Introduction
Plasma concentrations of cardiac-derived natriuretic
peptides, specifically N-terminal pro-brain natriuretic peptide
(NT-proBNP) and brain natriuretic peptide (BNP), have been firmly
associated with cardiac function (1,2). The
increased release of those natriuretic peptides by cardiac myocytes
into the bloodstream may be the result of increased ventricular
wall stress, hypertrophy or volume overload. Therefore, measurement
of the increased serum levels of these markers may have the
potential to facilitate the accuracy of diagnosis and prognosis for
patients with heart failure (HF), thereby improving the
effectiveness of treatment strategies (3). Thus, these peptides are recognized as
diagnostically and prognostically meaningful biomarkers for
patients with HF (4).
Previous studies have also suggested that NT-proBNP
may be an effective biomarker to diagnose left ventricular
hypertrophy (LVH). To date, numerous studies have aimed to
determine the associations of various natriuretic peptide levels
with the risk of LVH in cardiovascular disease (5,6). Most
studies have focused on the association between natriuretic
peptides and the diagnostic indices measured by echocardiography,
particularly left ventricular diastolic dysfunction and left
ventricular mass (LVM) index (LVMI) (7-9).
While the prognostic significance of the NT-proBNP
regarding the risk of LVH has been evaluated in multiple research
studies, the association of NT-proBNP quartiles with the risk of
LVH in HF-negative patients remains to be explored. In addition,
the association between the serum levels of NT-proBNP with measures
of LVH, including LVMI, LVM and relative wall thickness (RWT), has
been insufficiently investigated or reported. Therefore, the
purpose of the present study was to determine how NT-proBNP levels,
assessed in different quartiles, may affect the occurrence of LVH
and to investigate the association between NT-proBNP and measures
of LVH, including LVM, LVMI and RWT, in HF-negative patients.
Patients and methods
Patients
As the present study was a retrospective
observational study, the requirement for patient consent and
specifically ethical approval was waived. Medical records of 774
patients who received standard treatment at the Department of
Cardiology of Tianjin Medical University General Hospital (Tianjin,
China) between June 2015 and September 2015 were reviewed. The
following exclusion criteria were then applied to the sample pool:
i) Lack of critical clinical data; ii) serious primary conditions,
including liver and renal failure, iii) other critical illnesses.
The criteria for HF-negative status were established as the absence
of any symptom that may contribute to the diagnosis of acute HF
(AHF) or chronic HF (CHF). In terms of AHF, the diagnosis was
confirmed on the basis of chest X-ray, which indicated cardiac
congestion, as well as echocardiography revealing cardiac
dysfunction and abnormally upregulated cardiac markers (10). In terms of CHF, in addition to the
features of cardiac dysfunction, particularly echocardiography
suggesting systolic or diastolic dysfunctions, the confirmative
symptoms also included certain other somatic abnormalities,
including swollen ankle, respiratory arrest and fatigue in a
stationary body position or along with movements (10). Taking all of the above standards into
account, 622 patients (age range, 16-89 years; mean age, 61.5±13
years; 366 males and 296 females) were finally included in the
present study.
Assays for biochemical indices and
NT-proBNP
The biochemical indices and NT-proBNP were available
from the patients' medical records. The relevant detection methods
are described below. Directly after fasting for 10 h, early in the
morning, venipuncture of the antecubital vein was performed to
collect a blood sample. All of the patient samples were delivered
to the professional diagnostic laboratory for blood tests,
including triglycerides (TG), total cholesterol (TC), high-density
lipoprotein cholesterol (HDL-C), low-density lipoprotein
cholesterol (LDL-C), uric acid (UA), alanine aminotransferase
(ALT), fasting plasma glucose (FPG) and serum creatinine (Scr).
Serum NT-proBNP levels were measured with an available immunoassay
analyzer (Elecsys 2010; Roche Diagnostics). The quality control and
quality assurance were in accordance with a standardized protocol
and all the relevant parameters passed the professional assessments
by the Clinical Laboratory of Tianjin Medical University General
Hospital. The Clinical Laboratory of Tianjin Medical University
General Hospital supplied the normal reference values.
Echocardiography
A cardiology professional consistently performed all
transthoracic echocardiography measurements. Using M-mode
echocardiography, the cardiac indices were measured following
standardized procedures from the American Society of
Echocardiography, including ventricular internal dimension in
end-diastole (LVIDd), interventricular septum thickness (IVST) in
end-diastole and posterior wall thickness (PWT) in end-diastole
(11). Based on the measurements of
the above indices, the LVM was calculated depending on the Devereux
formula: LVM
(g)=1.04x[(LVIDd+IVST+PWT)3-LVIDd3]-13.6 g.
The calculation of LVMI was in accordance with the following
formula: LVMI (g/m2)=LVM/body surface area, while LVH
was accordingly defined as LVMI ≥125 g/m2 in males and
≥120 g/m2 in females (12). The calculation of the relative wall
thickness (RWT) was as follows: RWT=2xPWT/LVIDd (11). The RWT partition value was set at
0.45(13). All subjects were
stratified into four groups depending on LV characteristics: Normal
LV geometry (normal LVMI and RWT), concentric LV remodeling (normal
LVMI and increased RWT), eccentric LVH (increased LVMI and normal
RWT) or concentric LVH (increased LVMI and RWT) (14).
Statistical analysis
Statistical analyses were performed with SPSS 17.0
software (SPSS, Inc.). Continuous variables are presented as the
mean ± standard deviation or as the median with interquartile range
for those variables with a skewed distribution (assessed by
Kolmogorov-Smirnov test), while categorical variables are presented
as numbers and percentages. Differences between groups were
analyzed using an independent-samples t-test, as well as a
non-parametric test (Mann-Whitney U test) for sets of measurement
data or the χ2 test for sets of categorical data. To
normalize the concentration distribution, the logarithmic values of
serum NT-proBNP levels were calculated. Accordingly, the
association between Log(NT-proBNP) and LVM, LVMI and RWT were
analyzed using multivariate linear regression. For influencing
factors, including age, sex, hypertension, percutaneous coronary
intervention (PCI), diabetes, smoking, heart rate (HR), body mass
index (BMI), systolic blood pressure (SBP), diastolic blood
pressure (DBP), Scr, TC, TG, HDL-C, LDL-C, FPG, ALT and UA,
corresponding adjustments were made. Using logistic regression
analysis, the association between NT-proBNP quartiles and the risk
of LVH was interpreted. Risk ratios (RRs) were presented with 95%
CIs. All of the statistical comparisons were two-tailed and
P<0.05 was considered to indicate statistical significance.
