Introduction
Liver cancer is one of the most common malignancies
and is the second leading cause of morbidity and mortality
worldwide. Furthermore, the incidence of liver cancer has increased
in recent years (1). Surgery, as
well as chemotherapy, radiation, targeted therapy and immunotherapy
are currently the most common treatment strategies for liver
cancer. However, these therapeutic methods, used either alone or in
combination, have numerous limitations (2). Therefore, a number of previous studies
have attempted to identify novel therapeutic agents for liver
cancer derived from Traditional Chinese Medicine (TCM) (3,4). In
China, it has been claimed that TCM has therapeutic advantages,
including suppressing liver cancer progression, reducing surgical
complications and increasing the sensitivity of cells to
chemotherapy and radiotherapy (4,5).
Furthermore, previous studies have reported that specific TCMs
improve the function of the immune system in certain organisms and
limit the detrimental effects of surgery, chemotherapy and
radiotherapy (2,5). Therefore, TCM has gradually become a
major focus for antitumor drug research and development.
Hydroxysafflor yellow A (HSYA) is a water-soluble
component of the safflower (Carthamus tinctorius), which has
been reported to exert pharmacological effects (6). Due to its potency and minimal side
effects, the clinical use of HSYA has continued to increase since
2000(7). Previous studies have
reported that safflower exerts antitumor effects (8), and it has been reported that HSYA
prevented pulmonary metastasis in liver cancer cells (9), induced apoptosis in human gastric
carcinoma cells (BGC-823 cells) (10) and inhibited the growth of
transplanted BGC-823 tumors. Another study also reported that the
effect of HSYA on tumor capillary angiogenesis may be one of the
mechanisms underlying its antineoplastic effect (11). However, to the best of our knowledge,
the effects of HSYA on autophagy regulation in liver cancer cells
have not yet been established.
Autophagy allows cells to survive under conditions
of stress, including nutritional deficiency and injury, and also
serves a critical role in maintaining cellular energy production
(12). Autophagy has a two-way
effect on tumor progression. A number of studies have reported that
autophagy can inhibit tumorigenesis (13-15);
however, when tumors form under oxygen-deficient conditions,
autophagy is able to promote tumor growth and survival (16). Additionally, >30
autophagy-associated proteins have been identified, including light
chain 3 (LC3), which was the first autophagosome protein marker to
be discovered, the Beclin 1 complex and p62 (17-19).
The expression of the Beclin 1 complex is closely associated with
the occurrence and development of various tumors (18) and p62 is an autophagic substrate
protein (19). Therefore, the three
aforementioned proteins were selected as target proteins in the
present study. Additionally, transmission electron microscopy was
performed to detect the formation of autophagosomes. The results of
the present study presented a theoretical and experimental basis
for the development of novel antitumor drugs.
Materials and methods
Cell source
The Hep-G2 cell line was provided by the Internal
Medicine Laboratory of Dongzhimen Hospital and identified by
Beijing Microread Genetics Co., Ltd. Cells were maintained in
RPMI-1640 medium (HyClone; GE Healthcare Life Sciences)
supplemented with 10% fetal bovine serum (Zhejiang Tianhang
Biotechnology Co., Ltd.) and 1% penicillin-streptomycin. Cells were
cultured at 37˚C with 5% CO2.
HSYA preparation
A working solution of HSYA
(C27H32O16; 32 µM/ml; National
Institutes for Food and Drug Control) was prepared by dissolving 20
mg HYSA standard stock in 1.02 ml PBS. The solution was then
sterilized using a 0.22-µm filter and stored at -20˚C.
MTT assay
At 80-90% confluency, Hep-G2 cells were harvested
and seeded (3x104 cells/ml) into a 96-well plate. The
cells were incubated at 37˚C with 5% CO2 for 24 h. HSYA
was added to the cells to a final concentration of 0.5, 1, 2, 4, 8
or 16 µM; the concentrations were selected based on a preliminary
study, as well as IC50 values obtained from the
literature (64.0±4.6 µM) (20). At
24 h post-transfection, 10 µl MTT solution was added to each well
and the plate was incubated for a further 4 h at 37˚C.
Subsequently, 100 µl DMSO (Sigma-Aldrich; Merck KGaA) was added to
each well and the plate was incubated for 10 min with shaking at
room temperature. Absorbance was measured at a wavelength of 570 nm
(reference wavelength, 630 nm) using a multi-functional
full-wavelength microplate reader and the optimal inhibitory
concentration was recorded. 50 mM chloroquine (CQ) solution was
prepared by dissolving 0.016 g CQ (cat. no. KH-0005; Key Organics)
in 100 µl DMSO and 900 µl RPMI-1640 medium (supplemented with 10%
FBS and 1% penicillin streptomycin). The solution was then
sterilized using a 0.22-µm filter and stored at 4˚C and diluted to
10 µM as previously described (21-24).
Observation of cell morphology
Hep-G2 cells (3x104 cells/ml) were seeded
into 6-well plates and incubated for 24 h at 37˚C. Subsequently,
the RPMI-1640 medium was discarded, 2 µM HSYA was added and the
plates were incubated for 24 h at 37˚C. Cells were photographed in
three randomly selected fields under an inverted microscope (CKX41;
Olympus Corporation) at x400 magnification.
Transmission electron microscopy
Hep-G2 cells (3x104 cells/ml) were plated in
six-well plates and incubated for 24 h at 37˚C. Subsequently, 2 µM
HSYA was added and the cells were incubated for a further 6 h at
37˚C. Cells were harvested, fixed with 2.5% glutaraldehyde for 12 h
at 4˚C and washed with PBS three times for 5 min each time. The
cells were further treated with 1% osmium tetroxide for 3 h at room
temperature. Subsequently, cell samples were dehydrated using an
ascending ethanol and acetone series, and embedded using 1.5%
DMP-30 and epoxy resin, gradient polymerization was performed on
the cell samples using the following conditions: 35˚C for 12 h,
45˚C for 12 h and 60˚C for 24 h. Subsequently, the samples were
sliced into 70-nm thick sections using a microtome prior to
staining with uranyl acetate and lead citrate for 2 h at room
temperature. The samples were observed using a Tecnai™ Spirit
transmission electron microscope (magnification, x18,500 and
x30,000).
Immunofluorescence detection
Hep-G2 cells were treated with 2 µM HSYA and
incubated for 6 h at 37˚C. Subs equently, the cells were harvested,
fixed in 4% paraformaldehyde for 15 min at room temperature, and
washed in 0.1% Triton X-100 at room temperature prior to blocking
[5% FBS; 1% FGS (cat. no. SL038; Beijing Solarbio Science &
Technology Co., Ltd. and 0.3% Triton X-100 (cat. no. P0096;
Beyotime Institute of Biotechnology)] at room temperature for 1 h.
Cells were incubated with an anti-LC3A/B primary antibody (cat. no.
4108; 1:50; Cell Signaling Technology, Inc.) at 4˚C overnight,
followed by a second incubation step with a goat anti-rabbit
secondary antibody (cat. no. A-1108; 1:200; Thermo Fisher
Scientific, Inc.) IgG (H+L) at room temperature for 2 h.
Subsequently, the samples were sealed with a water-soluble mounting
media containing DAPI and three fields of view were observed under
an inverted fluorescence microscope (magnification, x400; Olympus
Corporation).
