Introduction
Heart failure (HF) may be chronic, i.e., develop
over a long duration, or acute, i.e., with a rapid onset of
clinical manifestations (1). While
chronic HF (CHF) is usually the end stage of a variety of
cardiovascular diseases, acute HF (AHF) may be caused by numerous
factors that exacerbate CHF in a short period or result in a sudden
heart attack (2). AHF, which most
commonly involves acute left ventricular failure, is potentially
life-threatening and requires immediate in-hospital treatment. Even
so, the in-hospital mortality rate and readmission rate after
discharge are relatively high (3).
Several cardiac functional indexes, including left ventricular size
and ejection fraction (EF), and certain cardiac functional
classification methods, including the New York Heart Association
(NYHA) classification, have been used to assess the severity and
outcomes of AHF, but each of them has certain limitations (4,5).
Furthermore, several biomarkers, including serum B-type natriuretic
peptide (BNP) and creatinine, have been identified and widely used
to assess the severity of AHF and predict the prognosis of patients
with AHF (6). However, the
circulating levels of BNP may be affected by certain other
diseases, including valvular heart disease (7), while the serum creatinine concentration
is more associated with kidney dysfunction (6). Thus, it remains imperative to identify
novel biomarkers for AHF that are reliable and specific.
Recently, the hemoconcentration (HCT), an index
reflecting a rapid increase in the concentration of red blood cells
in the blood, has received attention from clinicians. An elevated
HCT has been reported to be associated with more severe damage to
the brain in patients with hemolytic uremic syndrome (8). Correlations between HCT and heart
diseases have been explored, but conflicting results have been
reported. For instance, HCT was indicated to be closely associated
with a more extensive weight loss and elevated risk of exacerbated
kidney function in patients with AHF during hospitalization
(9), whereas the data from the
Korean Heart Failure Registry suggested that increased HCT may be
beneficial for patients with AHF without hyponatremia (10). However, the ANCHOR study indicated
that either high or low HCT predicted worse outcomes in HF
patients, including death and re-hospitalization (11). The PRAISE study indicated that
anemia, in which the HCT is decreased, is an independent risk
factor for patients with severe HF (12). Hence, the effectiveness of HCT to
predict the prognosis of AHF patients remains elusive.
In the present study, the associations of different
levels of HCT with the outcome of AHF were investigated, and the
prognostic value of HCT for patients with AHF was examined and
compared with that of other well-recognized traditional biomarkers
for AHF, including BNP and serum creatinine. The aim of the present
study was to clarify whether HCT may serve as a prognostic
biomarker in patients with AHF. The results suggest that HCT may be
applied as a valuable prognostic factor for patients with AHF.
Materials and methods
Ethics statement.
The protocol of the present study was approved by
the Ethics Committee of the Affiliated Hospital of Hangzhou Normal
University (Hangzhou, China). All patients who participated in the
study were informed of the purpose of the study and provided
written informed consent.
Patient selection.
523 consecutive patients diagnosed with any AHF at
the Department of Critical Care Medicine, Affiliated Hospital of
Hangzhou Normal University (Hangzhou, China) between June 2013 and
June 2015 were selected for the present retrospective study. All
cases met the diagnostic criteria for AHF based on the Guidelines
for the Diagnosis and Treatment of Acute Heart Failure of the
American Heart Association from 2010(13). Patients fulfilling all of the
following criteria were included in the present study: i) Diagnosis
of AHF established based on medical history, associated cause(s),
clinical manifestations and echocardiographic findings; ii) NYHA
classification grade III to IV; iii) systolic/diastolic blood
pressure of ≥90/60 mmHg (1 mmHg = 133 Pa); and iv) age between 45
and 85 years. Patients with one of the following were excluded from
the present study: i) Severe valvular stenosis, constrictive
pericarditis, restrictive or hypertrophic cardiomyopathy; ii)
severe pulmonary hypertension, severe ventricular arrhythmia,
cardiogenic shock or insufficient blood volume; iii) severe
hypotension, malignant tumors, trauma or infection; or iv) severe
liver or kidney dysfunction. After application of the inclusion and
exclusion criteria, a total of 188 patients were finally included
during the 2-year follow-up period of the present study.
Data collection.
The demographic and baseline clinical
characteristics of all patients were collected from the electronic
medical record system of the hospital that had been entered for
each patient on admission, including age, gender, body mass index
(BMI), current diseases, comorbidities, past medical history,
medication status and cardiac function according to the NYHA
cardiac function classification.
End-points.
The end-point event of the present study was either
cardiac-associated death or re-hospitalization due to AHF. All
patients were followed up for 2 years or until the end-point
occurred. According to the serum HCT values on admission, the
patients were divided into four groups as follows: ≥40% group,
36-39% group, 30-35% group and <30% group. The incidence of
end-point events during the 2-year follow-up period was compared
among these four groups.
