Introduction
Satisfactory pain management is important during
postoperative treatment as it can increase patient comfort and
improve recovery (1). Multimodal
analgesia techniques, and techniques combining general and local
anesthesia lead to greater pain control, with less reliance on
opioids and fewer side effects (2).
The optimal regional block should have a rapid onset, a long
duration of analgesia and high-quality pain control without adverse
side effects. Tranversus abdominis plane (TAP) block is novel
peripheral nerve block method, which is used for any surgery
technique involving the anterolateral abdominal wall (3). As an important part of multimodal
analgesia following abdominal surgery, TAP block can be performed
to provide effective analgesia (4).
However, a single-shot TAP block is limited in duration and
analgesia. Increasing the volume (dose) of local anesthetics (LAs)
may provide more effective analgesia duration and long-lasting
nerve block, but can also increase the risk of dose-associated
systemic toxicity (5). Although
continuous catheter-based nerve blocks can improve postoperative
analgesia, this procedure is expensive and requires skillful
placement techniques (6). Thus far,
a range of adjuvants, including dexamethasone, clonidine, fentanyl
and midazolam, have been used to prolong the duration of nerve
block analgesia with varying degrees of success (7). The roles of adjuvants on peripheral
nerve blocks are debated, and previous studies have suggested
active and passive impacts (7).
Dexmedetomidine (Dex) is a highly selective
α2-adrenergic agonist that has sedative, analgesic,
sympatholytic and antianxiety effects without respiratory
depression (8). Due to its multiple
beneficial properties, the systemic administration of Dex during
the perioperative period is prevalent as a favorable sedative and
analgesic agent (9). Additionally,
Dex has been described as an effective adjuvant for regional
anesthetic agents. However, clinical trials have produced
contradictory results. Some animal and clinical studies have
demonstrated that Dex administered as an adjuvant to LAs for
peripheral nerve block and plexus block results faster, with a
longer duration of block and improved analgesic efficacy without
neurologic complications (10-12).
Other results have indicated a delay in sensory and motor block
onset time, or no effect on sensory and motor block duration
(13).
The present study was designed to assess the
hypothesis that adding Dex to ropivacaine in an ultrasound-guided
TAP block could improve analgesic quality and promote recovery
following laparoscopic colectomy.
Materials and methods
Study protocol
This prospective, randomized, double blind clinical
study was approved by the Institutional Human Investigations
Committee of Yantai Yuhuangding Hospital of Qingdao University and
registered at the following clinical trial website: http://www.chictr.org.cn (ChiCTR-IOR-17014122).
Written informed consent was obtained from all participants. A
total of 64 patients (aged 38-72 years; weighing 52-83 kg) at
American Society of Anesthesiologists, with a physical status score
(ASA) II-III grade and were scheduled for elective laparoscopic
colectomy under general anesthesia, were recruited between February
2017 and March 2018.
Randomization and blinding
Patients were excluded from the present study due to
the following reasons: A history of allergic reactions to
ropivacaine, treatment with other amino-amide LAs or
dexmedetomidine, psychological disorders, infection at the
injection site or any other contraindications to TAP block,
tolerance to opioids or the use of opioids within 2 days prior to
the start of the current study. The patients and all staff involved
in patient management and data collection were blind to the group
assignment until the end of the study. All TAP blocks were
performed by experienced anesthesiologists who were not involved in
data collection. Patients agreed to receive TAP blocks while they
were under general anesthesia. An anesthesiologist, who did not
participate in patient grouping or the research, prepared the
therapeutic agents based on randomization. The prepared syringes
contained either 20 ml 0.375% ropivacaine (Qilu Pharmaceutical Co.,
Ltd.) plus 2 ml normal saline for the R group, or 20 ml 0.375%
ropivacaine plus 2 ml of Dex (0.5 µg/kg; Jiangsu Hengrui Medicine
Co., Ltd.) for the RD group.
Study procedures
One day before surgery, patients were instructed on
how to use patient-controlled intravenous analgesia (PCIA), and the
Visual Analogue Scale (VAS) scores were determined (0=no pain and
10=the worst possible pain). Patients were assigned to either the R
(n=31) or RD (n=31) group on arrival to the operating room using a
computer-generated randomization list. Blood pressure (BP),
electrocardiograms (ECG), heart rate (HR), blood oxygen saturation
(SpO2) and the bispectral index (BIS) were monitored in
all patients. A total of 0.2 mg penehyclidine hydrochloride was
infused intravenously 5 min prior to the initiation of anesthesia.
General anesthesia was induced using midazolam (0.05 mg/kg),
sufentanil (0.4 µg/kg), propofol (1.5-2.0 mg/kg) and cisatracurium
(0.15 mg/kg). Following tracheal intubation, anesthesia was
maintained with 3-12 mg/kg/h propofol and 0.06-0.1 µg/kg/min
remifentanil. Cisatracurium (0.03 mg/kg) was administered
intermittently to maintain muscle relaxation. The tidal volume was
set at 6-8 ml/kg and the respiratory rate at 12 times/min in order
to maintain the PETCO2 at 35-45 mmHg. Sufentanil (0.2
µg/kg) was administered prior to skin incision. A BIS value between
45 and 60 was maintained.
Following induction, a bilateral ultrasound-guided
TAP block was implemented with an ultrasound machine (Venue 50; GE
Healthcare) using the same method outlined in a previous study
(14). Following the preparation of
the skin with antiseptic solution, a 7-12 MHz high-frequency linear
probe was placed transversely on the anterolateral abdominal wall
between the costal margin and iliac crest on the mid-axillary line.
Optimal images captured the three layers of muscles: The external
oblique, the internal oblique and the transversus abdominis. The
needle was introduced in the plane of the ultrasound probe directly
under the probe and advanced until it reached the plane between the
internal oblique and transversus abdominis muscles. Correct
positioning of the needle tip was confirmed with an injection of 1
ml normal saline, which led to separation between the internal
oblique and transversus abdominis muscle. Subsequently, a total of
22 ml prepared solution was injected using the ultrasound for
guidance. From the ultrasound image, expansion of hypoechoic
liquid, which reflected the distribution of LAs during injection,
indicated a successful outcome. The same procedures were followed
on the opposite side.