Results
The occurrence of LVH increases
progressively along with individual NT-proBNP quartiles in
HF-negative patients without HF
To confirm the prognostic significance of NT-proBNP
levels regarding the prevalence of LVH in patients without HF, they
were stratified into groups of quartiles based on the NT-proBNP
concentration in the present study: Quartile 1 (≤56.67 pg/ml),
quartile 2 (56.95-119.50 pg/ml), quartile 3 (119.70-414.00 pg/ml)
and quartile 4 (≥415.60 pg/ml). The basic clinical features of the
testing groups are listed in Table
I. Among the four groups, the following parameters were
comparable without any significant variation: Sex, smoking history,
previously diagnosed PCI, hypertension and diabetes. Age, HR, FPG,
LVIDd, LVM and LVMI were statistically significant among the four
groups. The number of patients with LVH in quartiles 1-4 were 44,
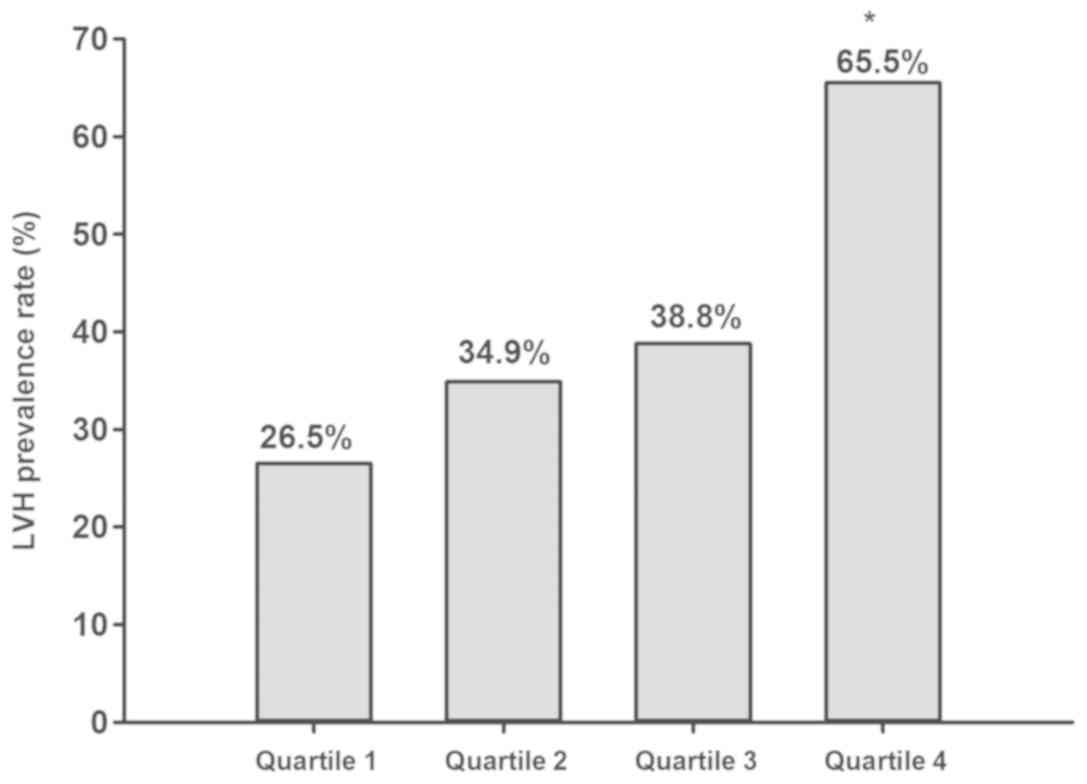
58, 64 and 108, respectively. The LVH prevalence rates in NT-proBNP
quartiles 1-4 were 28.5, 34.9, 38.8 and 65.5%, respectively, as
presented in Fig. 1. The patients in
quartile 4 had an increased LVH occurrence rate in comparison with
that in the other quartiles and the LVH risk increased across the
individual quartiles with a positive association with the NT-proBNP
level.
 | Table IClinical characteristics of the study
subjects in N-terminal pro-brain natriuretic peptide quartiles. |
Table I
Clinical characteristics of the study
subjects in N-terminal pro-brain natriuretic peptide quartiles.
