Introduction
The epidermal growth factor receptor (EGFR) is
expressed on the surface of normal epithelial cells and is
overexpressed in certain tumor cells. Overexpression of EGFR is
linked to the migration and invasion of tumor cells and patient
prognosis (1). Distant metastasis is
a complex process in which cancer cells leave the primary tumor,
invade the lymph and blood system and growing in distant organ
sites; each of these steps is closely associated with the
biological characteristics of the tumor (2). Lymph node metastasis is the most common
metastatic pathway of lung cancer, which affects its stage and
prognosis (3). Previous studies have
indicated that EGFR mutations are highly associated with ethnicity,
gender, adenocarcinoma and smoking status (4-9).
With the application of sequencing and PCR technology, EGFR
mutations have become the major predictors of the effectiveness of
EGFR-tyrosine kinase inhibitor (TKI) targeted therapy (10). Patients on EGFR-TKI treatment had an
objective response rate (ORR) of 70-80%, median progression-free
survival (mPFS) of 9-12 months and overall survival (OS) of 20-32
months, making EGFR-TKI become the first-line treatment for
patients with EGFR-mutant non-small cell lung cancer (NSCLC)
(9).
EGFR mutations mainly exist in exons 18-21, among
which exon 19 deletion mutation, exon 21 L858R point mutation and
exon 20 T790M mutation are the most common types, which account for
50-90% of the overall EGFR mutations and are referred to as
classical mutations (8,11); other sensitive mutations, referred to
as uncommon mutations in clinical practice, include G719X in
exon18, L861Q and G719C in exon 21 (12,13).
Uncommon mutations and common mutations have been indicated to have
similar clinicopathological features, but uncommon mutations have a
lower sensitivity and a lesser response to EGFR-TKI treatment
(14,15). Previous studies have suggested that
for EGFR-TKI treatment, NSCLC patients with uncommon mutations are
less responsive than those with common mutations, as indicated by
their shorter PFS and lower ORR after EGFR-TKI treatment (16,17).
However, due to the low incidence of uncommon mutations and the
partial overlap of the incidence of the two types of mutations, the
reasons for the differences in the therapeutic effect of EGFR-TKIs
across different ethnicities and mutation types have remained
elusive; furthermore, only a small number of relevant studies has
been published to date (18,19).
The frequency of EGFR mutation in NSCLC has been
documented to differ across ethnic groups and the occurrence was
observed to be markedly higher in East-Asian trials compared with
that in European studies (20,21).
China is a large country by area; Uygur people live between East
Asia and the European continent (20,22), but
little is known regarding the difference in EGFR mutation rates
between Xinjiang Uygur and Han people.
The present study was based on populations of
different genetic backgrounds and living habits, i.e., Xinjiang
Uygur and Han people. The ADx Amplification Refractory Mutation
System (ADx-ARMS) was used to determine EGFR gene expression,
compare the ethnic differences in EGFR mutations between Xinjiang
Uygur and Han people, analyze the distribution of uncommon mutation
types and evaluate the association between the clinicopathological
features of uncommon mutations and the efficacy of EGFR-TKI
treatment, which aimed to justify the clinical treatment of
patients with uncommon EGFR mutations.
Materials and methods
Patients
A total of 2,984 patients who were hospitalized at
the Affiliated Tumor Hospital of Xinjiang Medical University
(Urumqi, China) between February 2012 and February 2017 and had
been pathologically confirmed as having NSCLC were enrolled in the
present study, among whom 29 patients with uncommon mutations were
screened out after the ADx-ARMS test for EGFR gene mutations. The
study was approved by the Affiliated Tumor Hospital Xinjiang
Medical University (Urumqi, China). All patients provided written
informed consent prior to enrollment. The inclusion criteria were
as follows: i) Pathologically confirmed lung adenocarcinoma; ii)
advanced clinical stage (stage IIIB or IV) according to the seventh
edition American Joint Committee on Cancer (AJCC) staging system
(23); iii) age >18 years; iv)
all tissue samples had been tested for EGFR gene expression prior
to treatment; v) patients treated with EGFR-TKIs were defined as
patients receiving standard treatment with gefitinib, erlotinib and
icotinib for at least 30 days; vi) at least one measurable lesion
as defined by the Response Evaluation Criteria in Solid Tumors
(RECIST) 1.1(24); vii) clinical
stage was determined using the AJCC TNM staging system (VII)
(23); viii) regular follow-ups had
been performed with complete pathological data. The exclusion
criteria were as follows: i) Patients with known drug-resistant
EGFR mutations; ii) patients who had received no EGFR-TKI treatment
or had not been treated with EGFR-TKI as required; iii) patients
who stopped the medication or reduced the dose due to adverse
reactions; iv) patients who died from diseases not associated with
the disease studied (e.g. heart disease or severe pulmonary
infection); v) patients with their follow-up results lost.