Western blotting
Total protein was extracted from Hep-G2 cells using
RIPA buffer (Applygen Technology Co., Ltd.) supplemented with 2%
protease inhibitor and 1% protein phosphatase inhibitor according
to the manufacturer's instructions. Total protein was quantified
using a bicinchoninic acid assay and 20 µg of protein/lane were
separated via SDS-PAGE (7, 10 or 13% due to the different molecular
weights of the samples). Subsequently, separated proteins were
transferred to PVDF membranes and blocked with 5% non-fat milk at
room temperature for 1 h. The membranes were incubated at 4˚C
overnight with primary antibodies targeted against the following:
LC3A/B (1:500), Beclin 1 (cat. no. 11306; 1:5,000; ProteinTech
Group, Inc.), p62 (cat. no. 18420; 1:2,000; ProteinTech Group,
Inc.), ERK1/2 (cat. no. AF0155; 1:2,500; Affinity Biosciences),
phosphorylated (p)-ERK1/2 (cat. no. AF1015; 1:1,500; Affinity
Biosciences), GAPDH (cat. no. HRP-6004; 1:50,000; ProteinTech
Group, Inc.) and β-actin (cat. no. HRP-6008; 1:20,000; ProteinTech
Group, Inc.). After washing with TBS-Tween, the membranes were
incubated with a goat anti-mouse (cat. no. ZB-2305; 1:20,000;
Zhongshan Golden Bridge Bio-Technology) immunoglobulin G (IgG)
(H+L) or goat anti-rabbit (cat. no. CW0103S; 1:5,000; Kangwei
Century) IgG (H+L) secondary antibody for 1 h at room temperature.
Protein bands were visualized using the horseradish
peroxidiase-conjugated ECL Luminol substrate (Applygen
Technologies, Inc.). Protein expression was quantified using
AlphaView software (version 3.4; Alpha Innotech gel imager) with
GAPDH or β-actin as the loading control. Western blot experiments
for each protein were repeated at least three times.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical differences were calculated using the Student' t-test,
one-way ANOVA followed by Dunnett's post hoc test or two-way ANOVA
followed by Sidak's multiple comparison test. Statistical analyses
were performed using SPSS software (version 20.0; IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
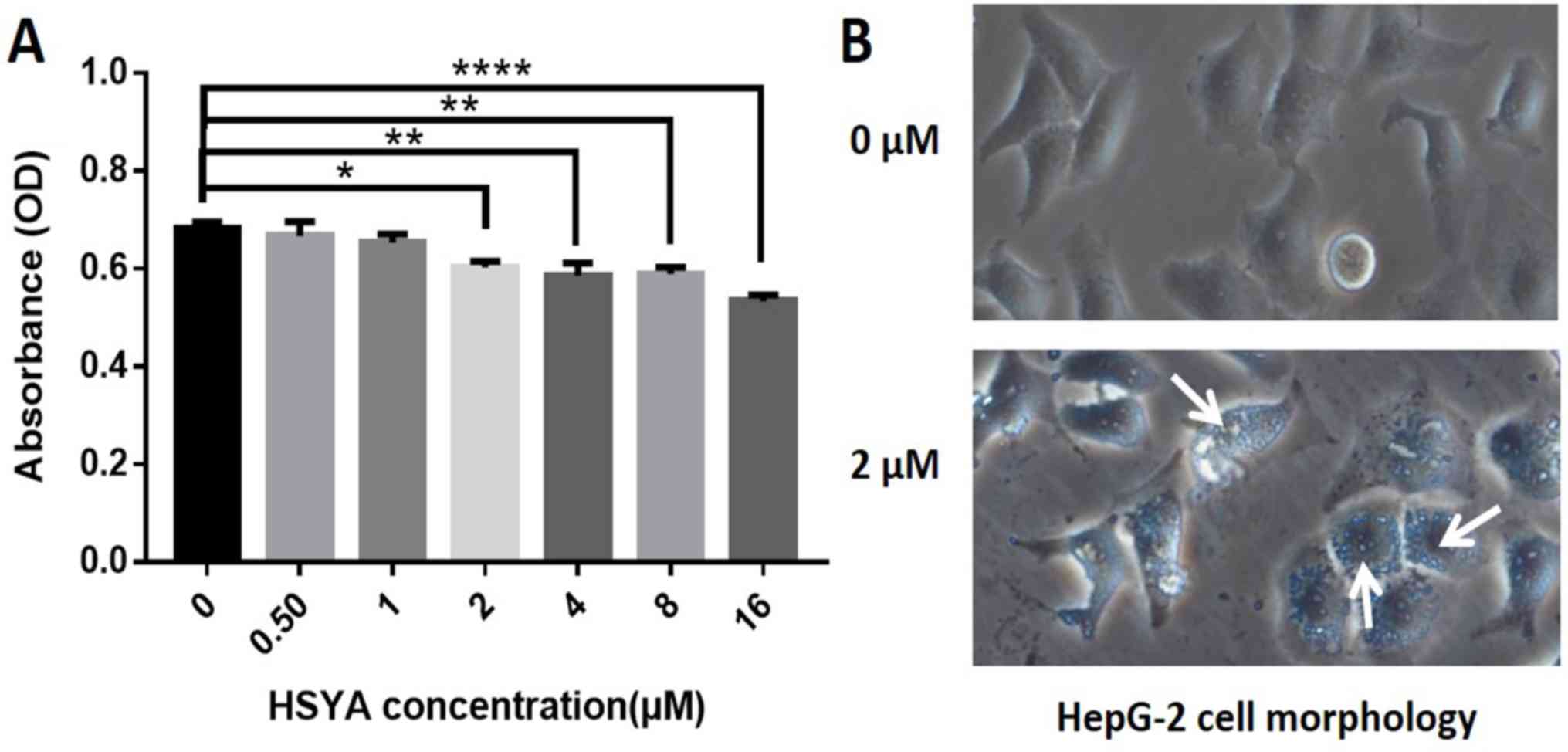
HSYA reduces the viability of Hep-G2
cells
Hep-G2 cells were treated with six different
concentrations of HSYA and cell viability was assessed using an MTT
assay. The results revealed that cell viability was decreased
following HSYA treatment compared with the control group, in a dose
dependent manner (Fig. 1A). The 2-16
µM HSYA groups exhibited a significant decrease in cell viability
compared with the control group (Fig.
1A). Therefore, 2 µM HSYA was selected for subsequent
experimentation based on the minimum effective dose.
Hep-G2 cells in the control group were
morphologically confirmed to be in good condition, displaying
fullness, clear cell boundaries and close adherence (Fig. 1B). After incubation for 24 h with 2
µM HSYA, Hep-G2 cells exhibited a large number of vacuoles and
cellular debris, suggesting that autophagy had occurred (Fig. 1B).