| Item | Total (n=622) | Quartile 1 ≤56.67
pg/ml (n=166) | Quartile 2
56.95-119.50 pg/ml (n=166) | Quartile 3
119.70-414.00 pg/ml (n=165) | Quartile 4 ≥415.60
pg/ml (n=165) | P-value |
|---|
| Male sex (%) | 366 (55.3) | 102 (61.4) | 81 (48.8) | 90 (54.5) | 93 (56.4) | 0.139 |
| Age (years) | 61.48±12.85 | 54.48±12.64 | 59.60±11.75 | 63.81±11.75 | 67.97±11.15 | <0.001 |
| Hypertension (%) | 446 (71.7) | 113 (68.1) | 108 (65.1) | 107 (64.8) | 118 (71.5) | 0.531 |
| PCI (%) | 193 (31.0) | 30 (18.1) | 36 (21.7) | 56 (33.9) | 71 (43.0) | 0.613 |
| Diabetes (%) | 171 (27.5) | 38 (22.9) | 41 (24.7) | 45 (27.3) | 47 (28.5) | 0.613 |
| Smokers (%) | 287 (46.1) | 76 (45.8) | 67 (40.4) | 69 (41.8) | 75 (45.5) | 0.692 |
| HR (bpm) (%) | 72.28±15.19 | 72.20±11.01 | 69.84±12.96 | 69.07±14.57 | 77.98±19.46 | <0.001 |
| BMI
(kg/m2) | 25.71±3.56 | 26.56±3,60 | 25.34±3.53 | 25.46±3.53 | 25.53±3.48 | 0.006 |
| SBP (mmHg) | 137.76±47.11 | 136.50±18.99 | 137.88±18.69 | 137.35±20.31 | 137.36±23.79 | 0.924 |
| DBP (mmHg) | 80.44±13.58 | 83.52±12.94 | 80.21±13.46 | 80.36±12.46 | 77.72±14.85 | 0.002 |
| Scr (umol/l)
(62-133)a | 72.60±35.95 | 66.23±14.53 | 71.17±15.07 | 72.52±20.42 | 80.71±37.10 | 0.003 |
| TC (mmol/l)
(3.59-5.17)a | 4.36±1.05 | 4.40±0.94 | 4.4.26±0.95 | 4.36±0.1.08 | 4.44±1.22 | 0.495 |
| TG (mmol/l)
(0.57-1.71)a | 1.60±1.24 | 1.75±1.26 | 1.48±1.10 | 1.56±1.04 | 1.62±1.51 | 0.272 |
| HDL (mmol/l)
(0.80-2.20)a | 1.07±0.33 | 1.04±0.26 | 1.13±0.43 | 1.08±0.33 | 1.05±0.28 | 0.050 |
| LDL (mmol/l)
(1.33-3.36)a | 2.61±0.89 | 2.62±0.82 | 2.49±0.82 | 2.60±0.89 | 2.73±1.02 | 0.109 |
| FPG (mmol/l)
(3.6-5.8)a | 5.82±2.03 | 5.44±1.62 | 5.56±1.62 | 5.76±1.84 | 6.50±2.68 | <0.001 |
| ALT (U/l)
(5-40)a | 25.95±38.72 | 25.96±18.7 | 23.81±20.38 | 23.99±20.80 | 26.85±21.83 | 0.538 |
| UA (µmol/l)
(140-414)a | 356.13±108.82 | 362.45±105.91 | 341.44±91.99 | 350.25±97.81 | 371.82±122.13 | 0.046 |
| LVIDd (mm) | 48.59±4.57 | 47.53±3.66 | 47.93±4.15 | 48.33±4.42 | 50.54±5.31 | <0.001 |
| IVST (mm) | 10.37±1.59 | 10.29±1.33 | 10.11±1.26 | 10.38±1.53 | 10.70±2.07 | 0.008 |
| PWT (mm) | 10.26±1.29 | 10.20±1.25 | 10.06±1.19 | 10.24±1.20 | 10.52±1.44 | 0.011 |
| LVM (g) | 215.60±59.91 | 205.57±52.31 | 203.84±52.13 | 212.67±55.18 | 240.54±71.26 | <0.001 |
| LVMI
(g/m2) | 121.11±31.02 | 111.28±23.87 | 114.66±25.52 | 121.71±29.78 | 136.87±37.10 | <0.001 |
| RWT | 0.42±0.06 | 0.43±0.05 | 0.42±0.06 | 0.42±0.05 | 0.42±0.06 | 0.376 |
Association between NT-proBNP
quartiles and LV geometric patterns
For an in-depth analysis of the correlation between
NT-proBNP quartiles and LV geometric patterns, all participants
were first stratified into four groups depending on their LVMI and
RWT values, namely the normal LV geometry group, concentric
remodeling group, eccentric LVH group and concentric LVH group. The
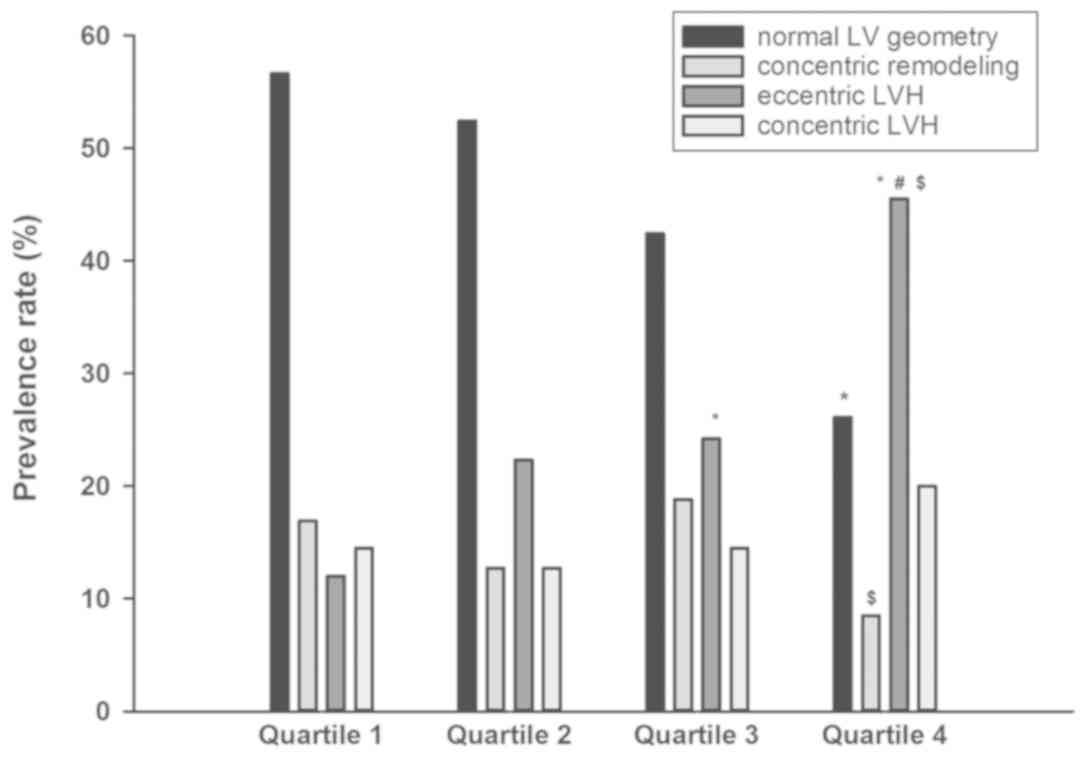
prevalence rates of abnormal LV geometric patterns are presented
for the concentric remodeling group, eccentric LVH group, eccentric
LVH group and eccentric LVH group in Fig. 2 and were 16.9, 22.3, 24.2 and 45.5%
in patients with NT-proBNP levels in quartiles 1, 2, 3 and 4,
respectively. The prevalence rates of eccentric LVH between the
lowest quartile and the highest quartile differed significantly.
