Introduction
Human skin has many unique properties, including its
function as a physicochemical barrier. This property protects the
body from dangerous pathogens however, it also complicates the
delivery of therapeutic agents and resists the penetration of a
number of molecules (1). Skin
penetration follows the ‘500 Dalton rule’, therefore, it is
difficult for hydrophilic therapeutic molecules of large molecular
weight to penetrate the normal skin barrier (2). This is problematic since it is often
important for dermatologists to deliver effective ingredients to a
targeted layer of skin.
To overcome this difficulty, a number of methods can
be used to temporarily increase the permeation of drugs through the
skin barrier (3,4). These methods include chemical,
biochemical and physical approaches. In particular, chemical
enhancers have been developed that increase the diffusibility of
the substance across the barrier, increase product solubility in
the vehicle and improve the partition coefficient (5). Furthermore, the manipulation of lipid
biosynthesis has allowed the modification of the barrier structure
itself to increase drug penetration. However, in some cases these
modifications result in skin irritations, therefore, these
formulations must be carefully evaluated in a variety of
preparations (6). The main physical
techniques that can increase the cutaneous penetration of
substances are: i) Iontophoresis, which increases the penetration
of ionized substances; ii) electroporation, which electrically
induces penetration through the barrier; and iii) sonophoresis,
which is based on 20 to 25 KHz ultrasound and induces alterations
in the skin barrier allowing the penetration of active drugs
(7,8). Recently, novel physical methods,
including fractional laser devices and micro-needle rollers have
also been developed (9). These
techniques promote drug absorption by inducing fine perforations
that mildly perturb the stratum corneum (SC), thereby creating
holes through which molecules can pass (10). However, the use of these modalities
may result in some pain or discomfort, and can also disrupt the
normal barrier function of the SC (11,12).
In contrast, a number of studies have suggested that
hydration of SC lipid lamellar regions or osmotic forces in the
skin can enhance the permeation of drugs through the skin (13). Water is the most natural and
biocompatible penetration enhancer that has been demonstrated to
improve skin permeability (14).
Furthermore, recent evidence has suggested that extensive hydration
using occlusion methods may disrupt lipid ultrastructure (15,16). The
SC has been indicated to be a dynamic structure, in which extended
hydration (>8 h) swells corneocytes, induces intercorneocyte
rupture, and causes microstructural changes in lipid self-assembly
(17). These disruptions allow
penetration through the SC barrier. However, these disruptions are
reversible, as removing the hydration source easily restores the
barrier (18).
Clothing is used daily and often comes into close
contact with human skin. Different types of fabric are used in
clothing and effect skin moisture conditions differently and may,
therefore, enable drug absorption for wound dressing, skin care and
cosmetic products (19). The
majority of cosmetic slimming creams contain a variety of
ingredients (including caffeine, centella asiatica, ruscus, mate,
retinol and Ginko biloba), which modulate fat storage in
adipocytes (20-22).
With the aim of overcoming current limitations in
transdermal delivery systems, the present study developed a novel
fabric for transdermal drug delivery and evaluated its ability to
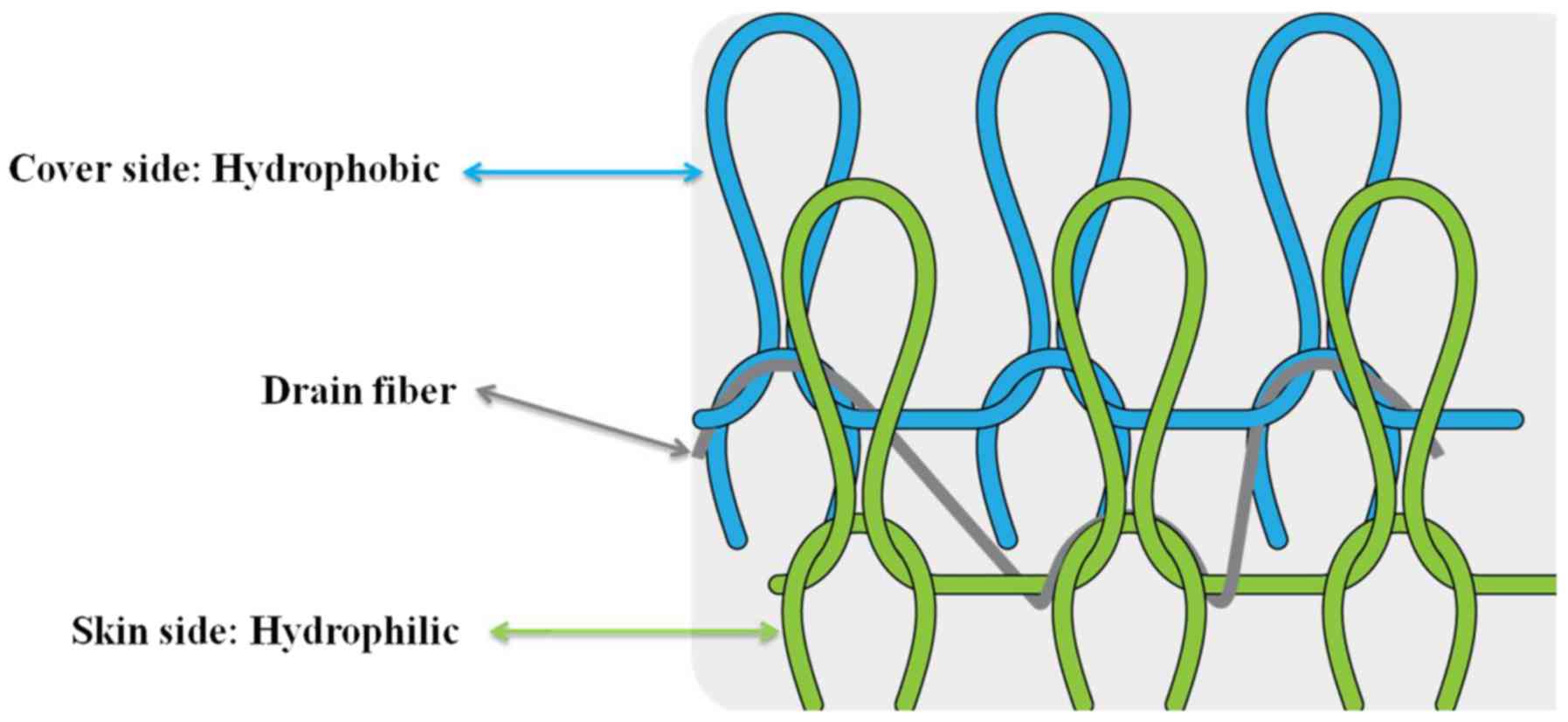
enhance the effect of slimming creams. The novel fabric used in the
present study consisted of two layers, an outer hydrophobic layer
of polypropylene and an inner hydrophilic layer of nylon with
polyurethane. This fabric creates a unique combination of
conditions at the skin surface in which the hydrophobic outer layer
prevents water evaporation and thus puts water in direct contact
with the skin, whereas the hydrophilic layer, which is also in
direct contact with the skin, maintains skin moisture (Fig. 1). Therefore, the aim of the present
study was to evaluate the efficacy of this novel fabric for the
enhancement of a transdermal drug delivery system in vivo
and in vitro.
