Introduction
Vitiligo is a common congenital or acquired
disfiguring skin disorder related to melanocyte destruction. The
incidence of vitiligo is 0.5-1.0% worldwide (1,2), and
causes the skin to lose its natural pigmentation (3). The incidence of vitiligo is not related
to age, sex, skin type or ethnicity (4). The endoplasmic reticulum (ER) is an
important organelle that is mainly responsible for protein
biosynthesis, folding and the maintenance of cell homeostasis
(5). However, under certain
physiological and pathological conditions, protein folding may be
severely impaired, causing ER stress (6). As a result, a specific ER stress
response pathway will be activated, which can lead to apoptosis
(7). Tyrosinase is a rate-limiting
enzyme that catalyzes the production of melanin in melanocytes
(8). Tyrosinase is critical for
melanogenesis and plays a key role in a number of pigment-deficient
diseases. Le Poole et al (9)
indicated that vitiligo-related gene 1 expression was decreased in
vitiligo patients compared with the healthy controls, which may be
due to the transfer of tyrosinase in the ER, but the specific
mechanism behind this process remain to be elucidated.
Receptor-interacting serine/threonine-protein kinase
1 (RIPK1) was first reported to serve a crucial role in necroptosis
(10). Necroptosis is a form of
programmed cell death in development, inflammation and tissue
homeostasis (11). The function of
necroptosis is to regulate downstream molecules through
post-transcriptional modifications, including phosphorylation and
ubiquitination (12). RIPK1 has a
major impact on liver pathogenesis and liver disease prognosis
(13,14). Previous research has indicated that
RIPK1-mediated necrotic apoptosis can also occur in neuronal cells,
leading to neurodegenerative disease (15). However, to the best of our knowledge,
the role of RIPK1 in vitiligo remains undetermined.
A previous study reported that the PI3K/AKT/mTOR
pathway is associated with cell survival in response to oxidative
stress (16). Growth factors may
protect against oxidative stress-induced apoptosis through the
activation of the AKT and mTOR pathways (17-19).
Furthermore, another study suggested that α-melanocyte-stimulating
hormone stimulated melanogenesis through activating the
mitogen-activated protein kinase kinase/ERK or PI3K/AKT pathways
(20). Regulation of the
PI3K/AKT/mTOR signaling pathway has been reported to be a novel
approach for the clinical treatment of vitiligo (21). Moreover, the association between
RIPK1 and the PI3K/AKT/mTOR pathway in melanocytes under ER stress
remains largely unclear. Therefore, the present study aimed to
explore the mechanisms of action of RIPK1 in ER-stressed human
melanocytes.
Materials and methods
Cell culture and treatment
Human primary epidermal melanocytes were acquired
from American Type Culture Collection. Cells were cultured in
Medium 254 (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with human melanocyte growth supplement (Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C and 5% CO2.
To induce ER stress, human primary epidermal
melanocytes (1x106 cells per well) were treated with 3
µM tunicamycin (TM; Sigma-Aldrich; Merck KGaA) (22) at 37˚C for 24, 48 and 72 h.
Primary epidermal melanocytes were transfected with
1 µg control plasmid (cat no. sc-437275; Santa Cruz Biotechnology,
Inc.) or 1 µg RIPK1 plasmid (cat no. sc-422681-ACT; Santa Cruz
Biotechnology, Inc.) for 24 h using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance
with the manufacturer's protocol. Reverse
transcription-quantitative PCR (RT-qPCR) and western blot analysis
were used to detect the efficiency of cell transfection. 24 h after
cell transfection, subsequent experiments were performed.
RT-qPCR
Total RNA was isolated from human primary epidermal
melanocytes using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific Inc.) and cDNA was synthesized using a
High-Capacity cDNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) following the manufacturer's
protocol. The following thermocycling conditions were used: 70˚C
for 5 min, 37˚C for 5 min and 42˚C for 60 min. Subsequently, qPCR
was performed using the SYBR Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95˚C for 5 min; 40 cycles of 95˚C for 10 sec, 60˚C
for 20 sec and a final extension at 72˚C for 30 sec. The following
primer pairs were used for the qPCR: GAPDH forward,
5'-TGTTGCCATCAATGACCCCTT-3' and reverse, 5'-CTCCACGACGTACTCAGCG-3';
RIPK1 forward, 5'-AGGCTTTGGGAAGGTGTCTC-3' and reverse,
5'-CGGAGTACTCATCTCGGCTTT-3'; protein kinase R-like endoplasmic
reticulum kinase (PERK) forward, 5'-TCCTGCTTTGCATCGTAGCC-3' and
reverse, 5'-GATGGAAAAGCCTGCGCA-3'; eukaryotic translation
initiation factor 2 subunit 1 (eIF2α) forward,
5'-CTCCTGAAAGCAGCAACCTC-3' and reverse, 5'-GACCGAGATGAAGCATCGTG-3'
and CCAAT/enhancer-binding protein epsilon (CHOP) forward,
5'-CTTCCATGTAGCGGAGTCCT-3' and reverse, 5'-GTGAGAGCCAGTCTCCCTTT-3'.
Relative gene expression was quantified using the 2-ΔΔCq
method (23). GAPDH was used as the
internal control.