Compared with quartile 3, the prevalence rates of concentric
remodeling and eccentric LVH were statistically different form
quartile 4.
Elevated NT-proBNP predicts LVH
risk
The association of the NT-proBNP level with LVH risk
was assessed using univariate and multivariate logistic regression
(Table II). Univariate logistic
regression indicated that the LVH risk values in quartiles 2, 3 and
4 were 1.489 (0.931-2.382), 1.757 (1.103-2.799) and 5.254
(3.281-8.413) fold of that in the quartile 1, respectively. The LVH
prevalence rates also significantly varied among the three
quartiles, as well as between the highest and lowest quartile
(quartiles 4 and 1, respectively). After the results were adjusted
for age, sex, hypertension, PCI, diabetes, smoking, HR, BMI, SBP,
DBP, Scr, TC, TG, HDL-C, LDL-C, FPG, ALT and UA, multivariate
logistic regression analysis was performed. Using the final
multivariate model, the RRs for the LVH risk of patients in
quartiles 2, 3 and 4 compared with those in quartile 1 were 1.683
(1.015-2.792), 1.800 (1.071-3.025) and 5.679 (3.225-9.999),
respectively. Overall, patients in quartiles 2, 3 and 4 had a
significantly higher LVH prevalence rate compared with patients in
quartile 1; in summary the results indicated that patients with
elevated NT-proBNP were significantly more likely to develop
LVH.
 | Table IIUni- and multivariate logistic
regression models describing the risk for the prevalence rate of
LVH in the study subjects. |
Table II
Uni- and multivariate logistic
regression models describing the risk for the prevalence rate of
LVH in the study subjects.
| Model 1 | Model 2 |
|---|
| Item | RR (95% CI) | P-value | RR (95% CI) | P-value |
|---|
| NT-pro BNP
quartile |
| 1 (≤56.67
pg/ml) | 1 | - | 1 | - |
| 2 (56.95-119.50
pg/ml) | 1.489
(0.931-2.382) | 0.097 | 1.683
(1.015-2.792) | 0.044 |
| 3 (119.70-414.00
pg/ml) | 1.757