Materials and methods
Experimental protocols
The clothing used in the present study was
constructed using double layered fabric, consisting of an outer
layer of polypropylene and an inner layer of nylon with
polyurethane. The proportions of each material were 15%
polypropylene, 72.5% nylon and 12.5% polyurethane. The fabric also
incorporated strategic spatial distribution of hydrophobic and
hydrophilic fiber materials. The hydrophobic fiber layer covered
the outside of the fabric to prevent water evaporation, whereas the
hydrophilic fiber layer was kept in direct contact with the skin to
keep it moist after the application of water to the skin (Fig. 1). The control clothing was made of
pure organic cotton (100%) and was designed to have a similar fit
and shape. All textiles were cut into 15x20 cm squares. To attach
the fabric to the four legs of each animal, each piece of fabric
was punched at four uniform sites and held together with adhesive
tape to prevent clothing removal during the study. All animals were
able to maintain a high level of activity while wearing the
clothing, but if worn for an entire day, normal activity became
difficult to maintain. After applying the topical slimming creams
(2 mg/cm2) on the abdominal fat of each guinea pig,
these procedures were repeated every eight hours per day for 28
days.
Animal model
A total of twelve female guinea pigs (six months of
age or older; weight, 150-250 g) were purchased from ORIENT BIO,
Inc. and used in the current study. All animals were housed
individually under controlled environmental conditions
(temperature, 18-22˚C; relative air humidity, 30-70%; 15 air
changes/h; 12-h light-dark cycle). All procedures involving animals
conform to internationally accepted standards and have been
reviewed and approved by the Institutional Animal Care and Use
Committee of Chung-Ang University, Republic of Korea (IRB number:
2018-9077).
After an acclimation period of 7 days, guinea pigs
with a healthy appearance (no abnormal eye movements) were randomly
allocated into four groups (n=3) as follows: i) Group 1, untreated
control; ii) group 2, topical cosmetic slimming cream alone (Hera
Glam Body Slite®; Amorepacific Co.); iii) group 3,
slimming cream with normal fabric (made of 100% cotton), and iv)
group 4, slimming cream with the novel drug delivery fabric (Doctor
Slim®; Ventex). After all treatments, to take skin
samples, guinea pigs were anesthetized using an intramuscular
injection of a mixture of ketamine HCL (45 mg/kg of body weight;
Ketalar; Yuhan Co., Ltd.) and xylazine (5 mg/kg; Bayer AG). All
animals were euthanized using exsanguination immediately after
terminal CO2 or ketamine HCL-xylazine administration on
days 0 and 28.
Ultrasound analysis, histological
examination and western blot analysis
The changes and reductions in fat layer thickness
were calculated with direct contact using by diagnostic ultrasound
(Bionet®), on day 28, on the abdominal skin. Ultrasound
measurements were performed on one representative guinea pig from
each group. The amount of fat loss in each guinea pig was evaluated
by histological staining with hematoxylin and eosin (H&E;
Sigma-Aldrich; Merck KGaA). Tissue biopsy was done after sacrifice
on day 0 and day 28. Adipose tissue sections (5 µm thick) were
fixed using 4% paraformaldehyde (PFA) for 24 h at room temperature,
embedded in paraffin, and transferred to probe-on-plus slides
(Thermo Fisher Scientific, Inc.). Deparaffinized skin sections were
then stained for 24 h at room temperature using H&E and
examined using light microscopy to assess histological changes
(magnification, x400). To evaluate the mechanisms underlying the
induction of lipid catabolism, western blot analysis was performed
on guinea pig skin specimens using antibodies specific for
peroxisome proliferator-activated receptor-γ (PPAR-γ) and actin.
PPARs are lipid-activated transcription factors that belong to the
nuclear hormone receptor family and serve key roles in lipid
homeostasis (23). PPAR-γ is part of
the PPAR family and is highly expressed in adipose tissue, and
serves a crucial role in lipid metabolism, adipocyte function and
fat storage (24). Therefore, the
expression of PPAR-γ was investigated in adipose tissue following
the application of slimming creams, to evaluate the efficacy of
drug delivery. Tissue biopsies were collected from abdominal
subcutaneous adipose tissue of one representative guinea pig from
each group and homogenized in lysis buffer [50 mM Tris-HCl (pH
8.0); 150 mM NaCl; 1 mM EDTA; 1% NP-40 and 0.25% deoxycholate acid]
containing a protease inhibitor cocktail (Roche Molecular
Diagnostics). The protein concentration of each lysate was
quantified using a Bio-Rad DC protein assay kit II (Bio-Rad
Laboratories, Inc.). The harvested tissues were homogenized in
Pro-prep solution (Intron Biotechnology, Inc.) and lysates were
centrifuged at 12,000 x g for 30 min at 4˚C. Total protein (40 µg)
was separated by electrophoresis on 10% SDS-polyacrylamide gels.