Western blot analysis
Total protein was extracted using ice-cold RIPA
buffer (Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. BCA assays (Thermo Fisher Scientific,
Inc.) were used to measure the protein concentrations. Protein
samples (40 µg/lane) were separated by 12% SDS-PAGE and transferred
to PVDF membranes (EMD Millipore). The membranes were blocked with
5% skim milk in TBS containing 0.1% Tween for 2 h at room
temperature. The membranes were then incubated with the following
primary antibodies: RIPK1 (cat. no. 3493; 1:1,000; Cell Signaling
Technology, Inc.), PERK (cat. no. 5683; 1:1,000; Cell Signaling
Technology, Inc.), eIF2α (cat. no. 5324; 1:1,000; Cell Signaling
Technology, Inc.), CHOP (cat no. 2895; 1:1,000; Cell Signaling
Technology, Inc.), caspase-3 (cat. no. 14220; 1:1,000; Cell
Signaling Technology, Inc.), Bcl-2 (cat. no. 3498; 1:1,000; Cell
Signaling Technology, Inc.), Bax (cat. no. 5023; 1:1,000; Cell
Signaling Technology, Inc.), phospho (p)-AKT (cat. no. 4060;
1:1,000; Cell Signaling Technology, Inc.), p-mTOR (cat. no. 5536;
1:1,000; Cell Signaling Technology, Inc.), p-PI3K (cat. no. BS4811;
1:1,000; Biogot Technology Co., Ltd.) and GAPDH (cat. no. 5174;
1:1,000; Cell Signaling Technology, Inc.) overnight at 4˚C.
Subsequently, the membranes were incubated with horseradish
peroxidase-conjugated anti-mouse/anti-rabbit Immunoglobulin G
secondary antibodies (cat. nos. 7076 and 7074; 1:1,000; Cell
Signaling Technology, Inc.) at room temperature for 2 h. Protein
bands were visualized by enhanced chemiluminescence (EMD
Millipore). GAPDH was used as the loading control. ImageJ version
2.0 software (National Institutes of Health) was used to quantify
the band intensity.
Flow cytometric analysis of
apoptosis
Cell apoptosis was detected using the
Annexin-V/propidium iodide (PI) Apoptosis Detection kit [cat. no.
70-AP101-100; Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.].
Human melanocytes were plated in six-well plates at a density of
2-3x105 cells per well overnight. Cells were then
transfected with control or RIPK1 plasmids for 24 h, followed by
treatment with 3 µM TM for 48 h. Cells were then collected by
centrifugation (1,000 x g; 5 min; 4˚C), and resuspended in 100 µl
of FITC-binding buffer. Subsequently, the buffer was added to 5 µl
ready-to-use Annexin V-FITC (BD Biosciences) and 5 µl PI. Cells
were incubated in the dark for 30 min at room temperature. Annexin
V-FITC and PI fluorescence were assessed using a BD FACSCalibur
