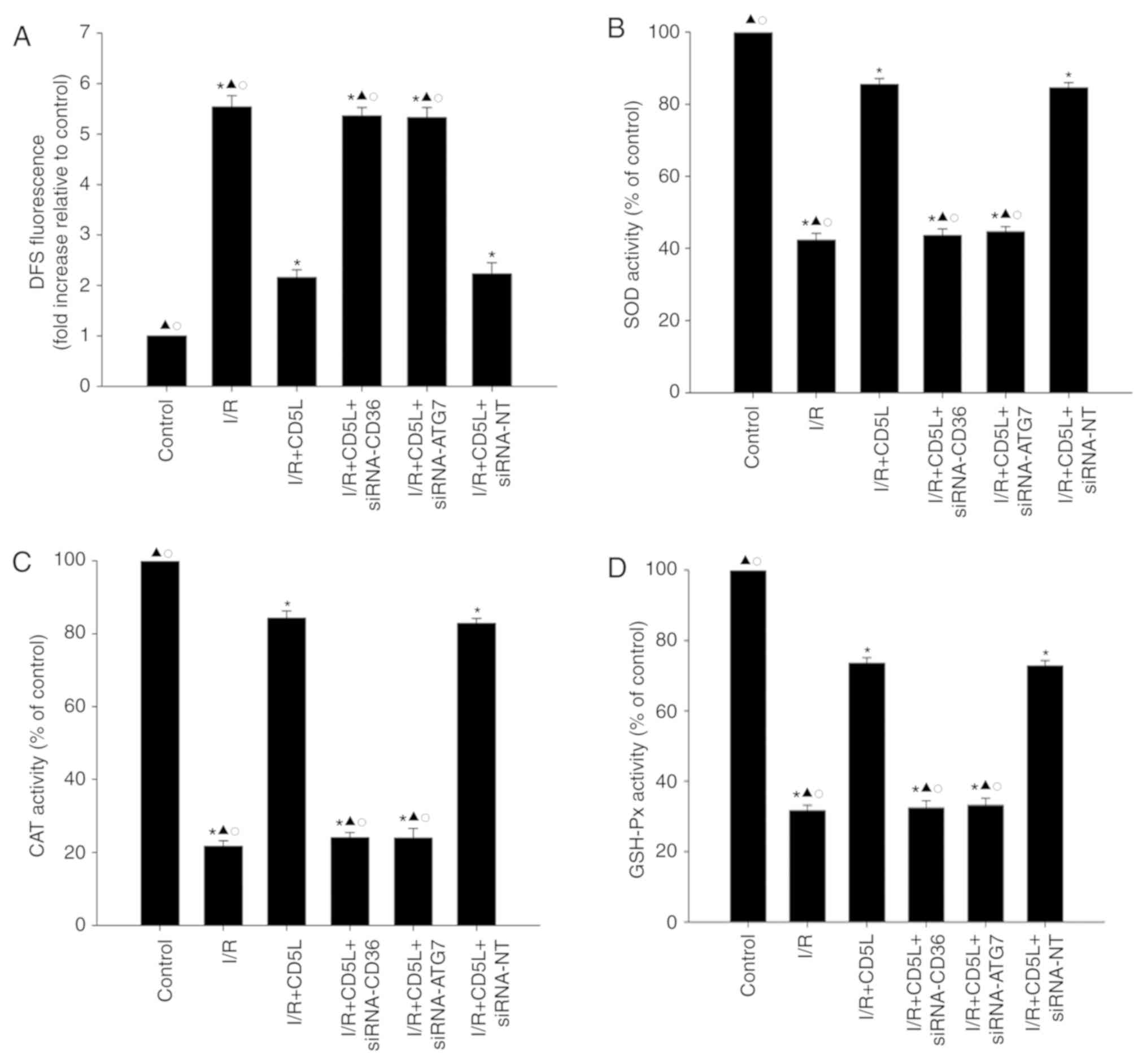 | Figure 5.CD36 and ATG7 reverse the antioxidant
effect of CD5L on hepatocytes. (A-D) Hepatocytes transfected with
siRNA-CD36, siRNA-ATG7 or siRNA-NT and cultured under I/R
conditions with or without CD5L. Hepatocytes without any treatment
were used as controls. (A) Intracellular ROS production was
analyzed by fluorescence spectrophotometry. Commercial kits were
used to determine the levels of (B) SOD, (C) CAT and (D) GSH-Px
activities in hepatocytes. Data represent the mean ± SD from three
independent experiments. *P<0.05 vs. control;
▲P<0.05 vs. I/R + CD5L; ○P<0.05 vs. I/R
+ CD5L + siRNA-NT. DFS, dual fluorescent staining; I/R,
ischemia/reperfusion; CD5L, CD5-like; CD36, cluster of
differentiation 36; ATG7, autophagy-related 7; siRNA, small
interfering RNA; NT, non-targeting; ROS, reactive oxygen species;
SOD, superoxide dismutase; CAT, catalase; GHS-Px, glutathione
peroxidase. |
Discussion
Liver ischemic injury is of paramount importance in
VIO applied during liver transaction (29). Liver hypoxia and ischemia affect
liver cell homeostasis and function, which leads to metabolic
alterations that may result in apoptosis (30). Ischemic stress-induced hepatocyte
damage and cell death subsequently impair liver function (31). The present study demonstrated that
hepatic injury induced cell damage and hepatocyte apoptosis.
