Introduction
Human immunodeficiency virus type 1 (HIV-1)
infection is followed by the integration of viral DNA with the host
genome leading to formation of proviral DNA (1). Viral replication in the human body is a
complex process regulated by several hosts as well as viral factors
(1). Transcription of HIV-1 is
facilitated by the 5' long terminal repeat (LTR) through the
formation of an integrated provirus (2). The process of HIV transcription is
suppressed by the inactivation of host chromatin through the
expression of inhibitors and remodeling molecules (3). Therefore, the organization of chromatin
material plays a vital role in the expression of the HIV-1 genes
involved in transcription (4).
Retinoblastoma binding protein 4 (RbAp48) maintains
chromatin organization and is associated with chromatin assembly
factor 1(5). It acts as a histone
de-acetylation and nucleosome remodeling agent, thereby inhibiting
the process of viral transcription (6). A previous study identified that RbAp48
interacts with H3-H4 histones, which makes it an important
component of various complexes that are involved in chromatin
remodeling (7). RbAp48 is also
involved in several other cellular processes, such as the
maintenance of pluripotent stem cells and the induction of
apoptosis in some selected tissues (8,9). Higher
expression of RbAp48 has been shown to induce autoimmune
exocrinopathy and inhibit the process of transcription (10). Therefore, transcription of genes is
regulated by RbAp48 through several pathways. In HIV infection, one
of the most important steps is transcription of genes to multiply
and spread the virus. In the present study, the effect of
thieno[3,4-d]pyrimidine (TEP) on the transcription of viral
genes and its association with RbAp48 was investigated. The present
study demonstrated that TEP inhibited HIV-1 infection through the
upregulation of RbAp48 expression and activation of the NF-κB
pathway. TEP also suppressed tumor necrosis factor-α (TNF-α) and
phorbol 12-myristate 13-acetate (PMA)-mediated upregulation of
HIV-1 transcription, thereby acting as an anti-HIV agent.
Materials and methods
Chemicals and reagents
TEP was purchased from Sigma Aldrich; Merck
KGaA.
Plasmid constructs
The pRbAp48 vector and the pCTL (control) vector
were purchased from OriGene Technologies, Inc. pRbAp48 was knocked
down using short hairpin RNA (shRNA; (Takara Bio, Inc.) using the
following gene sequences: Forward, 5'-CGAGGAAUACAAAAUAUGGTT-3' and
reverse, 5'-CCAUAUUUUGCUCGTT-3' (10).
Cell culture and transfections
293T and TZM-bl cell lines were supplied by American
Type Culture Collection. The cells were cultured in DMEM (Thermo
fisher Scientific, Inc.) mixed with 10% FBS (Thermo Fisher
Scientific, Inc.). The medium also contained antibiotics, 100 U/ml
penicillin-100 µg/ml streptomycin (Thermo Fisher Scientific, Inc.).
The cell culture was performed under an atmosphere of 5%
CO2 and 95% air at 37˚C. Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used for the
transfection of plasmids into 293T and TZM-bl cells.
Viral stock production and
infection
For the production of viral stocks, H9/HTLV-IIIB
cells (obtained from Zhejiang University, Hangzhou, China) were
used according to a previously published procedure (11). Detection of antigens corresponding to
viral p24 was performed using an ELISA kit (cat. no. ab218268;
Abcam) according to the manufacturer's instructions. For infection
with HIV-1, CEM cells (American Type Culture Collection) at a
concentration of 2.5x106 cells/well were cultured at one
multiplicity of infection using spinoculation for 2.5 h at 1,000 x
g at a temperature of 25˚C (12).
The free virus was removed from the cell cultures by centrifugation
at 25˚C for 10 min at 300 x g. TZM-bl cells were subjected to
infection for determination of infectious viruses
quantitatively.
Analysis of luciferase activity
293T cells at a density of 2x105
cells/well were distributed into a 96-well plate. After overnight
incubation the cells were subjected to firefly luciferase construct
and pRbAp48 construct transfection. The negative control cells were
transfected with pGL3-vector (Promega Corporation) and empty pCTL
vector (OriGene Technologies, Inc.). The cells, after treatment
with concentrations of 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0,
2.25 and 2.5 µM TEP, were exposed to 25 ng/ml PMA (Sigma Aldrich;
Merck KGaA) and 20 ng/ml TNF-α. The activity of luciferase was
determined after 48 h of transfection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from the cells was obtained using the
RNeasy mini kit (Qiagen GmbH), according to the manufacturer's
instructions. The total RNA (1 µg) samples were used for the
preparation of cDNA by employing oligo(dT) primers and SuperScript
III RT (Invitrogen; Thermo Fisher Scientific, Inc.). Detection of
HIV-1 DNA was carried out using the known primers for the LTR
(13). Determination of HIV-1 mRNA
was also performed using previously published procedures (14,15). The
ABI 7500 Sequencing Detection System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used for the amplification process.
The amplification was carried out using cDNA (1 ng), forward and
reverse primers (125 nM) and 2X SYBR®Premix Ex Taq™ (25
µl; Takara Bio, Inc.) in 50 µl reaction. The reactions were carried
out for 1 min at 95˚C, 40 cycles for 15 sec at 95˚C, 20 sec at 56˚C
and 30 sec at 70˚C. The quantification cycle (Cq) of each sample
was recorded as a quantitative measure of the amount of PCR product
in the sample. The relative mRNA expression levels of the genes
were calculated following normalization to β-actin mRNA using the
2-ΔΔCq method (16). The primer sequences were as follows:
RbAp48 forward, CGA GGAAUACAAAAUAUGGTT and reverse, CCAUAUUUU
GUAUUCCUCGTT; HIV-1 forward, 5'-TAGCAGTGGCGC CCGA-3' and reverse,
5'-TCTCTCTCCTTCTAGCCTCC GC-3'; β-actin forward, 5'-TCCTCTCCCAAG
TCCACACA GG-3' and reverse, 5'-GGGCACGAAGGCTCATCATTC-3'.