Propofol and remifentanil were terminated upon
completion of skin closure. Dezocine (5 mg) and palonosetron (0.25
mg) were administrated intravenously 30 min prior to termination of
the surgical procedure. The infusion of the cisatracurium was
stopped ~30 min prior to the end of surgery. Upon completion of
surgery, neuromuscular blockade was antagonized via intravenous
administration of 1.0 mg neostigmine and 0.5 mg atropine. Propofol
and remifentanil infusions were stopped at the end of surgery.
Patients were extubated once the extubation criteria
(T4/T1 ratio >0.9) were met, and the
extubation time was recorded (15).
The patients were subsequently sent to the ward, where they
received nasal O2 supplementation and had their vital
signs continuously monitored. The PCIA pump (WZ-6523C-4 Disposable
Infusion Pump; Royal Fornia Medical Equipment Co., Ltd., Zhuhai,
China) regimen consisted of 10 mg butorphanol. The PCIA pump was
connected to the intravenous line. Settings included a basal
infusion rate of 2 ml/h, 15 min lockout and self-controlled doses
at 0.5 ml each.
Hemodynamic indexes (HR, BP and SpO2)
were recorded at the following time-points: Arrival at the
operating room (baseline, T0); induction (T1); intubation (T2); 5
min following TAP block (T3); 60 min following intubation (T4); at
extubation (T5); and 1, 2, 6, 12 and 24 h post-surgery (T6-T10).
All patients were sent to the ward once they met the recovery room
criteria (15). Lactated Ringer's
solution was tailored to the requirements of the patients during
surgery. Clear fluids were allowed on the day of surgery and free
fluids were allowed from the first postoperative day. Ambulation
was encouraged on postoperative day 1 and the urinary catheter was
removed when the patient was mobile. Patients were discharged from
the hospital when the discharge criteria were met (15).
VAS scores were assessed at rest (VASR) and at
movement (VASM) at T6-T10 by an investigator blinded to the group
allocation. After surgery, the duration of sensory block was
assessed via pinprick testing in the area of the TAP block
(16). A 3-point scale was applied
to evaluate the level of pain by pinprick with a 23 G needle (0,
sharp sensation; 1, blunt sensation; 2, no sensation). The duration
of sensory block was defined as the time from completion of TAP
block to complete recovery of sensation. The duration of analgesia
was defined as the time from completion of TAP block to first
rescue analgesia (when the VAS score was ≥4 or on patient
demand).
The following 5-point scale was applied to assess
the level of sedation at T6-T10: Completely awake, 0; drowsy/closed
eyes, 1; asleep/easily aroused with light tactile stimulation or a
simple verbal command, 2; asleep/arousable only by strong physical
stimulation, 3; unarousable, 4. The degree of nausea severity was
evaluated using the following 4-point scale: None, 1; mild, 2;
moderate, 3; severe, 4. Bradycardia (HR <50 beats/min),
hypotension (BP <90/60 mmHg), somnolence (sedation score ≥3) and
respiratory depression (SpO2 <90% or respiratory rate
<10 bpm lasting 3 min or more) (17) were considered severe adverse events
and were treated appropriately. Postoperative nausea and vomiting
(PONV) following surgery was also recorded. When the VAS score was
≥4 or the patient demanded rescue analgesia, 5 mg dezocine was
intravenously administered as rescue analgesia. Ondansetron (4 mg)
was administrated intravenously for PONV when needed. At 48 h
following surgery, a 3-point scale was used to evaluate the patient
overall satisfaction with the postoperative pain management (1,
highly satisfied; 2, satisfied; 3, dissatisfied). The time of bowel
function recovery (passage of flatus or stool since the first time
of oral fluid and normal diet consumption) post-surgery (15) and the length of hospital stay (LOS)
were recorded.
Statistical analysis
All data in the present study were analyzed using
SPSS version 16.0 (SPSS, Inc.). Data are presented as the mean ±
standard deviation, and count (%). Patient parameters, including
age, weight, body mass index (BMI), operation time, blood loss,
infusion volume, urine output, duration of sensory block and
analgesia, time of bowel function recovery and LOS were compared
using an unpaired Student's t-test. HR and mean BP at different
time-points were compared between the two groups using a one-way
ANOVA, followed by Bonferroni's post hoc test. The male/female
ratio, ASA grade, incidence of adverse events and degree of
satisfaction were analyzed using the χ2 or
Fisher's exact test. The Mann-Whitney U test was performed to
compare pain and sedation scores between the groups. The present
study considered that block duration differences for ≥60 min
revealed the clinical significance between the two groups. To
achieve a statistical power of at least 90% using ANOVA with a
significance level of 0.05 and a predicted dropout of ~15%, at
least 32 subjects were recruited in each group. P<0.05 was
considered to indicate a statistically significant difference.
