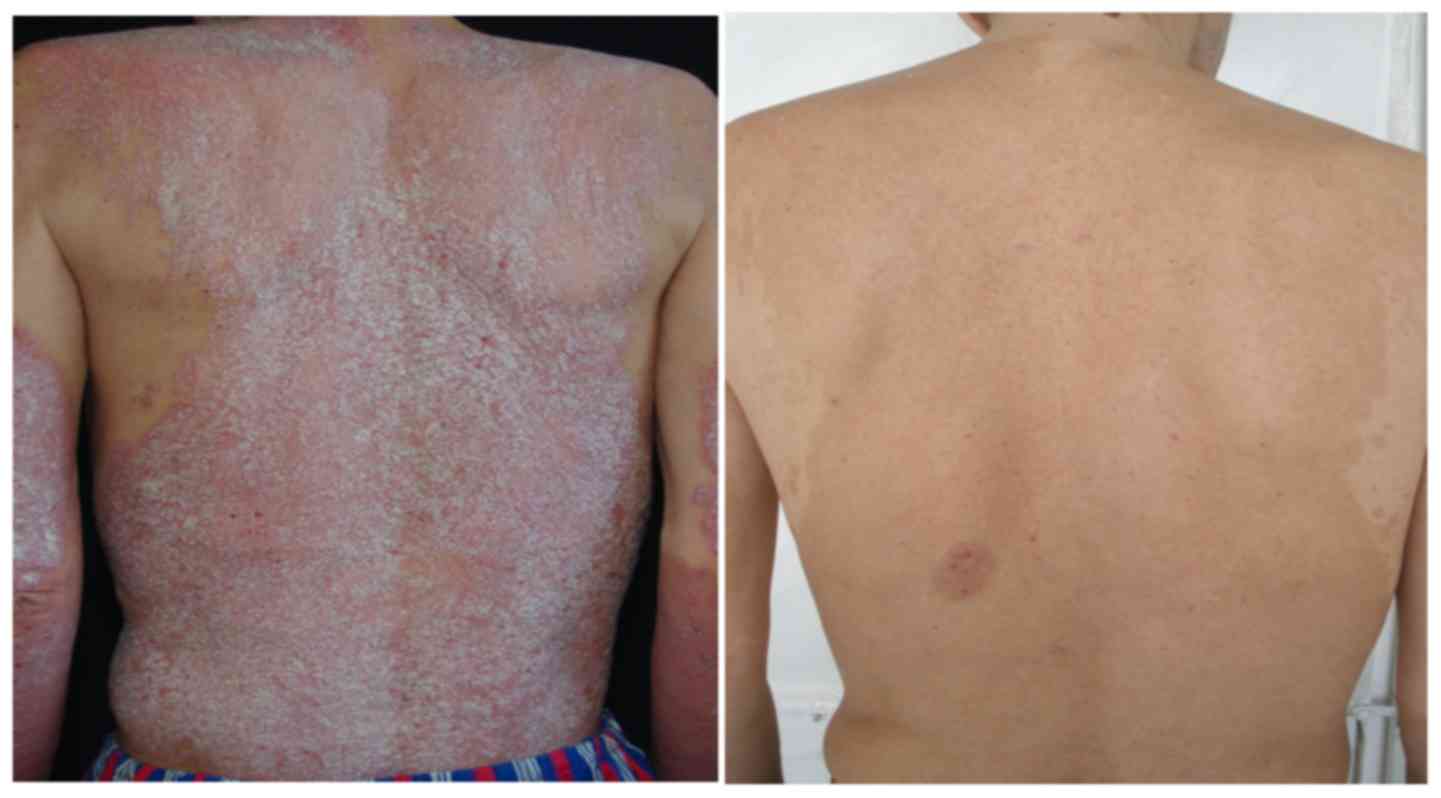
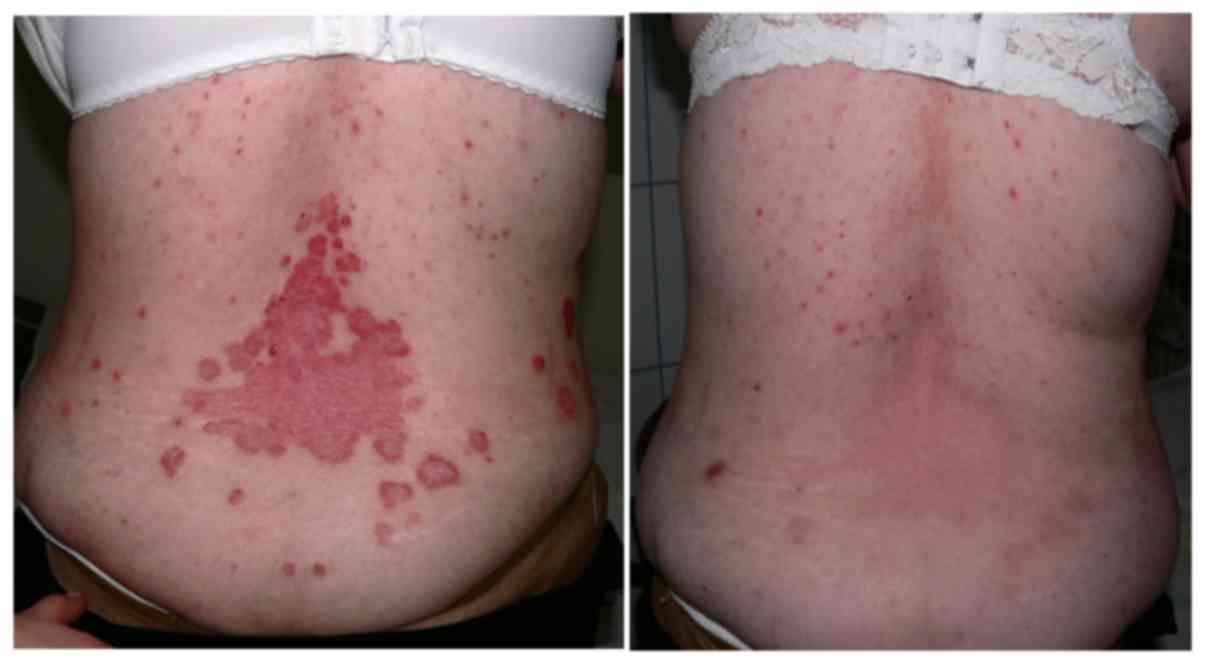
Introduction
Psoriasis is a chronic relapsing autoimmune disease,
in which genetic and epigenetic changes result in an altered immune
function, keratinocyte activation and hyperproliferation. It mainly
affects the skin and joints (1).
Some environmental factors, such as stress, are considered to lead
to an immune dysregulation, but only in patients with genetic
predisposition, resulting in an abnormal proliferation and
maturation of keratinocytes, proliferation of dermal blood vessels,
mast cell activation and skin infiltration by inflammatory cells
(2-4).
The inflammatory nature of psoriasis was illustrated by a systemic
and dermal secretion of cytokines such as interleukins (IL): IL-2,
IL-6, IL-8, IL-17, IL-18, IL-22, IL-23, IL-24, interferon-γ (INF-γ)
and tumor necrosis factor-α (TNF-α) (5). TNF-α is a cytokine secreted by
lymphocyte T, keratinocytes and dermal macrophages,
CD11+ dendritic cells and mastocytes (6). TNF-α increases the production of IL-6
and ICAM-1 expression. IL-6 secretion induced by TNF-α leads to
hepatic stimulation of production of acute phase reactants such as
C-reactive protein (CRP) and fibrinogen (7). CRP is an acute phase protein that
indirectly illustrates pro-inflammatory activity of cytokines. For
this reason, CRP is considered to be an inflammatory marker
(8). The study of Rocha-Pereira
et al (9) presented the
association between inflammation and psoriasis through increased
levels of CRP, fibrinogen, haptoglobin, C3, C4 and the fact that
the blood levels increase with the severity of the disease. They
also proposed that blood values of elastase, CRP,
elastase/α-macroglobulin and elastase/neutrophil ratios to be used
for prognosis regarding the worsening of psoriasis. Gisondi et
al (10) studied the correlation
between high blood levels of CRP, resistin, chemerin and psoriasis
before treatment and a reduction of these inflammatory marker
levels after biological therapy. It is very important to monitor
and control inflammation in order to chase-up the evolution of the
disease and its comorbidities. It has been proved that IL-6 induces
type two diabetes mellitus and cardiovascular diseases and that
INF-α could be involved in causing atherosclerosis (11). Devaraj et al (12) showed that CRP is a risk factor for
cardiovascular disease, as well as a predictor factor for vascular
events. They proposed treatment with statins for patients with CRP
>2 mg/l and metabolic syndrome.