After blocking with 5% non-fat milk, for 2 h at room temperature,
blots were probed with antibodies against PPAR-γ (1:1,000; LSBio;
cat. no. LS-B651) or actin (1:2,000, LSBio; cat. no. LS-C63547) and
incubated for 2 h at room temperature with HRP-conjugated
anti-mouse (1:1,000; cat. no. P0447; Dako; Agilent Technologies,
Inc.) or anti-rabbit secondary antibodies (1:1,000; cat. no. P0448;
Dako; Agilent Technologies, Inc.). Immunoreactive bands were
detected using an enhanced chemiluminescence system (GE
Healthcare). Protein levels were normalized to those of actin,
which was used as a loading control. The transferred proteins were
visualized with a Pierce ECL western blotting substrate (Pierce;
Thermo Fisher Scientific, Inc.) and quantified by scanning
densitometry using Image-Pro Plus 6.0 (Media Cybernetics,
Inc.).
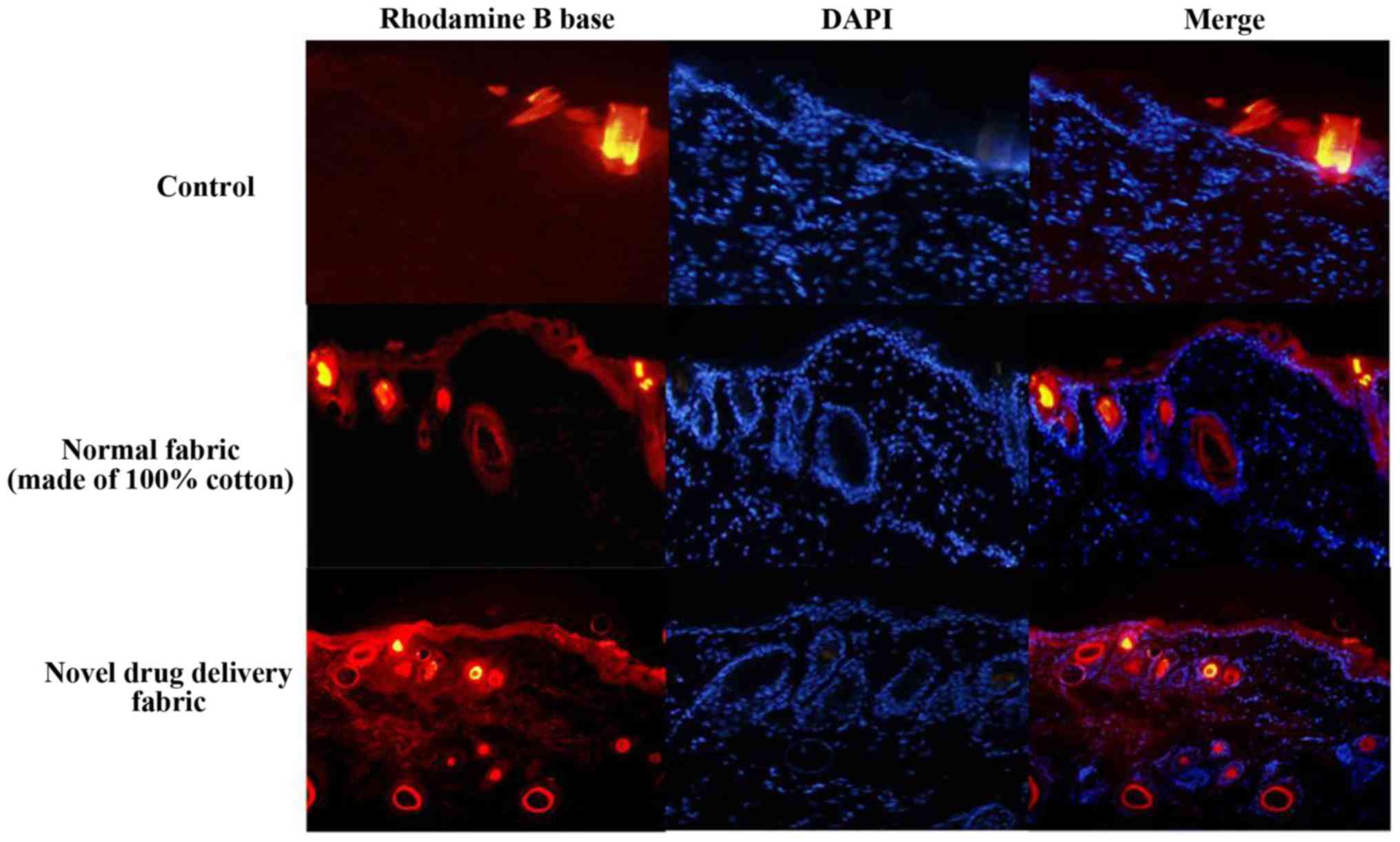
Fluorescence-based assay of rhodamine
B base skin penetration
To visualize the efficiency of the normal and test
fabrics on transdermal drug delivery, the topical application of a
lipophilic dye, rhodamine B, was performed on the back skin of all
group of each the guinea pigs. A confocal laser scanning microscope
(Leica SP5 white laser; Leica Microsystems GmbH; magnification,
x100) was then used to examine the dye delivery associated with
each fabric. Rhodamine B base dye (0.0005 M; Sigma-Aldrich; Merck
KGaA) was left to penetrate the skin for 3 h. Immediately after
treatment, skin samples were collected and then washed with PBS to
remove any residual rhodamine B and embedded in material at the
optimal cutting temperature. Fixed skin tissue was frozen by
immersion in liquid N2-cooled hexane and stored at
-80˚C. Transverse sections (60 µm), including the whole right and
left ventricles were obtained using a cryostat (Leica CM1325; Leica
Microsystems GmbH) and mounted on glass. A DAPI mounting medium kit
(OriGene Technologies, Inc.) was used to counterstain the nuclei
for 10 min at room temperature, and stained cells were visualized
using an Olympus FLUOVIEW FV10i confocal microscope (Olympus
Optical Co., Ltd.; magnification x100).