flow cytometer (BD Biosciences), and the data were analyzed using
FlowJo software (version 7.6.1; FlowJo LLC).
MTT assay
Human melanocyte viability was determined using an
MTT assay. Human melanocytes were plated in 96-well plates at a
density of 5x103 cells/well. Human melanocytes were
transfected with control or RIPK1 plasmids for 24 h and then
treated with 3 µM TM for 48 h. Subsequently, 20 µl MTT reagent
(Sigma-Aldrich; Merck KGaA) was added into each well for another 4
h at 37˚C. Subsequently, 150 µl DMSO (Sigma-Aldrich; Merck KGaA)
was added into each well and shaken for 15 min. The optical density
values were read at a wavelength of 490 nm using the
FLUOstar® Omega Microplate Reader (BMG Labtech
GmbH).
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three independent experiments. One-way ANOVA followed
by Tukey's post-hoc test was used for multiple comparisons.
Unpaired Student's t-test was used to analyze the statistical
significance between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of RIPK1 in human
melanocytes induces ER stress
To explore the role of RIPK1 in ER stress-induced
human melanocytes, cells were treated with 3 µM TM for 24, 48 and
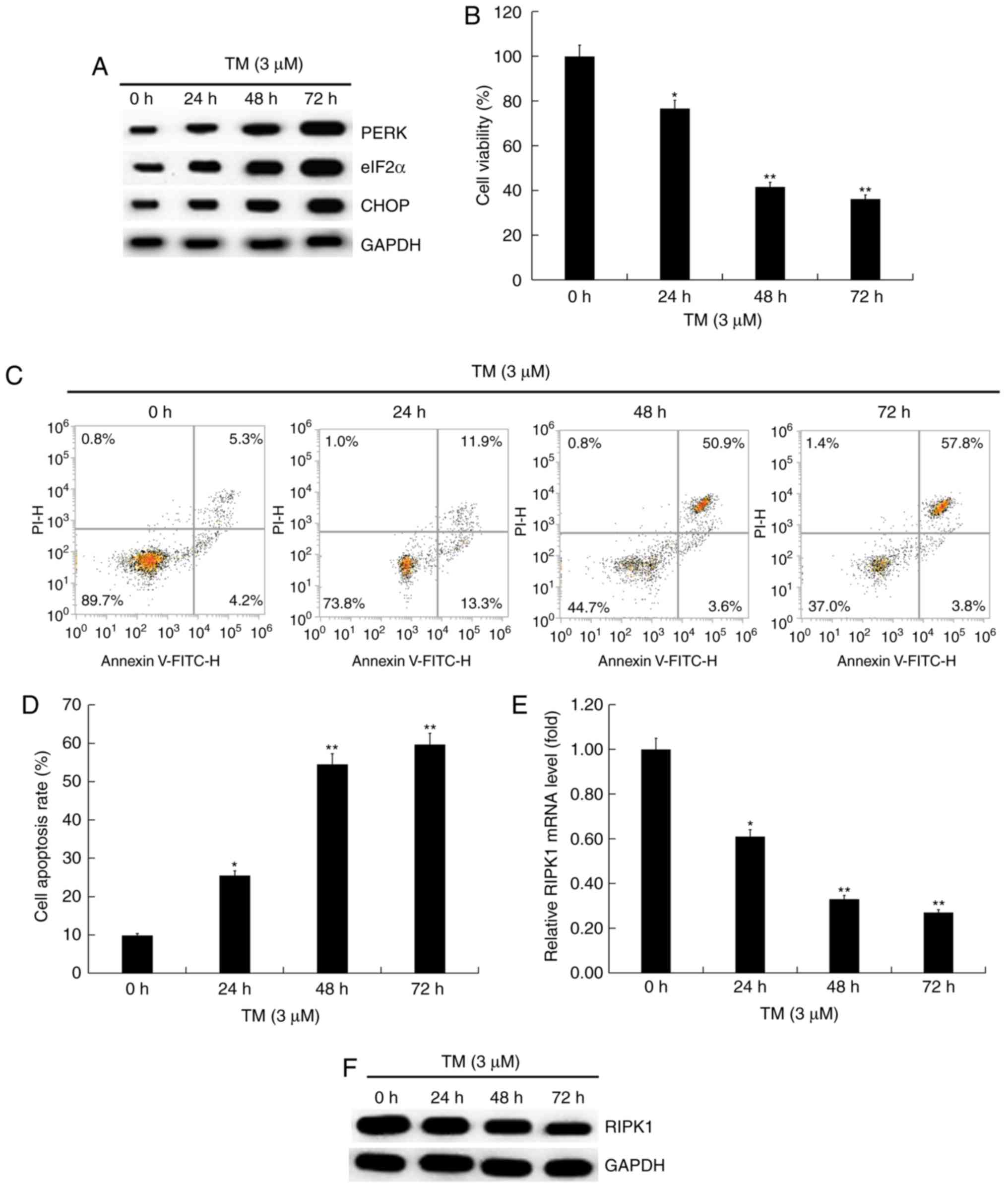
72 h. Firstly, the expression of ER stress-related proteins in
human melanocytes induced by ER stress was investigated. Western
blot analysis indicated that the expression of ER stress-related
proteins, including PERK, eIF2α and CHOP was upregulated in a
time-dependent manner (Fig. 1A),
indicating that 3 µM TM activated ER stress in human melanocytes.
MTT assay indicated that TM significantly inhibited cell viability
(Fig. 1B) and induced cell apoptosis
(Fig. 1C and D) in a time-dependent manner in human
melanocytes compared with the control. RT-qPCR and western blot
analysis results indicated that RIPK1 expression decreased with the
increase of TM treatment time (Fig.
1E and F). RIPK1 expression was
decreased in human melanocytes induced by ER stress.
Transfection efficiency of RIPK1
plasmid in human melanocytes
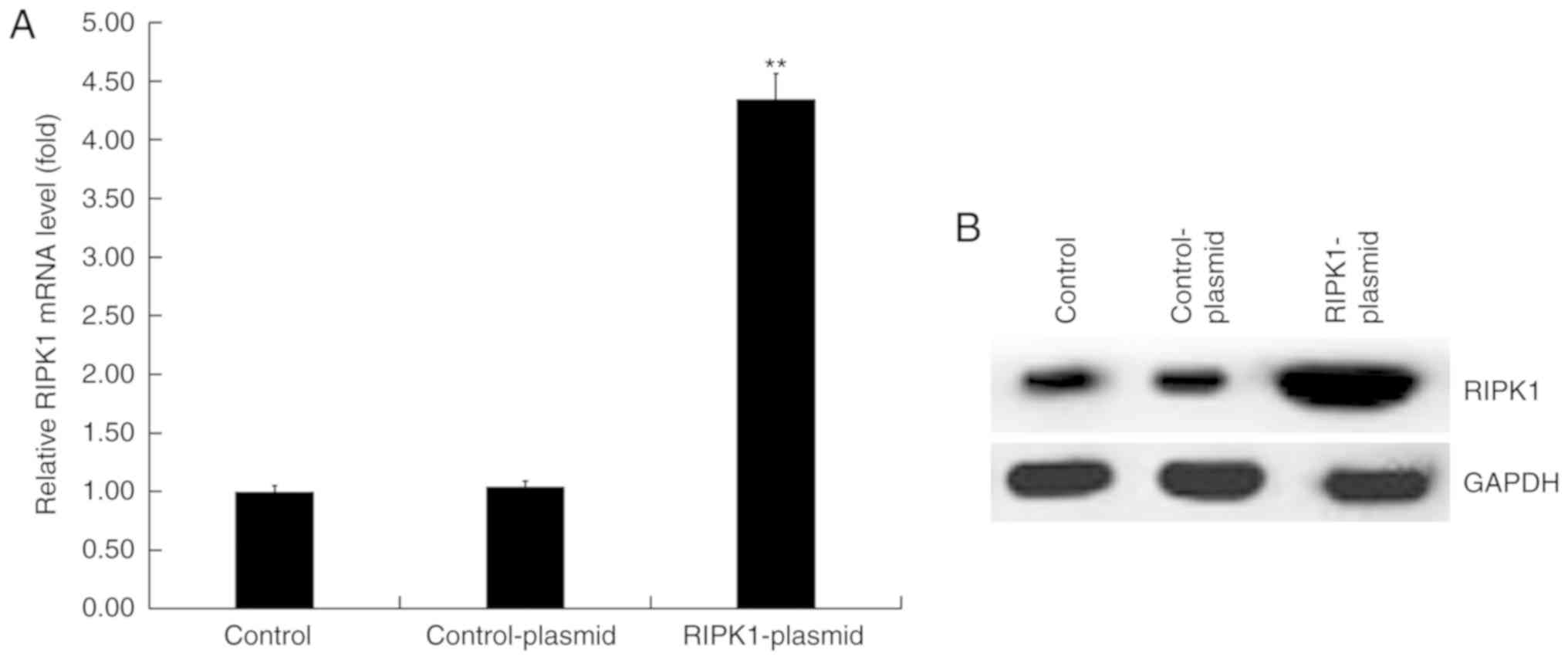
Human melanocytes were transfected with control or
RIPK1 plasmids for 24 h. RT-qPCR and western blot analysis were
performed to detect transfection efficiency. RT-qPCR results
demonstrated that compared with the control group, the mRNA
expression of RIPK1 significantly increased in RIPK1
plasmid-transfected human melanocytes (Fig. 2A). Similar results were observed in
the western blot analysis assay (Fig.