Western blotting
Lysates of the cells were prepared using a
radioimmunoprecipitation assay buffer mixed with phenyl
methylsulfonyl fluoride (Beyotime Institute of Biotechnology). The
lysates were centrifuged for 10 min at 12,000 x g at a temperature
of 4˚C. Concentrations of the proteins were determined using a
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). The proteins (20 µg samples/lane) were separated by
SDS PAGE on 12% gels and subsequently electro-transferred using
electro-blotting apparatus (Bio Rad Laboratories, Inc.) onto
nitrocellulose membranes. The membranes were blocked with
tris-buffered saline with 1/1,000 tween containing 5% non-fat milk
for 1 h at room temperature. Subsequently the membranes were
incubated overnight with antibodies against RbAp48 (cat. no.
ab79416; 1:1,000; Abcam), NF-κB (cat. no. 545380-34-5; 1:1,000;
Merck KGaA) and GAPDH (cat. no. D16H11; 1:1,000; Cell Signaling
Technology, Inc.) at 4˚C. After washing, membranes were incubated
for 1 h at room temperature with horseradish peroxidase-conjugated
secondary antibodies (1:2,000; cat. no. 7074; Cell Signaling
Technology, Inc.). After incubation, membrane washing was performed
with TBS and Tween-20 followed by enhanced chemiluminescence
reagent (EMD Millipore) treatment. For analysis, Gel Pro Analyzer
software version 4.0 (Media Cybernetics, Inc.) was used.
Electrophoretic mobility shift assay
(EMSA)
The 293T cells were subjected to incubation in6-well
plates containing DMEM for 24 h with 20 ng/ml TNF-α. The medium was
then replaced with new medium containing concentrations of 0.25,
0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 2.25 and 2.5 µM TEP and cells
were incubated for 48 h. Commercially available NE-PERTM nuclear
and cytoplasmic extractions kit (cat. no. 78833; Thermo Fisher
Scientific, Inc.) were used for extraction of nuclear fractions,
according to the manufacturer's protocol. NF-κB was probed in
nuclear and cytoplasmic extracts using the Light Shift
Chemiluminescent EMSA kit (Thermo Fisher Scientific, Inc.). The
nuclear extract samples (10 µg) were subjected to incubation at
room temperature with wild probes for 45 min in 20 µl reaction
buffer. The buffer contained 1X binding buffer, magnesium chloride
(5 mM), propantriol (2.5%), poly (dI.dC; 55 ng/µl) and NP-40
(0.08%). Electrophoresis of the binding reactions was performed in
a polyacrylamide gel (8%) for 1 h at 110 V in Tris-borate-EDTA
(TBE) buffer (0.5X) after the addition of 4 µl loading buffer. The
transfer unit was sandwiched with nylon membrane (+vely charged)
and gel, which was subsequently placed into ice-cold 0.5X TBE
buffer for 1 h at 370 V. The membranes were blocked with
tris-buffered saline with 1/1,000 tween containing 5% non-fat milk
for 1 h at room temperature. Then, membrane incubation was
performed with peroxidase conjugated streptavidin horseradish
antibody (1:300; cat. no. 7074; Thermo Fisher Scientific, Inc.) for
20 min at 4˚C. The membrane washing with 1X buffer was followed by
equilibration and incubation with substrate. The VersaDoc MP 5000
imaging system (Bio-Rad Laboratories, Inc.) was used for the
visualization of reaction bands.
Statistical analysis
The values are presented as the mean ± SEM. The
experiments were carried out in triplicates independently. The data
were compared using Student's t-test and one way ANOVA followed by
tukey's post hoc multiple comparison test. P<0.05was considered
to indicate a statistically significant difference. Analysis of the
data obtained was performed using SPSS version 18.0 (SPSS,
Inc.).
Results
TEP inhibits HIV-1 production in 293T
cells
In order to determine whether TEP can affect HIV-1
production, 293T cells were treated with 0.25, 0.5, 0.75, 1.0,
1.25, 1.5, 1.75, 2.0, 2.25 and 2.5 µM of TEP on day 2 after HIV-1
infection. A marked decrease was observed in the production of the
HIV-1 genome in 293T cells with increasing concentrations of TEP
≤2.5 µM (Fig. 1). At higher
concentrations of TEP there was no further decrease in HIV-1
replication was observed (data not shown). There was also no
significant change in HIV-1 production in 293T cells at
concentrations <0.25 µM by TEP (data not shown). RT-qPCR
analysis demonstrated a significant decrease (P<0.05) in HIV-1
genomic expression from 0.75 µM concentration of TEP compared with
the untreated cells. No significant change in the production of the
HIV-1 genome in 293T cells was induced by TEP at concentrations of
0.25 and 0.5 µM.
