Introduction
Conventional venography has been considered as the
gold standard in detecting deep venous thrombosis (DVT), even in
patients who are asymptomatic (1,2).
However, its clinical application has been limited due to several
disadvantages, including pain, the requirement of ionic contrast
agents, radiation exposure, the fact it is time consuming and the
lack of reliability in cases due to patient contraindication or
technical issues (3). CT allows the
evaluation of DVT with a ‘combined CT venography and pulmonary
angiography imaging procedure initially introduced by Loud et
al (4), but radiation exposure
of CT venography notably increases the gonadal dose (5). Further limitations include poor venous
enhancement and streak artifacts from orthopedic implants (6). Alternatively, duplex sonography is the
most widely used imaging modality for symptomatic DVT assessment
from the common femoral to the popliteal vein due to its high
sensitivity at 76-100% and specificity at 96-100%, compared with
conventional venography (7,8). However, the accurate evaluation of
pelvic and abdominal veins is operator-dependent and requires
extensive experience (9).
MRI has gained growing interest due to absence of
radiation exposure, improved ability for displaying soft tissues,
visualization of proximal and peripheral venous vasculatures with
high spatial resolution (6,7,10).
Magnetic resonance venography (MRV) offers superior evaluation for
venous thrombus in the abdomen, pelvis and lower limbs of patients
(10,11), despite complications including
swollen legs, edema, wounds, obesity, plaster involvement,
iodinated contrast agents and interference of bowel gas, which are
also technical limitations for conventional venography and duplex
sonography (12).
A variety of MRI techniques are available for the
evaluation of the venous system (13). Time-of-flight MRV is a
non-contrast-enhanced method that relies on the intrinsic
properties of flowing blood, but it requires longer image
acquisition time and is limited by flow artifacts and saturation
effect, which may decrease diagnostic accuracy (7). MR direct thrombus imaging involves
injecting dilute gadolinium directly into the distal extremity of
expected pathology (14-16).
However, MR direct thrombus imaging cannot be used if alternative
sites of intravenous access if thrombus or obstruction have been
identified, as this method requires venous cannulation of the
affected extremity (15,16).
Volume interpolated body examination (VIBE) is a
gradient echo MR sequence first introduced by Rofsky et al
(17), which can produce high
isotropic resolution images, combined with fat-suppression
technique, by minimizing partial volume effect and maximizing
tissue contrast in contrast-enhanced MR examinations. Visualization
of vascular structures maybe improved by administration of an
intravenous contrast agent, and contrast-enhanced MRV using
fat-suppressed VIBE is an efficient method with superior
visualization capability to depict thrombus in the abdomen, pelvis
and lower limbs (18-21).
VIBE with spectral fat saturation (VIBE-fs) in
previous MRV studies requires frequency-selective saturation pulse,
which must be equal to the fat resonance frequency (22). VIBE-fs is sensitive to static
magnetic field non-uniformity, thus is inadequate when used in
large field of view imaging (22),
and results in an unsatisfied fat saturation efficacy, which may
affect visualization and the evaluation of small vessels. In
contrast to spectral fat saturation, the Dixon fat-suppressed
technique does not require a fat-selective pulse (20,22). To
the best of our knowledge, there have been no previous studies
investigating the use of Dixon fat-suppressed VIBE (VIBE-Dixon) for
DVT detection in the lower leg.
The present study investigated the feasibility of
using high resolution MRV, breath-hold VIBE-fs for the
abdominopelvic region and VIBE-Dixon for the lower legs on a 1.5T
MR scanner, to evaluate DVT compared with duplex sonography.
Materials and methods
Study population
The current prospective study was approved by The
Medical Ethics Committee of Huazhong University of Science and
Technology. Patients were informed of procedures, and written
consent was obtained.
In total, 31 consecutive patients (men, 18; women,
13; mean age, 50.1±14.8 years; age range, 17-75 years) with DVT
identified by duplex sonography between January 2015 and March 2016
were included in the present study. All patients presented with leg
swelling or leg pain, and were recruited from Union Hospital,
Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China). Of the 31 patients, there were 29 in
patients and 2 out patients; a total of 10 patients (32.3%)
presented with DVT only, 21 (67.7%) exhibited pulmonary embolism
and DVT. D-Dimer is a marker of endogenous fibrinolysis and can be
detected in patients with DVT (23).
A total of 2 ml of blood was taken from the 20 patients who were
required to undergo D-Dimer testing, followed by duplex sonography,
and the results of the D-Dimer test ranged from 0.9-20.0 mg/l
(6.0±5.1 mg/l; reference value, <0.5 mg/l); the other 11
patients were not required to take the test as their DVT had
previously been confirmed using duplex sonography. The exclusion
criteria were as follows: i) Pregnant women; ii) inability to
accomplish MR imaging due to severe dyspnea, continuous cough and
shock; iii) contraindication to MR scanning; iv) acute or chronic
severe renal impairment (estimated glomerular filtration rate,
<30 ml/min/1.73 m2); and v) inability to establish
intravenous access.
Duplex sonography
Duplex sonography was performed by a specialized
radiologist, with >15 years of experience in duplex sonography,
using the same duplex sonography device (LOGIQ E9; GE Healthcare)
equipped with a 7.5-MHz probe for the lower leg, and a 3.0- or
5.0-MHz probe for the abdominal-pelvic region. The medical reports
were acquired via the electronic medical record of our hospital.