CD5L is a 40-kDa soluble glycoprotein that belongs
to the scavenger receptor cysteine rich superfamily (32). CD5L is involved in a variety of
biological processes, such as infection, atherosclerosis and
apoptosis (11,20). Various disease models, including
cancer, have demonstrated that CD5L participates in cellular
functions by preventing apoptosis (33,34).
Human CD5L has also been demonstrated to inhibit apoptosis in liver
cancer cell lines in response to cisplatin by inducing autophagy
(12). A previous study has
suggested that CD5L serves cytoprotective effects by binding to the
CD36 receptor (16). The results of
the present study demonstrated that recombinant CD5L exhibited an
antiapoptotic effect in I/R-induced apoptosis, and siRNA silencing
of CD36 reversed this effect, which suggested that CD36 may be
involved in the cytoprotective effects of CD5L.
Autophagy serves an important role in supporting
hepato-cellular viability following I/R injury (21). Promotion of autophagy prevents
mitochondrial dysfunction and cell death following reperfusion
(35). A recent study has
demonstrated that increasing the level of autophagy decreases
hepatic I/R injury (36). Previous
studies have reported that CD5L increases macrophage survival and
liver cancer cell survival by enhancing autophagy (12,16).
These data indicate the participation of CD5L in promoting
autophagy. In accordance with this, the results of the present
study revealed that CD5L enhanced the expression of LC3B-II and
ATG7 in hepatocytes. Additionally, silencing of ATG7, a key
component of the autophagy signaling network, impaired the
antiapoptotic effect of CD5L. These results support the hypothesis
that CD5L promotes autophagy mechanisms in hepatocytes to induce an
antiapoptotic effect.
Production of ROS, including superoxide, hydrogen
peroxide and hydroxyl radicals, has been implicated in I/R injury
(37). The results of the present
study demonstrated that I/R induced ROS generation and inhibited
antioxidative enzyme activity. A previous study has suggested that
CD5L induces anti-inflammatory effects by autophagy (20); therefore, the inhibitory effect of
CD5L on oxidative stress was examined in the present study. In
accordance with the result of previous study, CD5L inhibited the
oxidative stress induced by I/R. In addition, autophagy is a major
regulator of cell homeostasis and function through the modulation
of oxidative stress under ischemic conditions (38); consistent with this, the present
study revealed that when autophagic flux was blocked by ATG7
silencing in hepatocytes, the inhibition of oxidative stress by
CD5L was partially reversed, which suggested that the CD5L-induced
inhibition of oxidative stress may be autophagy-dependent.
In summary, the results of the present study
demonstrated a protective effect of CD5L on I/R-induced hepatic
injury through a CD36-dependent autophagic pathway, as well as
inhibition of oxidative stress. These results indicate that CD5L
may be a potential candidate for the treatment of I/R injury.
Acknowledgements
Not applicable.
Funding
The current study was supported by The Natural
Science Foundation of Zhejiang Province (grant no. LQ13H160022 to
JL), the Science and Technology Program of Wenzhou Municipality
(grant no. Y20140712 to JL) and the Science and Technology Program
of Wenzhou Municipality (grant no. Y20190445 to LZ).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JJL and WL made substantial contributions to the
acquisition of data, analysis and interpretation of data. LZ was
involved in conception and design of the study, and drafting the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Wenzhou Medical University, Wenzhou, China (approval
no. WMU18825).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Starlinger P, Assinger A, Haegele S, Wanek
D, Zikeli S, Schauer D, Birner P, Fleischmann E, Gruenberger B,
Brostjan C and Gruenberger T: Evidence for serotonin as a relevant
inducer of liver regeneration after liver resection in humans.