Results
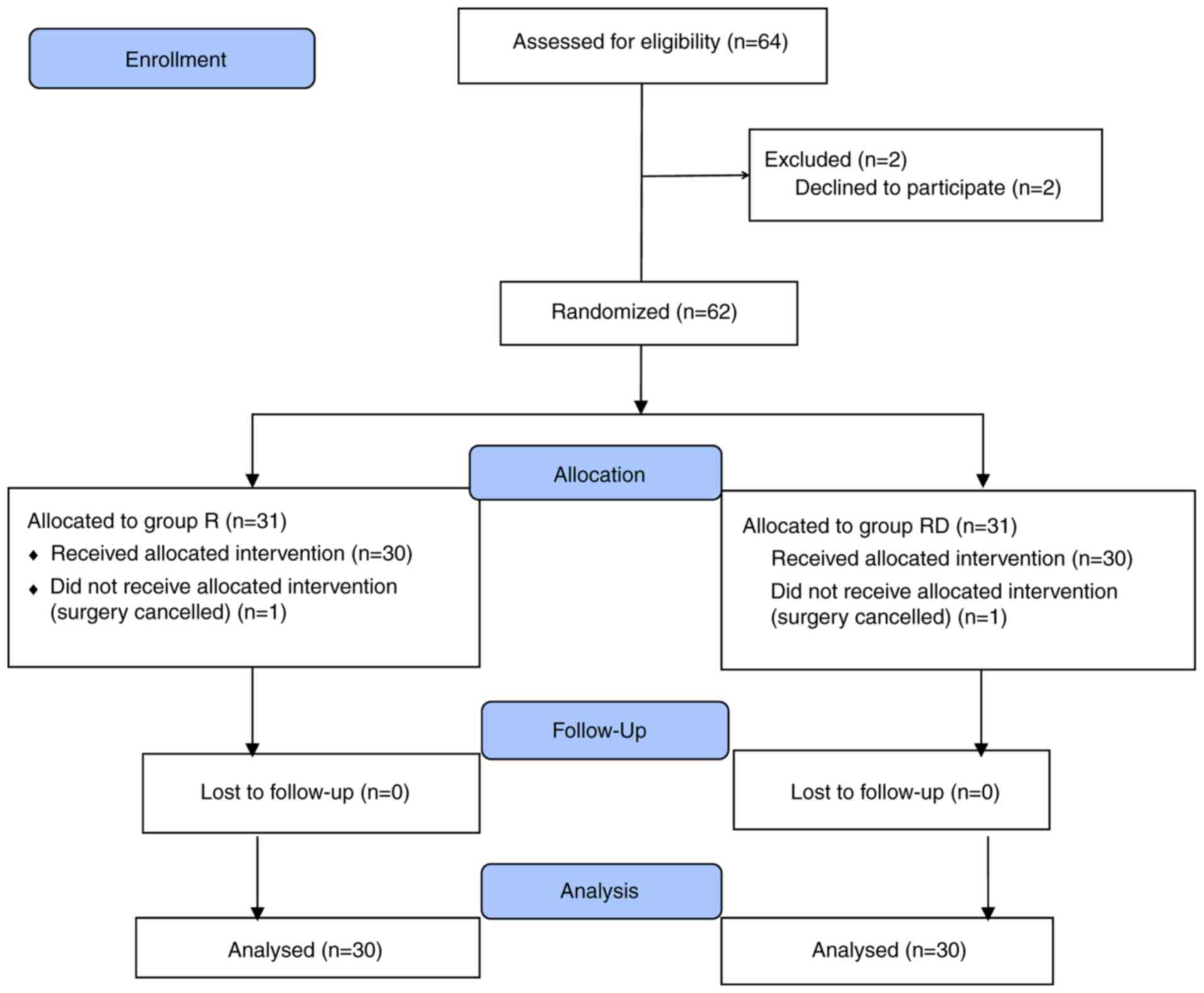
A total of 64 patients were assessed for
eligibility. However, a total of 4 patients were excluded from the
study as 2 patients refused to participate and 2 surgeries were
cancelled. A total of 60 patients completed the trial (n=30 in each
group; Fig. 1). TAP block was
accurately localized under ultrasound guidance and blocks were
successfully completed in all patients. In each group, no patients
had any complications that were attributed to TAP block.
There were no significant differences between the
two groups regarding the clinical characteristics of patients,
including age, male/female ratio, ASA grade, weight, BMI and
intraoperative data (Table I). In
addition, hemodynamic variables were similar in both groups during
the surgery. The BP and HR were slightly reduced following
anesthesia induction in both groups. Furthermore, decreased HR was
indicated in the RD group following TAP block; however, there was
no significant difference between the two groups (Fig. 2).
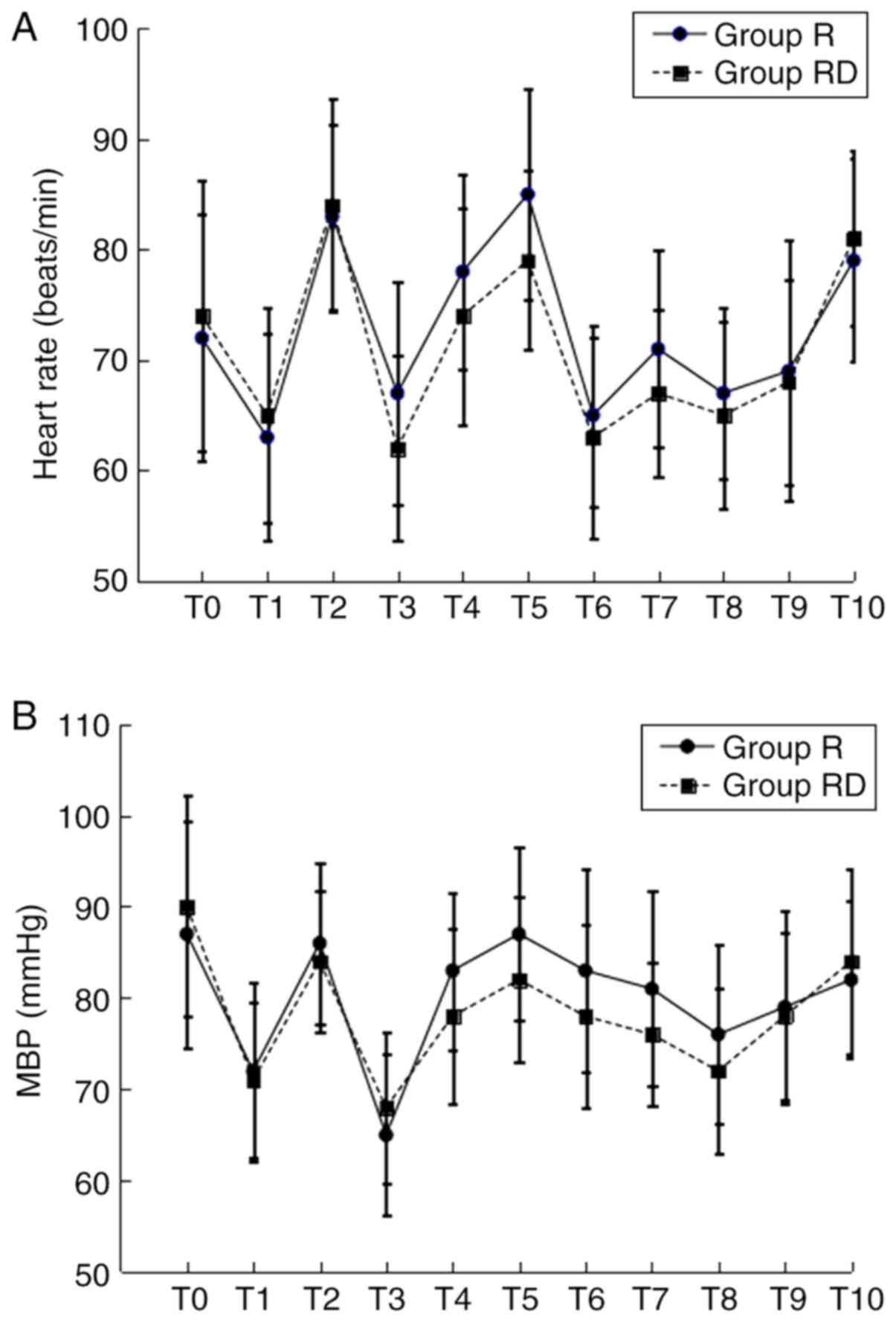 | Figure 2.HR and MBP at different time-points.