2B).
Effect of RIPK1 upregulation on the
expression of ER stress-related proteins in human melanocytes
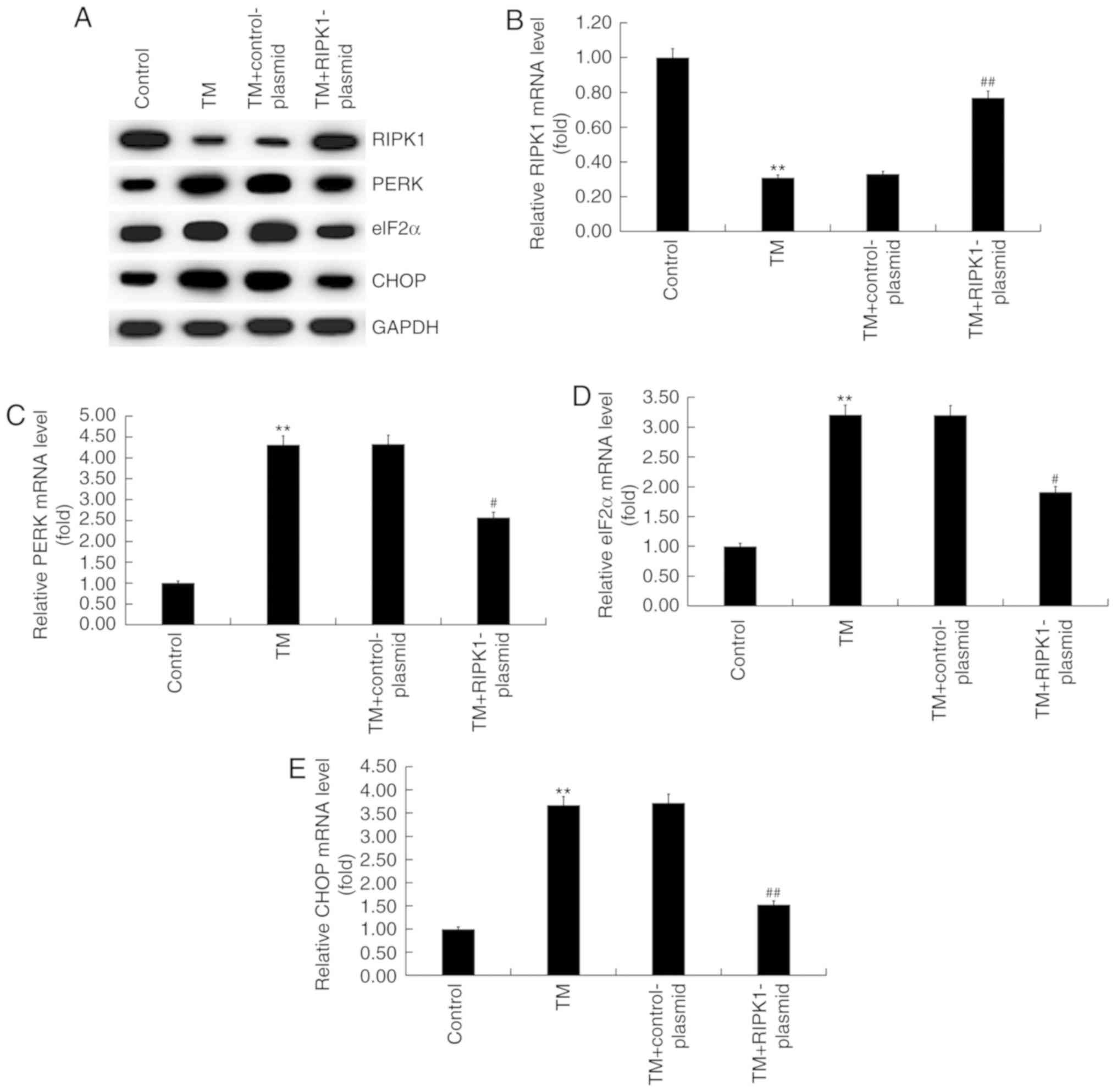
To investigate the effect of high RIPK1 expression
on the expression of ER stress-related proteins in human
melanocytes, the expression of PERK, eIF2α and CHOP were examined
using RT-qPCR and western blot analysis. The results revealed that
RIPK1 protein expression decreased while PERK, eIF2α and CHOP
protein expression increased in the TM-treated group compared with
the control group (Fig. 3A).
Additionally, RIPK1 expression increased (Fig. 3A) while protein expression of PERK,
eIF2α and CHOP decreased in the TM + RIPK1-plasmid group compared
with the TM-treated group (Fig. 3A).
Similar results were observed in the RT-qPCR assays (Fig. 3B-E).