(1.103-2.799) | 0.018 | 1.800
(1.071-3.025) | 0.027 |
| 4 (≥415.60
pg/ml) | 5.254
(3.281-8.413) | <0.001 | 5.679
(3.225-9.999) | <0.001 |
| Age (years) | | | 1.014
(0.997-1.031) | 0.115 |
| Male sex | | | 1.104
(0.694-1.756) | 0.677 |
| Hypertension | | | 1.524
(1.016-2.287) | 0.042 |
| PCI | | | 1.183
(0.791-1.769) | 0.414 |
| Diabetes | | | 1.488
(0.922-2.401) | 0.103 |
| Smoking | | | 1.637
(1.085-2.470) | 0.019 |
| HR (bpm) | | | 0.991
(0.980-1.003) | 0.142 |
| BMI
(kg/m2) | | | 0.991
(0.939-1.045) | 0.731 |
| SBP (mmHg) | | | 1.000
(0.996-1.004) | 0.975 |
| DBP (mmHg) | | | 1.025
(1.009-1.041) | 0.002 |
| Scr (mmol/l) | | | 0.997
(0.989-1.004) | 0.372 |
| TC (mmol/l) | | | 0.451
(0.187-1.090) | 0.077 |
| TG (mmol/l) | | | 1.242
(0.942-1.638) | 0.125 |
| HDL-C (mmol/l) | | | 1.486
(0.555-3.976) | 0.430 |
| LDL-C (mmol/l) | | | 2.327
(0.946-5.724) | 0.066 |
| FPG (mmol/l) | | | 1.033
(0.924-1.154) | 0.569 |
| ALT (U/l) | | | 1.000
(0.995-1.004) | 0.928 |
| UA (µmol/l) | | | 1.001
(0.999-1.003) | 0.600 |
Serum NT-proBNP is positively
correlated with LVM and LVMI, while it is inversely correlated with
RWT
To substantiate the association between NT-proBNP
and LVM, LVMI and RWT, two types of regression analyses were
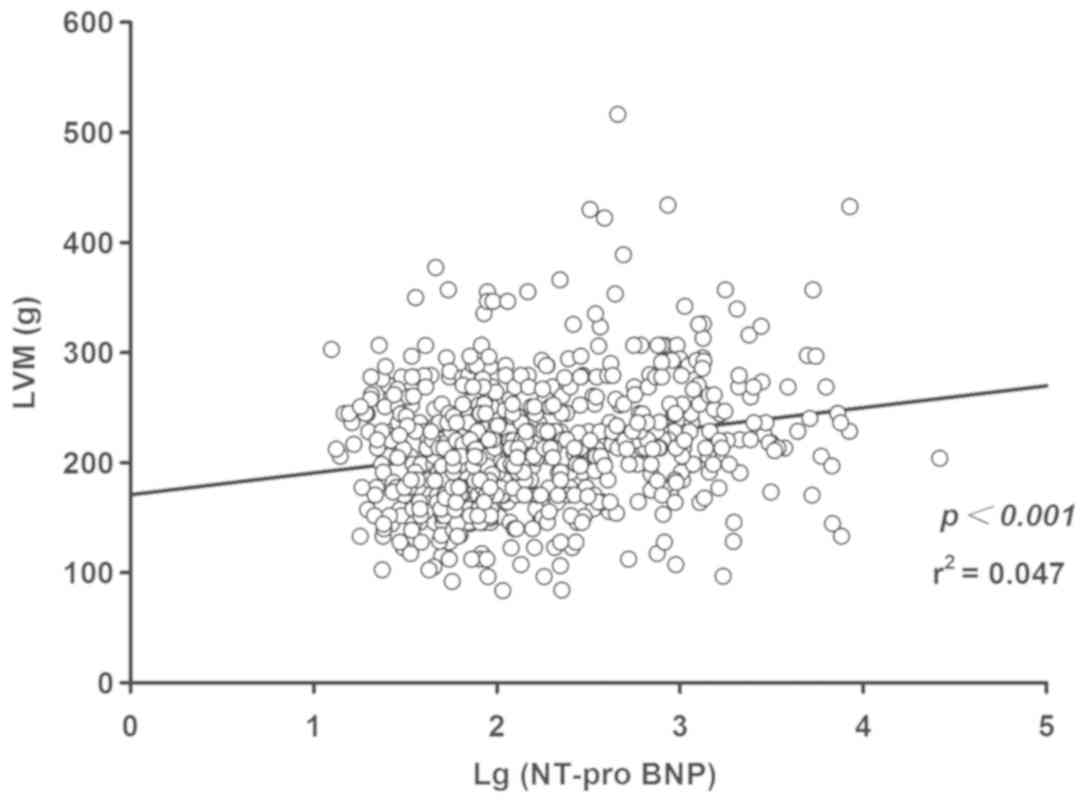
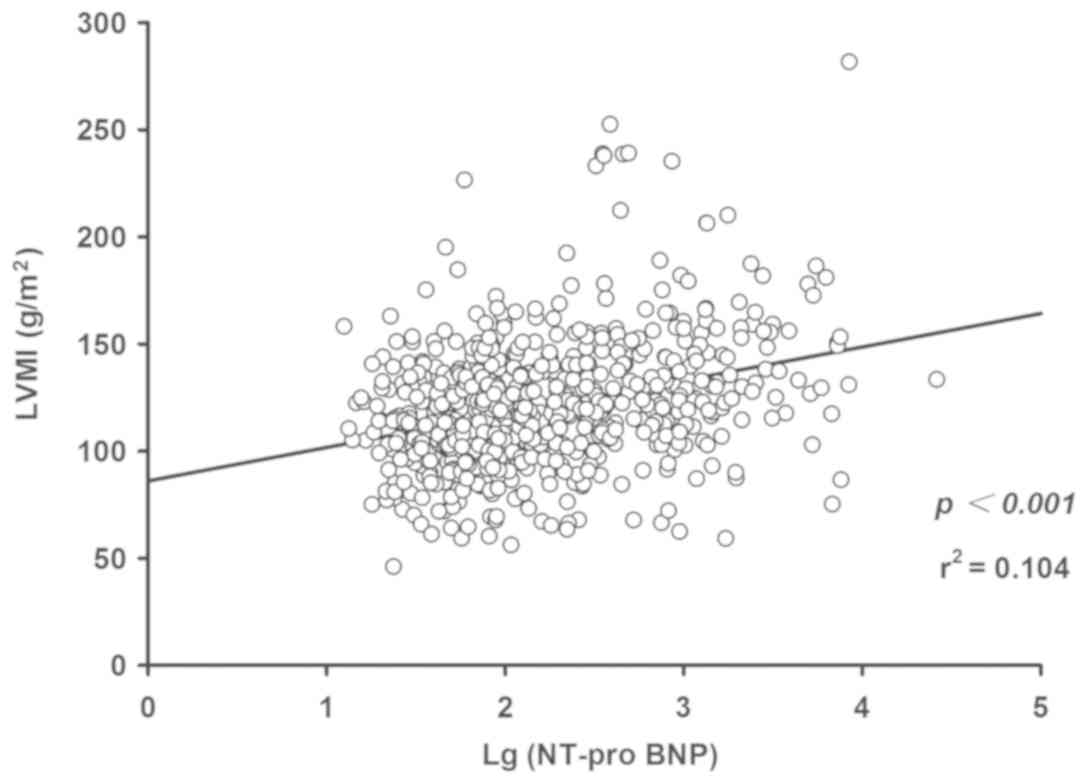
performed. The linear regression results indicated a positive
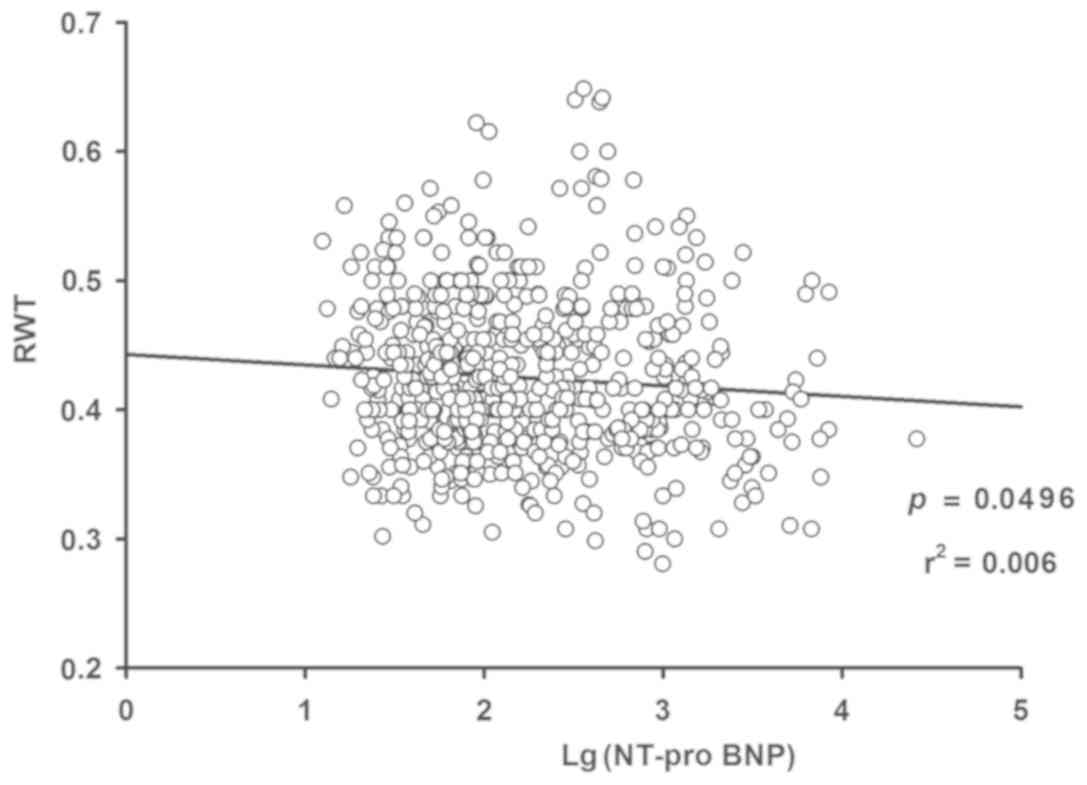
association between Log(NT-proBNP) and LVM (P<0.001; Fig. 3) and LVMI (P<0.001; Fig. 4), while an inverse correlation
between Log(NT-proBNP) and RWT was indicated (P<0.05; Fig. 5). Using the multivariate regression
model, significant associations between Log(NT-proBNP) and LVM,
LVMI and RWT were confirmed after adjustment for age, sex,
hypertension, PCI, diabetes, smoking history, HR, BMI, SBP, DBP,
Scr, TC, TG, HDL-C, LDL-C, FPG, ALT and UA, as presented in
Table III.