(A) HR and (B) MBP. T0, baseline; T1, induction; T2, intubation;
T3, 5 min following TAP block; T4, 60 min following intubation; T5,
extubation; T6-T10, 1, 2, 6, 12 and 24 h post-surgery,
respectively; MBP, mean blood pressure; HR, heart rate. |
 | Table IClinical characteristics of patients
in the R and RD groups. |
Table I
Clinical characteristics of patients
in the R and RD groups.
| Characteristic | R Group (n=30) | RD Group (n=30) | P-value |
|---|
| Age | 61.5±12.3 | 60.2±13.5 | 0.698 |
| Male/female | 16/14 | 17/13 | 0.795 |
| ASA grade
(II/III) | 22/8 | 20/10 | 0.573 |
| Weight (kg) | 69.2±15.6 | 71.3±14.4 | 0.593 |
| Height (cm) | 165.2±10.5 | 163.9±11.8 | 0.654 |
| BMI
(kg/m2) | 23.3±4.8 | 23.6±5.1 | 0.815 |
| Operative time
(min) | 172.6±52.5 | 178.8±48.6 | 0.637 |
| Blood loss
(ml) | 187±69 | 192±94 | 0.814 |
| Infusion volume
(ml) | 1738±421 | 1766±384 | 0.789 |
| Urine output
(ml) | 596±156 | 622±143 | 0.504 |
The duration of sensory block and analgesia was
significantly increased in the RD group compared with the R group
(P<0.05; Table II). However, the
highest level of sensory block achieved was similar in both groups.
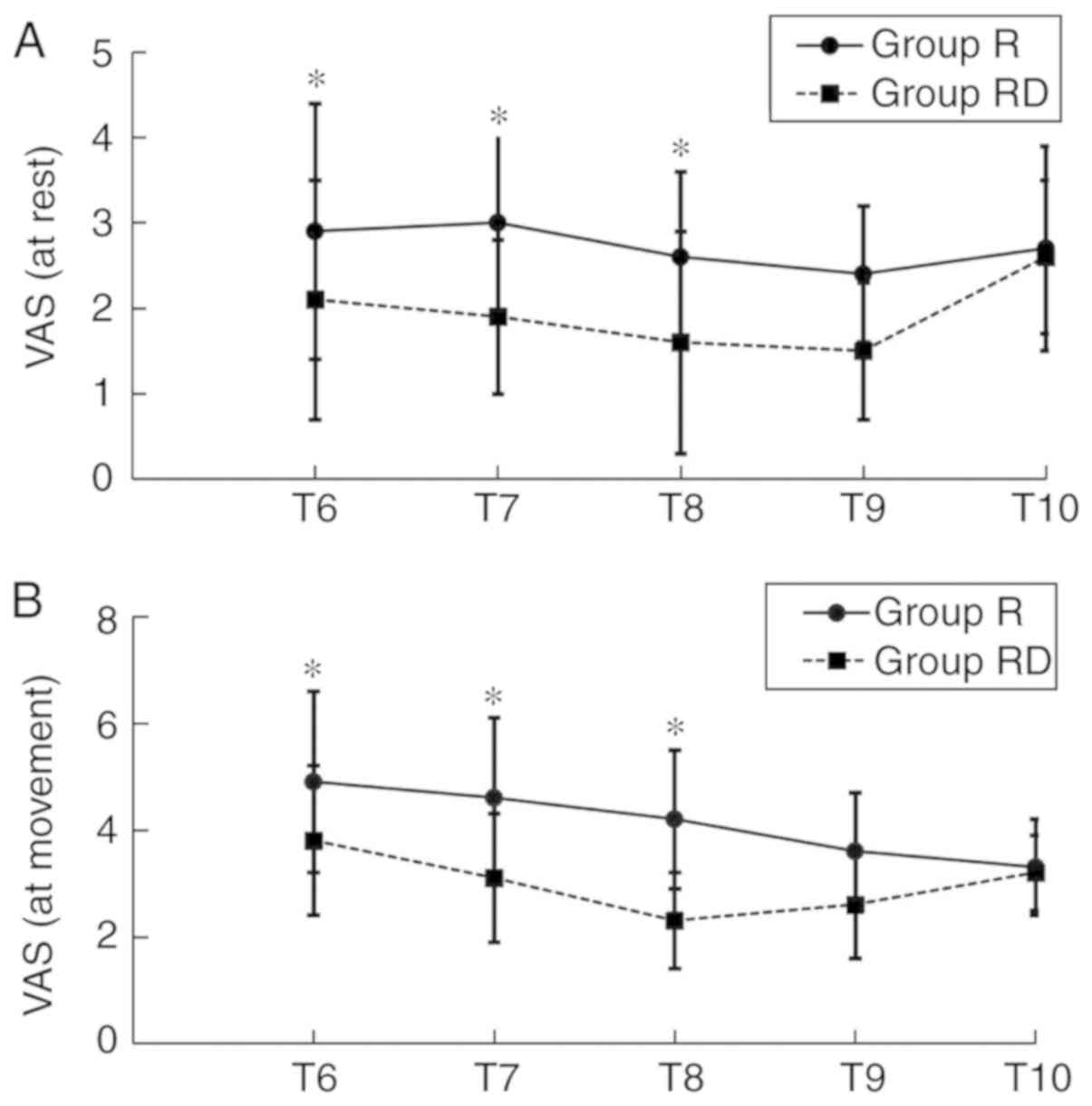
VAS scores at T6-T9 in the RD group, either at rest or movement,
were decreased compared with the Group R (Fig. 3). However, no significant difference
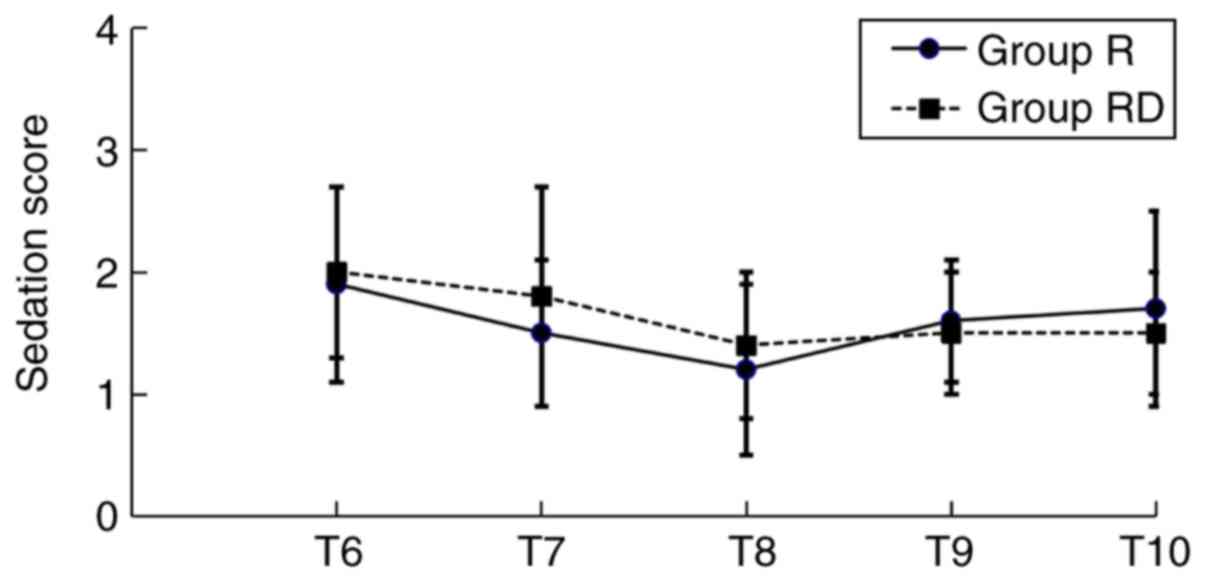
was observed regarding the levels of sedation between the two
groups at T6-T10. The majority of the patients in the present study
exhibited a sedation grade ≤3 (Fig.
4).
 | Table IIDuration of sensory block and
analgesia (h). |
Table II
Duration of sensory block and
analgesia (h).
| Duration
measure | R Group (n=30) | RD group
(n=30) | P-value |
|---|
| Sensory block
(h) | 9.4±3.5 | 13.5±4.1 | <0.001 |
| Analgesia (h) | 6.4±3.8 | 10.2±4.3 | 0.001 |
In the RD group, bradycardia was observed in 5
patients (4 did not require treatments and 1 patient was treated
with atropine). In the R group, bradycardia was observed in 2
patients who did not require treatments. Notably, the 0-24 h rates
of PONV in the RD group were significantly decreased compared with
the R group (P<0.05; Table