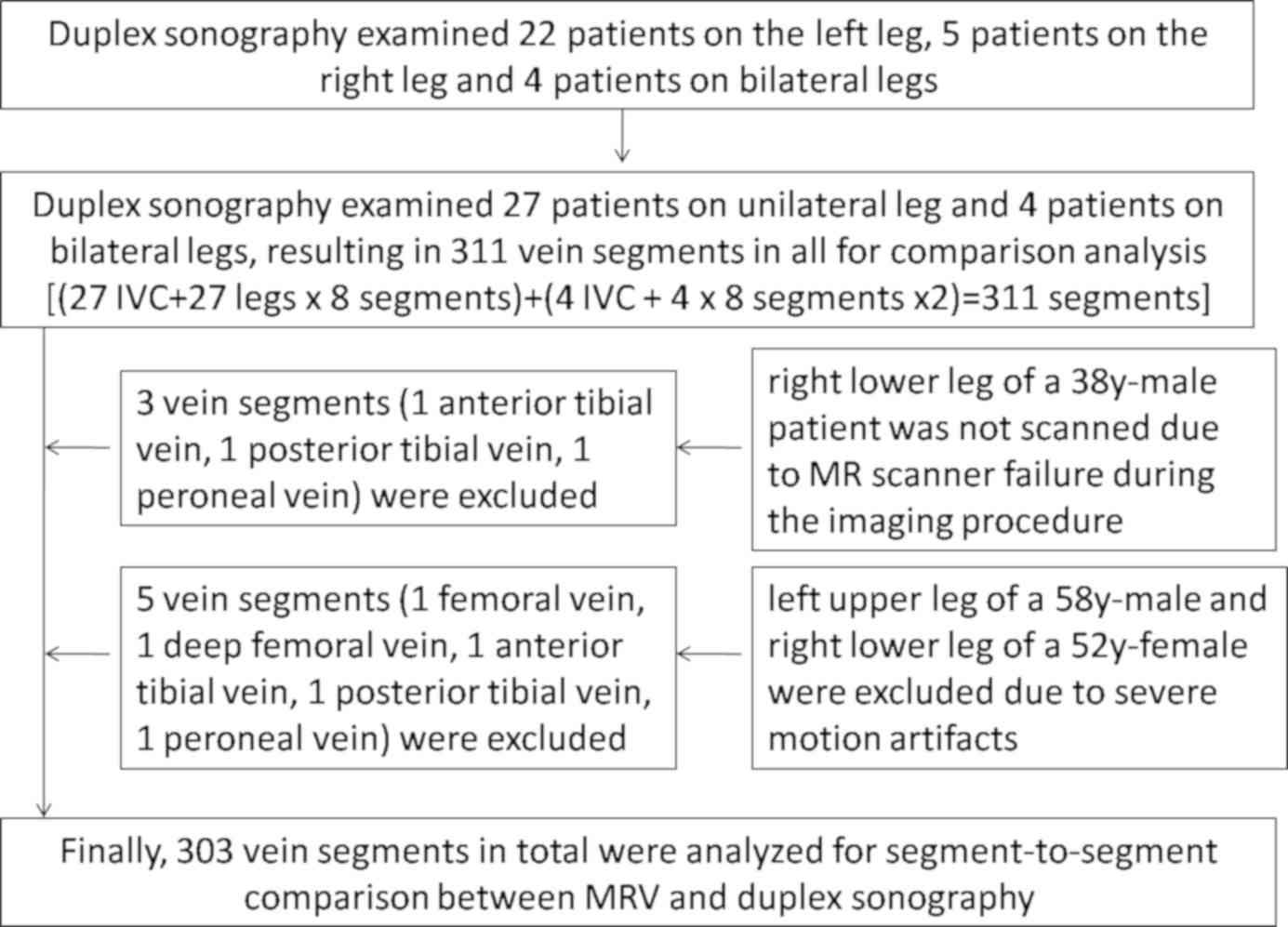
Duplex sonography examined and detected DVTs in the left leg of 22
patients, the right leg in five patients and on both sides of the
leg in four patients, according to clinical demands. Therefore,
duplex sonography examined 35 single legs in all [22+5+(4x2)=35];
the inclusion and exclusion of patients are presented in Fig. 1. Diagnostic standards for DVT were as
follows: i) The infrainguinal veins could be imaged by Color
Doppler flow imaging (CDFI) and compression sonography; ii) DVT
could be diagnosed with the visualization of intraluminal thrombus;
iii) venous lumen filled with weak echo; iv) venous lumen cannot be
completely flattened when being pressured; and v) the absence of
blood flow within the veins on CDFI. As suprainguinal veins are too
deep to be fully compressible, longitudinal and transverse sections
of the abdominal-pelvic veins were examined using Doppler for the
presence of spontaneous flow, and DVT was diagnosed by the absence
of flow using Doppler and the absence of blood flow on CDFI
(24).
MR scanning protocol
All patients were scanned using a 1.5T MR scanner
system (MAGNETOMAera; Siemens Healthineers) equipped with 45 mT/m
gradient strength and a 200 T/m/s maximum slew rate. The MR
scanning was carried out 1.7±1.8 days after duplex sonography. The
abdomen and pelvic regions were covered with two matrix coils with
12 channels. A peripheral angiography phased-array coil equipped
with 32 channels covered the lower extremities. Patients were
positioned in the supine position, head-first toward the bore of
the magnetic field. The arms of the patients were positioned on the
bilateral sides, the bilateral legs were tied up using the belts of
peripheral coil and ankles were elevated using a folded soft sponge
to avoid compression of leg veins and motion artifacts.