Blood tests.
Blood samples were collected from each patient on
admission and the HCT, as well as the levels of BNP, hemoglobin,
creatinine, uric acid, low-density lipoprotein (LDL) and total
cholesterol (TC) were measured.
Echocardiography.
Echocardiography was performed using an EPIQ 7
ultrasound machine (Philips) for all participants and cardiac
function indexes, including left ventricular EF (LVEF), left
ventricular mass index (LVMI) and left ventricular end diastolic
diameter (LVEDD) were determined.
Statistical analysis.
All statistical analyses were performed by SPSS 19.0
software (IBM, Corp.). Continuous variables, which are normally
distributed, were expressed as the mean and standard deviation,
comparisons of these data between groups were performed by a
student's t-test. In addition, median and interquartile ranges were
analyzed when the variables were non-normally distributed, such as
in the case of BNP. Correlations between the two sets were
performed using Spearman's correlation analysis. Categorical
variables were expressed as count and percentage, and were analyzed
by the chi-square test. Kaplan-Meier analysis was used to evaluate
survival curve, which were compared using a log-rank test. Cox
proportional hazard models were used to determine the risk factors
influencing the prognosis, including cardiac-associated death or
re-hospitalization due to AHF. The area under the receiver
operating characteristic (ROC) curve (AUC) was assessed to
determine the ability of factors to predict the prognosis of AHF
patients. P<0.05 was considered to indicate statistical
significance.
Results
Demographic and basic clinical
characteristics.
A total of 188 patients [mean age, 54.8 (±11.3)
years; 110 males and 78 females] were enrolled in the present study
during the 2-year follow-up period. Of these, 99 patients
experienced either cardiac-associated death or re-hospitalized due
to AHF within the study period, corresponding to a rate of 52.7%.
The average time from first hospitalization to the occurrence of
the end-point event was 10.5 months.
Comparison of clinical data among
patients in different HCT groups.
The 188 patients were divided into four groups based
on the HCT values: The ≥40% group (n=51), 36-39% group (n=46),
30-35% group (n=56) and the <30% group (n=35). The clinical data