III). During the postoperative period, no other side effects,
including respiratory depression, hypoxemia, hypotension or
somnolence, were indicated in either group.
 | Table IIIPatients' rates of PONV 0-24 h
following surgery. |
Table III
Patients' rates of PONV 0-24 h
following surgery.
| Measurement | R Group (n=30)
(%) | RD Group (n=30)
(%) | P-value |
|---|
| Nausea |
|
0-24 h | 18(60) | 5 (16.7) | 0.001 |
|
0-4 h | 8 (26.7) | 2 (6.7) | 0.038 |
|
4-24 h | 10 (33.3) | 3(10) | 0.028 |
| Vomiting |
|
0-24 h | 17 (56.7) | 3(10) | 0.001 |
|
0-4 h | 8 (26.7) | 1 (3.3) | 0.026 |
|
4-24 h | 9(30) | 2 (6.7) | 0.023 |
There were 90.0 and 66.7% patients who seemed
satisfied with the postoperative pain management in the RD and R
group, respectively (Table IV). The
mean time of first passage of flatus and stool, and time of oral
fluid and normal diet consumption was significantly reduced in the
RD group compared with the R group (P<0.05). Furthermore, the
LOS did not significantly differ between the study groups (Table V).
 | Table IVPatient overall satisfaction with
anesthesia. |
Table IV
Patient overall satisfaction with
anesthesia.
| Satisfaction | R Group (n=30)
(%) | RD Group (n=30)
(%) | P-value |
|---|
| Overall
satisfied | 20 (66.7) | 27(90) | 0.028 |
| Very satisfied | 6 (20.0) | 10 (33.3) | 0.243 |
| Satisfied | 7 (23.3) | 12(40) | 0.165 |
| Moderately
satisfied | 7 (23.3) | 5 (16.7) | 0.519 |
| Not satisfied | 10 (33.3) | 3(10) | 0.028 |
 | Table VTime to recovery of bowel function
and hospital LOS. |
Table V
Time to recovery of bowel function
and hospital LOS.
| Recovery
measure | R Group (n=30) | RD Group
(n=30) | P-value |
|---|
| Time to first
flatus (h) | 2.8±0.4 | 2.2±0.3 | 0.001 |
| Time to first stool
(days) | 4.2±1.7 | 3.3±1.4 | 0.029 |
| Time to oral fluids
(days) | 1.2±0.2 | 0.9±0.1 | 0.036 |
| Time to normal diet
(days) | 3.6±0.8 | 2.9±1.1 | 0.007 |
| Hospital LOS
(days) | 9.5±0.9 | 9.2±0.8 | 0.178 |
Discussion
Based on the observations of the current study, it
was suggested that adding 0.5 µg/kg Dex to ropivacaine in bilateral
TAP block was able to prolong the analgesic duration and improve
recovery in patients undergoing laparoscopic colectomy.