Gadopentetate dimeglumine (Magnevist; Bayer Schering Pharma AG) was
administered via a right intravenouscatheter at the rate of 2.5
ml/s, with a total volume 0.15 mmol/kg using a power injector
(Stellant injection system MedRad; Bayer AG). A 15 ml saline chaser
bolus was also administered at the same aforementioned rate.
MRV examinations were performed by applying
fat-suppressed VIBE in the coronal projection. The craniocaudal
scanning range from diaphragm to ankle level was divided into three
stacks, the abdominal and pelvic region, the upper leg region and
the lower leg region. Imaging acquisition was initiated 3 min after
gadolinium injection via a right intravenous antecubital catheter.
The total imaging matrix technique (TIM) was applied, which
eliminated the need for patient positioning and manual coil changes
during MR scans. The abdominal and pelvic region was scanned from
the diaphragm to the iliac vein region for the inferior vena cava
(IVC), bilateral common, external and internal iliac veins.
Breath-hold VIBE-fs was performed to avoid motion artifacts in the
abdominal-pelvic region, and patients held their breath for 18 sec
during each examination. Upper and lower legs from the iliac vein
region to ankle level were analyzed in two consecutive regions
using high-resolution VIBE-Dixon without breath-holding; these two
regions were acquired with voxel size of 0.5x0.5x1 mm. Detailed
parameters of gradient echo VIBE are listed in Table I. The diagnostic criterion for DVT in
MRV were defined as visualization of direct hypointensity filling
defects in venous lumen or non-visualization of a venous segment
(13).
 | Table IParameters of gradient echo VIBE for
abdominal, pelvic and lower extremities. |
Table I
Parameters of gradient echo VIBE for
abdominal, pelvic and lower extremities.
| Imaging region | Abdominal and
pelvic region | Upper and lower leg
regions |
|---|
| Sequence | VIBE with spectral
fat saturation | VIBE with
Dixon |
| Repetition
time/echo time, ms | 3.47/1.27 | 6.95/2.39 |
| Field of view,
mm2 | 400 | 400 |
| Slice thickness,
mm | 1.50 | 1.00 |
| Slice interval,
mm | 0.30 | 0.20 |
| Number of
slices | 88 | 104 |
| Flip angle, ˚ | 10 | 10 |
| Bandwidth,
Hz/px | 450 | 450 |
| Phase encoding
direction | R/L | R/L |
| iPAT acceleration
factor | 3 | 2 |
| Matrix size | 237x320 | 384x384 |
| Acquisition
time | 18 sec | 2 min 27 sec for
upper or lower legregions, 4 min 54 sec for whole legs |
MR image processing
The generated coronal images were transferred to the
Syngo MR workstation (Siemens AG) for analysis and review. Coronal
images and reconstructed axial images were used for evaluation in a
fixed order: IVC, pelvic veins, upper leg veins and lower leg
veins. Multiplanar reconstruction, curved planar reconstruction and
maximum intensity projection were reconstructed as required
according to diagnostic demands.
Subjective image quality
evaluation
In the present study, two radiologists with 12 years
and 5 years of MR experience, who were unaware of duplex sonography
results or D-Dimer test results, analyzed the MR images for DVT
detection. The venous segment-to-segment comparison analysis
between MRV and duplex sonography for DVT detection was based on
vein segments of i) IVC; ii) common iliac vein; iii) external iliac
vein; iv) internal iliac vein; v) femoral vein; vi) deep femoral
vein; vii) popliteal vein; viii) anterior tibial veins; ix)
posterior tibial veins; and x) peroneal veins. The venous segments
i-x) were evaluated independently for MRV image quality by the two
experienced radiologists using a modified 5-point scaling system
(21). The following point system
was used: 5, Excellent, without blurring/artifacts, sharply defined
vessel borders; 4, good, with minimal blurring/artifacts, good
sharply of the vessel borders; 3, moderate, with vessel segments
clearly definable with moderate blurring/artifacts, sharpness of
vessel border is insufficient: 2, poor, with vessel segments
definable but with significant blurring/artifacts, vessel borders
only suspected but not clearly visible; and 1, non-diagnostic.
Statistical analysis
Statistical analysis was performed using Cohen's κ
coefficient statistic in MRV and duplex sonography diagnostic
agreement of DVT for venous segment-to-segment comparison.
According to the Fleiss classification (25) (poor, <0.40; moderate, 0.40-0.59;
good, 0.60-0.75; excellent, >0.75), inter-observer variability
between two attending radiologists was analysed using Wilcoxon rank
sum test. Intraclass correlation coefficient (ICC) estimates and
95% CI were calculated using SPSS software (SPSS 22; IBM Corp.)
based on absolute agreement, a two-way random model was used for
the reliability analysis (poor reliability, <0.5; moderate
reliability, 0.5-0.75; good reliability, 0.75-0.9; excellent
reliability, >0.9). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient data
All 31 enrolled patients finished MRV successfully
without allergic reactions. A total of eight venous segments were
excluded due to MR scanner failure during the imaging procedure
(three venous segments), and severe motion artifacts (five venous
segments; Fig. 1). A total of 303
vein segments were analyzed for segment-to-segment comparison
between MRV and duplex sonography in the present study. Of the 303
evaluated vein segments, duplex sonography identified 119 (39.3%)
vein segments with thrombus, while MRV detected 170 (56.1%) vein
segments with thrombus (Table II).