Effect of RIPK1 upregulation on the
survival of ER stress-induced human melanocytes
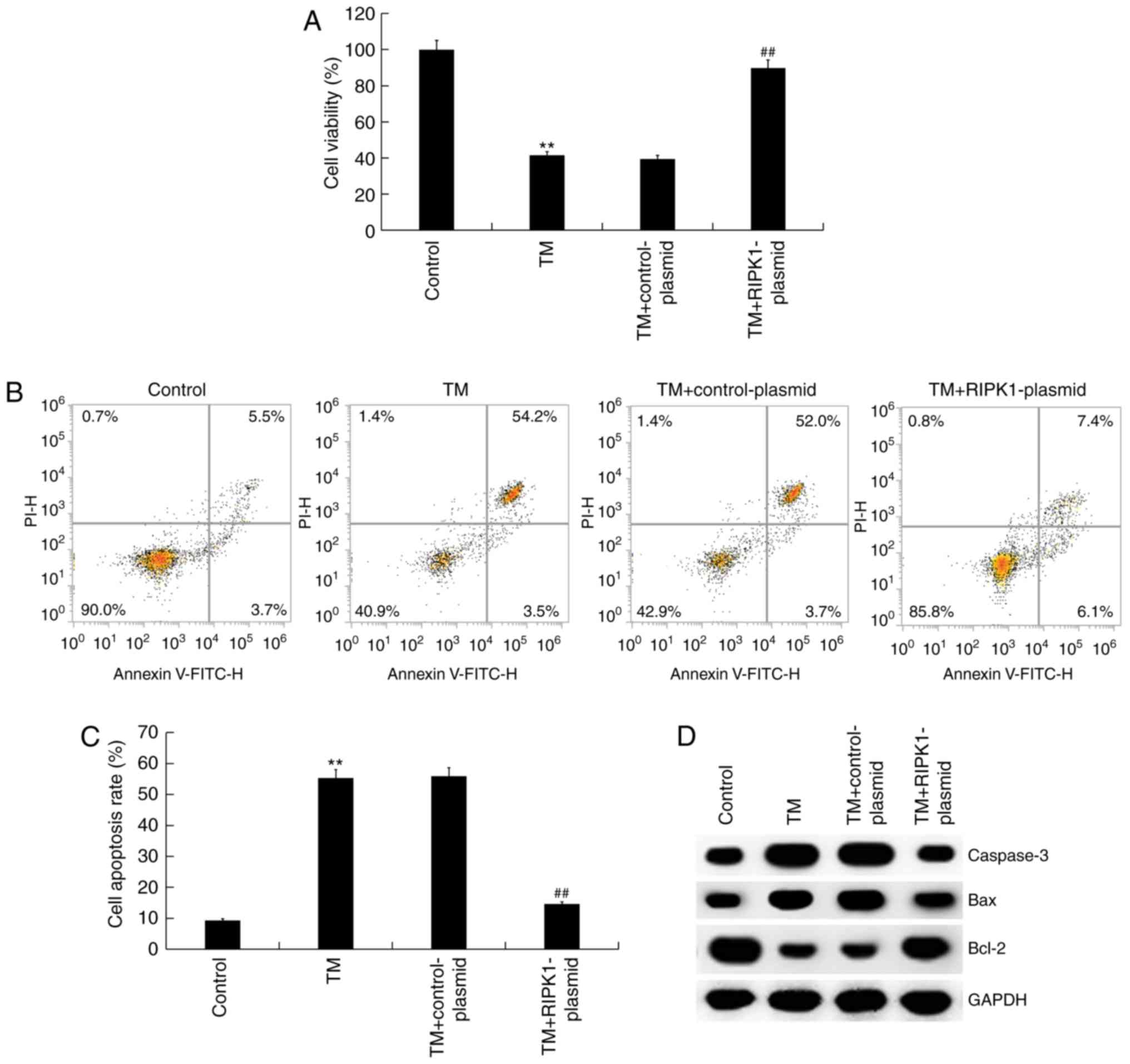
The effect of high RIPK1 expression on the survival
of ER stress-induced human melanocytes was investigated. MTT and
flow cytometry assays revealed that compared with the control
group, the cell viability of human melanocytes was significantly
reduced, while cell apoptosis significantly increased in the TM
treatment groups. RIPK1 plasmid transfection was indicated to
significantly increase cell viability (Fig. 4A) and decrease cell apoptosis
compared with the TM treatment groups (Fig. 4B and C). The expression of apoptosis-related
proteins was also assessed. The results of western blot analysis
indicated that compared with the control group, the protein
expression of Bax and caspase-3 increased while Bcl-2 expression
decreased in the TM treatment group. RIPK1 plasmid transfection
decreased Bax and caspase-3 protein expression and increased Bcl-2
protein expression compared with the TM treatment group (Fig. 4D). Therefore, overexpression of RIPK1
reversed cell growth inhibition induced by TM treatment.