The aim of the present study was to emphasize the
role of the inflammatory syndrome in psoriasis [via CRP,
fibrinogen, and erythrocyte sedimentation rate (ESR)], its
evolution after treatment and its role in psoriasis
comorbidities.
Patients and methods
This study included 194 patients with psoriasis aged
between 7 and 83 years. It has been observed that psoriasis is
frequent in middle-aged adults and elderly, in comparison to
children and young adults, where there is a lower prevalence.
The 194 patients were treated differently, according
to the severity of the disease: 51 patients with moderate psoriasis
were treated with methotrexate (group I) and 143 patients with
severe psoriasis were treated with biological therapy (group
II).
In this study, the patients with moderate and severe
psoriasis were selected defined by the extent of body surface area
(BSA) involvement. This method was chosen because it is currently
the preferred severity assessment instrument used in clinical
practice. Using BSA, the patients were divided into three
categories: Mild psoriasis (<3% BSA), moderate psoriasis (3-10%
BSA) and severe psoriasis (>10% BSA). The evolution of the
inflammatory syndrome was followed via gradation of the blood
levels for ESR, CRP and fibrinogen before and after treatment. Two
groups of patients were established. Group I included 51 patients
with moderate psoriasis treated with methotrexate 7.5 mg/week.
Group II included 143 patients with severe psoriasis treated with
biological therapy according to the specific features of each
patient: Etanerceptum (Enbrel) 50 mg x2/week was administered
subcutaneously x12 weeks, then 50 mg/week x12 weeks (n=51),
infliximab (Remicade) 5 mg/kg intravenously 0, 2, 6 week, then
every 8 weeks (n=48), adalimumab (Humira) initial 80 mg, followed
by 40 mg subcutaneously every 2 weeks, starting one week after the
initial dose (n=42), ustekinumab (Stelara) 45 mg subcutaneously 0.4
week, then every 12 weeks (n=2).
This study was approved by the Clinical Research
Ethics Committee of ‘Sf. Spiridon’ Clinical Emergency County
Hospital (Iasi, Romania) and the Ethics Committee of ‘Gr. T. Popa’
University of Medicine and Pharmacy (Iasi, Romania). Written
informed consents were obtained by the patients and/or
guardians.
Results
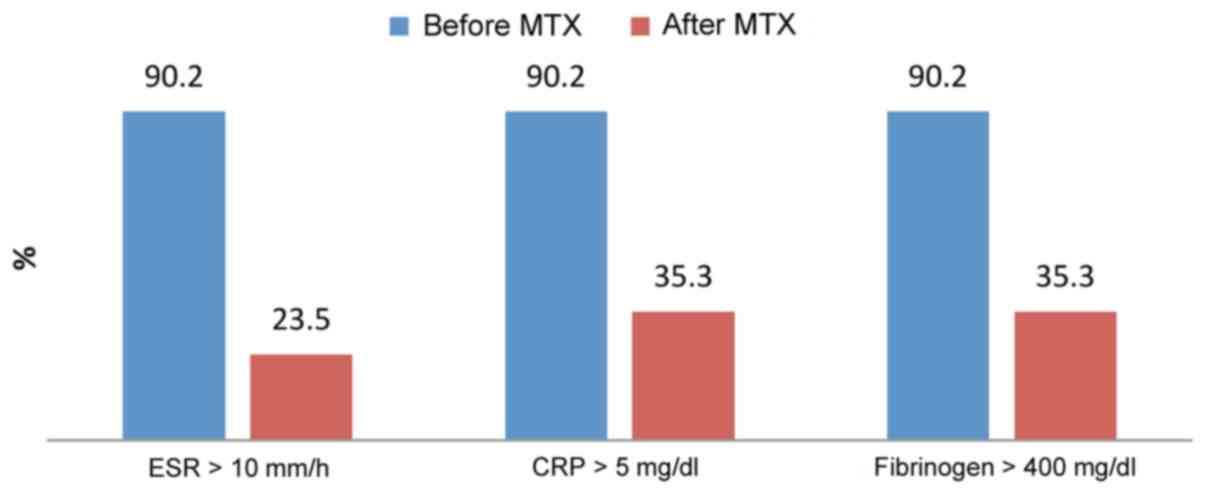
In group I, 46 out of 51 patients (90.2%) had
elevated levels of acute phase reactants before treatment. After
treatment with methotrexate 7.5 mg/week, 12 out of 51 patients
(23.5%) had elevated blood levels of ESR and 18 out of 51 patients
had elevated CRP and fibrinogen (35.3%) (Fig. 1).