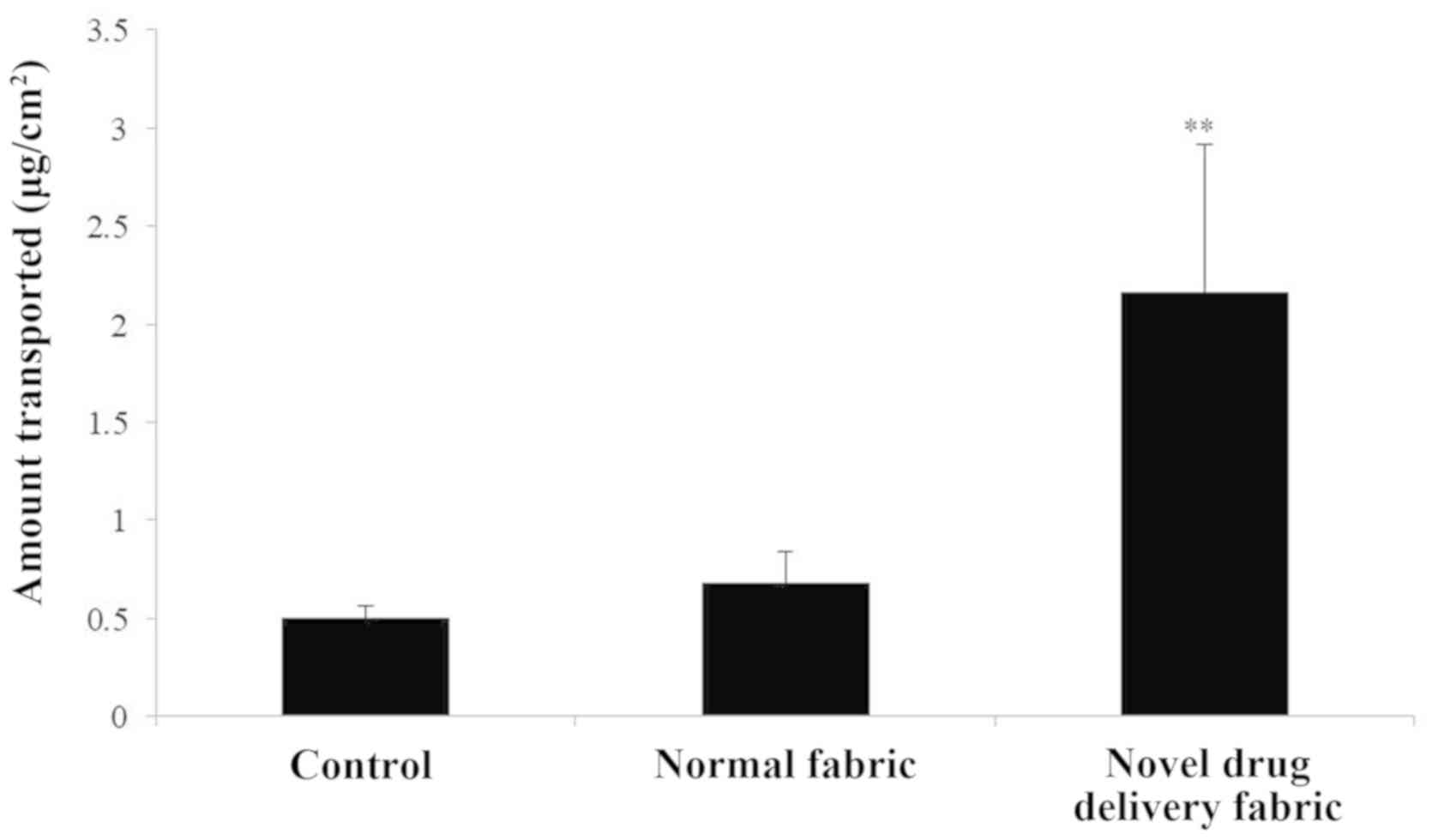
In vitro caffeine penetration
study
To assess the ability of each fabric to mediate the
penetration of a 4% caffeine solution into excised skin samples,
the samples were covered with the hydrophobic surface of the normal
fabric or the novel drug delivery fabric. Vertically assembled
Franz-type diffusion cells were used (5 experiments; 3
cells/experiment). The caffeine release experiment used an FDC-6
transdermal diffusion cell drive console (Logan Instrument Corp.).
This system is fitted with a VTC-200 heater circulator and is
equipped with a jacketed vertical glass Franz diffusion cell as its
main unit. These cells provided a diffusion area of 0.57
cm2 and the volume of the receptor compartment was 1 ml.
The test formulation (4% caffeine solution; 200 µl/cm2)
was loaded into the donor compartment before occluding the donor
compartments using normal fabric or the test (novel drug delivery)
fabric. To maintain sink conditions, PBS (pH 7.4) was used as a
receptor. Receptor samples (1 ml) were taken periodically, after
which the cells were replenished up to their marked volumes with
fresh receptor solution. Receptor solution samples were withdrawn
through the receptor sampling port every 2 h. The receptor phase
was immediately replenished with an equal volume of fresh receptor
phase. Samples were analyzed using high performance liquid
chromatography on a Futecs system. The amount of drug released was
calculated as a function of time. Each experiment was performed at
least five times.
Statistical analysis
Statistical comparisons between the treated and
untreated groups were performed using one-way ANOVA and a post-hoc
Tukey's test (SPSS software version 12.0; SPSS Inc.). Results are
expressed as the mean of at least 5 repeats and of at least three
independent experiments. *P<0.05 was considered to
indicate a statistically significant difference.