TAP block is an ultrasound-guided regional
anesthetic technique used for analgesia, which is designed to block
the abdominal wall neural afferents (T7-L1) by injection of a local
anesthetic between the internal oblique and transversus abdominis
muscles. Since first being proposed in 2001 by Rafi (18), TAP block has become increasingly
popular. A meta-analysis of 9 studies, which included 413 patients,
investigated the efficacy of TAP block. The study revealed that
that TAP block reduced the postoperative morphine requirements,
opioid associated side-effects and the severity of pain, and
enhanced recovery in patients following abdominal surgery (19). Previous research has demonstrated
that a single-shot TAP block improved post-cesarean analgesia and
the duration of analgesia was limited when compared with
intrathecal morphine (20). Many
analgesic adjuvants, including opioids, epinephrine and
α2 agonists (including clonidine), have been added to
LAs in various peripheral nerve blocks to prolong the analgesic
duration. A previous study of healthy volunteers revealed that
epinephrine, as an adjuvant to TAP block, was effective in
decreasing peak plasma concentrations of levobupivacaine, with
limited value on block characteristics or duration (21). The addition of dexamethasone (8 mg)
to 30 ml levobupivacaine (0.25%) in a TAP block for analgesia
following a cesarean delivery prolonged effective analgesia
duration, and decreased deep and superficial pain scores (22). In contrast, Bollag et al
(23) reported no benefit of a TAP
block with or without clonidine following cesarean delivery for a
low-risk population receiving a spinal anesthetic with morphine and
multimodal analgesia as the analgesic consumption and pain scores
were low overall.
Dex is a commonly used sedative for anesthesia and
intensive care medicine (24). The
clinical effect of Dex as an adjuvant to LAs for peripheral nerve
blocks has been investigated in experimental and clinical studies
(25-28).
The addition of Dex to LAs in various nerve blocks can increase the
duration of block and improve analgesia effects, including ulnar
nerve block, palatine nerve block, infraorbital nerve block,
axillary brachial plexus and cervical plexus, which has been
described in scientific literature and applied in the daily
clinical practice (12,25-28).
Previous studies have indicated that various doses of Dex (20 to
150 µg) can be added to LAs. Dex combined with ropivacaine produces
a dose-dependent (50, 100 and 150 µg) prolongation of ulnar nerve
sensory block and dose-dependent sedation (29). The addition of 0.75 µg/kg Dex to 0.5%
levobupivacaine for supraclavicular plexus block shortens sensory
and motor block onset time, and extends sensory block, motor block
and analgesia duration (30). A
dosage of 0.5 µg/kg Dex in bilateral TAP block was chosen in
present study, which was based from data obtained in previous
studies and on doses that have been proven safe for sedation
(31). Furthermore, this dose was
chosen due to concerns regarding potential dose-dependent adverse
systemic events that have been associated with Dex. Dex has been
broadly applied as an additive to LAs under clinical settings.
To the best of our knowledge, the impact of Dex with
ropivacaine in TAP block for postoperative analgesia and recovery
has not been reported. The current study, which performed the TAP's
block prior to surgery, indicated that this method may be helpful
in decreasing opioid dosage and stabilizing BP and HR during
operation. Furthermore, patients were not indicated to feel pain
under general anesthesia. The present study demonstrated that the
addition of Dex to ropivacaine was able to prolong the analgesic
duration and enhance analgesic effects. The mechanism of action of
Dex in peripheral nerve blocks is not completely understood. A
number of mechanisms have been proposed, including central
analgesia, vasoconstriction and anti-inflammatory effects (32). Dalle et al (33) indicated that clonidine (an α-2
adrenoreceptor agonist) improves the sensory blockade by blocking
the inhibiting hyperpolarization-activated cation (Ih) current,
which enhances the level of hyperpolarization and inhibits
subsequent action potentials. Other research has suggested that
adding Dex to LAs increases the time required for analgesia by
decreasing the release of norepinephrine and causing α-2 receptor
independent inhibitory effects on nerve fiber action potentials
(28). However, further studies are
required to investigate the underling effects of Dex in TAP
block.
A previous study suggested a balanced anesthesia
with Dex decreased PONV following laparoscopic surgery via the
addition of Dex to ropivacaine (34). This may be due to the antiemetic
properties of Dex. Pain itself is considered a significant risk
factor for PONV (34,35). Intense pain may result in PONV and
enhanced analgesia in the RD group in turn decreased PONV.
One concern about the use of Dex in TAP block is
that Dex may be systemically absorbed and induce bradycardia and
excessive sedation (25-28).
However, in the current study, the hemodynamic variables that were
assessed did not reveal significant differences between two groups
during or after surgery. Furthermore, there was no excessive
sedation post-surgery and no significant difference in the levels
of sedation between the two groups. This may be primarily due to
lower doses of Dex used in the present study. Additionally, Dex may
be absorbed slowly in the TAP, so the plasma concentration of Dex
would be too low to induce bradycardia and excessive sedation.
In the present study, although the HR was not
significantly different between the two groups following surgery,
bradycardia was observed in 5 patients post-surgery in the RD
group, which may be explained by systemic absorption and cannot be
ignored. Bradycardia could potentially be life-threatening if not
detected and treated in time (26,28).
Therefore, when Dex is used as an adjuvant to ropivacaine, patients
should be monitored during the operation for potential side
effects, including bradycardia, hypotension and sedation. The lack
of other side effects, including PONV and respiratory depression,
make Dex an appealing adjuvant for the TAP block.