were compared among these different HCT groups. As presented in
Table I, there were significant
differences among these groups in the BNP level, hemoglobin level,
creatinine concentration, NYHA grade, and 1-year and 2-year
survival rates (P<0.05), but no differences were present with
regard to age, gender, comorbidities and BMI. However, there were
no significant differences in the proportion of patients treated
with angiotensin-converting enzyme inhibitor vs. angiotensin
receptor blocker, or in the serum lipid levels, uric acid
concentration, LVEF, LVMI or LVEDD among these groups (P>0.05;
Table I). In addition, Spearman
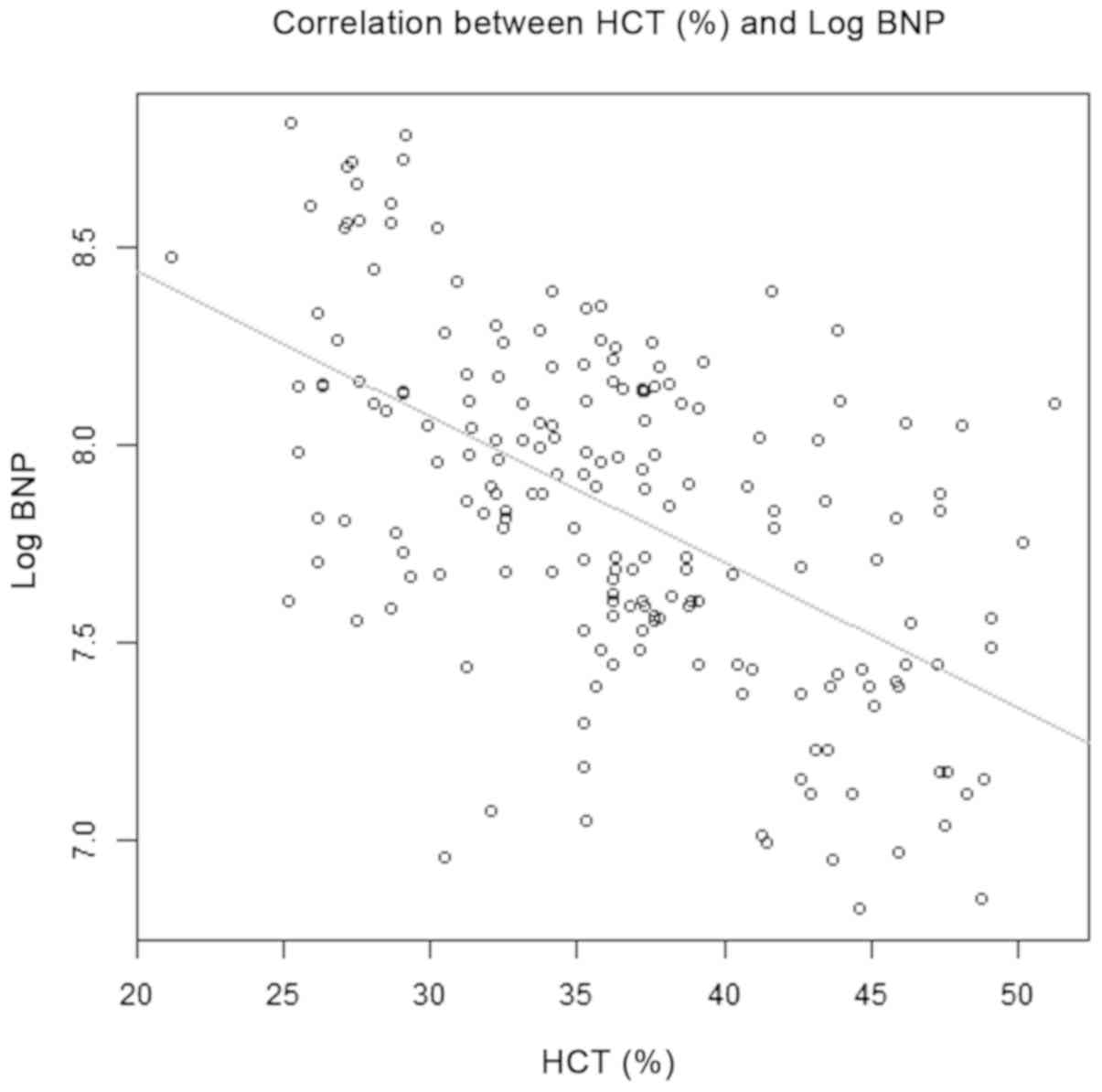
correlation analysis suggested that HCT was negatively correlated
with Log BNP (r=-0.629, P=0.024; Fig.
1).
 | Table IComparison of clinical data of
patients of different HCT groups. |
Table I
Comparison of clinical data of
patients of different HCT groups.
| | HCT (%) | |
|---|
| Item | ≥40 (n=51) | 36-39 (n=46) | 30-35 (n=56) | <30 (n=35) | P-value |
|---|
| Age (years) | 56.8±9.3 | 55.4±10.2 | 53.6±11.7 | 53.4±14.2 | 0.893 |
| Sex
(male/female) | 26/25 | 28/18 | 35/21 | 21/14 | 0.638 |
| Diabetes
(yes/no) | 11/40 | 10/36 | 10/46 | 6/29 | 0.919 |
| ACEI/ARB
(yes/no) | 17/34 | 14/32 | 18/38 | 12/23 | 0.984 |
| BMI
(kg/m2) | 22.9±5.4 | 22.3±3.8 | 23.1±4.1 | 23.7±3.5 | 0.934 |
| Hemoglobin (g/l) | 121.8±12.5 | 116.8±13.4 | 110.8±12.4 | 104.8±16.9 | 0.032 |
| BNP (pg/ml) | 2,470
(1,249-4,535) | 2,684
(1,361-4,883) | 3,125
(1,530-5,187) | 3,470
(1,919-6,735) | 0.015 |
| Creatinine
(µmol/l) | 105.4±24.5 | 113.8±21.6 | 118.6±30.2 | 123.9±35.7 | 0.026 |
| Uric acid
(µmol/l) | 324.5±56.9 | 346.2±67.5 | 339.4±44.5 | 347.1±58.2 | 0.763 |
| LDL (mmol/l) | 3.25±0.47 | 3.41±0.75 | 3.47±0.83 | 3.38±0.91 | 0.682 |
| TC (mmol/l) | 5.37±1.27 | 5.61±1.62 | 5.17±0.54 | 5.38±1.31 | 0.863 |
| NYHA classification
(II/III/IV) | 32/14/5 | 23/15/8 | 30/17/9 | 8/16/11 | <0.001 |
| EF (%) | 39.3±7.2 | 35.6±10.1 | 32.4±8.4 | 29.9±10.6 | 0.641 |
| LVMI (mm) | 151.7±18.2 | 153.6±23.4 | 154.3±19.9 | 153.7±20.5 | 0.932 |
| LVEDD (mm) | 65.1±9.4 | 65.2±11.3 | 66.2±9.2 | 67.0±11.9 | 0.857 |
| 1-year survival rate
(%) | 74.5 | 71.7 | 66.0 | 45.7 | 0.033 |
| 2-year survival rate
(%) | 60.8 | 52.2 | 41.1 | 28.6 | 0.036 |
Comparison of cardiac event-free rates
among different HCT groups.