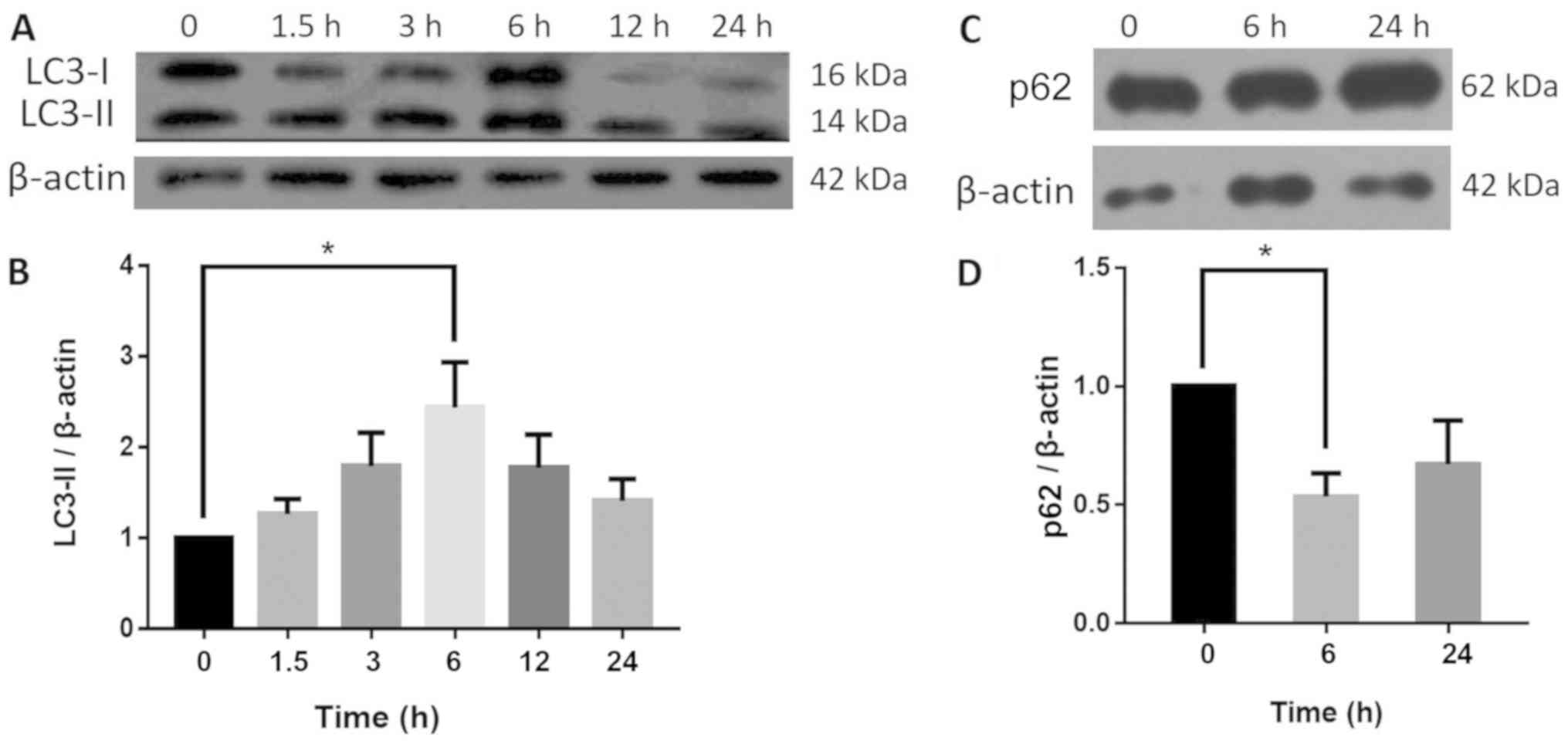
HSYA affects the expression of
autophagy-associated proteins
To identify autophagy, the autophagy marker protein,
LC3 and its substrate protein, p62, were detected using western
blotting. The results revealed that the LC3-II content increased in
a time-dependent manner in the HSYA cells, peaking at the 6-h time
point to a level significantly higher compared with the control
group. Furthermore, LC3-II content decreased between 6 and 24 h in
HSYA groups. By contrast, the protein expression of p62 was
significantly reduced by 46% at 6 h, and there was no significant
difference at 24 h compared with the control group (Fig. 2).
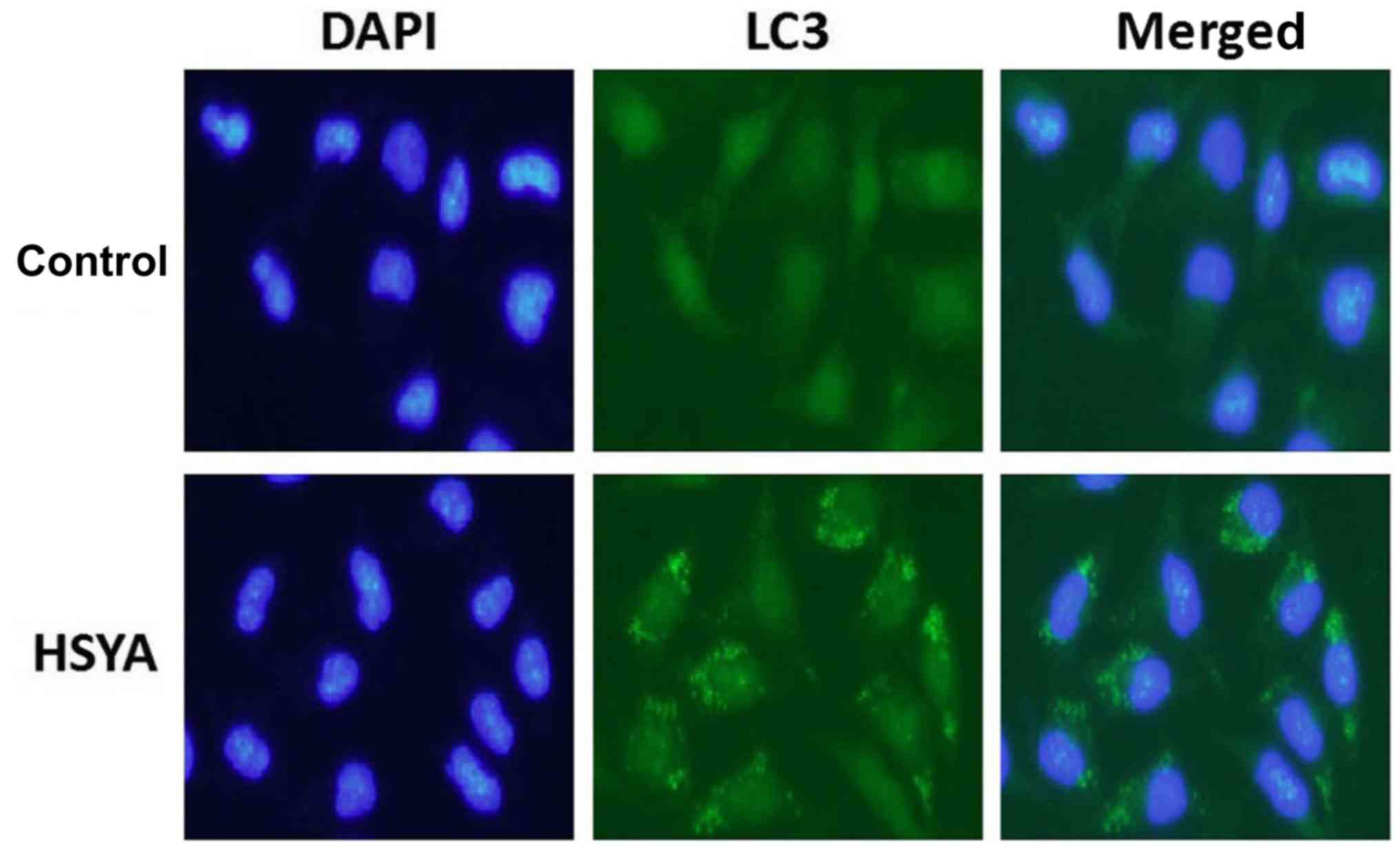
HSYA induces autophagy in Hep-G2
cells
Following the addition of 2 µM HSYA for 6 h, the
distribution of LC3 protein in Hep-G2 cells was observed by
immunofluorescence staining. The nucleus exhibited blue coloration
and LC3 protein expression was indicated in green (Fig. 3). The results suggested that the
green fluorescence in the control group was diffusely distributed;
however, a large number of green clusters were visible within the
cells of the HYSA group, indicating an increase in LC3 protein
expression. At 6 h, the increase in autophagy marker protein LC3
and decrease in autophagy substrate p62 expression further
suggested that HSYA-induced autophagy had occurred (Fig. 2B and D).