A study by Cheung et al (36) indicated that intraoperative Dex in
colorectal surgery did not improve recovery in patients, which was
due to inhibition of Dex on intestinal motility via the suppression
of acetylcholine release. A few studies have investigated the
quality of postoperative recovery following the use of TAP block
(37). In the present study, TAP
block with Dex and ropivacaine shortened the time to first passage
of flatus and first stool, promoted bowel movement and increased
patient satisfaction. Multiple factors are considered for recovery
from surgery, including pain, and surgery-induced metabolic,
endocrine and immune changes, which are also known as ‘stress
responses’ (36,37). Acute postoperative pain will reduce
movement motivation and restrict patients at a relatively
‘comfortable position’, which impairs the ability of responding to
stress physically and mentally, and defer recovery. It has
previously been speculated that improvement of recovery in the RD
group was primarily associated with promoted analgesia following
surgery. However, further investigations are required to verify
this hypothesis. No significant difference was observed in the mean
LOS between the two groups. It is well know that there are a number
of factors (including the surgical floor capacity, discharge
criteria and patient- and institution-associated factors) other
than the difference in TAP block in the current study that have an
influence on the LOS.
The onset time of TAP block was not assessed in the
current study, since the procedure was performed when patients were
under general anesthesia. Some trials have indicated that adding
Dex to LAs reduces the onset time of peripheral nerve or plexus
block (25,28). Therefore, it was speculated that
adding Dex to ropivacaine could also reduce the onset time of TAP
block. However, this requires further study. Additionally, a number
of anatomical structures can cause pain following abdominal
surgeries, and may be considered superficial and deep, or visceral
and somatic. Different analgesic techniques may not indicate a
similar pain-relieving effect on different causes of pain. The
effect of TAP block on visceral pain that causes deep pain
sensations may not be expected to be efficient. In the present
study, even though a significant difference in pain score was
observed between the two groups, visceral and somatic pain
components were not separately assessed. Opioid dosage during and
after operation was also not recorded, because this may be an
important factor for PONV and bowel function recovery. The majority
of postoperative parameters assessment were subjective, however,
all parameters were assessed by an investigator blinded to the
group allocation. Finally, a relatively low dosage of Dex was used
in the present study. Therefore, there is a possibility that a
prolongation may be observed if the dosage of Dex had been higher.
Therefore, whether a higher dosage of Dex may have better analgesic
efficacy of the TAP block should be the focus of future studies.
Additional analgesic agents can impact VAS scores. If patients
required additional analgesic agents (for example, at 1 h
post-surgery), then it would be expected to have an impact on the
VAS score at the 2 h time-point.
In conclusion, data from the present study
demonstrated that adding Dex to ropivacaine could improve
postoperative analgesia and the duration of TAP block, and promote
recovery of bowel function following laparoscopic colectomy.
Acknowledgements
Not applicable.
Funding
The current study was financed by the Science and
Technology Program Foundation of Yantai, China (grant no.
2019YD022).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WP and GZL performed the study and also were major
contributors in writing the manuscript. MJ and GGL performed the
study and collected the data. TL and QS analyzed data. JM and HL
designed the study and edited the manuscript. All authors approved
the final manuscript.
Ethics approval and consent to
participate
Approval was obtained from the Institutional Human
Investigations Committee of Yantai Yuhuangding Hospital of Qingdao
University. All patients provided written informed consent for
participation.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ong CK, Lirk P, Seymour RA and Jenkins BJ:
The efficacy of preemptive analgesia for acute postoperative pain
management: A meta-analysis. Anesth Analg. 100:757–773, table of
contents. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Buvanendran A and Kroin JS: Multimodal
analgesia for controlling acute postoperative pain. Curr Opin
Anaesthesiol. 22:588–593. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
McDonnell JG, O'Donnell B, Curley G,
Heffernan A, Power C and Laffey JG: The analgesic efficacy of
transversus abdominis plane block after abdominal surgery: A
prospective randomized controlled trial. Anesth Analg. 104:193–197.
2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yu N, Long X, Lujan-Hernandez JR, Succar
J, Xin X and Wang X: Transversus abdominis-plane block versus local
anesthetic wound infiltration in lower abdominal surgery: A
systematic review and meta-analysis of randomized controlled
trials. BMC Anesthesiol. 14(121)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schoenmakers KP, Wegener JT and Stienstra
R: Effect of local anesthetic volume (15 vs. 40 ml) on the duration
of ultrasound-guided single shot axillary brachial plexus block: A
prospective randomized, observer-blinded trial. Reg Anesth Pain
Med. 37:242–247. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ilfeld BM: Continuous peripheral nerve
blocks: A review of the published evidence. Anesth Analg.
113:904–925. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kirksey MA, Haskins SC, Cheng J and Liu
SS: Local anesthetic peripheral nerve block adjuvants for
prolongation of analgesia: A systematic qualitative review. PLoS
One. 10(e0137312)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gerlach AT and Dasta JF: Dexmedetomidine:
An updated review. Ann Pharmacother. 41:245–252. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Blaudszun G, Lysakowski C, Elia N and
Tramer MR: Effect of perioperative systemic α2 agonists on
postoperative morphine consumption and pain intensity: Systematic
review and meta-analysis of randomized controlled trials.
Anesthesiology. 116:1312–1322. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brummett CM, Hong EK, Janda AM, Amodeo FS
and Lydic R: Perineural dexmedetomidine added to ropivacaine for
sciatic nerve block in rats prolongs the duration of analgesia by
blocking the hyperpolarization-activated cation current.
Anesthesiology. 115:836–843. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brummett CM, Norat MA, Palmisano JM and
Lydic R: Perineural administration of dexmedetomidine in
combination with bupivacaine enhances sensory and motor blockade in
sciatic nerve block without inducing neurotoxicity in rat.
Anesthesiology. 109:502–511. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Marhofer D, Kettner SC, Marhofer P, Pils
S, Weber M and Zeitlinger M: Dexmedetomidine as an adjuvant to
ropivacaine prolongs peripheral nerve block: A volunteer study. Br
J Anaesth. 110:438–442. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gandhi R, Shah A and Patel I: Use of
dexmedetomidine along with bupivacaine for brachial plexus block.
National J Med Res. 2:67–69. 2012.
|
|
14
|
Hebbard P, Fujiwara Y, Shibata Y and Royse
C: Ultrasound-guided transversus abdominis plane (TAP) block.