Hepatology. 60:257–266. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schiergens TS, Stielow C, Schreiber S,
Hornuss C, Jauch KW, Rentsch M and Thasler WE: Liver resection in
the elderly: Significance of comorbidities and blood loss. J
Gastrointest Surg. 18:1161–1170. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Duval H, Mbatchi SF, Grandadam S, Legendre
C, Loyer P, Ribault C, Piquet-Pellorce C, Guguen-Guillouzo C,
Boudjema K and Corlu A: Reperfusion stress induced during
intermittent selective clamping accelerates rat liver regeneration
through JNK pathway. J Hepatol. 52:560–569. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Olthof PB, Reiniers MJ, Dirkes MC, Gulik
TMV and Golen RFV: Protective mechanisms of hypothermia in liver
surgery and transplantation. Mol Med. 21:833–846. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Castellaneta A, Yoshida O, Kimura S,
Yokota S, Geller DA, Murase N and Thomson AW: Plasmacytoid
dendritic cell-derived IFN-α promotes murine liver
ischemia/reperfusion injury by induction of hepatocyte IRF-1.
Hepatology. 60:267–277. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zuo S, Kong D, Wang C, Liu J, Wang Y, Wan
Q, Yan S, Zhang J, Tang J, Zhang Q, et al: CRTH2 promotes
endoplasmic reticulum stress-induced cardiomyocyte apoptosis
through m-calpain. EMBO Mol Med. 10(e8237)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun P, Zhang P, Wang PX, Zhu LH, Du Y,
Tian S, Zhu X and Li H: Mindin deficiency protects the liver
against ischemia/reperfusion injury. J Hepatol. 63:1198–1211.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rao J, Qian X, Li G, Pan X, Zhang C, Zhang
F, Zhai Y, Wang X and Lu L: ATF3-mediated NRF2/HO-1 signaling
regulates TLR4 innate immune responses in mouse liver
ischemia/reperfusion injury. Am J Transplant. 15:76–87.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang Y, Liu X, She ZG, Jiang DS, Wan N,
Xia H, Zhu XH, Wei X, Zhang XD and Li H: Interferon regulatory
factor 9 is an essential mediator of heart dysfunction and cell
death following myocardial ischemia/reperfusion injury. Basic Res
Cardiol. 109(434)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ozawa T, Maehara N, Kai T, Arai S and
Miyazaki T: Dietary fructose-induced hepatocellular carcinoma
development manifested in mice lacking apoptosis inhibitor of
macrophage (AIM). Genes Cells. 21:1320–1332. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sanjurjo L, Aran G, Roher N, Valledor AF
and Sarrias MR: AIM/CD5L: A key protein in the control of immune
homeostasis and inflammatory disease. J Leukoc Biol. 98:173–184.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Aran G, Sanjurjo L, Bárcena C, Simon-Coma
M, Téllez É, Vázquez-Vitali M, Garrido M, Guerra L, Díaz E,
Ojanguren I, et al: CD5L is upregulated in hepatocellular carcinoma
and promotes liver cancer cell proliferation and antiapoptotic
responses by binding to HSPA5 (GRP78). FASEB J. 32:3878–3891.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang C, Yosef N, Gaublomme J, Wu C, Lee Y,
Clish CB, Kaminski J, Xiao S, Meyer Zu Horste G, Pawlak M, et al:
CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell
pathogenicity. Cell. 163:1413–1427. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xiao J, Ke ZP, Shi Y, Zeng Q and Cao Z:
The cardioprotective effect of thymoquinone on ischemia-reperfusion
injury in isolated rat heart via regulation of apoptosis and
autophagy. J Cell Biochem. 119:7212–7217. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li X, Huang Q, Wang M, Yan X, Song X, Ma
R, Jiang R, Zhao D and Sun L: Compound K inhibits
autophagy-mediated apoptosis through activation of the PI3K-Akt
signaling pathway thus protecting against ischemia/reperfusion
injury. Cell Physiol Biochem. 47:2589–2601. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sanjurjo L, Amézaga N, Aran G,
Naranjo-Gómez M, Arias L, Armengol C, Borràs FE and Sarrias MR: The
human CD5L/AIM-CD36 axis: A novel autophagy inducer in macrophages
that modulates inflammatory responses. Autophagy. 11:487–502.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
van Golen RF, van Gulik TM and Heger M:
Mechanistic overview of reactive species-induced degradation of the
endothelial glycocalyx during hepatic ischemia/reperfusion injury.
Free Radic Biol Med. 52:1382–1402. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
van Golen RF, van Gulik TM and Heger M:
The sterile immune response during hepatic ischemia/reperfusion.