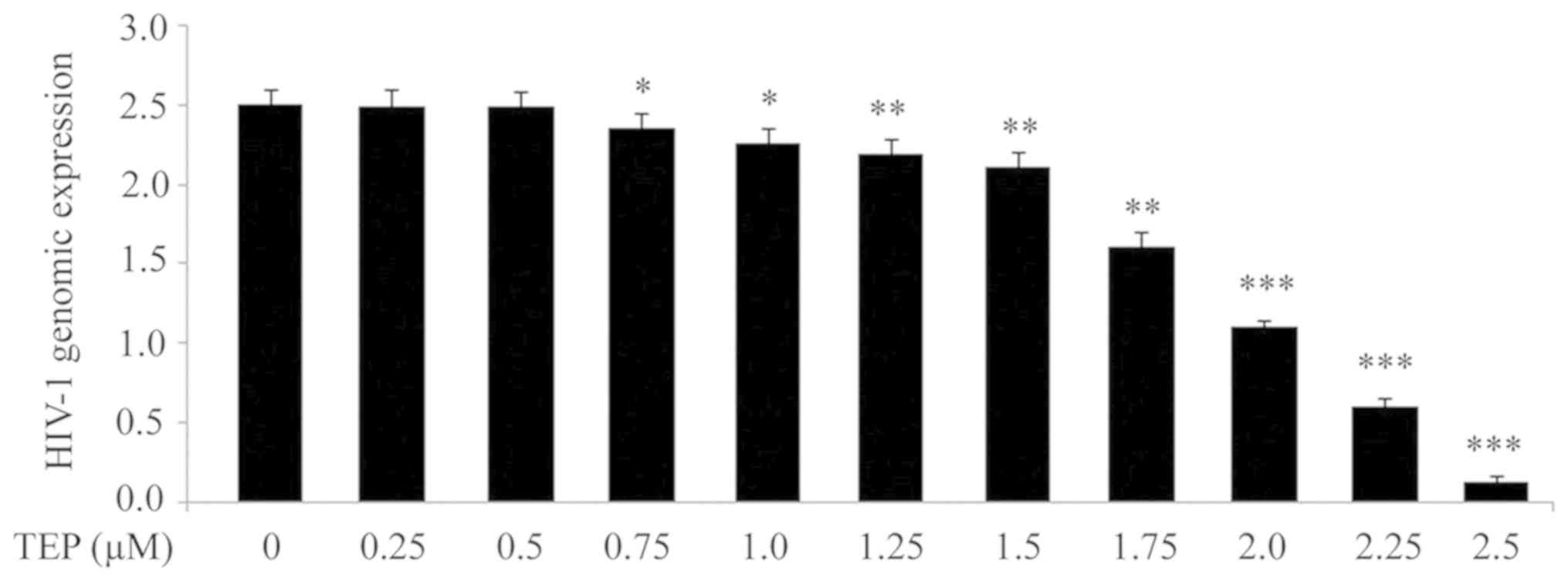 | Figure 1Effect of TEP on HIV-1 production in
293T cells. TEP at concentrations of 0.25, 0.5, 0.75, 1.0, 1.25,
1.5, 1.75, 2.0, 2.25 and 2.5 µM was added to 293T cells after 48 h
HIV infection (multiplicity of infection of 1 by spinoculation at
1,200 x g for 2 h at 25˚C). The cell supernatants were collected
after 48 h of TEP treatment to examine the HIV-1 genome expression
by reverse transcription-quantitative PCR. *P<0.05,
**P<0.02 and
***P<0.01 vs. untreated cells.
TEP, thieno[3,4-d]pyrimidine; HIV-1, human immunodeficiency
virus type 1. |
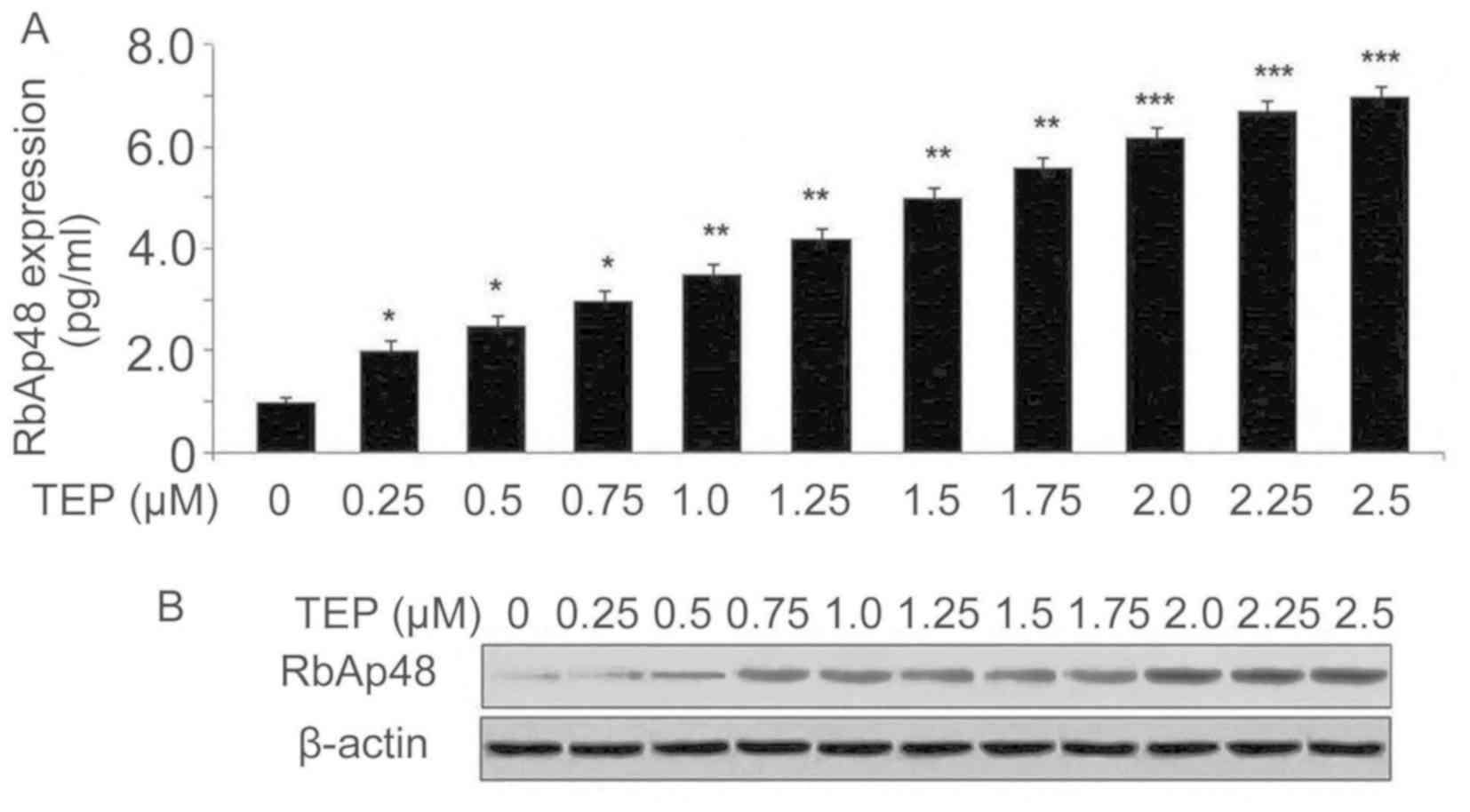
TEP increases expression of RbAp48 in
293T cells
The expression levels of RbAp48 mRNA and protein in
293T cells following HIV-1 infection were examined using RT-qPCR
and western blotting assays, respectively. TEP treatment of HIV-1
infected 293T cells led to upregulation of RbAp48 mRNA and protein
in a concentration-dependent manner (Fig. 2). The level of RbAp48 mRNA at 0.25,
0.5, 0.75, 1.0, 1.25, 1.5, 1.75, 2.0, 2.25 and 2.5 µM TEP on day 2
after HIV-1 infection increased to 2.0-, 2.5-, 3.0-, 3.5-, 4.2-,
5.0-, 5.6-, 6.2-, 6.7- and 7-fold, respectively, compared with the
untreated cells (Fig. 2A). Western
blotting also demonstrated that the RbAp48 protein level increased
in 293T cells with TEP treatment (Fig.