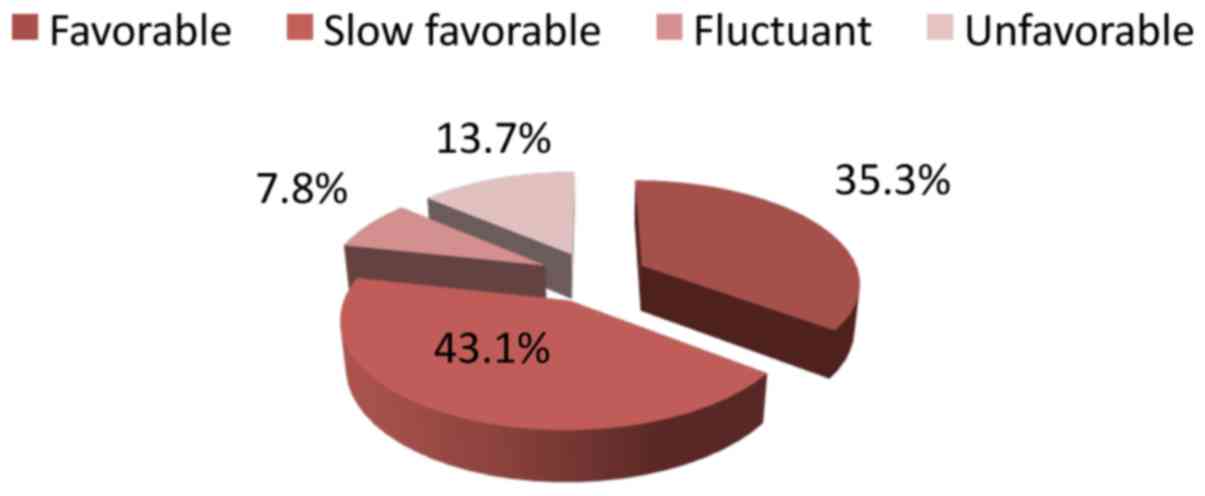
Eighteen patients out of 51 (35.3%) presented a
favorable evolution, 22 patients had a slow favorable evolution,
four patients had a fluctuant evolution (43.1%) and seven patients
had an unfavorable evolution (13.7%) (Fig. 2).
A reduction of blood levels was observed in
inflammatory markers after treatment with methotrexate and an
improvement of the well-being of patients-index measured by quality
of life questionnaire (QOL). The clinical evolution was assessed
using the Psoriasis Area Severity Index questionnaire and clinical
symptoms (pruritus and pain, which are known to negatively impact
the patients' lives).