Results
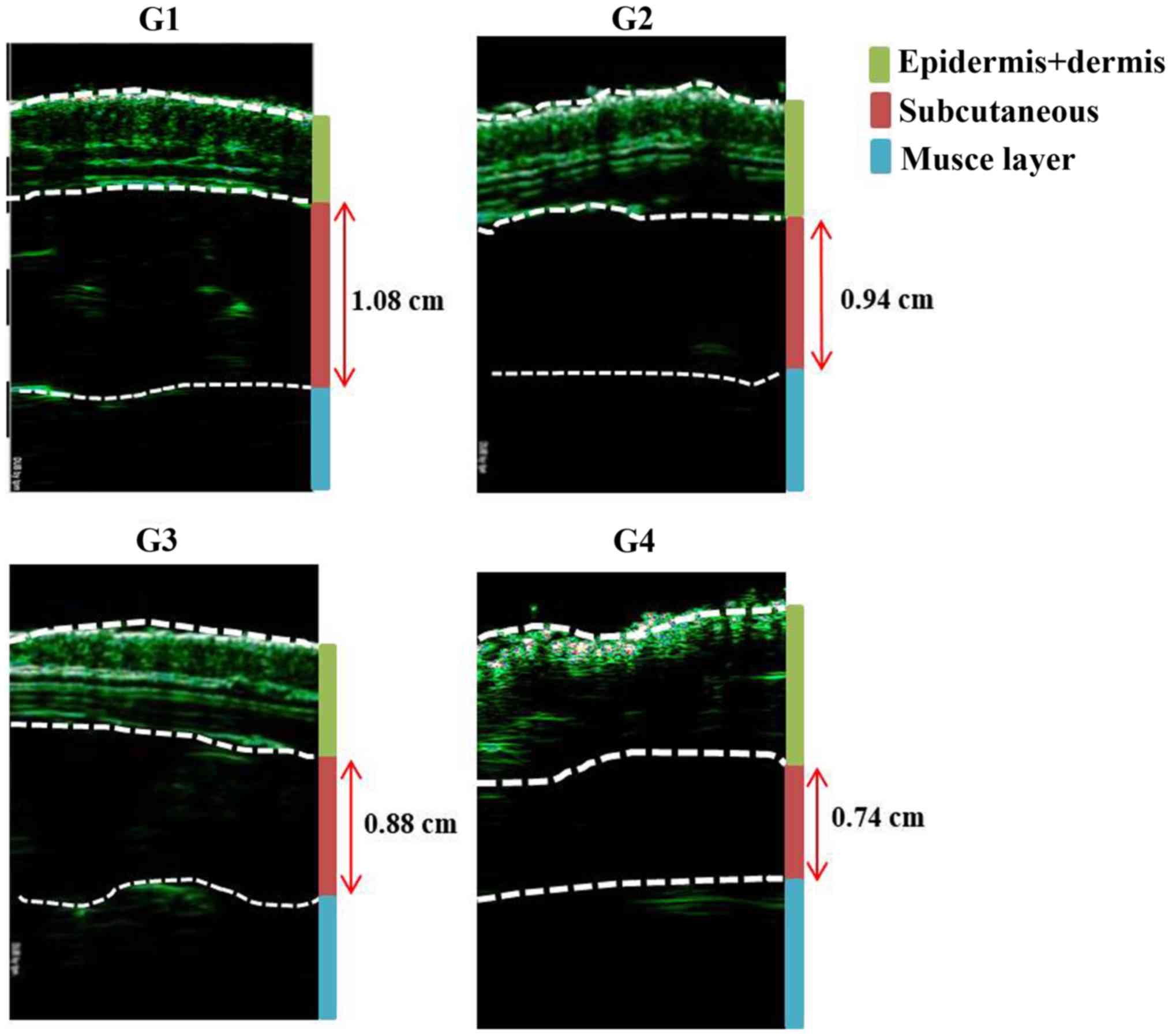
The reduction in thickness of the subcutaneous fat
was confirmed using ultrasound examination (thickness of fat layer;
group 1, 1.08 cm; group 2, 0.94 cm; group 3, 0.88 cm; group 4, 0.74
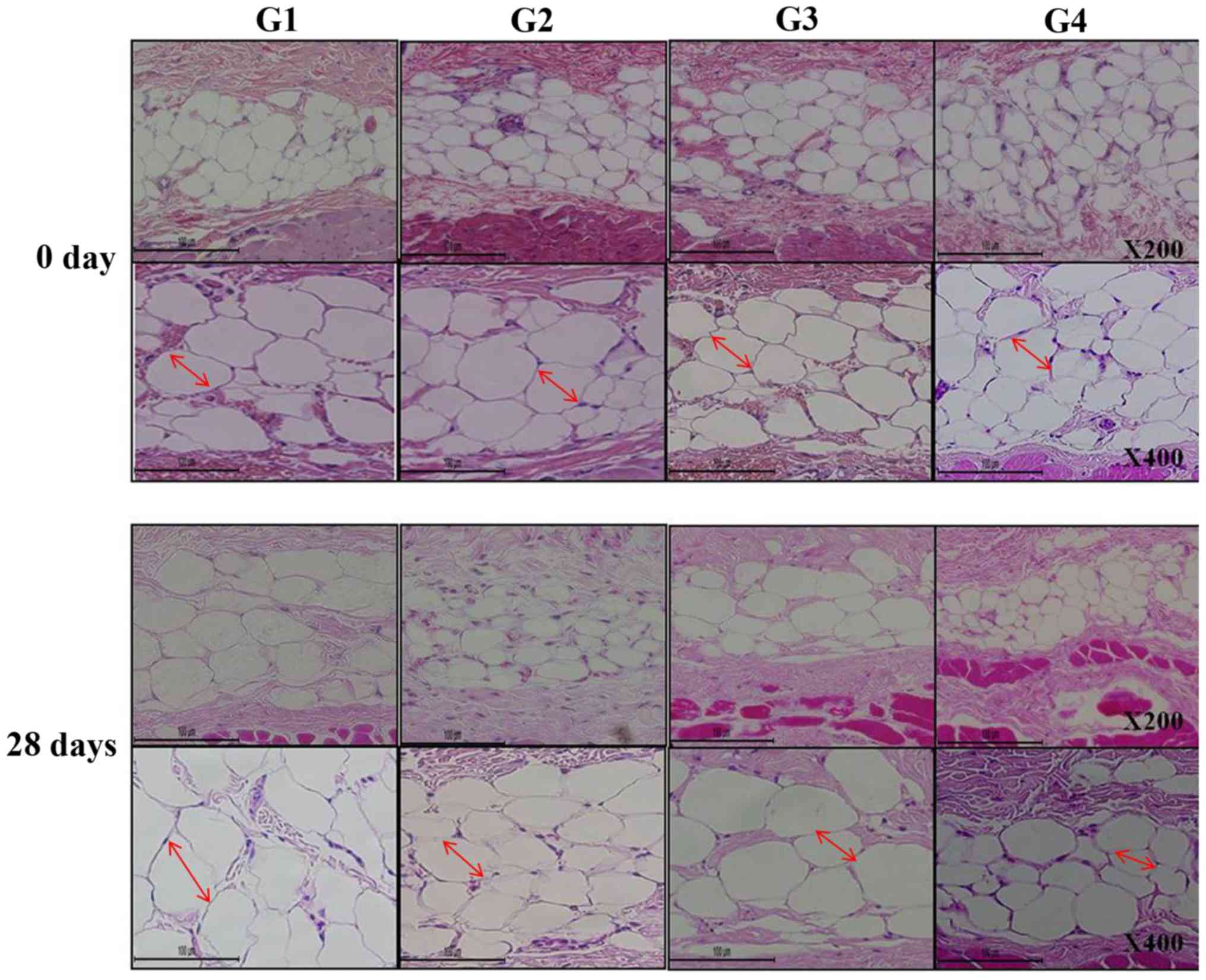
cm; Fig. 2). Furthermore, as
presented in Fig. 3, histological
analysis of the subcutaneous fat indicated that the reduction in
fat mass associated with the novel fabric was primarily due to
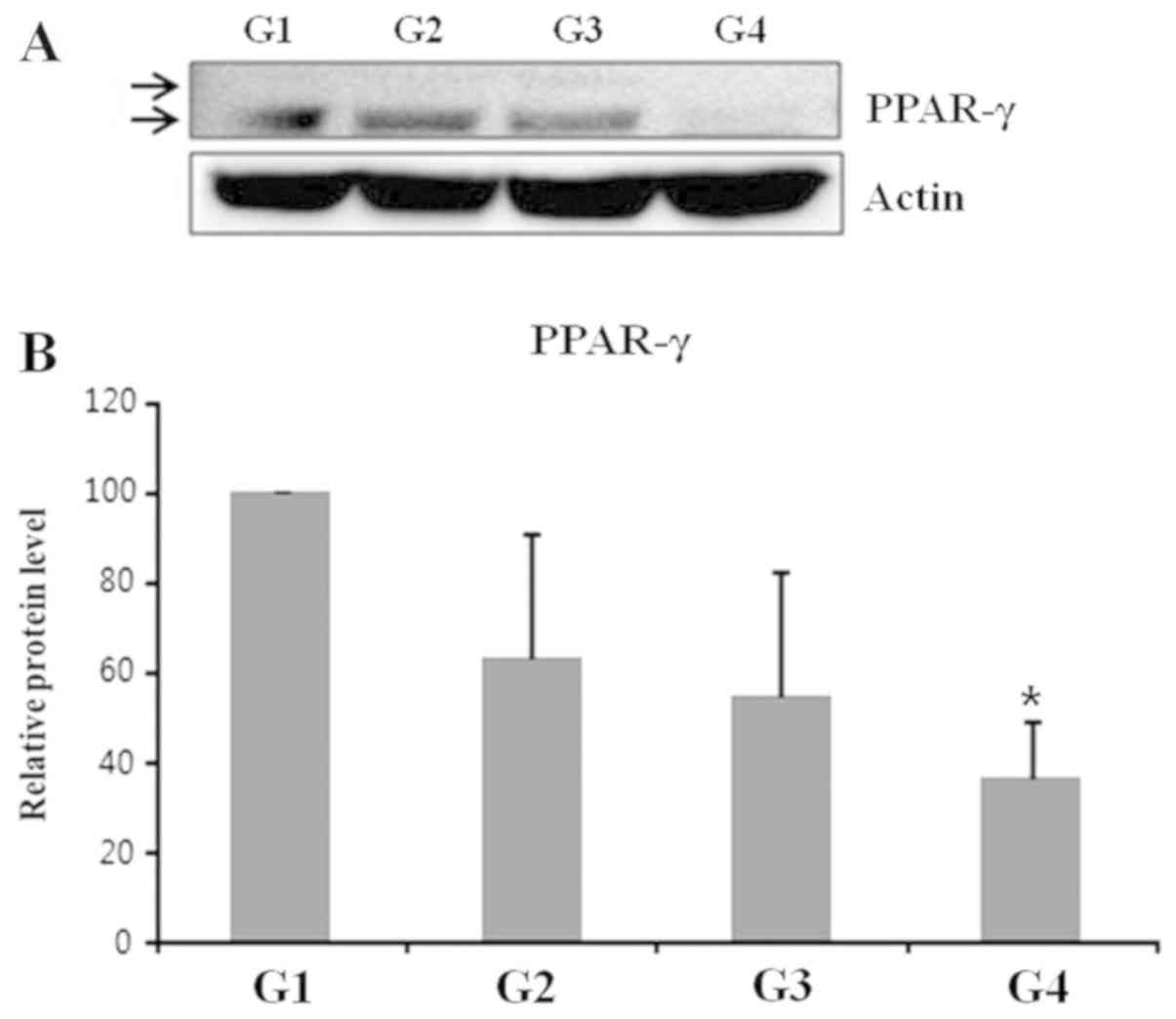
decreased adipocyte size. Additionally, the novel fabric exhibited
lower levels of PPAR-γ compared with those from control guinea pigs
(Fig. 4). In the fluorescence-based
assay of rhodamine B base skin penetration, the novel drug delivery
fabric was indicated to enhance the passage of rhodamine B through
the guinea pig skin (Fig. 5). For
animals in the control group with normal fabric, weak rhodamine B
fluorescence was observed in the upper epidermis. In contrast,
animals in the novel drug delivery fabric group exhibited strong
fluorescence in the subcutaneous and deeper dermis layer (Fig. 5). Furthermore, in the in vitro
caffeine penetration study, the quantities of caffeine that had
permeated the excised skin samples 120 min after application to the
normal fabric or the novel drug delivery fabric, are presented in
Fig. 6. The novel drug delivery
fabric enabled the permeation of 3.18-fold (2.16 µg/cm2)
more caffeine compared with normal fabric (0.68
µg/cm2).