2B).
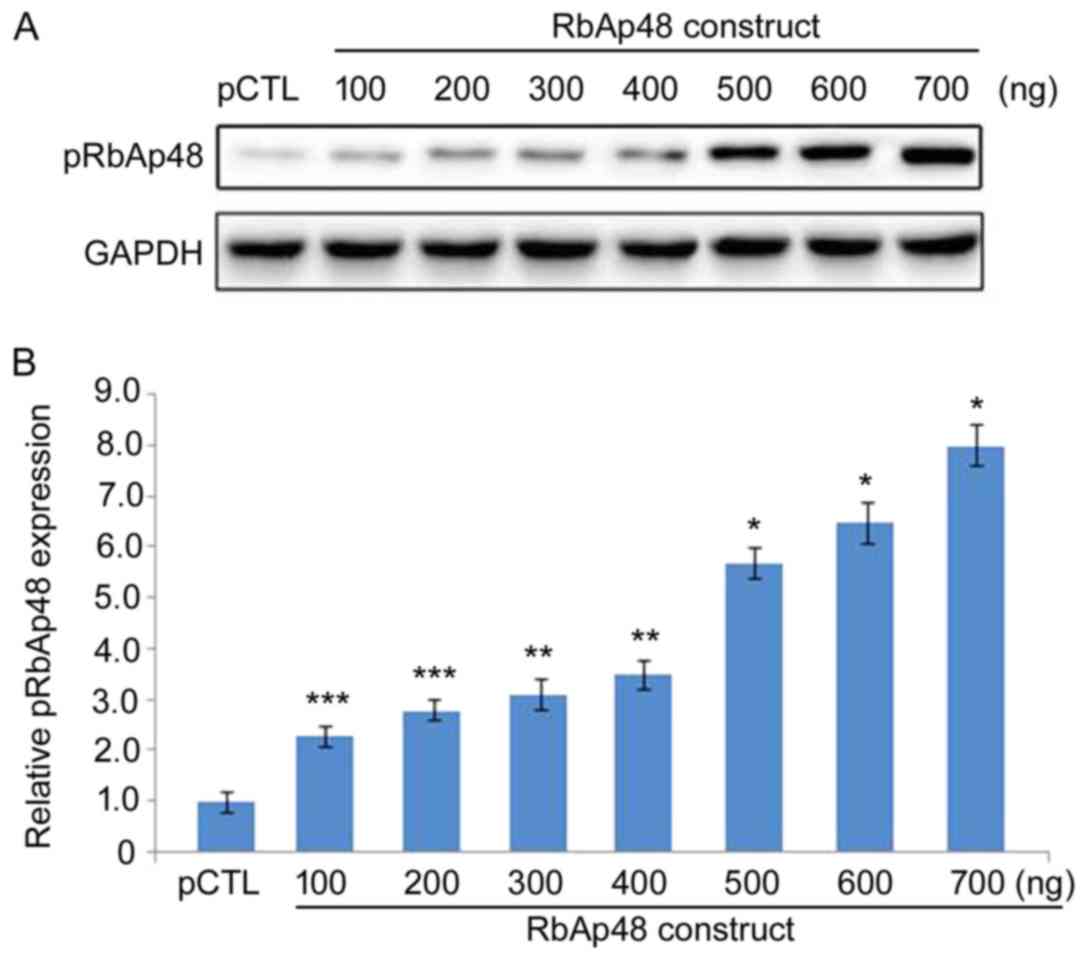
pRbAp48 transfection increases the
pRbAp48 protein level in 293T cells
The pRbAp48 protein level was markedly upregulated
in 293T cells following pRbAp48 transfection in a dose-dependent
manner (Fig. 3). Transfection with
100, 200, 300, 400, 500, 600 and 700 ng pRbAp48 markedly
upregulated the pRbAp48 protein expression. The pRbAp48 protein
expression was not elevated in 293T cells transfected with the
negative control, pCTL. These results demonstrated successful
transfection of pRbAp48 in 293T cells.
TEP and pRbAp48 inhibit LTR activity
in 293T cells
pRbAp48 transfection of 293T cells decreased the LTR
activity in a concentration-dependent manner. When the cells were
transfected with 100, 200, 300, 400, 500, 600 and 700 ng pRbAp48,
the activity of LTR was decreased by 21, 26, 34, 43, 51, 68 and
79%, respectively, in comparison with cells transfected with the
empty vector (Fig. 4A). These
concentrations of pRbAp48 were selected based on preliminary
screening (data not shown). TEP treatment of 293T cells also
decreased LTR activity in a concentration-dependent manner. The LTR
activity was decreased by 24, 29, 34, 41, 52, 63 and 92% in 293T
cells upon treatment with 0.5, 0.75, 1.0, 1.5, 1.75, 2.0 and 2.5 µM
TEP, respectively (Fig. 4A). The
effect of RbAp48, TEP and pCTL on TNF-α and PMA-induced activity of
LTR was analyzed by determining the relative luciferase activity.