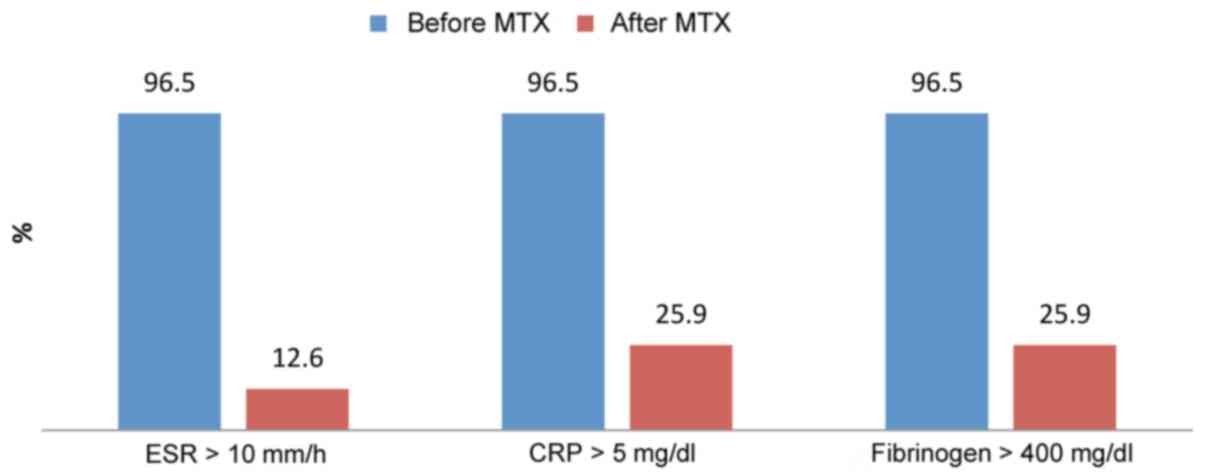
Group II contained 143 patients with severe
psoriasis treated with biological therapy. Before treatment, 138
patients out of 143 presented abnormal high range for acute phase
reactants (96.5%). After treatment, a dramatic decrease of acute
phase reactants blood levels was observed. After treatment, 18
patients out of 138 had elevated blood levels of ESR (12.6%) and 37
patients out of 138 elevated CRP and fibrinogen (25.9%) (Fig. 3).
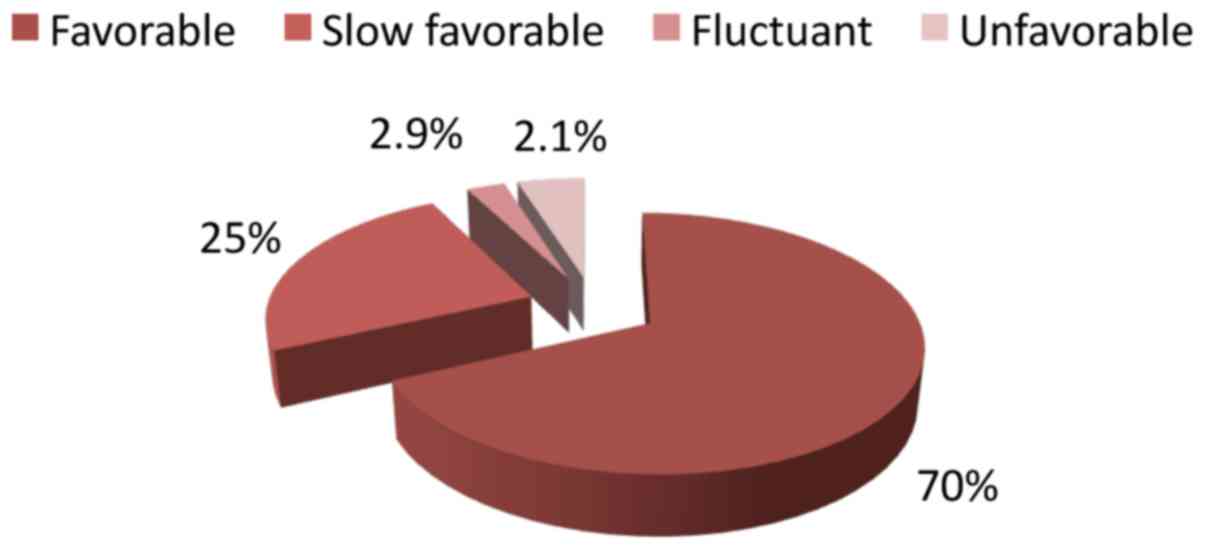
A favorable evolution was noticed in 98 patients out
of 143 (70%), a slow favorable evolution in 35 patients (25%), a
fluctuant evolution in only 3 patients (2.9%) and an unfavorable
evolution in 7 patients (2.1%) (Fig.