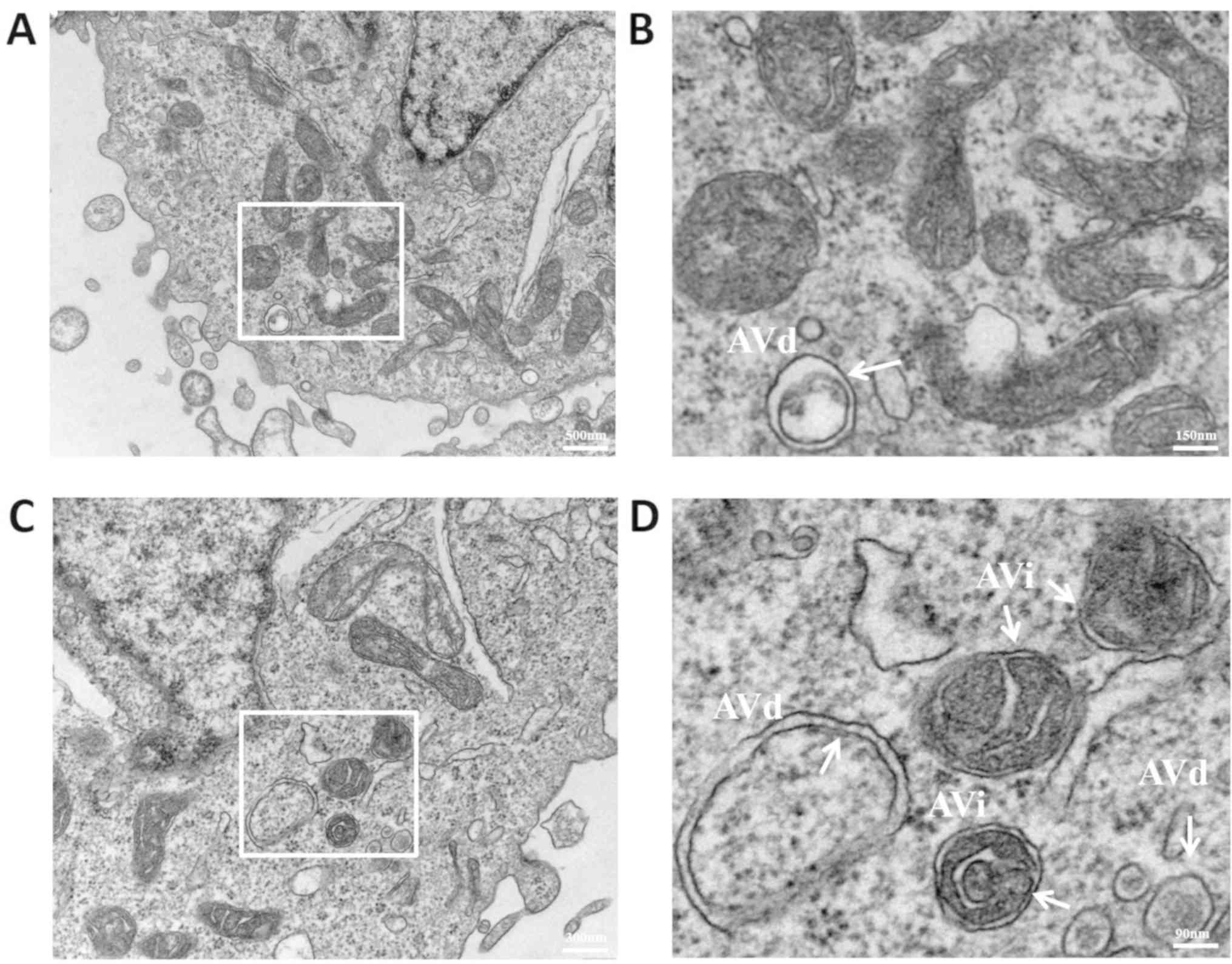
To further investigate autophagy, the cell
morphology assays were visualized under a transmission electron
microscope. Due to the higher resolution of transmission electron
microscopy compared with fluorescence microscopy, the
microstructures of pre-autophagosomes, autophagosomes and
autolysosomes that were present during autophagy formation were
clear. When autophagy begins, the phagocytic vacuoles of the
bowl-shaped bilayer membrane structure form and continuously extend
into the cytoplasm, enclosing the organelles to form autophagosomes
with a vesicular structure and the encapsulated organelles are
subsequently degraded (25). In the
present study, the early (AVi) and late (AVd) autophagosomes were
broadly classified depending on whether the envelope structure in
the vesicles remained intact. The two structures distinguished the
different stages of autophagy, to determine whether autophagy had
occurred. After treatment with 2 µM HSYA for 6 h, Hep-G2 cells were
observed under a transmission electron microscope. Part of the
bilayer membrane structure of AVi enveloped the contents prior to
degradation (Fig. 4A). At the same
time, autophagosomes were fused with lysosomes to form
autolysosomes and the encapsulated contents were degraded to form a
single-layer membrane structure, the AVd. The control group