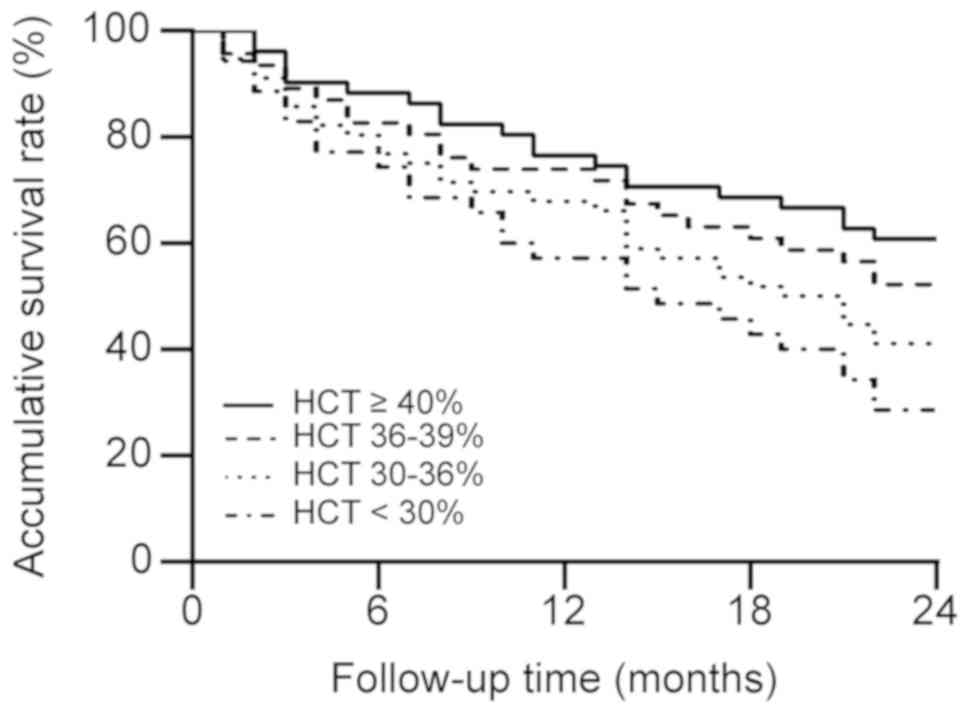
Kaplan-Meier analysis was performed to compare the
cumulative survival rates of patients among the four HCT groups.
The log-rank test result was χ2=9.442 with P=0.024 and
the cumulative survival rate was significantly higher in the higher
HCT groups than in the lower HCT group (Fig. 2).
Predictive value of HCT regarding
cardiac event-free of patients.
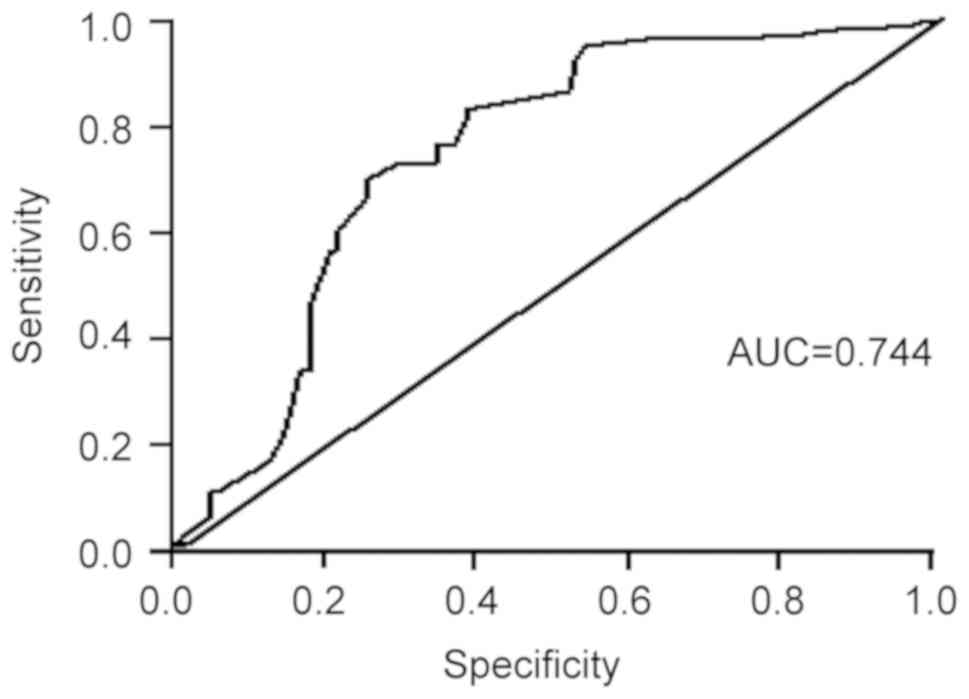
A ROC curve analysis was performed to evaluate the
predictive value of the HCT regarding the survival outcome for
patients with AHF. As presented in Fig.