Discussion
Although a number of methods have been previously
evaluated as transdermal drug delivery enhancers, these methods are
associated with a number of drawbacks, including low efficacy,
limitations regarding molecular weight (<500 Da), high
lipophilicity and skin irritation (25,26).
Consequently, overcoming the skin barrier in a safe and effective
way remains to be a difficulty in the development of novel
transdermal drug delivery systems. An aqueous pore pathway has been
proposed to mediate the diffusion of active therapeutic agents
across the skin under high stress conditions, including excessive
hydration (15). This class of
permeation enhancers has been demonstrated to increase skin
permeability by disordering or ‘fluidizing’ the lipid structure of
the SC and forming micro cavities within the lipid bilayers, which
increases the diffusion coefficient of a drug (16,17).
Excessive hydration utilizes the preexisting scattered lacunae that
are embedded in the lipid bilayer. These lacunae expand and form
continuous water channels that facilitate the diffusion of
hydrophilic and lipophilic permeants (27). Therefore, occlusion can increase
absorption, especially for hydrophilic compounds (28). Furthermore, a previous study assessed
the effect of clothing on physiological parameters, including skin
hydration (17). Occlusion, which
can be produced by fabric that maintains hydrophilic conditions for
the skin, partially hinders the loss of skin humidity by increasing
the water content of the horny layer (29).
Based on these observations, a novel fabric was
developed for transdermal drug delivery and an in vivo and
in vitro study was performed to assess its efficiency. The
results indicated that components of the slimming cream exhibited
increasing permeation into deeper skin layers when administered
with the novel fabric compared with a different fabric.
Although the fabric was not tested on human skin,
this fabric's ability to enhance drug penetration may be explained
by is the skin hydration effect. Fabric is known to serve an
important role in the maintenance of liquid and moisture levels
near the skin surface (30). By
placing a novel fabric on the surface of the skin, a film can form
over the skin surface. This film leads to high levels of hydration
in the SC. This hydration causes swelling of the SC and induces
openings that enhance the penetration of the given permeant.
Therefore, the fabric enables the drug to pass through the lipid
bilayers of the SC, consequently increasing its permeation. Another
possible explanation is that the skin hydration that is performed
by the fabric may also affect skin temperature. Water evaporation
normally absorbs heat and helps regulate body temperature in
response to environmental changes (30). Furthermore, fabric with hydrophilic
properties has been proposed to have physiological significance by
reducing heat strain and maintaining skin temperature (31). Therefore, clothing serves an
important role in the regulation of body temperature and heat loss.
Local increases in skin temperature have also been reported to
increase blood flow, which in turn increase the rate of permeation
or transport of active substances into the skin (26). A previous study concluded that the
application of heat (42-44˚C) for 4 h was sufficient to decrease
the time required for the patch to deliver steady-state serum
concentrations of fentanyl, from 14-18 to 3-4 h (26). Although the skin temperature was not
measured in the current study, this explanation is one possible
mechanism by which the novel fabric was observed to enhance drug
delivery.
The small sample size of the current study limited
the statistical power to detect significant differences. Skin
hydration levels were also not measured using a corneometer and the
amount of transepidermal water loss was also not determined, which
would have aided in clarifying the effect of the novel fabric on
skin hydration. Additionally, as aforementioned, if skin hydration
can contribute to the enhanced transcutaneous penetration, an
additional control experiment using a film or membrane occlusive
environment is required. The measurement of PPAR-γ, which was
conducted in the current study, is limited in explaining the
mechanism. However, the antibodies commercially available that are
reactive against guinea pig adipose tissue are limited. Therefore,
experiments using guinea pigs usually utilize the measurement of
PPAR-γ to analyze changes in fat and adipocyte layer. In future
studies, further tests that are based on a variety of results
should be conducted in the future. Finally, the novel fabric had
yet to be tested on human skin. This limitation could be overcome
in the future after producing sufficient amounts of fabric and
conducting large-scale laboratory experiments or clinical trials on
humans. Nevertheless, compared with transdermal delivery systems,
the use of this novel fabric may exhibit advantages: i) It
increases patient compliance by providing a simple route of
administration, and ii) it minimizes the risk of trauma or any
other tissue injury. Therefore, enhancing delivery of bioactive
molecules through the skin with fabrics including the novel fabric
characterized in the present study, promises to create exciting new
opportunities in the development of novel and improved techniques
for drug delivery through the skin.
In conclusion, the current study demonstrated that a
novel fabric for a transdermal drug delivery system enhances
penetration of molecules through the skin. Additional studies
investigating the potential of using drug delivery fabrics to
administer drugs are required to support the findings of the
present study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BJK and KHY designed the study. TRK, CTO and WJO
performed the research and analyzed the data. KCK and YHN analyzed
and interpreted data and contributed essential reagents or tools.