Effect of RIPK1 upregulation on the
PI3K/AKT/mTOR signaling pathway in human melanocytes
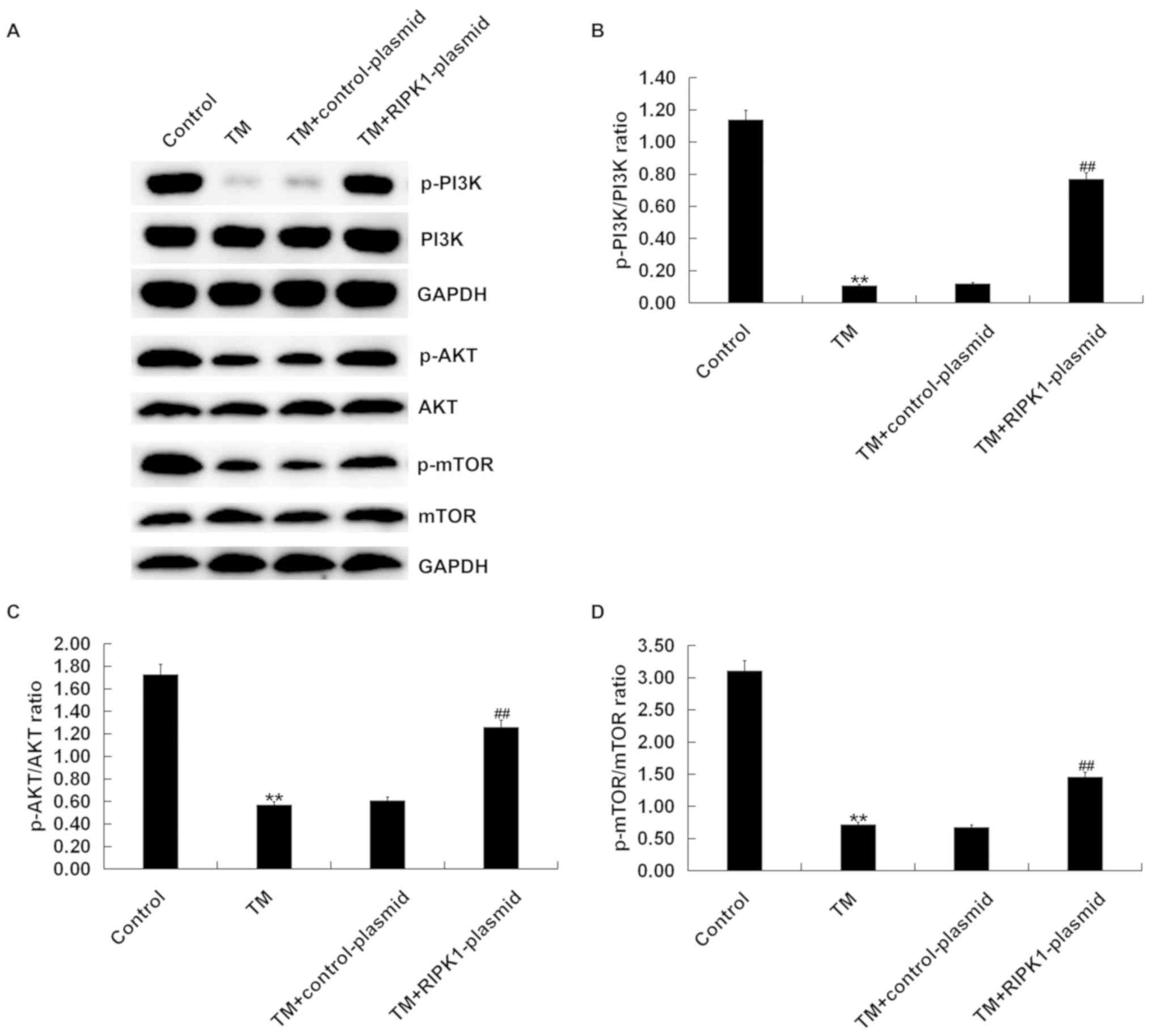
Western blot analysis demonstrated that compared
with the control group, the protein expression of p-PI3K (Fig. 5A and B), p-AKT (Fig.
5A and C) and p-mTOR (Fig. 5A and D) significantly decreased in the TM treated
group, but this effect was reversed by RIPK1 plasmid transfection
(Fig. 5). Taken together, the
results indicated that the effect of RIPK1 overexpression on human
melanocyte growth may be associated with the PI3K/AKT/mTOR
signaling pathway.
Discussion
Vitiligo is a common congenital or acquired skin
disease that is characterized by loss of melanocytes, causing
progressive skin depigmentation (24). Currently, vitiligo treatment mainly
prevents disease development and achieves repigmentation in
non-pigmented areas (25,26). Phototherapy is currently the
preferred method of vitiligo treatment, but corticosteroids,
surgery or local immunomodulators are also used (27-29).
The ER stress response is a cellular process that
can be aroused by different conditions that cause homeostatic
imbalance (5). ER stress was
reported to relate to the pathogenesis of a variety of diseases,
including neurodegeneration, inflammation or cancer (30-33).
Emerging evidence has suggested that pharmacological targeting of
ER stress can be an effective therapeutic strategy for treating
tumors (34-36).
Different natural compounds induced ER stress-mediated death in
cancer cells (37). ER stress was
also identified to serve a critical role in the pathogenesis of
vitiligo (38-40).
However, to the best of our knowledge, the mechanism behind
vitiligo pathogenesis caused by ER stress remains to be determined.
In the present study, TM enhanced the protein expression of ER
stress-related proteins PERK, eIF2α and CHOP in a time-dependent
manner. TM inhibited cell viability and induced apoptosis in human
melanocytes.
RIPK1 is a crucial regulator of tumor necrosis
factor receptor 1 signaling (41).
RIPK1 regulates the balance between cell survival, apoptosis and
necrotic apoptosis after the stimulation of tumor necrosis factor-α
(42). In addition, several studies
have indicated that RIPK1 promotes or inhibits the effector
functions of caspase-8 and RIPK3 (43-45).
In the present study, RIPK1 expression was demonstrated to be
downregulated in human melanocytes induced by ER stress.
Previous studies have demonstrated that RIPK1
overexpression may lead to apoptosis in a number of cell types
(16,46). Luan et al (22) demonstrated that RIPK1 is important
for the survival of melanoma cells undergoing pharmacological ER
stress. The results of the present study showed that TM inhibited
the survival of human melanocytes, but this effect was reversed by
RIPK1 plasmid transfection. The PI3K/AKT/mTOR pathway has been
indicated to be associated with cell survival in response to
oxidative stress (20) and
melanogenesis (17). Activation of
the PI3K/AKT/mTOR pathway could reduce oxidative stress-induced
apoptosis (18,19). The present study explored whether the
role of RIPK1 in melanocyte damage induced by oxidative stress was
associated with the PI3K/AKT/mTOR pathway. ER stress-induced
inhibition of the PI3K/AKT/mTOR signaling pathway in human
melanocytes was significantly suppressed by RIPK1
overexpression.