3, the ROC curve analysis indicated that serum HCT values were
predictive of survival of patients with AHF, with an AUC of 0.610
[95% confidence interval (CI): 0.528-0.691, P<0.001], at cutoff
point of HCT is 35.45%, the sensitivity and specificity were 54.5
and 65.2%, respectively (Fig.
3).
Determination of the association
between HCT and recurrent cardiac events or re-hospitalization due
to AHF.
Cox proportion hazards regression analysis was
performed to determine the influence of various factors, including
the HCT, BNP, serum creatinine and NYHA classification, on the
recurrent cardiac or re-hospitalization due to AHF. Demographic
variables including age, sex and BMI, and clinical variables
including HCT, ACEI/ARB, hemoglobin, BNP, serum creatinine, uric
acid, LDL, TC, NYHA classification, and diabetes were analyzed
using the Cox proportional hazards model. The results revealed that
HCT, sex, ACEI/ARB, hemoglobin, log of BNP, serum creatinine and
NUHA classification were significantly associated with recurrent
cardiac events (Table II; Model 1). Males [hazard ratio (HR), 0.08;
P<0.001; reference, female], with ACEI/ARB (HR, 0.01;
P<0.001; reference, without ACEI/ARB), higher HCT (HR, 0.96;
P=0.019), higher hemoglobin (HR, 0.98; P=0.009), lower BNP (log
BNP's HR, 2.38; P<0.001), lower serum creatinine (HR, 1.01;
P=0.003) and lower classes of NYHA [HR (class III), 26.82;
P<0.001; HR (class IV), 98.10; P<0.001; reference, class II]
had a lower risk of cardiac event recurrence (Table II; Model
1).
After adjustment for sex, ACEI/ARB, hemoglobin, Log
of BNP, serum creatinine, uric acid, LDL, TC, NYHA classification
and diabetes, it revealed that a higher HCT value was associated
with a lower risk of recurrent cardiac events (HR, 0.48; P=0.098;
Table II; Model 2).
 | Table IIAnalysis of the factors affecting the
recurrent cardiac events of patients with acute heart failure. |
Table II
Analysis of the factors affecting the
recurrent cardiac events of patients with acute heart failure.
| | Model
1a | Model
2b |
|---|
| Variable | Crude HR (95%
CI) | P-value | Adjusted HR (95%
CI) | P-value |
|---|
| Age | 0.99
(0.98-1.01) | 0.549 | - | - |
| Sex |
|
Male vs.
female | 0.08
(0.05-014) | <0.001 | 0.35
(0.18-0.67) | 0.002 |
| ACEI/ARB |
|
Yes vs.
no | 0.01
(0.001-0.08) | <0.001 | 0.54
(0.01-0.43) | 0.006 |
| BMI
(kg/m2) | 0.97
(0.92-1.02) | 0.266 | - | - |
| HCT (%) | 0.96
(0.93-0.99) | 0.019 | 0.48
(0.20-1.14) | 0.098 |
| Hemoglobin
(g/l) | 0.98
(0.97-0.99) | 0.009 | 1.02
(0.92-1.12) | 0.737 |
| Log BNP
(pg/ml) | 2.38
(1.50-3.77) | <0.001 | 0.17
(0.006-4.93) | 0.302 |
| Serum creatinine
(µmol/l) | 1.01
(1.00-1.02) | 0.003 | 0.90
(0.94-1.04) | 0.685 |
| Uric acid
(µmol/l) | 1.00
(0.99-1.00) | 0.845 | 1.00
(0.99-1.01) | 0.07 |
| LDL (mmol/l) | 0.85
(0.65-1.11) | 0.239 | 0.83
(0.60-1.13) | 0.238 |
| TC (mumol/l) | 1.03
(0.88-1.21) | 0.697 | 0.92
(0.76-1.12) | 0.422 |
| NYHA classification
(II/III/IV) |
|
II vs.
III | 26.82
(12.45-57.78) | <0.001 | 0.01
(0.00-9.29) | 0.191 |
|
III vs.
IV | 98.10
(42.85-224.59) | <0.001 | 0.02
(0.00-23.39) | 0.284 |
| Diabetes |
|
No vs.
yes | 0.00
(0.00-infc) | 0.994 | 0.00
(0.00-infc) | 0.996 |
| LVMI
(g/m2) | 1.00
(0.99-1.01) | 0.659 | - | - |
| LVEDD (mm) | 1.00
(0.98-1.02) | 0.823 | - | - |
Discussion
Prior research has indicated that HCT is closely
linked to the health status of patients with CHF. For instance, a
previous study reported a significant correlation between increased
levels of HCT and a better quality of life in patients with AHF
(14), and other studies suggested
that a decreased HCT and impaired kidney function are risk factors
for mortality in patients with CHF (11). However, the association of HCT with
the short-term outcomes of acute decompensated HF has remained
largely elusive. In the present study, the association of HCT with
the outcomes of AHF patients, i.e., cardiogenic death or
re-hospitalization, were examined, and a higher HCT was indicated
to be linked to a reduced risk of cardiac-associated events in
patients with AHF. The present results also suggest that, similar
to BNP and serum creatinine, HCT may serve as an independent
prognostic factor for patients with AHF.