Methods
A retrospective analysis was performed on the
clinical data of the patients with uncommon mutations (n=29),
including gender, age, smoking status, ethnicity, pathological
type, TNM stage and primary lesion location. In order to explore
whether uncommon EGFR mutations were associated with specific
metastases, patients with uncommon mutations were examined for
organ and lymph node metastasis. MRI was used to determine brain
metastasis and CT to determine other metastases. In addition, the
EGFR mutation type, optimal efficacy of targeted therapy, time of
disease progression, time of last follow-up and time of death were
recorded.
ADx-ARMS
The ARMS was adopted to determine EGFR gene
expression in the tissue samples of all patients (n=2,984), and all
of the tissue samples were obtained during surgery. The ARMS is a
highly sensitive real-time PCR-based test system that covers the 29
EGFR mutation hotspots from exon 18 to exon 21(25). The assay was performed with the
MX3000P (Stratagene) real-time PCR system according to the
manufacturer's protocol for the ADx EGFR29 Mutation Kit (Amoy
Diagnostics). The 25-µl RT-PCR system consisted of 0.4 µl of
template DNA, 3.6 µl of deionized water and 16 µl of other reaction
components (26). PCR was performed
by initial denaturation at 95˚C for 10 min, followed by 40 cycles
of amplification (95˚C for 30 sec and 61˚C for 1 min). The results
were analyzed according to the criteria defined by the
manufacturer's protocol.
Efficacy evaluation indexes
According to the RECIST, the efficacy was divided
into complete response (CR), partial response (PR), stable disease
(SD) and progressive disease. From the numbers of patients with CR,
PR and SD, the following parameters were calculated:
ORR=(nCR+nPR)/total number of cases x100%.
Disease control rate
(DCR)=(nCR+nPR+nSD)/total number
of cases x100%.
Statistical analysis
SPSS (version 21.0; IBM Corp.) was used for data
analysis. The χ2 test was used to analyze the
differences in the uncommon EGFR mutations between Uygur and Han
people. The normally distributed data were expressed as the mean ±
standard deviation, while the non-normally distributed data were
expressed as the median with interquartile range. P<0.05 was
considered to indicate statistical significance.
Results
Differences in EGFR gene mutations
between Xinjiang Uygur and Han people
A total of 2,984 patients (542 Uygur people and
2,442 Han people) with stage IIIb/IV NSCLC were enrolled in the
study. Among the 542 Uygur people, 298 were male and 244 were
female, with a median age of 63 (range, 36 to 89 years). Among the
2,442 Han people, 1018 were male and 1424 were female, with a
median age of 66 (ranging from 23 to 87 years). The clinical and
pathological features of Xinjiang Uygur and Han ethnicities were
showed in Table SI. There were
significant differences in EGFR mutations between Xinjiang Uygur
and Han people (P<0.001): Uygur and Han people with
adenocarcinoma had an EGFR mutation rate of 10.79 and 72.22%,
respectively, and those with squamous cell carcinoma had an EGFR
mutation rate of 3.26 and 10.13%, respectively. The differences in
uncommon EGFR mutations were significant between Uygur and Han
people with lung adenocarcinoma (P<0.05), but not significant
between those with lung squamous cell carcinoma. Uygur and Han
people with lung adenocarcinoma had an uncommon EGFR mutation rate
of 0.25 and 1.96%, respectively, and those with lung squamous cell
carcinoma had an uncommon EGFR mutation rate of 0 and 0.26%,
respectively. The differences in the EGFR gene mutations between
Xinjiang Uygur and Han people are presented in Table I.
 | Table IDifferences in EGFR gene mutations
between Xinjiang Uygur and Han ethnicities. |
Table I
Differences in EGFR gene mutations
between Xinjiang Uygur and Han ethnicities.