 | Table IIIResults of multivariate modelling for
Lg(NT-proBNP) (n=662). |
Table III
Results of multivariate modelling for
Lg(NT-proBNP) (n=662).
| RWT | LVM | LVMI |
|---|
| Item | β | t | P-value | β | t | P-value | β | t | P-value |
|---|
| Lg(NT-proBNP) | -0.009 | -2.138 | 0.033 | 26.388 | 7.604 | <0.001 | 16.674 | 8.065 | <0.001 |
| Male sex | 0.004 | 0.667 | 0.505 | -32.883 | -6.789 | <0.001 | -4.167 | -1.444 | 0.149 |
| Age (years) | <0.001 | 1.416 | 0.157 | -0.143 | -0.832 | 0.406 | 0.029 | 0.289 | 0.773 |
| Hypertension | 0.024 | 4.512 | <0.001 | 13.170 | 3.128 | 0.002 | 6.587 | 2.626 | 0.009 |
| PCI | -0.012 | -2.177 | 0.030 | -1.150 | -0.269 | 0.788 | 0.226 | 0.089 | 0.929 |
| Diabetes | -0.003 | -0.478 | 0.633 | 4.511 | 0.885 | 0.376 | 5.271 | 1.736 | 0.083 |
| Smoking | <0.001 | 0.021 | 0.983 | 13.252 | 3.051 | 0.002 | 6.514 | 2.517 | 0.012 |
| HR (bpm) | <0.001 | 1.055 | 0.292 | 0.039 | 0.318 | 0.750 | -0.010 | -0.131 | 0.896 |
| BMI
(kg/m2) | <0.001 | 0.530 | 0.596 | 4.617 | 8.252 | <0.001 | 0.110 | 0.330 | 0.742 |
| SBP (mmHg) | <0.001 | 1.344 | 0.179 | 0.016 | 0.403 | 0.687 | 0.011 | 0.469 | 0.639 |
| DBP (mmHg) | 0.001 | 2.943 | 0.003 | 0.501 | 3.142 | 0.002 | 0.341 | 3.587 | <0.001 |
| Scr (mmol/l) | <0.001 | 2.597 | 0.010 | -0.034 | -0.632 | 0.528 | -0.017 | -0.533 | 0.594 |
| TC (mmol/l) | 0.008 | 0.783 | 0.434 | -5.955 | -0.714 | 0.475 | -2.729 | -0.549 | 0.583 |
| TG (mmol/l) | -0.003 | -1.081 | 0.280 | 1.284 | 0.529 | 0.597 | 0.400 | 0.277 | 0.782 |
| HDL-C (mmol/l) | -0.014 | -1.191 | 0.234 | 1.355 | 0.141 | 0.888 | -1.782 | -0.311 | 0.756 |
| LDL-C (mmol/l) | -0.011 | -1.017 | 0.310 | 6.909 | 0.815 | 0.415 | 2.985 | 0.591 | 0.555 |
| FPG (mmol/l) | 0.002 | 1.185 | 0.237 | 0.947 | 0.815 | 0.416 | 0.223 | 0.322 | 0.748 |
| ALT (U/l) | <0.001 | -0.391 | 0.696 | -0.039 | -0.820 | 0.413 | -0.008 | -0.267 | 0.789 |
| UA (µmol/l) | <0.001 | -0.022 | 0.982 | 0.022 | 1.119 | 0.026 | 0.014 | 1.187 | 0.236 |
Discussion
Accumulating studies suggest that inherent and
acquired abnormalities within the natriuretic peptide system may
contribute to the occurrence of a series of systemic and cardiac
diseases, particularly cardiac hypertrophy (2). NT-proBNP is a BNP precursor released by
ventricular tissues in response to an aggravated ventricular
burden, which may induce ventricle remodeling. The preliminary
NT-proBNP has a relatively large molecular size, as well as a
longer half-life than the active form; therefore, its measurement
is comparably convenient and is less likely to be interfered with
by acute changes that may markedly affect the levels of other
natriuretic peptides (15). As the
relative stability of NT-proBNP enhances its reliability as a
ventricular stress indicator and as a potential prognostic
indicator, the serum levels of NT-proBNP were selected as the
candidate biomarker to be investigated in the present study.
Previous studies have proved that NT-proBNP is an
effective marker for LVH diagnosis. For instance, a population
study indicated that NT-proBNP levels were elevated in LVH
regardless of hypertension diagnosis (6). NT-proBNP measurements were also proved
to be significant to LVH diagnosis for patients in the 1st year
after renal transplantation (16)
and were correlated with left ventricular dysfunction in dialysis
patients (17). The present study
provided an in-depth insight into the risk of LVH predicted by
NT-proBNP quartiles and the complex association of NT-proBNP with
LVM, LVMI and RWT in HF-negative patients. The results indicated
that the prevalence rate of LVH increased progressively across
individual NT-proBNP quartiles in patients without HF. The
prevalence rate of abnormal LV geometric patterns was highest in
the concentric remodeling group in NT-proBNP quartile 1 and in the
eccentric LVH group in quartiles 2-4. According to univariate
logistic regression, patients in NT-proBNP quartile 4 and quartile
3 had a 5.254- and a 1.757-fold increased LVH risk compared to
patients in NT-proBNP quartile 1, respectively. Multivariate
logistic regression also indicated that, compared with that of
patients in NT-proBNP quartile 1, patients in NT-proBNP quartiles
2-4 had a significantly increased LVH risk. Furthermore,
significant positive linear correlations of NT-proBNP with LVM and
LVMI were identified, while an inverse correlation of NT-proBNP
with RWT was indicated. After adjustments with the consideration of
multiple potential interferences, including clinical parameters and
predisposing conditions, significant associations between NT-proBNP
and LVM, LVMI and RWT were further confirmed.