HF is a common condition and a leading cause of
hospitalization worldwide (15).
Compared with CHF, AHF is associated with higher cardiogenic
mortality, a higher re-hospitalization rate and more cardiovascular
events due to the rapid decrease in myocardial contractility and
increase in cardiac load, resulting in a sudden drop in acute
cardiac output, increased pulmonary circulation pressure and
elevated peripheral circulation resistance (16). Due to weakened left ventricular
systolic function accompanied by a decreased glomerular filtration
rate and increased reabsorption of water and sodium by renal
tubules, the blood volume is frequently increased, subsequently
resulting in sodium retention and dilute anemia, as well as
decreased hemoglobin and HCT (17).
Thus, HCT is an indicator reflecting the severity of anemia and
sodium retention, which are linked to kidney dysfunction and affect
the prognosis of patients with HF (18). HCT has therefore been considered as
another predictor of the prognosis of patients with AHF. Indeed,
the results of the present study support this notion: i) In
patients with decompensated AHF, a higher HCT value on admission
was significantly associated with a higher survival rate during the
2-year follow-up period compared with a lower HCT value, ii) ROC
curve analysis indicated that the sensitivity and specificity of
HCT for predicting cardiac events were 83.3 and 63.7%,
respectively, and iii) multivariate Cox analysis suggested that the
HCT value on admission is an independent predictor of mortality of
AHF patients. These observations were consistent with those of a
previous study indicating that HCT is a predictor of short-term
prognosis in AHF patients after adjustment for risk factors for
traditional cardiogenic events (19). Thus, it may be concluded that
patients with AHF with a higher HCT have a lower risk of any
recurrence, which are in turn linked to cardiac-associated death
and re-hospitalization, and this association is independent of
traditional risk factors, including Log BNP, NYHA classification
and serum creatinine. While the exact mechanisms underlying the
association between HCT and the prognosis of patients with AHF
remain elusive, multiple factors are likely to be implicated. For
instance, the association between HCT and kidney function may be
involved (20).
Of note, conflicting results were reported regarding
the association between HCT and the outcomes for patients with HF.
For instance, the ANCHOR study suggested that HCT, either high or
low, was predictive of unfavorable outcomes in patients with HF
(11), but a Korean study supported
a beneficial role of HCT in non-hyponatremic patients with AHF
(10). In addition, the PRAISE study
revealed an association between a decreased HCT and the severity of
HF (12). The exact reasons for
these discrepancies remain elusive, but they may, at least
partially, be attributed to the fact that the patients selected in
those studies had different comorbidities. The results of the
present study support an association between high HCT and favorable
outcomes in AHF patients.
BNP and serum creatinine are well-recognized
prognostic biomarkers for patients with HF. Regarding the
production of BNP, a precursor, NT-pro-BNP, is synthesized and
secreted mainly by the ventricular myocytes, and it exhibits a
variety of biological activities, including diuresis, suppression
of Na+ transport, dilation of blood vessels and
inhibition of the renin-angiotensin-aldosterone system (21). In a number of heart diseases,
including acute myocardial infarction, congenital heart disease and
heart failure, an elevated concentration of BNP in the blood stream
has been observed (22-24).
A meta-analysis of 40 prospective studies revealed that elevated
circulating levels of BNP were closely associated with an increased
risk of cardiovascular disease (25), and another study suggested that BNP,
but not left ventricular cardiac function, held a strong prognostic
value regarding outcomes for patients with HF (26). Consistent with the above results, the
present study indicated that BNP was an independent risk factor for
poor outcome in patients with AHF. It was also revealed that HCT,
as another independent prognostic factor in AHF patients, exhibited
a significant negative correlation with the Log BNP. Collectively,
these observations suggest that BNP and HCT may serve as prognostic
predictors for outcomes in patients with HF. However, compared with
the measurement of BNP, the detection of HCT is more convenient,
cost-efficient and technologically reliable. At present, HCT is one
of the widely used clinical indicators in a number of diseases.