exhibited fewer autophagosomes compared with the HSYA group
(Fig. 4A and B). Furthermore, AVi and AVd structures were
increased in the HYSA group, indicating that autophagy had occurred
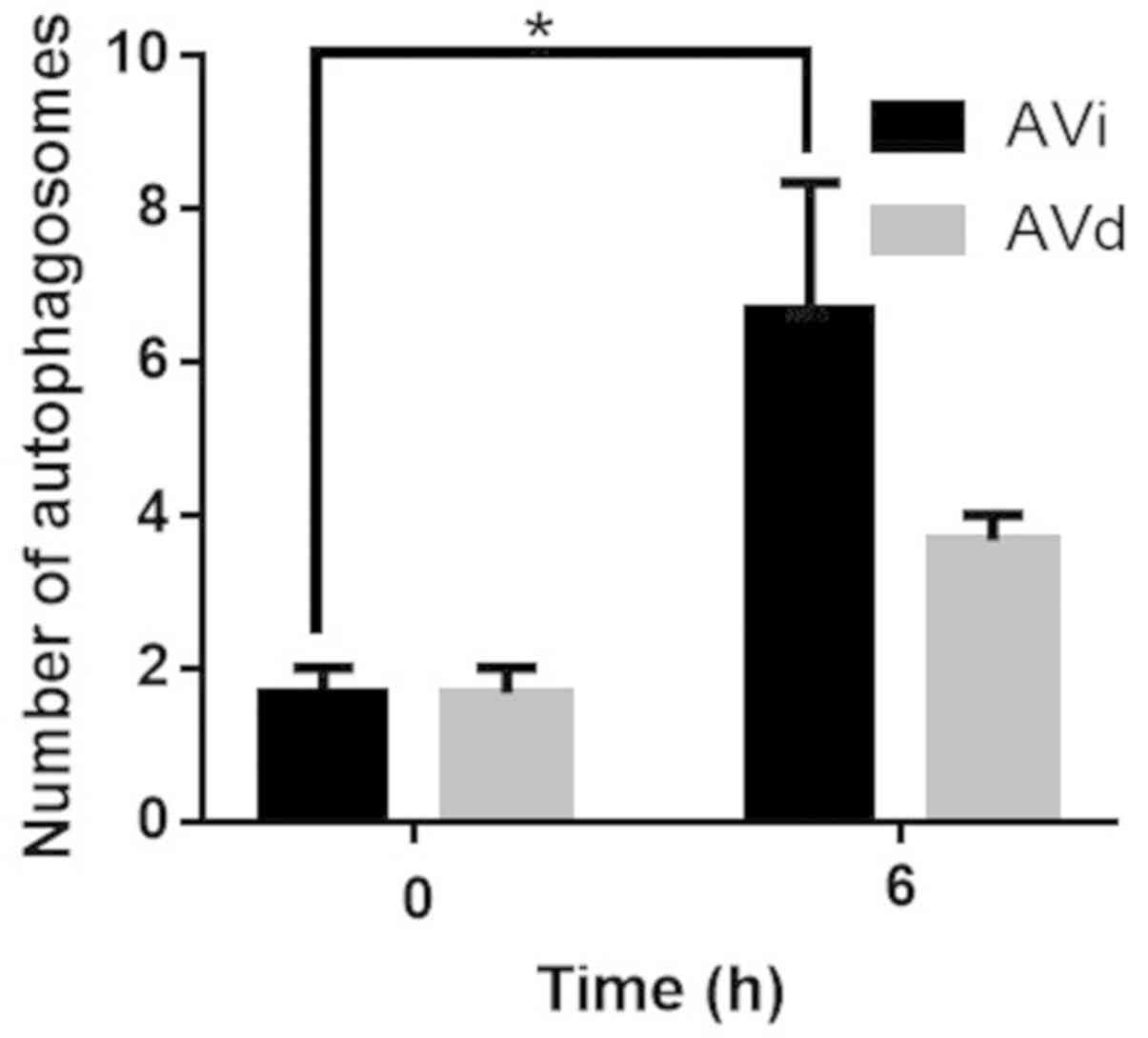
(Fig. 4C and D). Following quantitative analysis, the
degree of autophagy in the 6 h group, regardless of AVi or AVd
status, was higher compared with the 0 h group. The AVi status of
the 6 h group was significantly increased compared with the 0 h
group (Fig. 5). Collectively, the
results suggested that 2 µM HSYA induced autophagy in Hep-G2 cells,
most significantly at the 6 h time point.
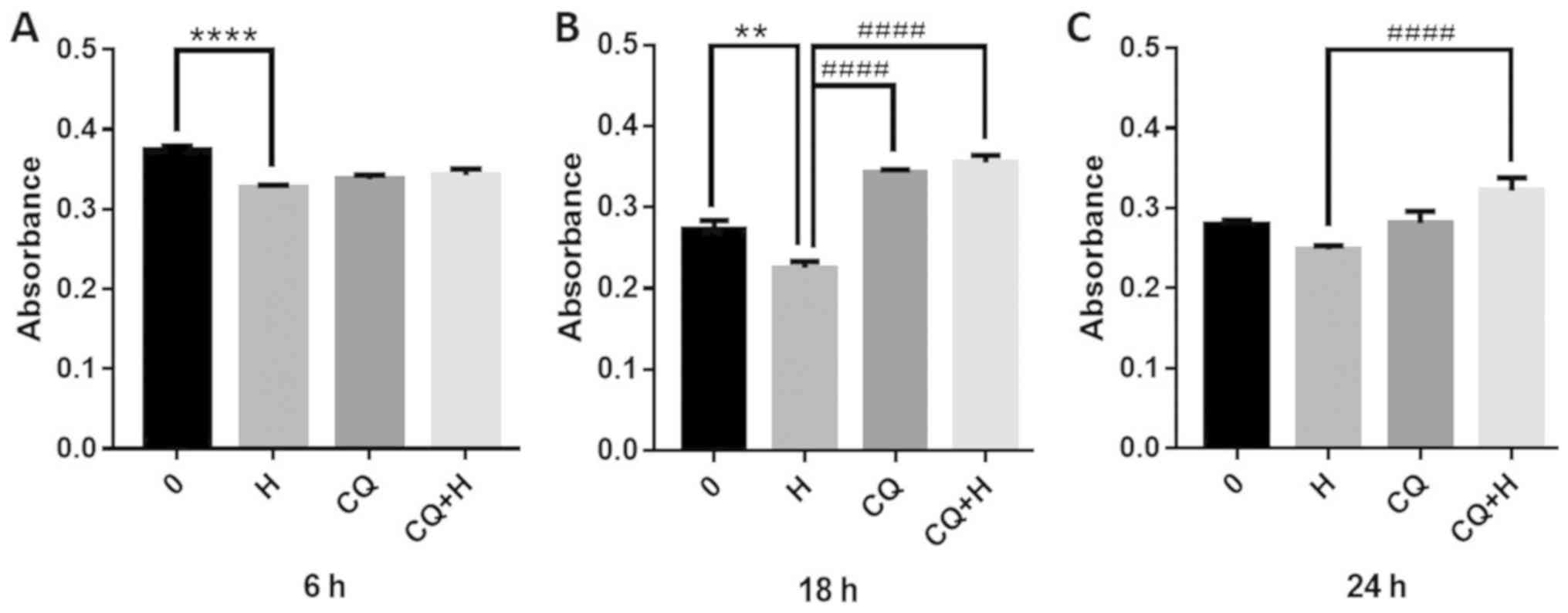
HSYA and CQ increase the viability of
Hep-G2 cells
After observing HSYA-induced autophagy, the effects
of the 10 µM autophagy inhibitor CQ on the viability of Hep-G2
cells were assessed. CQ was combined with HSYA and alterations in
hepatoma cell viability were determined using an MTT assay. The
results revealed that HSYA (2 µM for 6 h) treatment reduced
hepatoma cell viability, with a significant decrease of 13%
compared with the control group (Fig.
6A). However, the combination of CQ and HSYA increased cell
viability at 18 and 24 h, compared with the use of either reagent
alone (Fig. 6B and C). At 18 h, the viability of the
combination group was significantly increased by 58% compared with
the HSYA group (Fig. 6C).