Therefore, a total of 51 additional DVT vein segments were detected
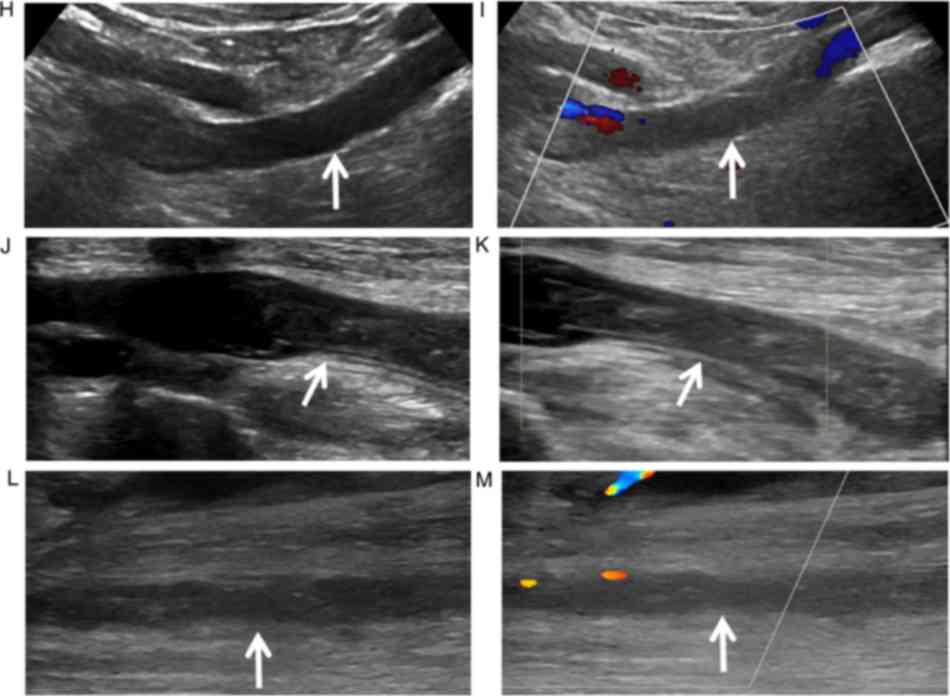
using MRV compared with duplex sonography (images of one
representative patient shown in Fig. 2A-M). In six patients,
separation of water-only and fat-only images were incorrectly
reconstructed, so that one leg demonstrated fat-only signal, the
other leg revealed water-only signal in the water-only image and
the same condition in the fat-only image (images of one
representative patient shown in Fig. 2E-G).
 | Table IIPer-vein-segment analysis of deep
vein thrombosis between magnetic resonance venography and duplex
sonography in 31 patients. |
Table II
Per-vein-segment analysis of deep
vein thrombosis between magnetic resonance venography and duplex
sonography in 31 patients.
| Vein segment | Duplex
sonography | Magnetic resonance
venography | Κ value | ICC | 95% CI |
|---|
| Inferior vena
cava | 4 | 5 | 0.89 | 0.87 | 0.76-0.94 |
| Common iliac
vein | 10 | 13 | 0.87 | 0.74 | 0.54-0.86 |
| External iliac
vein | 9 | 13 | 0.81 | 0.74 | 0.54-0.86 |
| Femoral vein | 13 | 14 | 0.96 | 0.94 | 0.89-0.97 |
| Deep femoral
vein | 2 | 12 | 0.28 | 0.21 | 0.07-0.48 |
| Popliteal vein | 25 | 27 | 0.96 | 0.86 | 0.73-0.92 |
| Anterior tibial
veins | 4 | 23 | 0.28 | 0.13 | 0.09-0.38 |
| Posterior tibial
veins | 26 | 31 | 0.90 | 0.55 | 0.27-0.75 |
| Peroneal veins | 26 | 32 | 0.89 | 0.43 | 0.13-0.67 |
Agreement evaluation
The detection agreement rate of DVT between MRV and
duplex sonography was poor in deep femoral vein (κ value=0.28) and
anterior tibial veins (κ value=0.28), while excellent in other vein
segments (Table II). Excellent
reliability was detected in the femoral vein, good reliability in
IVC and popliteal vein, moderate reliability in common iliac vein,
external femoral vein and posterior tibial veins, and poor
reliability in the deep femoral vein, anterior tibial veins and
peroneal veins (Table II). As for
the 35 internal iliac veins, duplex sonography only detected 18
internal iliac vein segments, including one DVT involved, 16 were
diagnosed to be negative and one was not assessible. However, MRV
examined all the 35 internal iliac vein segments and found thrombus
in nine internal iliac veins, while the other 26 were negative.