4).
Biological therapy showed the best results and our
results correlated with the scientific data that support the
beneficial effect of biological therapy in decreasing inflammation
in psoriasis (Table I and Figs. 5 and 6).
 | Table IStatistical analysis between the two
groups of therapy. |
Table I
Statistical analysis between the two
groups of therapy.
| | Group I (n=51) | Group II (n=143) |
|---|
| Parameters | Before treatment | After treatment | P-value for paired
samples t-test | Favorable
evolution | Before treatment | After treatment | P-value for paired
samples t-test | Favorable
evolution |
|---|
| ESR | 0.92±0.13 | 0.33±0.25 | 0.001 | 26.1%a | 1.17±1.19 | 0.59±0.16 | 0.001 | 13.0% |
| CRP | 476±20.4 | 365±54.4 | 0.174 | 39.1% | 485±38.6 | 363±76.5 | 0.001 | 26.8% |
| Fibrinogen | 20.7±2.89 | 8.67±8.14 | 0.045 | 39.1% | 18.8±3.04 | 6.10±5.05 | 0.001 | 26.8% |
Discussion
This study sought to assess blood levels for CRP,
ESR and fibrinogen before and after treatment with methotrexate and
biological therapy, the respective blood level fluctuation
according to the treatment, the association between CRP, ESR,
fibrinogen and inflammatory activity of psoriasis and the evolution
of the disease after treatment. There are different reports
illustrating that blood levels of CRP, ESR and fibrinogen are
indirectly related to the inflammatory activity of TNF-α and other
cytokines, such as IL-6 that lead to hepatic stimulation of
production for acute phase reactants such as CRP and fibrinogen
(11). Biljan et al (13) and Isha et al (14) also observed that high levels of CRP
are correlated with inflammatory activity of psoriasis.
After treatment with methotrexate and biological
therapy, an important decrease in blood levels of CRP, ESR, and
fibrinogen was observed leading to reduction of the inflammatory
activity of the psoriasis.
After treatment with methotrexate, the inflammatory
markers were reduced in 34 patients: From the initial number of 46
patients with ESR high levels to 12, and in 28 patients from the
initial number of 46 patients with CRP and fibrinogen with elevated
blood levels to 18 patients. Methotrexate inhibits dihydrofolate
reductase, leading to a blockade of purines and pyrimidines
synthesis, hence the DNA. Methotrexate inhibits AICAR
transformylase as well, leading to an accumulation of adenosine
with a strong pro inflammatory effect (15). When used long-term, methotrexate can
cause side effects, such as pancytopenia, megaloblastic anaemia,
nausea, vomiting, hepatic fibrosis, pulmonary fibrosis, allergies
and foetal malformation (16).
Biological therapy showed the best results. Eighteen
patients out of 138 for ESR and 37 patients out of 138 for CRP and
fibrinogen presented elevated blood levels after treatment.
Zamanian et al (17) reported a decline of ESR and CRP
levels after treatment with infliximab. Strober et al
(18) illustrated that patients with
psoriasis have a significant systemic inflammation trough high CRP
levels. They observed that after Etanercept treatment, the CRP
levels plummeted. This therapy is superior compared to others, but
not all patients could be treated with it because of the selection
criteria that had to be strictly followed. Kanelleas et al
(19) studied 41 psoriasis patients
treated with etanercept. They concluded that CRP, fibrinogen and
ESR in association with PASI score show inflammatory status in
psoriasis. They purposed these three markers to be used in order to
follow up the response of treatment in psoriasis patients.
Systemic therapies, such as biological or
conventional systemic therapy, allow a decrease in the inflammatory
activity of psoriasis. These results correlate with the scientific
data, which support the beneficial effect of biological therapy in
decreasing inflammation in psoriasis (20,21).
Coimbra and Santos-Silva (22)
recommend CRP as the most sensitive biomarker for severity
evaluation and monitoring of treatment response in psoriasis
patients. Gokalp (23) also
suggested that CRP is a marker not only for psoriasis activity but
also for systemic inflammation.