Cytokine Growth Factor Rev. 23:69–84. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
de Graaf W, Heger M, Spruijt O, Maas A, de
Bruin K, Hoekstra R, Bennink RJ and van Gulik TM: Quantitative
assessment of liver function after ischemia-reperfusion injury and
partial hepatectomy in rats. J Surg Res. 172:85–94. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sanjurjo L, Aran G, Téllez É, Amézaga N,
Armengol C, López D, Prats C and Sarrias MR: CD5L promotes M2
macrophage polarization through autophagy-mediated upregulation of
ID3. Front Immunol. 9(480)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Biel TG, Lee S, Flores-Toro JA, Dean JW,
Go KL, Lee MH, Law BK, Law ME, Dunn WA Jr, Zendejas I, et al:
Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse
livers in a mitofusin 2-dependent manner. Cell Death Differ.
23:279–290. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yuan J, Chen M, Xu Q, Liang J, Chen R,
Xiao Y, Fang M and Chen L: Effect of the diabetic environment on
the expression of MiRNAs in endothelial cells: Mir-149-5p
restoration ameliorates the high glucose-induced expression of
TNF-α and ER stress markers. Cell Physiol Biochem. 43:120–135.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu Y, Xiong Y, Xing F, Gao H, Wang X, He
L, Ren C, Liu L, So KF and Xiao J: Precise regulation of miR-210 is
critical for the cellular homeostasis maintenance and
transplantation efficacy enhancement of mesenchymal stem cells in
acute liver failure therapy. Cell Transplant. 26:805–820.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Szabó K, Gesztelyi R, Lampé N, Kiss R,
Remenyik J, Pesti-Asbóth G, Priksz D, Szilvássy Z and Juhász B:
Fenugreek (trigonella foenum-graecum) seed flour and diosgenin
preserve endothelium-dependent arterial relaxation in a rat model
of early-stage metabolic syndrome. Int J Mol Sci.
19(E798)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo WZ, Fang HB, Cao SL, Chen SY, Li J,
Shi JH, Tang HW, Zhang Y, Wen PH, Zhang JK, et al: Steap3
deficiency in hepatocytes protects the liver against
ischemia/reperfusion injury by suppressing TAK1. Hepatology: Aug 8,
2019 (Epub ahead of print).
|
|
27
|
Geng J and Klionsky DJ: The Atg8 and Atg12
ubiquitin-like conjugation systems in macroautophagy ‘Protein
modifications: Beyond the usual suspects’ review series. EMBO Rep.
9:859–864. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mu H and Wang Y: Collagen peptide modified
carboxymethyl cellulose as both antioxidant drug and carrier for
drug delivery against retinal ischaemia/reperfusion injury. J Cell
Mol Med. 22:5008–5019. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Abt P, Crawford M, Desai N, Markmann J,
Olthoff K and Shaked A: Liver transplantation from controlled
non-heart-beating donors: An increased incidence of biliary
complications. Transplantation. 75:1659–1663. 2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jaeschke H and Lemasters JJ: Apoptosis
versus oncotic necrosis in hepatic ischemia/reperfusion injury.
Gastroenterology. 125:1246–1257. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bilzer M and Gerbes AL: Preservation
injury of the liver: Mechanisms and novel therapeutic strategies. J
Hepatol. 32:508–515. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gebe JA, Kiener PA, Ring HZ, Li X, Francke
U and Aruffo A: Molecular cloning, mapping to human chromosome 1
q21-q23, and cell binding characteristics of Spalpha, a new member
of the scavenger receptor cysteine-rich (SRCR) family of proteins.
J Biol Chem. 272:6151–6158. 1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li Y, Qu P, Wu L, Li B, Du H and Yan C:
Api6/AIM/Spα/CD5L overexpression in alveolar type II epithelial
cells induces spontaneous lung adenocarcinoma. Cancer Res.
71:5488–5499. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zou T, Garifulin O, Berland R and
Boyartchuk VL: Listeria monocytogenes infection induces prosurvival
metabolic signaling in macrophages. Infect Immun. 79:1526–1535.
2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chun SK, Lee S, Flores-Toro J, U RY, Yang
MJ, Go KL, Biel TG, Miney CE, Pierre Louis S, Law BK, et al: Loss
of sirtuin 1 and mitofusin 2 contributes to enhanced
ischemia/reperfusion injury in aged livers. Aging Cell.
17(e12761)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tan R, Tian H, Yang B, Zhang B, Dai C, Han
Z, Wang M, Li Y, Wei L, Chen D, et al: Autophagy and Akt in the
protective effect of erythropoietin helix B surface peptide against
hepatic ischaemia/reperfusion injury in mice. Sci Rep.
8(14703)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jaeschke H: Molecular mechanisms of
hepatic ischemia-reperfusion injury and preconditioning. Am J
Physiol Gastrointest Liver Physiol. 284:G15–G26. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nishida K, Kyoi S, Yamaguchi O, Sadoshima
J and Otsu K: The role of autophagy in the heart. Cell Death
Differ. 16:31–38. 2009. View Article : Google Scholar
|