Image quality
Image quality was poor in five vein segments due to
severe motion artifacts, which were excluded from the
segment-to-segment comparison mentioned above. The present results
suggested that the scores of venous segments in MRV between two
radiologists indicated no statistical difference. The overall mean
score for visualization of vein segments was 4.86±0.35, indicating
excellent image quality (Table
III). No statistical difference in the interobserver
variability was observed between the two radiologists. In addition,
the ICC value was 0.62, with 95% CI 0.56-0.66 (P<0.001), thus
indicating moderate interobserver reliability.
 | Table IIIImage quality scores of magnetic
resonance venography based on per-vein-segment, based on a 5-point
scale. |
Table III
Image quality scores of magnetic
resonance venography based on per-vein-segment, based on a 5-point
scale.
| Vein segment | Observer A | Observer B | P-value |
|---|
| Inferior vena
cava | 4.74±0.45 | 4.77±0.43 | 0.71 |
| Common iliac
vein | 4.82±0.38 | 4.81±0.40 | 0.76 |
| External iliac
vein | 4.84±0.37 | 4.87±0.34 | 0.41 |
| Internal iliac
vein | 4.90±0.30 | 4.84±0.37 | 0.16 |
| Femoral vein | 4.97±0.18 | 4.98±0.13 | 0.32 |
| Deep femoral
vein | 4.98±0.13 | 5.00±0.00 | 0.32 |
| Popliteal vein | 4.95±0.22 | 5.00±0.00 | 0.08 |
| Anterior tibial
veins | 4.69±0.50 | 4.74±0.44 | 0.18 |
| Posterior tibial
veins | 4.71±0.50 | 4.76±0.43 | 0.32 |
| Peroneal veins | 4.76±0.47 | 4.81±0.40 | 0.18 |
| Overall mean
scores | 4.85±0.37 | 4.87±0.33 | 0.05 |
Additional findings
Along with the vein segments used for
segment-to-segment comparison, MRV additionally examined 27 other
single legs, which were not identified by duplex sonography, and
indicated 38 vein segments with thrombosis. This included one
common iliac vein, one internal iliac vein, one external iliac
vein, two femoral veins, three popliteal veins, four anterior
tibial veins, 14 posterior tibial veins and 12 peroneal veins. In
addition, except for the thrombus detected in the deep venous
system, duplex sonography incidentally identified thrombus in five
great saphenous vein segments and five small saphenous vein
segments, while MRV detected thrombus in 11 great saphenous vein
segments and six small saphenous vein segments. Furthermore, duplex
sonography identified varicosities with thrombus in the great
saphenous vein in three patients, which were also clearly visible
in MRV images, and had been identified by high ligation of great
saphenous vein and varicose vein stripping surgery after duplex
sonography and MRV (Fig. 3).
Discussion
To the best of our knowledge, the present study is
the first to demonstrate the application of high resolution MRV
compared with duplex sonography for DVT assessment, using VIBE-fs
for abdominopelvic DVT (acquisition time, 18 sec) and VIBE-Dixon
for lower leg DVT (acquisition time, 4 min 54 sec). The deep venous
system was assessed using per-vein-segment analysis rather than
per-embolus or per-patient analysis, despite the limited number of
patients enrolled in the present study. Overall, MRV detected more
DVT-involved vein segments compared with duplex sonography,
especially in the deep femoral vein and anterior tibial veins.
MRV VIBE has been reported to be a faster and more
efficient method for identification of a normal venous system of
legs (18). Hansch et al
(19) introduced a combined protocol
for pulmonary arteries and abdominal/pelvic/leg veins by scanning
VIBE-fs after a blood pool contrast agent injection; in this
protocol venous system from ankle to IVC were visualized at a high
level of image quality. In addition, Bashir et al (26) used VIBE-fs to compare iron-based
ferumoxytol and gadolinium-based gadofosveset for abdominopelvic
and lower extremity venous enhancement, from the central IVC to
popliteal vessels, in patients with end-stage renal diseases.
However, differing from these previous MRV studies using VIBE-fs,
the present study included high resolution VIBE-Dixon for
infrainguinal DVT in the MRV protocol.
Unlike spectral fat saturation, Dixon fat-suppressed
technique used in present study is a multi-echo modulating
technique based on difference of the frequency between fat and
water molecules. Dixon fat-suppressed technique produces four
tissue-contrast images: In-phase, opposed-phase, fat-only and
water-only images; fat-only and water-only images can be
subsequently reconstructed by complex signal addition or
subtraction calculation by signals of in-phase and opposed-phase
images; the water-only image obtained by the Dixon's technique can
be used for fat suppression (20,27-29).
A previous mouse study reported that Dixon fat separation provided
a more reliable and homogenous fat suppression compared with a
chemical saturation method in phantoms and in vivo
experiments (27). In previous
phantom studies, signal-to-noise-ratio is higher in Dixon fat
saturation T1 weighted images compared with conventional fat
saturation (28,29). In the present study, the separation
of fat and water was not achieved completely in six patients, which
may be related with B0-inhomogeneity phase differences (20). However, this did not reduce the
observations of the venous system of the lower legs, as fat
suppressed images could be obtained by fat-only and water-only
images. Therefore, the venous vasculatures could be visualized
continuously by combining the two images.
Although the gadolinium-based blood pool contrast
agent gadofosveset trisodium was not applied in the present study,
the conventional extracellular gadolinium-based contrast agent
gadopentetate dimeglumine used had been proven to be feasible in
previous studies (21,30). Furthermore, the MRV VIBE sequence was
initiated 3 min after contrast administration in the present study,
in order to achieve veins fully filled with contrast material in
the venous system.
Sensitivity for distal DVT of MRV has been reported
to be lower than proximal DVT (62.1% vs. 93.9%) in a previous
meta-analysis (13). High signals
caused by intravenous gadolinium-based contrast agent in the
vessels allows for better depiction of venous characterization in
contrast enhanced MRV, and the more homogenous fat suppression
using the Dixon fat saturation technique may improve the
visualization of small vessels (28). Therefore, the present study used
VIBE-Dixon with a higher resolution (voxel size, 0.5x0.5x1) in the
lower leg.