Hence, determination of HCT in patients with AHF should be more
acceptable for patients and their families and may be widely
performed at primary hospitals. Serum creatinine is linked to the
glomerular filtration rate and mainly reflects the kidney function,
and high levels of serum creatinine may indicate the severity of HF
(27). In the present study, all
three factors, BNP, serum creatinine and HCT, were determined to be
independent predictors of the prognosis of patients with AHF. It
has been reported that BNP and serum creatinine are valuable
factors for risk stratification of patients with HF (28-30).
In the future, it may be worthwhile to explore whether inclusion of
BNP, serum creatinine and HCT into risk assessment models together
with other traditional cardiovascular risk factors increases the
accuracy of disease discrimination.
The limitations of the present study should be
pointed out. First, the study cohort was small and due to the
retrospective nature of the study, it was not possible to determine
any cause-effect associations. Large-cohort multi-center
prospective studies are required in order to corroborate the
present results and conclusions. Furthermore, the follow-up time
was 2 years, which was relatively short, and it has been well
documented that the incidence of cardiac events in patients with
AHF increases significantly over time. Therefore, dynamic changes
in HCT and their correlation with the long-term outcomes for
patients with AHF warrant further exploration in the future. In
addition, the present study focused on the association of HCT with
acute heart failure, and thus, troponin levels and HCT were not
compared in the present study. However, troponin levels will be
included in future studies by our group and there are plans to
expand the present study to further corroborate the major findings
from the present results.
In conclusion, patients with AHF with a low HCT have
a higher risk of recurrent cardiac events and the HCT is an
effective predictor of outcomes for patients with AHF.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QY analyzed and interpreted the patient data
regarding the hematological disease and the transplant. SFC
performed the histological examinations of the kidneys, and was a
major contributor in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Hangzhou Normal University Affiliated Hospital. All
patients who participated in the study were aware of the purpose of
the study and provided written informed consent.
Patients' consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gheorghiade M, Zannad F, Sopko G, Klein L,
Piña IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, et
al: Acute heart failure syndromes: Current state and framework for
future research. Circulation. 112:3958–3968. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kurmani S and Squire I: Acute heart
failure: Definition, classification and epidemiology. Current Heart
Fail Rep. 14:385–392. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Felker GM, Lee KL, Bull DA, Redfield MM,
Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL,
Ofili EO, et al: Diuretic strategies in patients with acute
decompensated heart failure. N Engl J Med. 364:797–805.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fonarow GC: Refining classification of
heart failure based on ejection fraction. JACC Heart Fail.
5:808–809. 2017. View Article : Google Scholar
|
|
5
|
Raphael C, Briscoe C, Davies J, Ian
Whinnett Z, Manisty C, Sutton R, Mayet J and Francis DP:
Limitations of the New York Heart Association functional
classification system and self-reported walking distances in
chronic heart failure. Heart. 93:476–482. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Richards AM: Biomarkers in acute heart
failure-cardiac and kidney. Card Fail Rev. 1:107–111.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ray SG: Natriuretic peptides in heart
valve disease. Heart. 92:1194–1197. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ardissino G, Daccò V, Testa S, Civitillo
CF, Tel F, Possenti I, Belingheri M, Castorina P,
Bolsa-Ghiringhelli N, Tedeschi S, et al: Hemoconcentration: A major
risk factor for neurological involvement in hemolytic uremic
syndrome. Pediatr Nephrol. 30:345–352. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Davila C, Reyentovich A and Katz SD:
Clinical correlates of hemoconcentration during hospitalization for
acute decompensated heart failure. J Card Fail. 17:1018–1022.
2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Oh J, Kang SM, Kim IC, Han S, Yoo BS, Choi
DJ, Kim JJ, Jeon ES, Cho MC, Oh BH, et al: The beneficial
prognostic value of hemoconcentration is negatively affected by
hyponatremia in acute decompensated heart failure: Data from the
Korean Heart Failure (KorHF) Registry. J Cardiol. 69:790–796.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Go AS, Yang J, Ackerson LM, Lepper K,
Robbins S, Massie BM and Shlipak MG: Hemoglobin level, chronic
kidney disease, and the risks of death and hospitalization in
adults with chronic heart failure: The Anemia in Chronic Heart
Failure: Outcomes and Resource Utilization (ANCHOR) Study.
Circulation. 113:2713–2723. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mozaffarian D, Nye R and Levy WC: Anemia
predicts mortality in severe heart failure: The prospective
randomized amlodipine survival evaluation (PRAISE). J Am Coll
Cardiol. 41:1933–1939. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lloyd-Jones D, Adams RJ, Brown TM,
Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K,
Gillespie C, et al: Executive summary: Heart disease and stroke
statistics-2010 update: A report from the American Heart
Association. Circulation. 121:948–954. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Testani JM, Brisco MA, Chen J, McCauley
BD, Parikh CR and Tang WH: Timing of hemoconcentration during
treatment of acute decompensated heart failure and subsequent
survival: Importance of sustained decongestion. J Am Coll Cardiol.