Anaesth Intensive Care. 35:616–617. 2007.PubMed/NCBI
|
|
15
|
Ris F, Findlay JM Hompes R, Rashid A,
Warwick J, Cunningham C, Jones O, Crabtree N and Lindsey I:
Addition of transversus abdominis plane block to patient controlled
analgesia for laparoscopic high anterior resection improves
analgesia, reduces opioid requirement and expedites recovery of
bowel function. Ann R Coll Surg Engl. 96:579–585. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nader A, Doty R Jr, Brodskaia A, Kendall
MC and McCarthy RJ: Sensory testing of distal sural and posterior
tibial nerves provides early prediction of surgical anesthesia
after single-injection infragluteal-parabiceps sciatic nerve block.
Anesth Analg. 110:951–957. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Overdyk FJ, Carter R, Maddox RR, Callura
J, Herrin AE and Henriquez C: Continuous oximetry/capnometry
monitoring reveals frequent desaturation and bradypnea during
patient-controlled analgesia. Anesth Analg. 105:412–418.
2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rafi AN: Abdominal field block: A new
approach via the lumbar triangle. Anaesthesia. 56:1024–1026.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hain E, Maggiori L, Prost À la Denise J
and Panis Y: Transversus abdominis plane (TAP) block in
laparoscopic colorectal surgery improves postoperative pain
management: A meta-analysis. Colorectal Dis. 20:279–287.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kanazi GE, Aouad MT, Abdallah FW, Khatib
MI, Adham AM, Harfoush DW and Siddik-Sayyid SM: The analgesic
efficacy of subarachnoid morphine in comparison with
ultrasound-guided transversus abdominis plane block after cesarean
delivery: A randomized controlled trial. Anesth Analg. 111:475–481.
2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Corvetto MA, Echevarria GC, De La Fuente
N, Mosqueira L, Solari S and Altermatt FR: Comparison of plasma
concentrations of levobupivacaine with and without epinephrine for
transversus abdominis plane block. Reg Anesth Pain Med. 37:633–637.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Akkaya A, Yildiz I, Tekelioglu UY,
Demirhan A, Bayir H, Ozlu T, Bilgi M and Kocoglu H: Dexamethasone
added to levobupivacaine in ultrasound-guided tranversus abdominis
plain block increased the duration of postoperative analgesia after
caesarean section: a randomized, double blind, controlled trial.
Eur Rev Med Pharmacol Sci. 18:717–722. 2014.PubMed/NCBI
|
|
23
|
Bollag L, Richebe P, Siaulys M, Ortner CM,
Gofeld M and Landau R: Effect of transversus abdominis plane block
with and without clonidine on post-cesarean delivery wound
hyperalgesia and pain. Reg Anesth Pain Med. 37:508–514.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Carollo DS, Nossaman BD and Ramadhyani U:
Dexmedetomidine: A review of clinical applications. Curr Opin
Anaesthesiol. 21:457–461. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Obayah GM, Refaie A, Aboushanab O,
Ibraheem N and Abdelazees M: Addition of dexmedetomidine to
bupivacaine for greater palatine nerve block prolongs postoperative
analgesia after cleft palate repair. Eur J Anaesthesiol.
27:280–284. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Khandaitkar S, Kolte V, Shenoi SR and
Budhraja N: A clinical study to determine the efficacy of 7ppm
dexmedetomidine as an adjuvant to 2% lignocaine in infraorbital
nerve block. Br J Oral Maxillofac Surg. 54:997–1000.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Esmaoglu A, Yegenoglu F, Akin A and Turk
CY: Dexmedetomidine added to levobupivacaine prolongs axillary
brachial plexus block. Anesth Analg. 111:1548–1551. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin YN, Li Q, Yang RM, Mao ZX and Liu JC:
Addition of dexmedetomidine to ropivacaine improves cervical plexus
block. Acta Anaesthesiol Taiwan. 51:63–66. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Brummett CM, Padda AK, Amodeo FS, Welch KB
and Lydic R: Perineural dexmedetomidine added to ropivacaine causes
a dose-dependent increase in the duration of thermal
antinociception in sciatic nerve block in rat. Anesthesiology.
111:1111–1119. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bisui B, Samanta S, Ghoshmaulik S,
Banerjee A, Ghosh TR and Sarkar S: Effect of locally administered
dexmedetomidine as adjuvant to levobupivacaine in supraclavicular
brachial plexus block: Double-blind controlled Study. Anesth Essays
Res. 11:981–986. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rancourt MP, Albert NT, Côté M, Létourneau
DR and Bernard PM: Posterior tibial nerve sensory blockade duration
prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg.
115:958–962. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Iskandar H, Benard A, Ruel-Raymond J,
Cochard G and Manaud B: The analgesic effect of interscalene block
using clonidine as an analgesic for shoulder arthroscopy. Anesth
Analg. 96:260–262. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dalle C, Schneider M, Clergue F, Bretton C
and Jirounek P: Inhibition of the I(h) current in isolated
peripheral nerve: A novel mode of peripheral antinociception?
Muscle Nerve. 24:254–261. 2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Massad IM, Mohsen WA, Basha AS, Al-Zaben
KR, Al-Mustafa MM and Alghanem SM: A balanced anesthesia with
dexmedetomidine decreases postoperative nausea and vomiting after
laparoscopic surgery. Saudi Med J. 30:1537–1541. 2009.PubMed/NCBI
|
|
35
|
Schnabel A, Meyer-Frießem CH, Reichl SU,
Zahn PK and Pogatzki-Zahn EM: Is intraoperative dexmedetomidine a
new option for postoperative pain treatment? A meta-analysis of
randomized controlled trials. Pain. 154:1140–1149. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cheung CW, Qiu Q, Ying AC, Choi SW, Law WL
and Irwin MG: The effects of intra-operative dexmedetomidine on
postoperative pain, side-effects and recovery in colorectal
surgery. Anaesthesia. 69:1214–1221. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bekker A, Haile M, Kline R, Didehvar S,
Babu R, Martiniuk F and Urban M: The effect of intraoperative
infusion of dexmedetomidine on the quality of recovery after major
spinal surgery. J Neurosurg Anesthesiol. 25:16–24. 2013.PubMed/NCBI View Article : Google Scholar
|