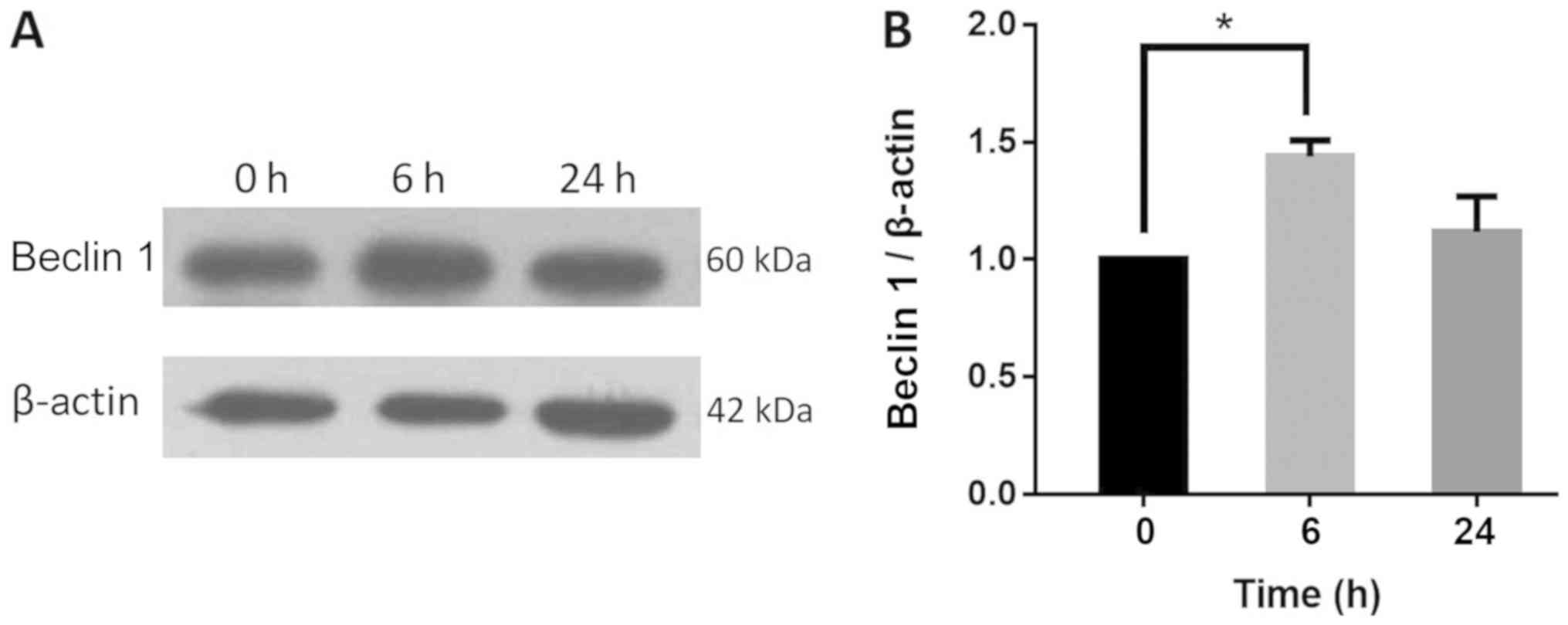
HSYA affects autophagy by regulating
the expression of Beclin 1 and ERK
To determine the possible mechanisms of autophagy
regulation, western blot analysis was performed to detect the
expression of the autophagy regulator proteins Beclin 1 and ERK in
Hep-G2 cells. Beclin 1 is an important protein involved in the
regulation of autophagy and the activation of ERK1/2 can be
detected in numerous autophagic processes (26,27). The
protein expression of Beclin 1 increased significantly by 44% in
Hep-G2 cells following the addition of 2 µM HSYA for 6 h. The
levels of Beclin 1 protein expression increased slightly at 24 h
compared with the control group (Fig.
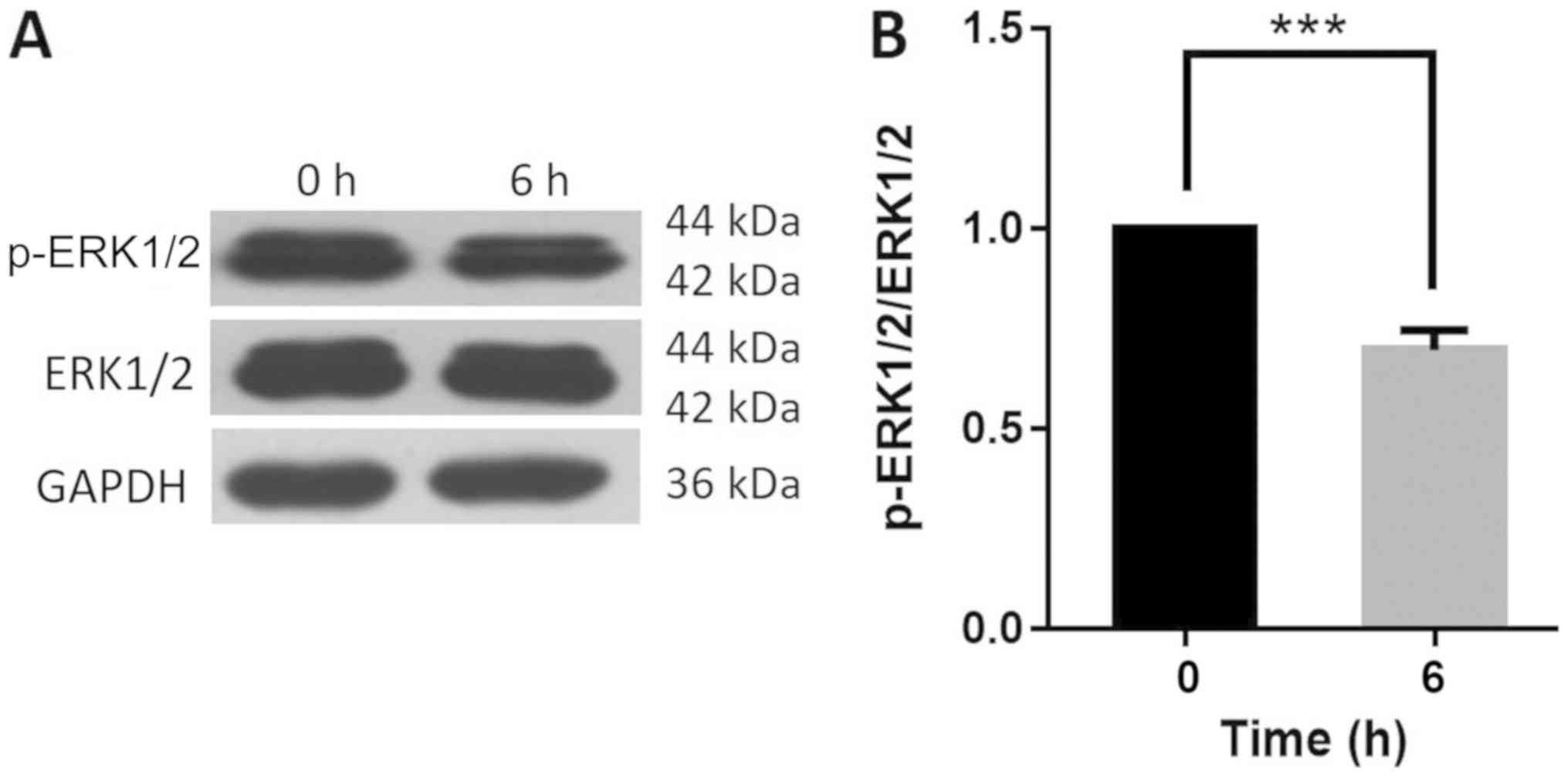
7A and B). Additionally, the
ratio of p-ERK1/2 to total ERK1/2 decreased significantly by 31% in
the HSYA group at 6 h compared with the control group (Fig. 8A and B). The results indicated that HSYA
influenced autophagy by regulating Beclin 1 and ERK protein
expression.
Discussion
Liver cancer is the second most common cause of
cancer-associated death worldwide, and the incidence and mortality
rates have steadily increased (28).
At present, surgery and western pharmaceutical drugs are the two
primary strategies for treating tumors. However, unwanted side
effects and drug resistance have led to increased research into
novel multi-faceted and multi-targeting antitumor drugs, including
a focus on TCM (4,5).