62:516–524. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
O'Connell JB and Bristow MR: Economic
impact of heart failure in the United States: Time for a different
approach. J Heart Lung Transplant. 13(S107-S112)1994.PubMed/NCBI
|
|
16
|
Yancy CW, Lopatin M, Stevenson LW, De
Marco T and Fonarow GC: ADHERE Scientific Advisory Committee and
Investigators: Clinical presentation, management, and in-hospital
outcomes of patients admitted with acute decompensated heart
failure with preserved systolic function: A report from the Acute
Decompensated Heart Failure National Registry (ADHERE) Database. J
Am Coll Cardiol. 47:76–84. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Waldum B, Westheim AS, Sandvik L, Flønæs
B, Grundtvig M, Gullestad L, Hole T and Os I: Baseline anemia is
not a predictor of all-cause mortality in outpatients with advanced
heart failure or severe renal dysfunction. Results from the
Norwegian Heart Failure Registry. J Am Coll Cardiol. 59:371–378.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Alexandrakis MG and Tsirakis G: Anemia in
heart failure patients. ISRN Hematol. 2012(246915)2012. View Article : Google Scholar
|
|
19
|
Oh J, Kang SM, Hong N, Youn JC, Han S,
Jeon ES, Cho MC, Kim JJ, Yoo BS, Chae SC, et al: Hemoconcentration
is a good prognostic predictor for clinical outcomes in acute heart
failure: Data from the Korean Heart Failure (KorHF) Registry. Int J
Cardiol. 168:4739–4743. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ter Maaten JM, Valente MA, Damman K,
Cleland JG, Givertz MM, Metra M, O'Connor CM, Teerlink JR,
Ponikowski P, Bloomfield DM, et al: Combining diuretic response and
hemoconcentration to predict rehospitalization after admission for
acute heart failure. Circ Heart Fail. 9:2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cataliotti A, Boerrigter G,
Costello-Boerrigter LC, Schirger JA, Tsuruda T, Heublein DM, Chen
HH, Malatino LS and Burnett JC Jr: Brain natriuretic peptide
enhances renal actions of furosemide and suppresses
furosemide-induced aldosterone activation in experimental heart
failure. Circulation. 109:1680–1685. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Durak-Nalbantić A, Džubur A, Dilić M,
Pozderac Z, Mujanović-Narančić A, Kulić M, Hodžić E, Resić N,
Brdjanović S and Zvizdić F: Brain natriuretic peptide release in
acute myocardial infarction. Bosn J Basic Med Sci. 12:164–168.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Eindhoven JA, van den Bosch AE, Jansen PR,
Boersma E and Roos-Hesselink JW: The usefulness of brain
natriuretic peptide in complex congenital heart disease: A
systematic review. J Am Coll Cardiol. 60:2140–2149. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Roberts E, Ludman AJ, Dworzynski K,
Al-Mohammad A, Cowie MR, McMurray JJ and Mant J: NICE Guideline
Development Group for Acute Heart Failure: The diagnostic accuracy
of the natriuretic peptides in heart failure: Systematic review and
diagnostic meta-analysis in the acute care setting. BMJ.
350(h910)2015.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Di Angelantonio E, Chowdhury R, Sarwar N,
Ray KK, Gobin R, Saleheen D, Thompson A, Gudnason V, Sattar N and
Danesh J: B-type natriuretic peptides and cardiovascular risk:
Systematic review and meta-analysis of 40 prospective studies.
Circulation. 120:2177–2187. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
van Veldhuisen DJ, Linssen GC, Jaarsma T,
van Gilst WH, Hoes AW, Tijssen JG, Paulus WJ, Voors AA and Hillege
HL: B-type natriuretic peptide and prognosis in heart failure
patients with preserved and reduced ejection fraction. J Am Coll
Cardiol. 61:1498–1506. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Iacoviello M, Leone M, Antoncecchi V and
Ciccone MM: Evaluation of chronic kidney disease in chronic heart
failure: From biomarkers to arterial renal resistances. World J
Clin Cases. 3:10–19. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Koglin J, Pehlivanli S, Schwaiblmair M,
Vogeser M, Cremer P and vonScheidt W: Role of brain natriuretic
peptide in risk stratification of patients with congestive heart
failure. J Am Coll Cardiol. 38:1934–1941. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shlipak MG, Chertow GC and Massie BM:
Beware the rising creatinine level. J Card Fail. 9:26–28.
2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Arakawa K, Ando Y, Matsunaga S and
Nishiura A: Diagnostic ability of natriuretic peptides for heart
failure according to eGFR level: Comparison between brain
natriuretic peptide and amino-terminal probrain natriuretic
peptide. Rinsho Byori. 64:251–257. 2016.(In Japanese). PubMed/NCBI
|