Numerous clinical studies have reported an
association between upregulated natriuretic peptides and abnormally
increased blood pressure. For instance, according to a
cross-sectional study involving 202 participants with a previous
history of dyspnea, the median NT-proBNP level was increased by 60%
in hypertension-positive participants in comparison with
hypertension-negative participants (18). A prospective analysis involving 1,801
participants reported that upregulated BNP plasma levels were
significantly correlated with an elevated risk of aggravated
hypertension occurring four years later for males rather than
females (19). Bower et al
(20) reported that participants in
the lowest NT-proBNP quartile had a minimum risk of hypertension in
comparison with any of the upper quartiles. In detail, the
hypertension risks in the order from the lowest to the highest
NT-proBNP quartiles were 1.00 at baseline (reference), 1.10 (95%
CI: 0.97-1.24), 1.08 (95% CI: 0.95-1.24), and 1.24 (1.08-1.42),
respectively. It also indicated that the log-unit increase of
NT-proBNP corresponded to an 8% increase of the hypertension risk
(95% CI: 1.03-1.13) (20). In
addition, hypertension was generally considered to be the
predominant predisposing factor for LVH, which was suggested to be
the result of excessive left ventricular afterload. Thus, it may be
speculated that individuals with upregulated NT-proBNP were at
increased risk of LVH, which may be associated with
hypertension.
Accumulating studies suggested that inflammatory
reactions may have a critical influence on the pathophysiological
mechanisms of LVH (21,22). Animal studies also indicated a
critical role of inflammatory cytokines in the pathogenesis of LVH.
Zhao et al (23) reported
that genetic deletion of interleukin (IL)-6 attenuates transverse
aortic constriction-induced LVH and LV dysfunction in mice.
According to previous research, in a population of asymptomatic
patients with high blood pressure, upregulated BNP was correlated
with increased levels of inflammatory cytokines, including tumor
necrosis factor-α, IL-6 and IL-8 and also associated with increased
LVMI and left atrial volume index (24). Furthermore, according to certain
animal studies, BNPs have important roles in the regulation of
myocardial fibrosis (25,26). Excessive interstitial fibrosis was
observed even in patients at the early stage of hypertension with
only moderately upregulated LVH (27,28). In
addition, certain studies have reported that regression of the
fibrosis degree resulted in improved LV function (29,30).
Thus, it may be speculated that the natriuretic peptide levels are
clinically significant markers for the preliminary subclinical
pathological process involving inflammation, myocardial fibrosis
and cardiac remodeling.
In conclusion, the present study examined the
association between NT-proBNP and LVH risk and the association
between NT-proBNP and LVH hallmarks in patients without HF.
Therefore, whether NT-proBNP levels have potential diagnostic,
prognostic and epidemiological implications regarding LVH in
patients without HF still requires in-depth investigation by
further studies. However, the present study had several
limitations. First, the study was observational. Furthermore, only
patients who were admitted to the cardiology department were
enrolled and due to lack of their NT-proBNP data, no healthy
subjects were included for reference. More importantly, the sample
size was limited and the results of the present study require
further verification by studies with an extended scope.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LH and XW designed the study and performed the
experiments. JZ and JY collected the data, LH and LFH analyzed the
data and LH and XW prepared the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
As the present study was a retrospective
observational study, the requirement for patient consent and
specifically ethical approval was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Lemos JA, McGuire DK and Drazner MH:
B-type natriuretic peptide in cardiovascular disease. Lancet.
362:316–322. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gupta DK and Wang TJ: Natriuretic peptides
and cardiometabolic health. Circ J. 79:1647–1655. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Santaguida PL, Don-Wauchope AC, Oremus M,
McKelvie R, Ali U, Hill SA, Balion C, Booth RA, Brown JA, Bustamam
A, et al: BNP and NT-probNP as prognostic markers in persons with
acute decompensated heart failure: A systematic review. Heart Fail
Rev. 19:453–470. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ponikowski P, Voors AA, Anker SD, Bueno H,
Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP,
Jankowska EA, et al: 2016 ESC guidelines for the diagnosis and
treatment of acute and chronic heart failure. Kardiol Pol.
74:1037–1147. 2016.(In Polish). PubMed/NCBI View Article : Google Scholar
|
|
5
|
Morillas P, Castillo J, Quiles J, Nuñez D,
Guillén S, Maceira A, Rivera M and Bertomeu V: Usefulness of
NT-probNP level for diagnosing left ventricular hypertrophy in
hypertensive patients. A cardiac magnetic resonance study. Rev Esp
Cardiol. 61:972–975. 2008.(In English, Spanish). PubMed/NCBI
|
|
6
|
Rivera Otero JM, Taléns-Visconti R,
Salvador A, Bertomeu V, Miró V, Jordán A, Sogorb F, Cortés R, Payá
R, Diago JL, et al: Ventricular hypertrophy increases NT-proBNP in
subjects with and without hypertension. Int J Cardiol. 96:265–271.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Parekh N and Maisel AS: Utility of
β-natriuretic peptide in the evaluation of left ventricular
diastolic function and diastolic heart failure. Curr Opin Cardiol.
24:155–160. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lubien E, DeMaria A, Krishnaswamy P,
Clopton P, Koon J, Kazanegra R, Gardetto N, Wanner E and Maisel AS:
Utility of B-natriuretic peptide in detecting diastolic
dysfunction: Comparison with doppler velocity recordings.
Circulation. 105:595–601. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Santosa YP, Tjandrawati A, Noormartany
Erwinanto, Yahya AF, Martanto E, Tedjokusumo P, Purnomowati A and
Antono E: Comparison of pro β-natriuretic peptide in hypertensive
patients with and without diastolic dysfunction. Acta Med Indones.