MRV VIBE-Dixon in the present study detected 13 and
30 more vein segments with DVT compared with duplex sonography in
femoropopliteal veins and distal veins, especially for deep femoral
vein and anterior tibial veins. The poor visualization of deep
femoral vein may be related to the depth and the relative
inaccessibility of duplex sonography, due to the limited range of
ultrasound waves in tissues. Anterior tibial veins are not
recommended to be investigated routinely as they have been
previously reported to be rarely affected by DVT (31-33).
In the present study, the patients enrolled were all
symptomatic with leg swelling and leg pain, so the anterior tibial
veins were examined and evaluated. The inferiority of duplex
sonography in anterior tibial veins observed in the present study
may be related to the edema in the legs, and the inexperienced
operators for detecting the anterior tibial veins which is not
performed routinely in clinical practice. Moreover, benefitting
from higher spatial resolution, MRV for visualizing subtle vein
segments and hypointensity filling defects could be displayed
clearly with the surrounding high intravascular enhancement. In
addition, homogeneous fat suppression allows MRV to be useful for
visualizing these vein segments (20).
With regards to the abdominopelvic region, duplex
sonography has a limited ability to detect pelvic and abdominal
thrombus due to complications, including obesity, bowel gas,
impeded acoustic penetration and its operator dependent feature
(9,32). In the present study, MRV VIBE-fs used
for the abdominopelvic region detected eight more DVT involved vein
segments compared with duplex sonography, including one in the IVC,
three in common iliac veins and four in the external iliac vein.
Although the internal iliac vein was not used for
segment-to-segment comparison, the present results suggested that
MRV may also be suitable for displaying the anatomy of internal
iliac veins, related thrombus inside the IVC and pelvic veins,
which is consistent with previous studies (10,11,21).
Evaluation of abdominopelvic veins, especially for
the IVC scanned with VIBE-fs, was assessed as contrast-enhanced MRV
offers good tissue contrast between thrombus and intravascular
blood in the IVC despite the interference of bowels, which is
essential to determine the accurate site for IVC filter placement
in clinical practice. Moreover, in the present study, MRV could
display the bilateral venous system of lower extremities and the
bilateral pelvic vessels simultaneously with the help of the
peripheral angiography phased-array coil.
Despite the limitations of duplex sonography, it has
advantages for DVT diagnosis. Duplex sonography is non-invasive,
cost effective treatment, which is available for critically ill
patients and has largely replaced conventional venography as the
standard for clinically suspected DVT diagnosis with a high
sensitivity and specificity (12).
Compression sonography alone would be an appropriate test when
identifying proximal DVT as it can achieve an optimal specificity
of 97.8%, while combined color-doppler method is the appropriate
technique for identifying distal DVT (12). Ultrasound elastography (UE) can
estimate elastic properties of soft tissues such as stiffness, and
it has been reported that UE may be a promising technique to
distinguish between acute and chronic DVT (34). However the clinical applicability of
UE to DVT has not yet been fully investigated (34).
With the advantages of MRV, this technique may be
considered as an alternative method for DVT assessment to duplex
sonography. However, it is not feasible in patients with
contraindications to gadolinium-contrast MR imaging, such as
patients with renal insufficiency with an estimated glomerular
filtration rate<30, pregnant women, those with a history of
allergic reaction to gadolinium-based contrast material, critically
ill patients who cannot tolerate MR scanning, claustrophobic
patients or those unable to establish venous access, which is
necessary for contrast-enhanced MRV (35). Moreover, compared with duplex
sonography, MR examination has a relatively higher cost with the
use of contrast material and a higher acoustic noise, and the main
reason for the noise is related to mechanical vibrations of
gradient coil during rapid switching of gradients (36).
The present study had limitations. First, while
conventional venography is generally accepted as the gold standard
for imaging DVT, it is rarely performed in clinical practice
(6). It is also a limitation to use
duplex sonography alone as the reference standard. Additionally,
whilst acquisition time for lower leg is only 4 min 54 sec,
VIBE-Dixon with a higher resolution in the present study requires
enormous reconstruction data, which consumed 6 min for each
Dixon-region to obtain coronal images. Therefore, the five vein
segments excluded from the comparative evaluation, related with
severe motion artifacts, were not scanned again in a timely manner,
despite a dedicated reconstruction computer available in the
department. Due to this reason, the present study had scanned the
abdominopelvic region with breath-hold VIBE-fs, as the images could
be visualized simultaneously after the scanning, which is helpful
to scan the sequence again if the images are affected by motion
artifacts related with the breath-hold. Moreover, since thrombus
detection in the deep venous system was the primary aim of the
present study, there was no systematic analysis of the superficial
veins between duplex sonography and MRV, thus further studies are
required. Another limitation was that the time used in duplex
sonography and MRV was not recorded, as acquisition time for MRV
was ~6 min in the present study, and time for patient-positioning
and image reconstruction was not included. In addition, although CT
venography (CTV) has considerable ionizing radiation exposure, a
lower kVp setting with a modified reconstruction technique can help
to decrease the radiation dose while maintaining the image quality
in CTV (37). Therefore, a
limitation of the present study includes the absence of a
comparison between MRV and CTV.
In conclusion, the results of the present study
suggested that contrast enhanced MRV scanned by VIBE-fs for
suprainguinal and VIBE-Dixon for infrainguinal regions may be a
feasible method for venous system depiction and DVT detection in
abdomen, pelvic and lower extremities. Agreement rate analysis
results suggested that MRV, compared with duplex sonography, may be
a good alternative imaging modality for DVT assessment when duplex
sonography is inadequate or not feasible.