In conclusion, RIPK1 may protect human melanocytes
from cell damage induced by ER stress by regulating the
PI3K/AKT/mTOR and ER stress signaling pathways. The results of the
current study indicated that RIPK1 might protect melanocytes from
ER stress induced damage. Therefore, RIPK1 might serve a protective
role in the occurrence and development of vitiligo. The present
research provides potential therapeutic targets and theoretical
basis for the treatment of vitiligo. However, the present study is
a preliminary study exploring the role of RIPK1 in vitiligo. To
elucidate the role of RIPK1 in vitiligo further, future in-depth
research is required. For example, the effect of RIPK1 on
melanocytes from vitiligo patients should be investigated. The
relationship between RIPK1 and PI3K/AKT/mTOR signaling pathway in
human melanocytes also requires more in-depth research. The effect
of RIPK1 in vitiligo should be investigated in vivo in the
future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81773335, 81803131
and 81602755), Zhejiang Provincial Natural Science Foundation
(grant no. LY18H110001) and Zhejiang Basic Public Welfare Research
Project (grant no. LGF18H110002).
Availability of data and materials
All datasets used and/or generated during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
XS and TW contributed to study design, data
collection, statistical analysis, data interpretation and
manuscript preparation. BH, GR and AX contributed to data
collection and statistical analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ezzedine K, Eleftheriadou V, Whitton M and
van Geel N: Vitiligo. Lancet. 386:74–84. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ezzedine K, Lim HW, Suzuki T, Katayama I,
Hamzavi I, Lan CC, Goh BK, Anbar T, Silva de Castro C, Lee AY, et
al: Revised classification/nomenclature of vitiligo and related
issues: The Vitiligo Global Issues Consensus Conference. Pigment
Cell Melanoma Res. 25:E1–E13. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gawkrodger DJ, Ormerod AD, Shaw L,
Mauri-Sole I, Whitton ME, Watts MJ, Anstey AV, Ingham J and Young
K: Therapy Guidelines and Audit Subcommittee, British Association
of Dermatologists. et al Guideline for the diagnosis and management
of vitiligo. Br J Dermatol. 159:1051–1076. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Speeckaert R and van Geel N: Vitiligo: An
update on pathophysiology and treatment options. Am J Clin
Dermatol. 18:733–744. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schwarz DS and Blower MD: The endoplasmic
reticulum: Structure, function and response to cellular signaling.
Cell Mol Life Sci. 73:79–94. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tabas I: Consequences of cellular
cholesterol accumulation: Basic concepts and physiological
implications. J Clin Invest. 110:905–911. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gunia-Krzyżak A, Popiol J and Marona H:
Melanogenesis inhibitors: Strategies for searching for and
evaluation of active compounds. Curr Med Chem. 23:3548–3574.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Le Poole IC, Sarangarajan R, Zhao Y,
Stennett LS, Brown TL, Sheth P, Miki T and Boissy RE: ‘VIT1ʼ, A
novel gene associated with vitiligo. Pigment Cell Res. 14:475–484.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang H, Chen T, Zhou Y, Geng L, Shen T,
Zhou L and Zheng S: RIPK1 inhibition enhances pirarubicin cytotoxic
efficacy through AKT-P21-dependent pathway in hepatocellular
carcinoma. Int J Med Sci. 15:1648–1657. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Linkermann A and Green DR: Necroptosis. N
Engl J Med. 370:455–465. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Galluzzi L, Kepp O, Chan FK and Kroemer G:
Necroptosis: Mechanisms and relevance to disease. Annu Rev Pathol.
12:103–130. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schneider AT, Gautheron J, Feoktistova M,
Roderburg C, Loosen SH, Roy S, Benz F, Schemmer P, Büchler MW,
Nachbur U, et al: RIPK1 suppresses a TRAF2-dependent pathway to
liver cancer. Cancer Cell. 31:94–109. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Saeed WK and Jun DW: Necroptosis: An
emerging type of cell death in liver diseases. World J
Gastroenterol. 20:12526–12532. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shan B, Pan H, Najafov A and Yuan J:
Necroptosis in development and diseases. Genes Dev. 32:327–340.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cao C and Wan Y: Parameters of protection
against ultraviolet radiation-induced skin cell damage. J Cell
Physiol. 220:277–284. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cao C, Huang X, Han Y, Wan Y, Birnbaumer
L, Feng GS, Marshall J, Jiang M and Chu WM: Galpha(i1) and
Galpha(i3) are required for epidermal growth factor-mediated
activation of the Akt-mTORC1 pathway. Sci Signal.
2(ra17)2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cao C, Lu S, Jiang Q, Wang WJ, Song X,
Kivlin R, Wallin B, Bagdasarian A, Tamakloe T, Chu WM, et al: EGFR
activation confers protections against UV-induced apoptosis in
cultured mouse skin dendritic cells. Cell Signal. 20:1830–1838.
2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cheng LB, Cheng L, Bi HE, Zhang ZQ, Yao J,
Zhou XZ and Jiang Q: Alpha-melanocyte stimulating hormone protects
retinal pigment epithelium cells from oxidative stress through
activation of melanocortin 1 receptor-Akt-mTOR signaling. Biochem
Biophys Res Commun. 443:447–452. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kadekaro AL, Kavanagh R, Kanto H, Terzieva
S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G,
Shertzer HG, et al: Alpha-Melanocortin and endothelin-1 activate
antiapoptotic pathways and reduce DNA damage in human melanocytes.