40:19–23. 2008.PubMed/NCBI
|
|
10
|
Chinese Society of Cardiology of Chinese
Medical Association; Editorial Board of Chinese Journal of
Cardiology: Chinese guidelines for the diagnosis and treatment of
heart failure 2014. Zhonghua Xin Xue Guan Bing Za Zhi 42: 98-122,
2014 (In Chinese).
|
|
11
|
Lang RM, Bierig M, Devereux RB,
Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward
J, Shanewise JS, et al: Recommendations for chamber quantification:
A report from the American society of echocardiography's guidelines
and standards committee and the chamber quantification writing
group, developed in conjunction with the European association of
echocardiography, a branch of the European society of cardiology. J
Am Soc Echocardiogr. 18:1440–1463. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu LS: Writing Group of Chinese
Guidelines for the Management of Hypertension: 2010 Chinese
guidelines for the management of hypertension. Zhonghua Xin Xue
Guan Bing Za Zhi. 39:579–615. 2011.(In Chinese). PubMed/NCBI
|
|
13
|
Gebker R, Mirelis JG, Jahnke C, Hucko T,
Manka R, Hamdan A, Schnackenburg B, Fleck E and Paetsch I:
Influence of left ventricular hypertrophy and geometry on
diagnostic accuracy of wall motion and perfusion magnetic resonance
during dobutamine stress. Circ Cardiovasc Imaging. 3:507–514.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang L, Teng T, Bian B, Yao W, Yu X, Wang
Z, Xu Z and Sun Y: Zinc levels in left ventricular hypertrophy.
Biol Trace Elem Res. 176:48–55. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yamazaki M, Ogawa T, Tamei N, Ando Y and
Nitta K: Relation of N-terminal pro-B-type natriuretic peptide
(NT-proBNP) and left atrial volume index to left ventricular
function in chronic hemodialysis patients. Heart Vessels.
26:421–427. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Slubowska K, Sadowska A, Kwiatkowski A and
Durlik M: N-terminal pro-B-type natriuretic peptide (NT-proBNP)
assessment in the first year after renal transplantation and its
relationship with graft function and left ventricular hypertrophy.
Transplant Proc. 46:2729–2732. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
David S, Kümpers P, Seidler V, Biertz F,
Haller H and Fliser D: Diagnostic value of N-terminal pro-B-type
natriuretic peptide (NT-proBNP) for left ventricular dysfunction in
patients with chronic kidney disease stage 5 on haemodialysis.
Nephrol Dial Transplant. 23:1370–1377. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rivera M, Taléns-Visconti R, Salvador A,
Bertomeu V, Miró V, García de Burgos F, Climent V, Cortés R, Payá
R, Pérez-Boscá JL, et al: NT-proBNP levels and hypertension. Their
importance in the diagnosis of heart failure. Rev Esp Cardiol.
57:396–402. 2004.(In Spanish). PubMed/NCBI
|
|
19
|
Freitag MH, Larson MG, Levy D, Benjamin
EJ, Wang TJ, Leip EP, Wilson PW and Vasan RS: Framingham Heart
Study: Plasma brain natriuretic peptide levels and blood pressure
tracking in the framingham heart study. Hypertension. 41:978–983.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bower JK, Lazo M, Matsushita K, Rubin J,
Hoogeveen RC, Ballantyne CM and Selvin E: N-terminal pro-brain
natriuretic peptide (NT-proBNP) and risk of hypertension in the
atherosclerosis risk in communities (ARIC) study. Am J Hypertens.
28:1262–1266. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Masiha S, Sundstrom J and Lind L:
Inflammatory markers are associated with left ventricular
hypertrophy and diastolic dysfunction in a population-based sample
of elderly men and women. J Hum Hypertens. 27:13–17.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Erten Y, Tulmac M, Derici U, Pasaoglu H,
Altok Reis K, Bali M, Arinsoy T, Cengel A and Sindel S: An
association between inflammatory state and left ventricular
hypertrophy in hemodialysis patients. Ren Fail. 27:581–589.
2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao L, Cheng G, Jin R, Afzal MR, Samanta
A, Xuan YT, Girgis M, Elias HK, Zhu Y, Davani A, et al: Deletion of
interleukin-6 attenuates pressure overload-induced left ventricular
hypertrophy and dysfunction. Circ Res. 118:1918–1929.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Phelan D, Watson C, Martos R, Collier P,
Patle A, Donnelly S, Ledwidge M, Baugh J and McDonald K: Modest
elevation in BNP in asymptomatic hypertensive patients reflects
sub-clinical cardiac remodeling, inflammation and extracellular
matrix changes. PLoS One. 7(e49259)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kapoun AM, Liang F, O'Young G, Damm DL,
Quon D, White RT, Munson K, Lam A, Schreiner GF and Protter AA:
B-type natriuretic peptide exerts broad functional opposition to
transforming growth factor-beta in primary human cardiac
fibroblasts: Fibrosis, myofibroblast conversion, proliferation, and
inflammation. Circ Res. 94:453–461. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tamura N, Ogawa Y, Chusho H, Nakamura K,
Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M,
et al: Cardiac fibrosis in mice lacking brain natriuretic peptide.
Proc Natl Acad Sci USA. 97:4239–4244. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rossi MA: Pathologic fibrosis and
connective tissue matrix in left ventricular hypertrophy due to
chronic arterial hypertension in humans. J Hypertens. 16:1031–1041.
1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ciulla M, Paliotti R, Hess DB, Tjahja E,
Campbell SE, Magrini F and Weber KT: Echocardiographic patterns of
myocardial fibrosis in hypertensive patients: Endomyocardial biopsy
versus ultrasonic tissue characterization. J Am Soc Echocardiogr.
10:657–664. 1997.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Diez J, Querejeta R, López B, González A,
Larman M and Martínez Ubago JL: Losartan-dependent regression of
myocardial fibrosis is associated with reduction of left
ventricular chamber stiffness in hypertensive patients.
Circulation. 105:2512–2517. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Brilla CG, Funck RC and Rupp H:
Lisinopril-mediated regression of myocardial fibrosis in patients
with hypertensive heart disease. Circulation. 102:1388–1393.
2000.PubMed/NCBI View Article : Google Scholar
|