TCM adjuvant treatments may have significant
advantages in improving the quality of life and prolonging the
survival time of patients with liver cancer compared with standard
treatment strategies (2). HSYA is
one of the primary water-soluble, active ingredients of the TCM
safflower. HSYA has been reported to promote apoptosis in abnormal
human umbilical vein endothelial cells (29) and to suppress the inflammatory
responses of BV2 microglial cells after oxygen-glucose deprivation
(30). In the present study, an MTT
assay revealed that HSYA reduced the viability of liver cancer
cells and a large number of vacuoles and a small amount of cell
debris were microscopically observed in HSYA-treated cells,
suggesting that HSYA induced autophagy. A previous study had
determined that the IC50 value for HSYA was 64.0±4.6 µM
(20); therefore, a final
concentration of 2 µM HSYA was used in the present study, and the
effects on cellular viability were considered to be the result of
the pharmacological properties, rather than toxic side effects.
Autophagy is referred to as cellular self-digestion.
The autophagic membrane envelops areas of the cytoplasm to form an
autophagosome, encompassing organelles and proteins for degradation
(25). The autophagosome then fuses
with a lysosome to form an autolysosome, which subsequently
degrades the encapsulated contents, the products of degradation
provide raw materials and are a means of recycling existing
cellular material (25). AVi
structures contain morphologically intact cytoplasm, which is
comparable to the surrounding cytoplasm (25). By contrast, AVd structures contain
material that can still be recognized as cytoplasmic. However, the
ribosomes are partially degraded, resulting in a more electron
dense cytoplasm compared with the surroundings. Only vacuoles
containing cytoplasmic material can be considered autophagic
(25). In addition, an autophagy
landmark LC3 protein test is required to determine whether
autophagy has occurred (17).
LC3 was the first autophagosome marker protein to be
identified in LC3 synthesis (17)
and exists in a soluble form (LC3-I) in the cytosol. When autophagy
occurs, LC3-I is converted to LC3-II with the help of autophagy
complexes. LC3-II is an autophagosome marker with membrane-binding
ability, which is also localized to autophagic membranes and its
expression is related to the number of autophagosomes (31). Theoretically, increases in the
LC3-II/LC3-I ratio indicate the occurrence of autophagy (32). However, LC3-II and LC3-I have
different sensitivity to antibodies thus the LC3-II/LC3-I ratio was
different than expected. Therefore, to determine the degree of
autophagic viability, it is necessary to observe the entire
process, including the degradation of the autophagy substrate
p62(32).
In the present study, treatment of Hep-G2 cells with
2 µM HSYA for 6 h increased the LC3-II content and decreased the
p62 content. In the HYSA group, immunofluorescence detection
suggested that the LC3 protein aggregated within the cells at 6 h
and electron microscopy revealed that AVi and AVd structures were
present, confirming that autophagy had occurred. In summary, the
results demonstrated that HSYA induced autophagy in Hep-G2 cells
and that autophagy occurred at the highest rate at the 6 h time
point.
He et al (33)
reported that the use of a chalcone derivative, Chal-24, induced
autophagy and promoted cancer cell apoptosis, indicating that
autophagic induction may serve as a novel treatment strategy for
tumors. In the present study, 2 µM HSYA significantly reduced cell
viability and CQ, a common autophagy inhibitor, increased cell
viability. CQ + HSYA treatment also increased liver cancer cell
viability compared with control cells. The results suggested that
CQ inhibited HSYA-induced autophagy and increased cell viability.
Furthermore, it could be suggested that HSYA reduced the viability
of cells by inducing autophagy, further suggesting that autophagy
inducers may serve as a potential treatment strategy to enhance the
effects of HSYA in liver cancer.
Beclin 1 is an important protein involved in the
regulation of autophagy (34), which
promotes autophagy by localizing to autophagic precursors (35). In the present study, the addition of
2 µM HSYA to Hep-G2 cells significantly increased Beclin 1 protein
expression levels at the 6 h time point, indicating that autophagy
was induced by regulating Beclin 1 in liver cancer cells.
ERK1/2 activation can be detected in various
autophagic processes and can influence the proliferation of tumor
cells via autophagy (26,27). p-ERK1/2 proteins directly
phosphorylate the S664 residue of the tuberin protein, thereby
inhibiting autophagy (36).
Furthermore, upregulating the p-ERK/ERK ratio can inhibit autophagy
in the hippocampus of mice (37).
Therefore, it was hypothesized that p-ERK/ERK was associated with
autophagy. In the present study, HSYA significantly decreased the
p-ERK/ERK content of Hep-G2 cells. The results indicated that HSYA
significantly inhibited the phosphorylation of ERK1/2 and
subsequently induced autophagy and inhibited the viability of liver
cancer cells.
During the process of autophagosome-to-autolysosome
formation, the Beclin 1 complex and ERK1/2 serve an important role
in regulating autophagy (38). The
results of the present study indicated an increase in LC3-II and a
decrease in p62 content following HYSA treatment of Hep-G2 cells,
suggesting an induction of autophagy. Therefore, the present study
suggested that the Beclin 1 complex may promote the occurrence of
autophagy; a process that may be indirectly inhibited by
ERK1/2.
In combination with previous research, the results
of the present study indicated that HSYA had various effects on
liver and gastric cancer cells, including preventing metastasis,
inducing apoptosis and reducing viability (10). The results of the current study also
suggested that HSYA induced autophagy by increasing the expression
of Beclin 1 and inhibiting the phosphorylation of ERK in human
liver cancer cells, therefore indicating that HSYA exhibited
antitumor viability. To conclude, HSYA may serve as a potential
therapeutic for liver cancer by enhancing autophagy.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 31500640, 30572436
and 31800652).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ and PW conceived and designed the study. ZC, LL
and YM performed the experiments. YL, SZ and JW analyzed the data.
SW, RD and MX provided reagents and performed immunofluorescence
and microscopy experiments. ZC and LL wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang
Z and Han J: The advantages of using traditional Chinese medicine
as an adjunctive therapy in the whole course of cancer treatment
instead of only terminal stage of cancer. Biosci Trends. 9:16–34.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gao X, Wang Y, Li Y, Wang Y, Yan M, Sun H,
Chen S and Pan X: Huganpian, a traditional Chinese medicine,
inhibits liver cancer growth in vitro and in vivo by inducing
autophagy and cell cycle arrest. Biomed Pharmacother.
120(109469)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liao X, Bu Y and Jia QA: Traditional
Chinese medicine as supportive care for the management of liver
cancer: Past, present, and future. Genes Dis.
2019.doi.org/10.1016/j.gendis.2019.10.016. View Article : Google Scholar
|
|
5
|
Xie G, Cui Z, Peng K, Zhou X, Xia Q and Xu
D: Aidi injection, a traditional Chinese medicine injection, could
be used as an adjuvant drug to improve quality of life of cancer
patients receiving chemotherapy: A propensity score matching
analysis. Integr Cancer Ther. 18(1534735418810799)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jin M, Xue CJ, Wang Y, Dong F, Peng YY,
Zhang YD, Zang BX and Tan L: Protective effect of hydroxysafflor
yellow a on inflammatory injury in chronic obstructive pulmonary
disease rats. Chin J Integr Med. 25:750–756. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fan L, Pu R, Zhao HY, Liu X, Ma C, Wang BR
and Guo DA: Stability and degradation of hydroxysafflor yellow A
and anhydrosafflor yellow B in the safflower injection studied by
HPLC-DAD-ESI-MSn. J Chin Pharm Sci. 20:47–56. 2011. View Article : Google Scholar
|
|
8
|
Ao H, Feng W and Peng C: Hydroxysafflor
yellow A: A promising therapeutic agent for a broad spectrum of
diseases. Evid Based Complement Alternat Med.