Cancer Res. 65:4292–4299. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wan J, Lin F, Zhang W, Xu A, DeGiorgis J,
Lu H and Wan Y: Novel approaches to vitiligo treatment via
modulation of mTOR and NF-κB pathways in human skin melanocytes.
Int J Biol Sci. 13:391–400. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Luan Q, Jin L, Jiang CC, Tay KH, Lai F,
Liu XY, Liu YL, Guo ST, Li CY, Yan XG, et al: RIPK1 regulates
survival of human melanoma cells upon endoplasmic reticulum stress
through autophagy. Autophagy. 11:975–994. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Le Poole IC, Das PK, van den Wijngaard RM,
Bos JD and Westerhof W: Review of the etiopathomechanism of
vitiligo: A convergence theory. Exp Dermatol. 2:145–153.
1993.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Speeckaert R and van Geel N: Vitiligo: An
update on pathophysiology and treatment options. Am J Clin
Dermatol. 18:733–744. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gawkrodger DJ, Ormerod AD, Shaw L,
Mauri-Sole I, Whitton ME, Watts MJ, Anstey AV, Ingham J and Young
K: Vitiligo: Concise evidence based guidelines on diagnosis and
management. Postgrad Med J. 86:466–471. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gianfaldoni S, Wollina U, Tirant M,
Tchernev G, Lotti J, Satolli F, Rovesti M, França K and Lotti T:
Herbal compounds for the treatment of vitiligo: A review. Open
Access Maced J Med Sci. 6:203–207. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lotti T, Wollina U, Tchernev G, Valle Y,
Lotti J, França K, Satolli F, Rovesti M, Tirant M, Lozev I, et al:
An innovative therapeutic protocol for vitiligo: Experience with
the Use of Fraxel Herbium laser, topical latanoprost and successive
irradiation with UVA-1 laser. Open Access Maced J Med Sci. 6:49–51.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang M and Kaufman RJ: Protein misfolding
in the endoplasmic reticulum as a conduit to human disease. Nature.
529:326–335. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bu Y and Diehl JA: PERK integrates
oncogenic signaling and cell survival during cancer development. J
Cell Physiol. 231:2088–2096. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ivanova EA and Orekhov AN: The role of
endoplasmic reticulum stress and unfolded protein response in
atherosclerosis. Int J Mol Sci. 17(pii: E193)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Keestra-Gounder AM, Byndloss MX, Seyffert
N, Young BM, Chávez-Arroyo A, Tsai AY, Cevallos SA, Winter MG, Pham
OH, Tiffany CR, et al: NOD1 and NOD2 signalling links ER stress
with inflammation. Nature. 532:394–397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Schonthal AH: Pharmacological targeting of
endoplasmic reticulum stress signaling in cancer. Biochem
Pharmacol. 85:653–666. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Maurel M, McGrath EP, Mnich K, Healy S,
Chevet E and Samali A: Controlling the unfolded protein
response-mediated life and death decisions in cancer. Semin Cancer
Biol. 33:57–66. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schonthal AH: Endoplasmic reticulum
stress: Its role in disease and novel prospects for therapy.
Scientifca (Cairo). 2012(857516)2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pereira DM, Valentão P, Correia-da-Silva
G, Teixeira N and Andrade PB: Translating endoplasmic reticulum
biology into the clinic: A role for ER-targeted natural products?
Nat Prod Rep. 32:705–722. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Park K, Lee SE, Shin KO and Uchida Y:
Insights into the role of endoplasmic reticulum stress in skin
function and associated diseases. FEBS J. 286:413–425.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Manga P, Elbuluk N and Orlow SJ: Recent
advances in understanding vitiligo. F1000Res. 5:pii: F1000 Faculty
Rev-2234. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guan C, Xu W, Hong W, Zhou M, Lin F, Fu L,
Liu D and Xu A: Quercetin attenuates the effects of H2O2 on
endoplasmic reticulum morphology and tyrosinase export from the
endoplasmic reticulum in melanocytes. Mol Med Rep. 11:4285–4290.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Pasparakis M and Vandenabeele P:
Necroptosis and its role in inflammation. Nature. 517:311–320.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Silke J, Rickard JA and Gerlic M: The
diverse role of RIP kinases in necroptosis and inflammation. Nat
Immunol. 16:689–697. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
42
|
Amin P, Florez M, Najafov A, Pan H, Geng
J, Ofengeim D, Dziedzic SA, Wang H, Barrett VJ, Ito Y, et al:
Regulation of a distinct activated RIPK1 intermediate bridging
complex I and complex II in TNFα-mediated apoptosis. Proc Natl Acad
Sci USA. 115:E5944–E5953. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Orozco S, Yatim N, Werner MR, Tran H,
Gunja SY, Tait SW, Albert ML, Green DR and Oberst A: RIPK1 both
positively and negatively regulates RIPK3 oligomerization and
necroptosis. Cell Death Differ. 21:1511–1521. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rickard JA, O'Donnell JA, Evans JM,
Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL,
Anderton H, et al: RIPK1 regulates RIPK3-MLKL-driven systemic
inflammation and emergency hematopoiesis. Cell. 157:1175–1188.
2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tenev T, Bianchi K, Darding M, Broemer M,
Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K
and Meier P: The Ripoptosome, a signaling platform that assembles
in response to genotoxic stress and loss of IAPs. Mol Cell.
43:432–448. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Park S, Hatanpaa KJ, Xie Y, Mickey BE,
Madden CJ, Raisanen JM, Ramnarain DB, Xiao G, Saha D, Boothman DA,
et al: The receptor interacting protein 1 inhibits p53 induction
through NF-kappaB activation and confers a worse prognosis in
glioblastoma. Cancer Res. 69:2809–2816. 2009.PubMed/NCBI View Article : Google Scholar
|