In 293T cells RbAp48 and TEP decreased TNF-α and PMA-induced LTR
activity in comparison with pCTL (Fig.
4B).
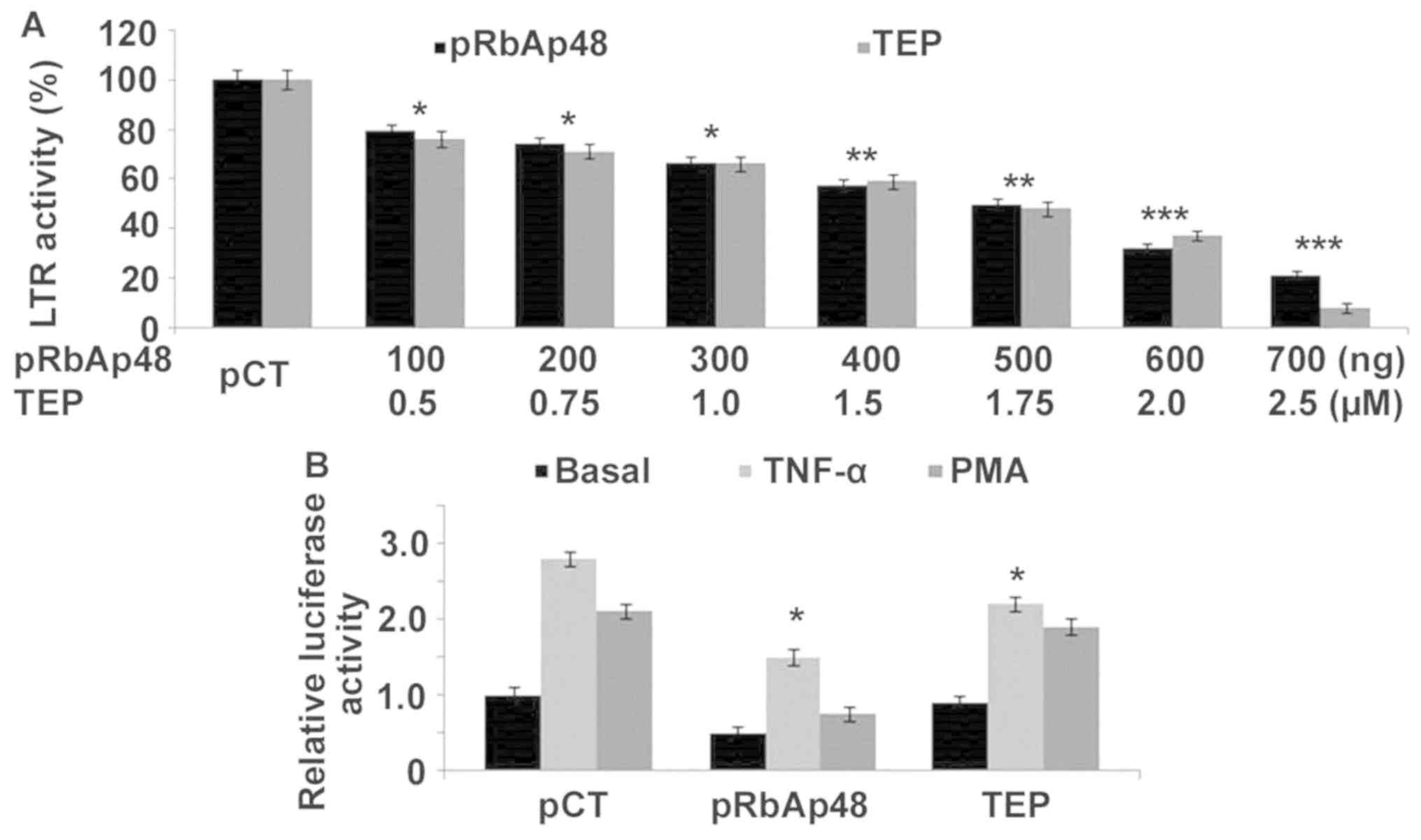 | Figure 4Effect of TEP and pRbAp48 on LTR
activity. (A) Cells were treated with 100, 200, 300, 400, 500, 600
and 700 ng pRbAp48 to analyze the LTR activity after 48 h. (B)
TNF-α and PMA-stimulated 293T cells were incubated with pRbAp48
(700 ng) and TEP (2.5 µM) for determination of the luciferase
activity. The experiments were performed in triplicates. The 0 ng
pRbAp48 group was transfected with the negative control, pCTL.
*P<0.05, **P<0.02 and
***P<0.01 vs. respective
control cells. TEP, thieno[3,4-d]pyrimidine; TNF-α, tumor
necrosis factor-α; RbAp48, retinoblastoma binding protein 4; LTR,
long terminal repeat; PMA, phorbol 12-myristate 13-acetate. |
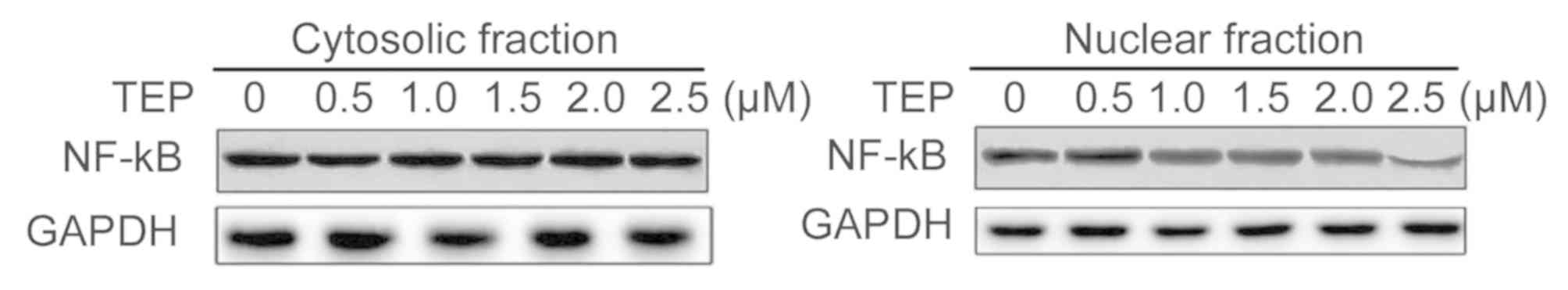
TEP reduces nuclear translocation of
NF-κB via stabilization of IκBα
The NF-κB translocation to the nucleus was markedly
reduced in 293T cells after incubation for 48 h with TEP compared
with the untreated cells (Fig. 5).