| | EGFR mutation | | | Uncommon EGFR
mutation | | |
|---|
| Ethnicity | + | - | Mutation rate | P-value | + | - | Mutation rate | P-value |
|---|
| Adenocarcinoma | | | | <0.001 | | | | <0.05 |
| Uygur | 42 | 347 | 10.79 | | 1 | 388 | 0.25 | |
| Han | 923 | 355 | 72.22 | | 25 | 1253 | 1.96 | |
| Squamous cell
carcinoma | | | | <0.001 | | | | 1.0 |
| Uygur | 5 | 148 | 3.26 | | 0 | 153 | 0 | |
| Han | 118 | 1046 | 10.13 | | 3 | 1161 | 0.26 | |
Clinicopathological features of
patients with uncommon mutations
The clinicopathological features of the 29 patients
with uncommon mutations (25 were Han people, 4 was Uygur) are
presented in Table II. The patients
with uncommon mutations consisted of 19 men and 10 women, and 17
were nonsmokers. A total of 3 (10.3%) patients had Squamous cell
carcinoma and 26 (89.7%) had Adenocarcinoma. A total of 8 (27.6%)
patients were classified as at stage IIIB, and 21 (72.4%) at stage
IV. A total of 13 patients had the primary tumor in left lung. The
most common site of lymph node metastasis in patients with uncommon
mutations was the hilar lymph node, supraclavicular/subclavian
lymph node, cervical lymph node and mediastinal lymph node; the
most common distant metastatic organs were the lung, bone, brain,
liver and adrenal gland.
 | Table IIClinicopathological features of
patients with uncommon mutations (n=29). |
Table II
Clinicopathological features of
patients with uncommon mutations (n=29).
|
Characteristics | Value (%) |
|---|
| Sex | |
|
Male | 19 (65.5) |
|
Female | 10 (34.5) |
| Median age
(years) | 68 (38-82) |
| Smoking | |
|
Smoker | 12 (41.3) |
|
Non-smoker | 17 (58.7) |
|
Average
smoking index (patients with smoking habits) | 495.8±23.7 |
|
Smoking
index interval | 100-900 |
| Ethnicity | |
|
Han | 25 (86.2) |
|
Uygur | 4 (13.8) |
| Pathological
type | |
|
Squamous
cell carcinoma | 3 (10.3) |
|
Adenocarcinoma | 26 (89.7) |
| Stage | |
|
IIIB | 8 (27.6) |
|
IV | 21 (72.4) |
| Primary tumor
location | |
|
Left
lung | 13 (44.8) |
|
Right
lung | 16 (55.2) |
| Metastatic
organ | |
|
Lung | 14 (48.3) |
|
Bone | 10 (34.5) |
|
Brain | 9 (31.0) |
|
Liver | 5 (17.2) |
|
Adrenal
gland | 4 (13.8) |
|
Other | 5 (17.2) |
| Lymph node
metastasis | |
|
Hilar lymph
node | 8 (27.6) |
|
Clavicular
lymph node | 5 (17.2) |
|
Cervical
lymph node | 3 (10.3) |
|
Mediastinal
lymph node | 1 (3.4) |
|
Other | 2 (6.9) |
Pathological types of uncommon
mutations
Among the 29 patients with uncommon mutations, 16
had single mutations, 11 had double mutations and 2 had triple
mutations (Table III).
 | Table IIIDistribution of uncommon EGFR
mutations by type. |
Table III
Distribution of uncommon EGFR
mutations by type.
| EGFR mutation | Mutant exon | No. of patients
(%) |
|---|
| G719X | 18 | 5 (17.2) |
| 20ins | 20 | 4 (13.8) |
| S768I | 20 | 2 (6.9) |
| L861Q | 21 | 5 (17.2) |
| G719X+20ins | 18+20 | 1 (3.4) |
| G719X+T790M | 18+20 | 2 (6.9) |
| G719X+L861Q | 18+21 | 2 (17.2) |
| 19Del+S768I | 19+20 | 1 (3.4) |
| S768I+20ins | 20 | 4 (13.8) |
| S768I+L858R | 20+21 | 1 (3.4) |
|
G719X+S768I+20ins | 18+20 | 1 (3.4) |
|
G719X+L861Q+L858R | 18+21 | 1 (3.4) |
EGFR-TKI treatments for uncommon
mutations
A total of 29 patients with uncommon mutations were
treated with EGFR-TKIs, among whom 16 were treated with gefitinib,
12 with erlotinib and 1 with icotinib. The number of patients
treated with first-line, second-line and third-line EGFR-TKIs was
16, 7 and 6, respectively. Their clinicopathological features and
outcomes are presented in Tables
IV-VI.