Acknowledgements
The authors would like to thank Dr Qing-Chang Chen,
(Department of Ultrasound, Union Hospital, Tongji Medical College,
Huazhong University of Science and Technology, Wuhan, China) for
his assistance in providing images of duplex sonography.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QF and QGC performed the scans, prepared the figures
and wrote the manuscript. SW performed the statistical analysis. SW
and XCK analyzed the images. All authors approved the final
manuscript.
Ethics approval and consent to
participate
This prospective study was approved by The Medical
Ethics Committee of Tongji Medical College, Huazhong University of
Science and Technology. Patients were informed of procedure and
purpose of this study, and written consent was obtained.
Patient consent for publication
All the patients provided written informed consent
for the publication of the associated data and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kearon C, Ginsberg JS, Douketis J,
Crowther MA, Turpie AG, Bates SM, Lee A, Brill-Edwards P, Finch T
and Gent M: A randomized trial of diagnostic strategies after
normal proximal vein ultrasonography for suspected deep venous
thrombosis: D-dimer testing compared with repeated ultrasonography.
Ann Intern Med. 142:490–496. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gaitini D: Multimodality imaging of the
peripheral venous system. Int J Biomed Imaging.
2017(54616)2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Eikelboom JW, Hirsh J, Spencer FA, Baglin
TP and Weitz JI: Antiplatelet drugs: Antithrombotic therapy and
prevention of thrombosis, 9th ed: American College of chest
physicians evidence-based clinical practice guidelines. Chest. 141
(2 Suppl):e89S–e119S. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Loud PA, Grossman ZD, Klippenstein DL and
Ray CE: Combined CT venography and pulmonary angiography: A new
diagnostic technique for suspected thromboembolic disease. AJR Am J
Roentgenol. 170:951–954. 1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rademaker J, Griesshaber V, Hidajat N,
Oestmann JW and Felix R: Combined CT pulmonary angiography and
venography for diagnosis of pulmonary embolism and deep vein
thrombosis: Radiation dose. J ThoracImag. 16:297–299.
2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Karande GY, Hedgire SS, Sanchez Y, Baliyan
V, Mishra V, Ganguli S and Prabhakar AM: Advanced imaging in acute
and chronic deep vein thrombosis. Cardiovasc Diagn Ther. 6:493–507.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carpenter JP, Holland GA, Baum RA, Owen
RS, Carpenter JT and Cope C: Magnetic resonance venography for the
detection of deep venous thrombosis: Comparison with contrast
venography and duplex Doppler ultrasonography. J Vasc Surg.
18:734–741. 1993.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ozbudak O, Eroğullari I, Öğüş C, Çilli A,
Türkay M and Özdemir T: Doppler ultrasonography versus venography
in the detection of deep vein thrombosis in patients with pulmonary
embolism. J Thromb Thrombolys. 21:159–162. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Labropoulos N, Borge M, Pierce K and
Pappas PJ: Criteria for defining significant central vein stenosis
with duplex ultrasound. J Vasc Surg. 46:101–107. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang SY, Kim CY, Miller MJ, Gupta RT,
Lessne ML, Horvath JJ, Boll DT, Evans PD, Befera NT, Krishnan P, et
al: Abdominopelvic and lower extremity deep venous thrombosis:
Evaluation with contrast-enhanced MR venography with a blood-pool
agent. AJR Am J Roentgenol. 201:208–214. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gary T, Steidl K, Belaj K, Hafner F,
Froehlich H, Deutschmann H, Pilger E and Brodmann M: Unusual deep
vein thrombosis sites: Magnetic resonance venography in patients
with negative compression ultrasound and symptomatic pulmonary
embolism. Phlebology. 29:25–29. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Goodacre S, Sampson F, Thomas S, van Beek
E and Sutton A: Systematic review and meta-analysis of the
diagnostic accuracy of ultrasonography for deep vein thrombosis.
BMC Med Imaging. 5(6)2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sampson FC, Goodacre SW, Thomas SM and van
Beek EJ: The accuracy of MRI in diagnosis of suspected deep vein
thrombosis: Systematic review and meta-analysis. Eur Radiol.
17:175–181. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ruehm SG, Wiesner W and Debatin JF: Pelvic
and lower extremity veins: Contrast-enhanced three-dimensional MR
venography with a dedicated vascular coil-initial experience.
Radiology. 215:421–427. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tan M, Mol GC, van Rooden CJ, Klok FA,
Westerbeek RE, Iglesias Del Sol A, van de Ree MA, de Roos A and
Huisman MV: Magnetic resonance direct thrombus imaging
differentiates acute recurrent ipsilateral deep vein thrombosis
from residual thrombosis. Blood. 124:623–627. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Westerbeek RE, Van Rooden CJ, Tan M, Van
Gils AP, Kok S, De Bats MJ, De Roos A and Huisman MV: Magnetic
resonance direct thrombus imaging of the evolution of acute deep
vein thrombosis of the leg. J ThrombHaemost. 6:1087–1092.
2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rofsky NM, Lee VS, Laub G, Pollack MA,
Krinsky GA, Thomasson D, Ambrosino MM and Weinreb JC: Abdominal MR
imaging with a volumetric interpolated breath-hold examination.