With the increase in concentration of TEP from 0.5 to 2.5 µM a
marked decrease was observed in NF-κB nuclear translocation.
HIV-1 expression in 293T cells is
inhibited by RbAp48
Transfection of shRNA to knockdown RbAp48 or sh-NC
as control into 293T cells was followed by HIV 1 infection. RT-qPCR
analysis on days 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 demonstrated a
marked downregulation in RbAp48 expression (Fig. 6A). Knockdown of RbAp48 expression by
sh-RNA in 293T cells caused a marked increase in HIV-1 production
compared with the control cells (Fig.
6B). These results suggested that RbAp48 expression inhibits
HIV production.
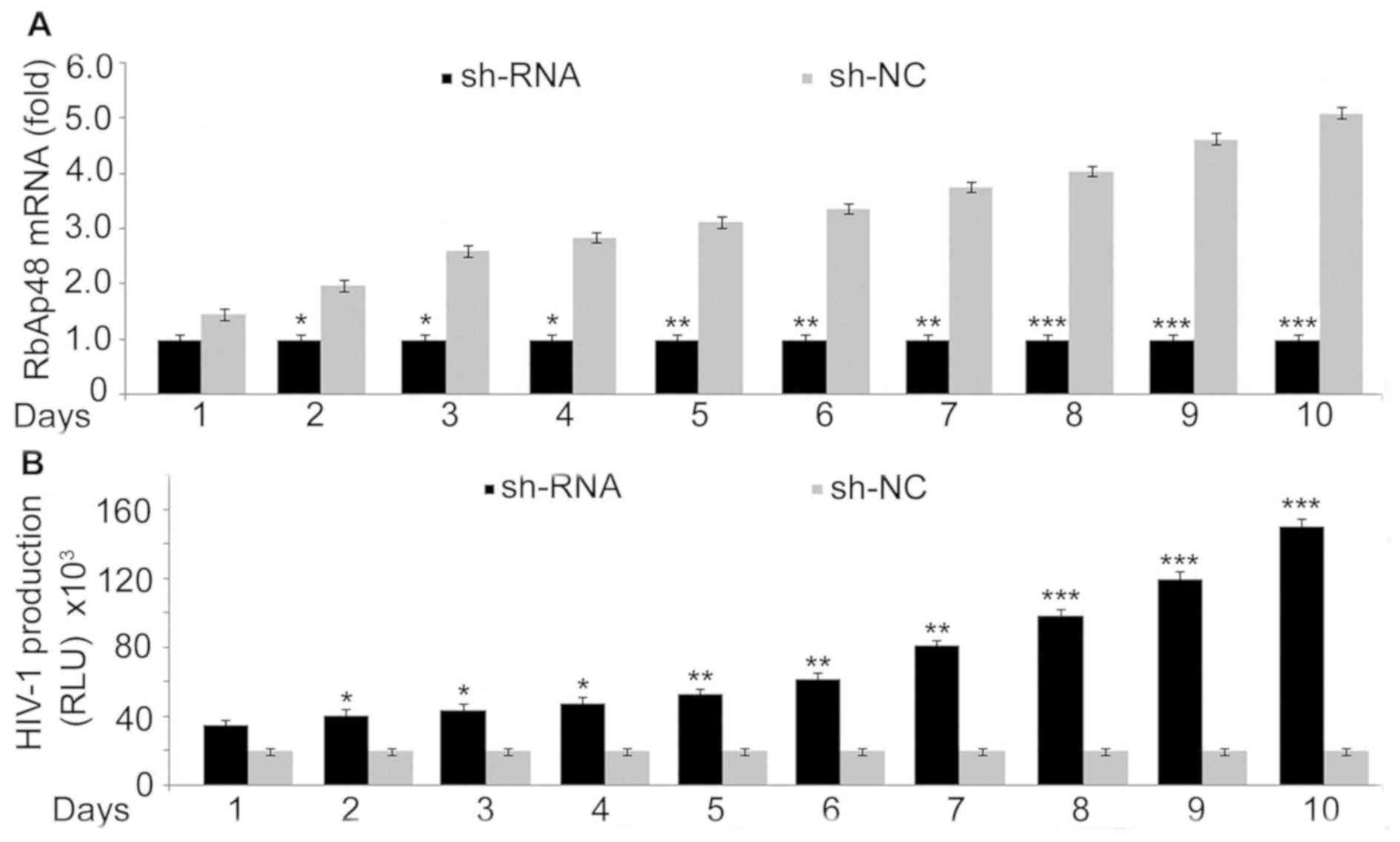 | Figure 6Effect of RbAp48 on HIV-1 production.