By evaluating the blood levels of CRP, ESR and
fibrinogen, according to the treatment the therapeutic efficacy of
the treatment was demonstrated using acute phase reactants, such as
ESR, CRP and fibrinogen.
Imaging techniques are also used to assess
non-invasive skin changes in psoriasis or high performance liquid
chromatography or cell membrane fluidity; however, this study aimed
to evaluate only the inflammatory activity (24-27).
Because psoriasis is a chronic condition, it is also
necessary to evaluate patients at the time of maximum symptoms and
consider evaluating its comorbidities.
In conclusion, this study illustrates a strong
correlation between plasmatic levels of acute phase reactants, such
as CRP, ESR, fibrinogen and the inflammatory activity of psoriasis.
By using these inflammatory markers, it was concluded that the best
results in reducing inflammatory activity were achieved by
biological therapy, which induces a decrease in blood levels of
CRP, ESR and fibrinogen. In addition, it indirectly points to a
reduction of complications associated with the inflammatory
syndrome that represents the basis of this disease. The authors
propose CRP, ESR and fibrinogen as prognostic indicators of the
unfavorable evolution of psoriasis. It is also less expensive to
evaluate these three markers in comparison with others and their
determination is easier.
Acknowledgements
Professional editing, linguistic and technical
assistance performed by Irina Radu, Individual Service Provider,
certified translator in Medicine and Pharmacy (certificate
credentials: series E no. 0048).
Funding
No funding was received.
Availability of data and materials
All data and materials supporting the results of the
present study are available in the published article.
Authors' contributions
DV, AIP and AIG contributed to the acquisition of
data. All authors contributed to the critical revision of
manuscript for important intellectual content. All authors
contributed to the acquisition of the data. CG, LGS, EPA were
responsible for the research design. CG, LGS and EPA were involved
in writing the manuscript. CG carried out the statistical analysis.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Clinical Research
Ethics Committee of ‘Sf. Spiridon’ Clinical Emergency County
Hospital (Iasi, Romania) and the Ethics Committee of ‘Gr. T. Popa’
University of Medicine and Pharmacy (Iasi, Romania). Written
informed consents were obtained by the patients and/or
guardians.
Patient consent for publication
Written informed consent was obtained from all
patients prior to publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin X and Huang T: Co-signaling molecules
in psoriasis pathogenesis: Implications for targeted therapy. Hum
Immunol. 76:95–101. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ghosh A and Panda S: Recent understanding
of the etiopathogenesis of psoriasis. Indian J Paediatr Dermatol.
18:1–8. 2017.
|
|
3
|
Caruntu C, Boda D, Dumitrascu G,
Constantin C and Neagu M: Proteomics focusing on immune markers in
psoriatic arthritis. Biomarkers Med. 9:513–528. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Căruntu C, Boda D, Musat S, Căruntu A and
Mandache E: Stress-induced mast cell activation in glabrous and
hairy skin. Mediators Inflamm. 2014(105950)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Farshchian M, Ansar A, Sobhan M and
Hoseinpoor V: C-reactive protein serum level in patients with
psoriasis before and after treatment with narrow-band ultraviolet
B. An Bras Dermatol. 91:580–583. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lowes MA, Suárez-Fariñas M and Krueger JG:
Immunology of psoriasis. Annu Rev Immunol. 32:227–255.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li R, Wang J, Wang X, Zhou J, Wang M, Ma H
and Xiao S: Increased βTrCP are associated with imiquimod-induced
psoriasis-like skin inflammation in mice via NF-κB signaling
pathway. Gene. 592:164–171. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pepys MB and Hirschfield GM: C-reactive
protein: A critical update. J Clin Invest. 111:1805–1812.
2003.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Rocha-Pereira P, Santos-Silva A, Rebelo I,
Figueiredo A, Quintanilha A and Teixeira F: The inflammatory
response in mild and in severe psoriasis. Br J Dermatol.