Radiology. 212:876–884. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pfeil A, Betge S, Poehlmann G, Boettcher
J, Drescher R, Malich A, Wolf G, Mentzel H and Hansch A: Magnetic
resonance VIBE venography using the blood pool contrast agent
gadofosveset trisodium-An interrater reliability study. Eur J
Radiol. 81:547–552. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hansch A, Betge S, Poehlmann G, Neumann S,
Baltzer P, Pfeil A, Waginger M, Boettcher J, Kaiser WA, Wolf G and
Mentzel HJ: Combined magnetic resonance imaging of deep venous
thrombosis and pulmonary arteries after a single injection of a
blood pool contrast agent. Eur Radiol. 21:318–325. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Michaely HJ, Attenberger UI, Dietrich O,
Schmitt P, Nael K, Kramer H, Reiser MF, Schoenberg SO and Walz M:
Feasibility of gadofosveset-enhanced steady-state magnetic
resonance angiography of the peripheral vessels at 3 Tesla with
Dixon fat saturation. Invest Radiol. 43:635–641. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kluge A, Mueller C, Strunk J, Lange U and
Bachmann G: Experience in 207 combined MRI examinations for acute
pulmonary embolism and deep vein thrombosis. AJR Am J Roentgenol.
186:1686–1696. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Delfaut EM, Beltran J, Johnson G, Rousseau
J, Marchandise X and Cotten A: Fat suppression in MR imaging:
Techniques and pitfalls. Radiographics. 19:373–382. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wells PS, Anderson DR, Rodger M, Forgie M,
Kearon C, Dreyer J, Kovacs G, Mitchell M, Lewandowski B and Kovacs
MJ: Evaluation of D-dimer in the diagnosis of suspected deep-vein
thrombosis. N Engl J Med. 349:1227–1235. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Killewich LA, Bedford GR, Beach KW and
Strandness DJ: Diagnosis of deep venous thrombosis. A prospective
study comparing duplex scanning to contrast venography.
Circulation. 79:810–814. 1989.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Landis JR and Koch GG: The measurement of
observer agreement for categorical data. Biometrics. 33:159–174.
1977.PubMed/NCBI
|
|
26
|
Bashir MR, Mody R, Neville A, Javan R,
Seaman D, Kim CY, Gupta RT and Jaffe TA: Retrospective assessment
of the utility of an iron-based agent for contrast-enhanced
magnetic resonance venography in patients with endstage renal
diseases. J Magn Reson Imaging. 40:113–118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ragan DK and Bankson JA: Two-point Dixon
technique provides robust fat suppression for multi-mouse imaging.
J Magn Reson Imaging. 31:510–514. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ma J, Vu AT, Son JB, Choi H and Hazle JD:
Fat-suppressed three-dimensional dual echo Dixon technique for
contrast agent enhanced MRI. J Magn Reson Imaging. 23:36–41.
2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Reeder SB, McKenzie CA, Pineda AR, Yu H,
Shimakawa A, Brau AC, Hargreaves BA, Gold GE and Brittain JH:
Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson
Imaging. 25:644–652. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fraser DG, Moody AR, Davidson IR, Martel
AL and Morgan PS: Deep venous thrombosis: Diagnosis by using venous
enhanced subtracted peak arterial MR venography versus conventional
venography. Radiology. 226:812–820. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Elias A, Cadène A, Elias M, Puget J,
Tricoire JL, Colin C, Lefebvre D, Rousseau H and Joffre F: Extended
lower limb venous ultrasound for the diagnosis of proximal and
distal vein thrombosis in asymptomatic patients after total hip
replacement. Eur J Vasc Endovasc Surg. 27:438–444. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Forbes K and Stevenson AJ: The use of
power Doppler ultrasound in the diagnosis of isolated deep venous
thrombosis of the calf. Clin Radiol. 53:752–754. 1998.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mattos MA, Melendres G, Sumner DS, Hood
DB, Barkmeier LD, Hodgson KJ and Ramsey DE: Prevalence and
distribution of calf vein thrombosis in patients with symptomatic
deep venous thrombosis: A color-flow duplex study. J Vasc Surg.
24:738–744. 1996.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mumoli N, Mastroiacovo D,
Giorgi-Pierfranceschi M, Pesavento R, Mochi M, Cei M, Pomero F,
Mazzone A, Vitale J, Ageno W and Dentali F: Ultrasound elastography
is useful to distinguish acute and chronic deep vein thrombosis. J
Thromb Haemost. 16:2482–2491. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pressacco J, Papas K, Lambert J, Paul Finn
J, Chauny JM, Desjardins A, Irislimane Y, Toporowicz K, Lanthier C,
Samson P, et al: Magnetic resonance angiography imaging of
pulmonary embolism using agents with blood pool properties as an
alternative to computed tomography to avoid radiation exposure. Eur
J Radiol. 113:165–173. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fischer S, Grodzki DM, Domschke M,
Albrecht M, Bodelle B, Eichler K, Hammerstingl R, Vogl TJ and
Zangos S: Quiet MR sequences in clinical routine: Initial
experience in abdominal imaging. Radiol Med. 122:194–203.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Baek HJ, Choo KS, Nam KJ, Hwang J, Lee JW,
Kim JY and Jung HJ: Comparison of image qualities of 80 kVp and 120
kVp CT venography using Model-Based iterative reconstruction at
same radiation dose. J Korean Soc Radiol. 78:235–241. 2018.
View Article : Google Scholar
|