(A) sh-RbAp48 (1 µg) or sh-NC transfection into 293T cells was
followed by administration of HIV-1 (multiplicity of infection of 1
by spinoculation at 1,200 x g for 2 h at 25˚C). Reverse
transcription-quantitative PCR was used to examine the expression
of RbAp48 mRNA on days 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10. (B)
Detection of viral production was analyzed using a commercially
available ELISA kit for HIV-1 p24. *P<0.05,
**P<0.02 and
***P<0.01 vs. respective sh-NC
transfected cells. RbAp48, retinoblastoma binding protein 4; HIV-1,
human immunodeficiency virus type 1; sh, short hairpin; NC,
negative control; RLU, relative light units. |
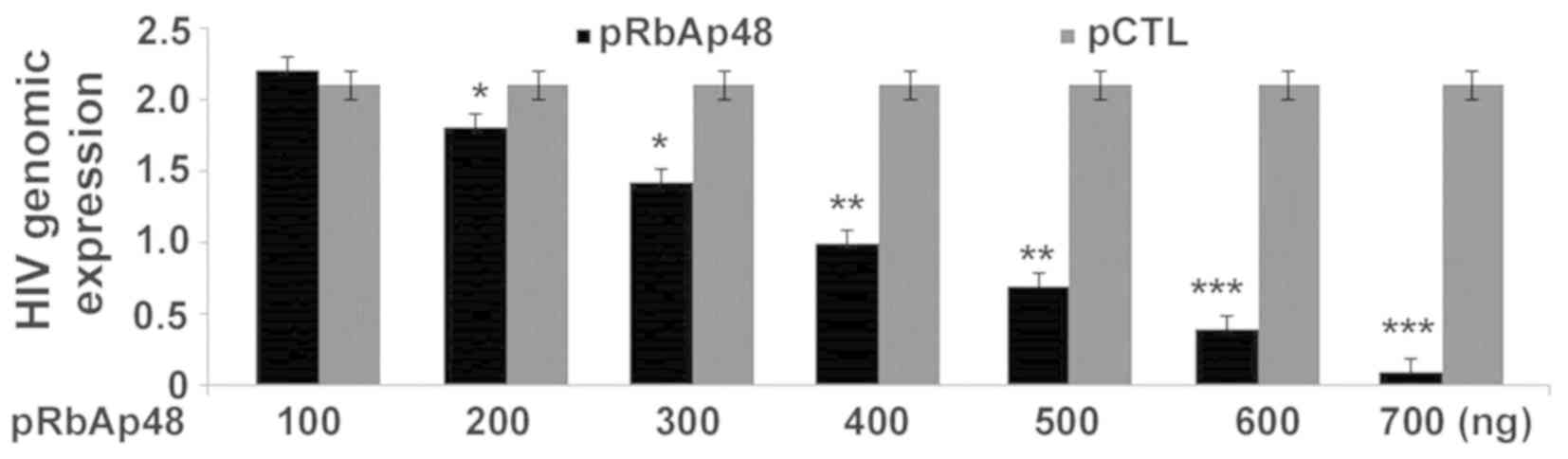
RbAp48 transfection inhibits HIV-1
production in 293T cells
In order to confirm whether RbAp48 transfection can
influence the HIV-1 production, 293T cells after HIV-1 infection
were administered various concentration of pRbAp48 (RbAp48
construct) or pCTL as a control vector. A marked decrease was
observed in the production of the HIV-1 genome in 293T cells with
upregulated pRbAp48 (Fig. 7).
RT-qPCR analysis showed a decrease in HIV-1 genomic expression at
100, 200, 300, 400, 500, 600 and 700 ng pRbAp48 compared with the
cells transfected with pCTL.
Discussion
HIV-1 infection leads to the upregulation of several
anti-HIV compounds such ascaveolin-1 which in turn inhibits
replication of HIV-1 (17,18). Infection with HIV-1 has been
demonstrated to induce the expression of NF-Iβ, which subsequently
downregulates HIV-1 replication (17,18). The
present study investigated the effect of TEP on transcription of
HIV-1 in 293T cells. Initial screening identified a significant
decrease in HIV-1 replication with TEP treatment in HIV-1 infected
293T cells. The present findings suggested that TEP possess
anti-HIV activity and requires further evaluation to understand the
underlying mechanism of its action.
The transcription of the viral genome is inhibited
by overexpression of RbAp48 through histone de-acetylation and
nucleosome remodeling (5,6). Therefore, the present study examined if
TEP interferes with the expression of the RbAp48 molecule in 293T
cells. The results from the present study demonstrated that TEP
treatment of HIV-1 infected 293T cells upregulated the RbAp48 mRNA
and protein levels in a concentration-dependent manner. A marked
increase in the level of RbAp48 mRNA and protein was observed in
HIV-1 infected 293T cells treated with TEP compared with the
control cells. Therefore, the present results suggested that TEP
exhibited anti-HIV effects in 293T cells through the upregulation
of RbAp48 expression.
Replication of viral particles is regulated by the
transcription of a provirus, which comprises an important phase in
the life cycle of HIV-1(19). The
transcription process is controlled by the HIV-1 LTR through a
well-organized pathway involving participation of several factors
(20). Many molecules bind to the
HIV-1 5' LTR and inhibit viral transcription (19). The molecules which bind and inhibit
viral transcription by targeting HIV-1 LTR include transcriptional
repressor protein YY1, transcription factor LSF, zinc finger
protein ZBRK1 and scaffold/matrix-associated region-1 binding
protein (21,22). In the present study, the effect of
exogenously transfected pRbAp48 and TEP treatment on LTR activity
in HIV-infected 293T cells was also analyzed. The present study
demonstrated that both pRbAp48 transfection and TEP treatment
significantly decreased LTR activity in a concentration-dependent
manner in HIV-1 infected 293T cells. The activity of LTR was lowest
in 293T cells treated with 2.5 µM TEP. It is known that the
triggering of LTR activity and activation of the NF-κB signaling
pathway is promoted by the administration of PMA and TNF-α
(23). In addition, PMA and TNF-α
also play an important role in the NF-κB signaling pathway
activation (23). In the present
study, the effect of RbAp48 and TEP on TNF-α- and PMA-induced LTR
activity in 293T cells was investigated. The present results
demonstrated that in 293T cells transfected with RbAp48 or treated
with TEP, TNF-α- and PMA-induced LTR activity was decreased. TEP
treatment of 293T cells suppressed the activity of luciferase
mediated by LTR following PMA- or TNF-α stimulation as well as in
basal states. A previous study identified inhibition of HIV genome
transcription and the induction of latency upon activation of NF-κB
p50 in HIV-infected cells (24). The
results from the present study demonstrated that nuclear
translocation of p65 was markedly reduced in HIV-infected 293T
cells treated with TEP compared with untreated cells.