KHY, WJO and BJK were involved in drafting of the manuscript and in
critical revision of the manuscript for important intellectual
content. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Animal experiments conform to internationally
accepted standards and have been reviewed and approved by the
Institutional Animal Care and Use Committee of Chung-Ang
University, Republic of Korea (IRB number: 2018-9077).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Carita AC, Eloy JO, Chorilli M, Lee RJ and
Leonardi GR: Recent advances and perspectives in liposomes for
cutaneous drug delivery. Curr Med Chem. 25:606–635. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bos JD and Meinardi MM: The 500 Dalton
rule for the skin penetration of chemical compounds and drugs. Exp
Dermatol. 9:165–169. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thong HY, Zhai H and Maibach HI:
Percutaneous penetration enhancers: An overview. Skin Pharmacol
Physiol. 20:272–282. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jampilek J and Brychtova K: Azone
analogues: Classification, design, and transdermal penetration
principles. Med Res Rev. 32:907–947. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Münch S, Wohlrab J and Neubert RHH: Dermal
and transdermal delivery of pharmaceutically relevant
macromolecules. Eur J Pharm Biopharm. 119:235–242. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cevc G: Lipid vesicles and other colloids
as drug carriers on the skin. Adv Drug Deliv Rev. 56:675–711.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Braun SA, Gerber PA and Hevezi PA:
Needling-assisted drug delivery: Enhanced response to ingenol
mebutate after microneedling. Dermatol Surg. 43:978–979.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ono A, Azukizawa H, Ito S, Nakamura Y,
Asada H, Quan YS, Kamiyama F, Katayama I, Hirobe S and Okada N:
Development of novel double-decker microneedle patches for
transcutaneous vaccine delivery. Int J Pharm. 532:374–383.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wenande E, Erlendsson AM and Haedersdal M:
Opportunities for laser-assisted drug delivery in the treatment of
cutaneous disorders. Semin Cutan Med Surg. 36:192–201.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Serrano-Castañeda P, Escobar-Chavez JJ,
Rodriguez-Cruz IM, Melgoza LM and Martinez-Hernandez J:
Microneedles as enhancer of drug absorption through the skin and
applications in medicine and cosmetology. J Pharm Pharm Sci.
21:73–93. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sabri AH, Ogilvie J, Abdulhamid K,
Shpadaruk V, McKenna J, Segal J, Scurr DJ and Marlow M: Expanding
the applications of microneedles in dermatology. Eur J Pharm
Biopharm. 140:121–140. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim HM, Lim YY, An JH, Kim MN and Kim BJ:
Transdermal drug delivery using disk microneedle rollers in a
hairless rat model. Int J Dermatol. 51:859–863. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Benson HA: Elastic liposomes for topical
and transdermal drug delivery. Curr Drug Deliv. 6:217–226.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Choi MJ and Maibach HI: Elastic vesicles
as topical/transdermal drug delivery systems. Int J Cosmet Sci.
27:211–221. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yousef S, Mohammed Y, Namjoshi S, Grice J,
Sakran W and Roberts M: Mechanistic evaluation of hydration effects
on the human epidermal permeation of salicylate esters. AAPS J.
19:180–190. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ogawa-Fuse C, Morisaki N, Shima K, Hotta
M, Sugata K, Ichihashi T, Oguri M, Yoshida O and Fujimura T: Impact
of water exposure on skin barrier permeability and ultrastructure.
Contact Dermatitis. 80:228–233. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tan G, Xu P, Lawson LB, He J, Freytag LC,
Clements JD and John VT: Hydration effects on skin microstructure
as probed by high-resolution cryo-scanning electron microscopy and
mechanistic implications to enhanced transcutaneous delivery of
biomacromolecules. J Pharm Sci. 99:730–740. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bogerd CP, Rechsteiner I, Wüst B, Rossi RM
and Brühwiler PA: The effect of two sock fabrics on physiological
parameters associated with blister incidence: A laboratory study.
Ann Occup Hyg. 55:510–518. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu B and Hu J: The application of
temperature-sensitive hydrogels to textiles: A review of Chinese
and Japanese investigations. Fiber Textiles East. Eur. 13:45–49.
2005.
|
|
20
|
Herman A and Herman AP: Caffeine's
mechanisms of action and its cosmetic use. Skin Pharmacol Physiol.
26:8–14. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Trauer S, Patzelt A, Otberg N, Knorr F,
Rozycki C, Balizs G, Buttemeyer R, Linscheid M, Liebsch M and
Lademann J: Permeation of topically applied caffeine through human
skin-A comparison of in vivo and in vitro data. Br J Clin
Pharmacol. 68:181–186. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shakeel F and Ramadan W: Transdermal
delivery of anticancer drug caffeine from water in-oil
nanoemulsions. Colloids Surf B Biointerfaces. 75:356–362.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Grygiel-Górniak B: Peroxisome
proliferator-activated receptors and their ligands: Nutritional and
clinical implications-A review. Nutr J. 13(17)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Janani C and Ranjitha Kumari BD: PPAR
gamma gene-A review. Diabetes Metab Syndr. 9:46–50. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gee CM, Nicolazzo JA, Watkinson AC and
Finnin BC: Assessment of the lateral diffusion and penetration of
topically applied drugs in humans using a novel concentric tape
stripping design. Pharm Res. 29:2035–2046. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tiwary AK, Sapra B and Jain S: Innovations
in transdermal drug delivery: Formulations and techniques. Recent
Pat Drug Deliv Formul. 1:23–36. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Prausnitz MR and Langer R: Transdermal
drug delivery. Nat Biotechnol. 26:1261–1268. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Marepally S, Boakye CH, Shah PP, Etukala
JR, Vemuri A and Singh M: Design, synthesis of novel lipids as
chemical permeation enhancers and development of nanoparticle
system for transdermal drug delivery. PLoS One.
8(82581)2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhong W, Ahmad A, Xing MM, Yamada P and
Hamel C: Impact of textiles on formation and prevention of skin
lesions and bedsores. Cutan Ocul Toxicol. 27:21–28. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhong W, Xing MM, Pan N and Maibach HI:
Textiles and human skin, microclimate, cutaneous reactions: An
overview. Cutan Ocul Toxicol. 25:23–39. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shah DK, Khandavilli S and Panchagnula R:
Alteration of skin hydration and its barrier function by vehicle
and permeation enhancers: A study using TGA, FTIR, TEWL and drug
permeation as markers. Methods Find Exp Clin Pharmacol. 30:499–512.
2008.PubMed/NCBI View Article : Google Scholar
|