2018(8259280)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ma L, Liu L, Ma Y, Xie H, Yu X, Wang X,
Fan A, Ge D, Xu Y, Zhang Q and Song C: The role of
E-cadherin/β-catenin in hydroxysafflor yellow A inhibiting
adhesion, invasion, migration and lung metastasis of hepatoma
cells. Biol Pharm Bull. 40:1706–1715. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu L, Si N, Ma Y, Ge D, Yu X, Fan A, Wang
X, Hu J, Wei P, Ma L, et al: Hydroxysafflor-yellow A induces human
gastric carcinoma BGC-823 cell apoptosis by activating peroxisome
proliferator-activated receptor gamma (PPARγ). Med Sci Monit.
24:803–811. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xi SY, Zhang Q, Liu CY, Xie H, Yue LF and
Gao XM: Effects of hydroxy safflower yellow-A on tumor capillary
angiogenesis in transplanted human gastric adenocarcinoma BGC-823
tumors in nude mice. J Tradit Chin Med. 32:243–248. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Levine B and Klionsky DJ: Development by
self-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu W, Zeng X, Yin Y, Li C, Yang W, Wan W,
Shi L, Wang G, Tao K and Zhang P: Targeting the WEE1 kinase
strengthens the antitumor activity of imatinib via promoting KIT
autophagic degradation in gastrointestinal stromal tumors. Gastric
Cancer. 23:39–51. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dent P, Booth L and Poklepovic A:
Metabolism of histone deacetylase proteins opsonizes tumor cells to
checkpoint inhibitory immunotherapies. Immunometabolism.
2(e200002)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Deng X, Guan W, Qing X, Yang W, Que Y, Tan
L, Liang H, Zhang Z, Wang B, Liu X, et al: Ultrafast
low-temperature photothermal therapy activates autophagy and
recovers immunity for efficient antitumor treatment. ACS Appl Mater
Interfaces. 12:4265–4275. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rautou PE, Mansouri A, Lebrec D, Durand F,
Valla D and Moreau R: Autophagy in liver diseases. J Hepatol.
53:1123–1134. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tanida I, Ueno T and Kominami E: LC3
conjugation system in mammalian autophagy. Int J Biochem Cell Biol.
36:2503–2518. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu J, Lin Y, Yang H, Deng Q, Chen G and
He J: The expression of p33(ING1), p53, and autophagy-related gene
Beclin1 in patients with non-small cell lung cancer. Tumour Biol.
32:1113–1121. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Masuda S, Mizukami S, Eguchi A, Ichikawa
R, Nakamura M, Nakamura K, Okada R, Tanaka T, Shibutani M and
Yoshida T: Immunohistochemical expression of autophagosome markers
LC3 and p62 in preneoplastic liver foci in high fat diet-fed rats.
J Toxicol Sci. 44:565–574. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Song H, Wang D, Li J, Yang Y, Mu X and Bai
X: Hydroxysafflor yellow A inhibits proliferation and migration of
human hepatocellular carcinoma cells and promotes apoptosis via
PI3K pathway. Tumor. 38:830–839. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang C, Jia XJ, Wang K, Bao JL, Li P,
Chen MW, Wan JB, Su HX, Mei ZN and He CW: Polyphyllin VII induces
an autophagic cell death by activation of the JNK pathway and
inhibition of PI3K/AKT/mTOR pathway in hepG2 cells. PLoS One.
11(e0147405)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li T, Tang ZH, Xu WS, Wu GS, Wang YF,
Chang LL, Zhu H, Chen XP, Wang YT, Chen Y and Lu JJ: Platycodin D
triggers autophagy through activation of extracellular
signal-regulated kinase in hepatocellular carcinoma HepG2 cells.
Eur J Pharmacol. 749:81–88. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li T, Wen L and Cheng B: Cordycepin
alleviates hepatic lipid accumulation by inducing protective
autophagy via PKA/mTOR pathway. Biochem Biophys Res Commun.
516:632–638. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lu Y, Zhang R, Liu S, Zhao Y, Gao J and
Zhu L: ZT-25, a new vacuolar H(+)-ATPase inhibitor, induces
apoptosis and protective autophagy through ROS generation in HepG2
cells. Eur J Pharmacol. 771:130–138. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Eskelinen EL: Fine structure of the
autophagosome. Methods Mol Biol. 445:11–28. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang M, Qiu S and Qin J: Baicalein induced
apoptosis and autophagy of undifferentiated thyroid cancer cells by
the ERK/PI3K/Akt pathway. Am J Transl Res. 11:3341–3352.
2019.PubMed/NCBI
|
|
27
|
Li HH, Song XX, Liu B and Yang WP:
UNBS5162 as a novel naphthalimide holds efficacy in human gastric
carcinoma cell behaviors mediated by AKT/ERK signaling pathway.
Drug Dev Ind Pharm. 45:1306–1312. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sia D, Villanueva A, Friedman SL and
Llovet JM: Liver cancer cell of origin, molecular class, and
effects on patient prognosis. Gastroenterology. 152:745–761.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Si N, Wang J, Xu Y, Liu L, Wang X, Sun H,
Lin Z, Wang X, Liu L, Zhang Q, et al: Inductive effect of hydroxyl
safflower yellow-A on apoptosis in abnormal HUVEC via the
mitochondrial pathway. J Trad Chin Med Sci. 2:25–31.
2015.PubMed/NCBI
|
|
30
|
Li J, Zhang S, Lu M, Chen Z, Chen C, Han
L, Zhang M and Xu Y: Hydroxysafflor yellow A suppresses
inflammatory responses of BV2 microglia after oxygen-glucose
deprivation. Neurosci Lett. 535:51–56. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hale AN, Ledbetter DJ and Gawriluk TR and
Rucker EB 3rd: Autophagy: Regulation and role in development.
Autophagy. 9:951–972. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang Y, He P and Li N: The antitumor
potential of extract of the oak bracket medicinal mushroom inonotus
baumii in SMMC-7721 tumor cells. Evid Based Complement Alternat
Med. 2019(1242784)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
He W, Wang Q, Srinivasan B, Xu J, Padilla
MT, Li Z, Wang X, Liu Y, Gou X, Shen HM, et al: A JNK-mediated
autophagy pathway that triggers c-IAP degradation and necroptosis
for anticancer chemotherapy. Oncogene. 33:3004–3013.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sutton MN, Huang GY, Liang X, Sharma R,
Reger AS, Mao W, Pang L, Rask PJ, Lee K, Gray JP, et al:
DIRAS3-Derived peptide inhibits autophagy in ovarian cancer cells
by binding to Beclin1. Cancers (Basel). 11(E557)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ma L, Chen Z, Erdjument-Bromage H, Tempst
P and Pandolfi PP: Phosphorylation and functional inactivation of
TSC2 by Erk implications for tuberous sclerosis and cancer
pathogenesis. Cell. 121:179–193. 2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhu J, Liao S, Zhou L and Wan L:
Tanshinone IIA attenuates Aβ25-35-induced spatial memory
impairment via upregulating receptors for activated C kinase1 and
inhibiting autophagy in hippocampus. J Pharm Pharmacol. 69:191–201.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pan H, Wang Y, Na K, Wang Y, Wang L, Li Z,
Guo C, Guo D and Wang X: Autophagic flux disruption contributes to
Ganoderma lucidum polysaccharide-induced apoptosis in human
colorectal cancer cells via MAPK/ERK activation. Cell Death Dis.
10(456)2019.PubMed/NCBI View Article : Google Scholar
|