150:917–928. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gisondi P, Lora V, Bonauguri C, Russo A,
Lippi G and Girolomoni G: Serum chemerin is increased in patients
with chronic plaque psoriasis and normalizes following treatment
with infliximab. Br J Dermatol. 168:749–755. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qu D, Liu J, Lau CW and Huang Y: IL-6 in
diabetes and cardiovascular complications. Br J Pharmacol.
171:3595–3603. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Devaraj S, Valleggi S, Siegel D and Jialal
I: Role of C-reactive protein in contributing to increased
cardiovascular risk in metabolic syndrome. Curr Atheroscler Rep.
12:110–118. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Biljan D, Situm M, Kostović K, Batinac T
and Matisić D: Acute phase proteins in psoriasis. Coll Antropol.
33:83–86. 2009.PubMed/NCBI
|
|
14
|
Isha, Jain VK and Lal H: C-reactive
protein and uric acid levels in patients with psoriasis. Indian J
Clin Biochem. 26:309–311. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Negrei C, Ginghină O, Căruntu C, Burcea
Dragomiroiu GT, Jinescu G and Boda D: Investigation relevance of
methotrexate polyglutamates in biological systems by high
performance liquid chromatography. Rev Chim. 66:766–768. 2015.
|
|
16
|
Boda D, Negrei C, Nicolescu F and Balalau
C: Assessment of some oxidative stress parameters in methotrexate
treated psoriasis patients. Farmacia. 62:704–710. 2014.
|
|
17
|
Zamanian A, Houshang Ehsani A, Bahareh
Darvari S, Mehran G and Azizpour A: Trend of C-reactive protein
anderythrocyte sedimentation rates in psoriatic patients on
treatment of standard protocol of infliximab. GMJ. 4:8–13.
2015.
|
|
18
|
Strober B, Teller C, Yamauchi P, Miller
JL, Hooper M, Yang YC and Dann F: Effects of etanercept on
C-reactive protein levels in psoriasis and psoriatic arthritis. Br
J Dermatol. 159:322–330. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kanelleas A, Liapi C, Katoulis A,
Stavropoulos P, Avgerinou G, Georgala S, Economopoulos T,
Stavrianeas NG and Katsambas A: The role of inflammatory markers in
assessing disease severity and response to treatment in patients
with psoriasis treated with etanercept. Clin Exp Dermatol.
36:845–850. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Olteanu R, Constantin MM, Zota A,
Dorobantu DM, Constantin T, Serban ED, Balanescu P, Mihele D and
Gheuca-Solovastru L: Original clinical experience and approach to
treatment study with interleukine 12/23 inhibitor in
moderate-to-severe psoriasis patients. Farmacia. 64:918–921.
2016.
|
|
21
|
Olteanu R, Zota A and Constantin M:
Biosimilars: An update on clinical trials (review of published and
ongoing studies). Acta Dermatovenerol Croat. 25:57–66.
2017.PubMed/NCBI
|
|
22
|
Coimbra S and Santos-Silva A: Biomarkers
of psoriasis severity and therapy monitoring. World J Dermatol.
3:15–27. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gocalp H: Effect of psoriasis severity on
inflammation parameters: Controlled study. Turk Arch Dermatol
Venereol. 52:91–94. 2018.
|
|
24
|
Căruntu C, Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morphol Embryol. 55 (Suppl 3):1191–1196.
2014.PubMed/NCBI
|
|
25
|
Ilie MA, Caruntu C, Lixandru D, Tampa M,
Georgescu SR, Constantin MM, Constantin C, Neagu M, Zurac SA and
Boda D: In vivo confocal laser scanning microscopy imaging of skin
inflammation: Clinical applications and research directions. Exp
Ther Med. 17:1004–1011. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Negrei C, Caruntu C, Ginghina O,
Dragomiroiu GT, Toderescu CD and Boda D: Qualitative and
quantitative determination of methotrexate polyglutamates in
erythrocytes by high performance liquid chromatography. Rev Chim.
66:607–610. 2015.
|
|
27
|
Negrei C, Arsene AL, Toderescu CD, Boda D
and Ilie M: Acitretin treatment in psoriasis may influence the cell
membrane fluidity. Farmacia. 60:767–771. 2012.
|