In summary, the present study suggested that TEP
inhibits transcription of HIV-1 through upregulation of RbAp48
expression and activation of the NF-κB pathway. Therefore, TEP may
be used for the development of a HIV treatment strategy either in
combination with other drugs or by structural modification.
However, further studies are required to fully elucidate the role
of TEP in inhibition of HIV replication.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by The
Zhejiang Provincial Department of Health Medical Health Project
(grant no. 2017 KY667).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ designed the study and wrote the manuscript. WX,
YW and JZ performed the experiments and compiled the data. JC
analyzed the data and performed literature survey. All the authors
approved the manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mohammadi P, di Iulio J, Muñoz M, Martinez
R, Bartha I, Cavassini M, Thorball C, Fellay J, Beerenwinkel N,
Ciuffi A, et al: Dynamics of HIV latency and reactivation in a
primary CD4+ T cell model. PLoS Pathog. 10(e1004156)2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mbonye U and Karn J: Transcriptional
control of HIV latency: Cellular signaling pathways, epigenetics,
happenstance and the hope for a cure. Virology. 454-455:328–339.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Maldarelli F, Wu X, Su L, Simonetti FR,
Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, et
al: HIV latency. Specific HIV integration sites are linked to
clonal expansion and persistence of infected cells. Science.
345:179–183. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Van Lint C, Bouchat S and Marcello A:
HIV-1 transcription and latency: An update. Retrovirology.
10(67)2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Loyola A and Almouzni G: Histone
chaperones, a supporting role in the limelight. Biochim Biophys
Acta. 1677:3–11. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Allen HF, Wade PA and Kutateladze TG: The
NuRD architecture. Cell Mol Life Sci. 70:3513–3524. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang W, Tyl M, Ward R, Sobott F, Maman J,
Murthy AS, Watson AA, Fedorov O, Bowman A, Owen-Hughes T, et al:
Structural plasticity of histones H3-H4 facilitates their
allosteric exchange between RbAp48 and ASF1. Nat Struct Mol Biol.
20:29–35. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
O'Connor MD, Wederell E, Robertson G,
Delaney A, Morozova O, Poon SSS, Yap D, Fee J, Zhao Y, McDonald H,
et al: Retinoblastoma-binding proteins 4 and 9 are important for
human pluripotent stem cell maintenance. Exp Hematol. 39:866–79.e1.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Creekmore AL, Walt KA, Schultz-Norton JR,
Ziegler YS, McLeod IX, Yates JR and Nardulli AM: The role of
retinoblastoma-associated proteins 46 and 48 in estrogen receptor α
mediated gene expression. Mol Cell Endocrinol. 291:79–86.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ishimaru N, Arakaki R, Yoshida S, Yamada
A, Noji S and Hayashi Y: Expression of the retinoblastoma protein
RbAp48 in exocrine glands leads to Sjögren's syndrome-like
autoimmune exocrinopathy. J Exp Med. 205:2915–2927. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang J, Yang Z, Lv H, Lou Y, Wang J and Wu
N: Bridging HIV-1 cellular latency and clinical long-term
non-progressor: An interactomic view. PLoS One.
8(e55791)2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
O'Doherty U, Swiggard WJ and Malim MH:
Human immunodeficiency virus type 1 spinoculation enhances
infection through virus binding. J Virol. 74:10074–10080.
2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Casabianca A, Gori C, Orlandi C, Forbici
F, Federico Perno C and Magnani M: Fast and sensitive quantitative
detection of HIV DNA in whole blood leucocytes by SYBR green I
real-time PCR assay. Mol Cell Probes. 21:368–378. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jablonski JA and Caputi M: Role of
cellular RNA processing factors in human immunodeficiency virus
type 1 mRNA metabolism, replication, and infectivity. J Virol.
83:981–992. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yedavalli VSRK and Jeang KT:
Trimethylguanosine capping selectively promotes expression of
Rev-dependent HIV-1 RNAs. Proc Natl Acad Sci USA. 107:14787–14792.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang XM, Nadeau PE, Lin S, Abbott JR and
Mergia A: Caveolin 1 inhibits HIV replication by transcriptional
repression mediated through NF-κB. J Virol. 85:5483–5493.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vemula SV, Veerasamy R, Ragupathy V,
Biswas S, Devadas K and Hewlett I: HIV-1 induced nuclear factor IB
(NF-IB) expression negatively regulates HIV-1 replication through
interaction with the long terminal repeat region. Viruses.
7:543–558. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Kumar A, Darcis G, Van Lint C and Herbein
G: Epigenetic control of HIV-1 post integration latency:
Implications for therapy. Clin Epigenetics. 7(103)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Coull JJ, Romerio F, Sun JM, Volker JL,
Galvin KM, Davie JR, Shi Y, Hansen U and Margolis DM: The human
factors YY1 and LSF repress the human immunodeficiency virus type 1
long terminal repeat via recruitment of histone deacetylase 1. J
Virol. 74:6790–6799. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sreenath K, Pavithra L, Singh S, Sinha S,
Dash PK, Siddappa NB, Ranga U, Mitra D and Chattopadhyay S: Nuclear
matrix protein SMAR1 represses HIV-1 LTR mediated transcription
through chromatin remodeling. Virology. 400:76–85. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nishitsuji H, Abe M, Sawada R and Takaku
H: ZBRK1 represses HIV-1 LTR-mediated transcription. FEBS Lett.
586:3562–3568. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rom S, Reichenbach NL, Dykstra H and
Persidsky Y: The dual action of poly(ADP-ribose) polymerase -1
(PARP-1) inhibition in HIV-1 infection: HIV-1 LTR inhibition and
diminution in Rho GTPase activity. Front Microbiol.
6(878)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Williams SA, Chen LF, Kwon H, Ruiz-Jarabo
CM, Verdin E and Greene WC: NF-kappaB p50 promotes HIV latency
through HDAC recruitment and repression of transcriptional
initiation. EMBO J. 25:139–149. 2006.PubMed/NCBI View Article : Google Scholar
|