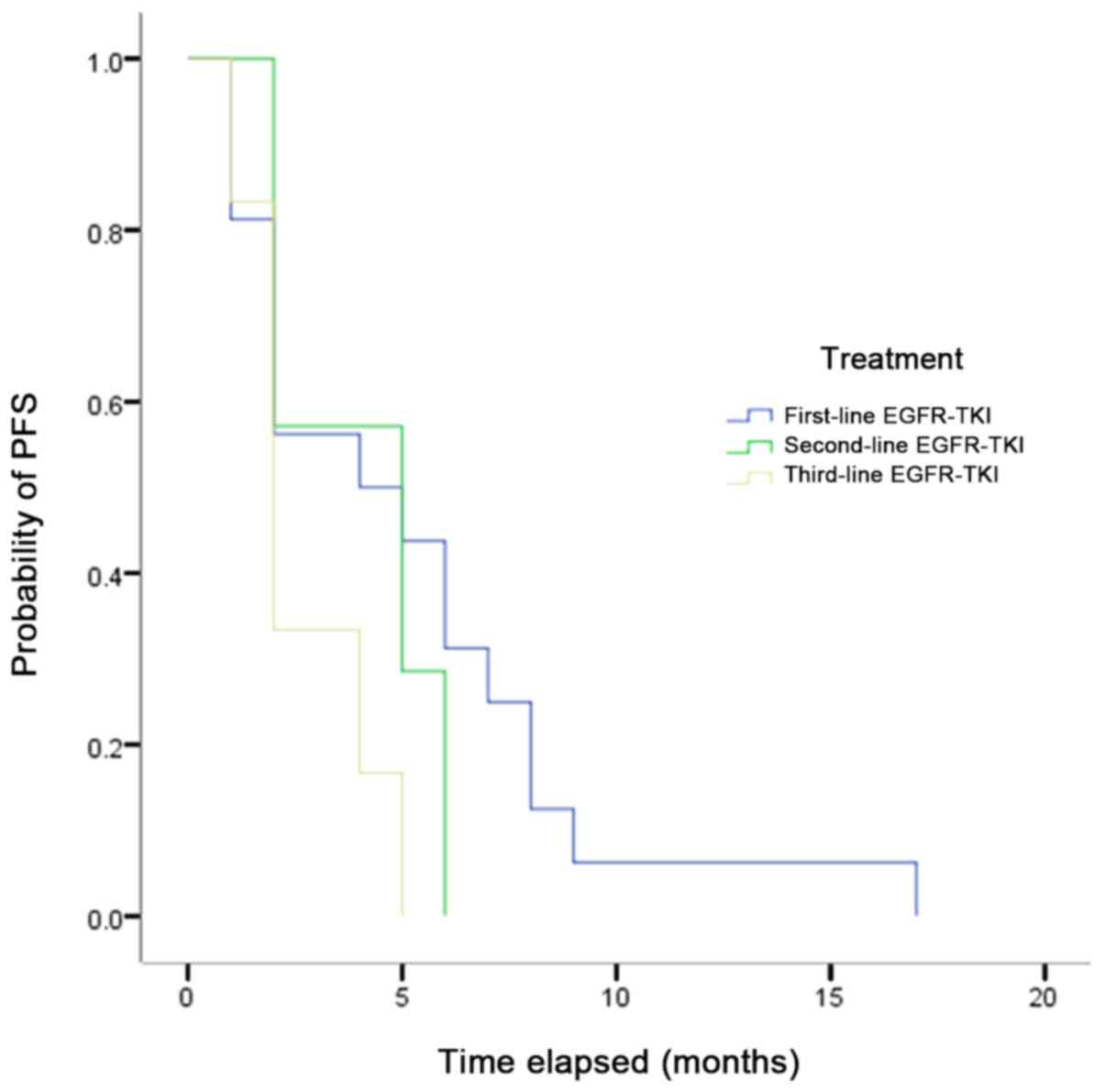
The ORRs, DCRs and mPFS on treatment with
first-line, second-line and third-line EGFR-TKIs are provided in
Table VII, second-line EGFR-TKIs
have a lower ORR and DCR while had a longer mPFS.
 | Table VIISummary of short-term outcomes of 29
patients with uncommon mutations on EGFR-TKI treatment. |
Table VII
Summary of short-term outcomes of 29
patients with uncommon mutations on EGFR-TKI treatment.
| EGFR-TKI
treatment | Cases (n) | CR (%) | PR (%) | SD (%) | PD (%) | ORR (%) | DCR (%) | mPFS (months) |
|---|
| First-line | 16 | 0 (0.0) | 7 (43.8) | 1 (6.3) | 8 (50.0) | 43.8 | 50.0 | 5.5 |
| Second-line | 7 | 0 (0.0) | 2 (28.6) | 1 (14.3) | 4 (57.1) | 28.6 | 42.9 | 4.0 |
| Third-line | 6 | 0 (0.0) | 2 (33.3) | 1 (16.7) | 3 (50.0) | 33.3 | 50.0 | 2.7 |
The mPFS of all patients with uncommon mutations who
received EGFR-TKIs ranged from 5.5 to 2.7 months (first-line,
second-line and third-line EGFR-TKIs was 5.5, 4.0 and 2.7 months,
respectively), with no significant differences observed (Fig. 1), but the result indicated that the
mPFS was shortened with the increasing lines of EGFR-TKIs.
Discussion
Lung cancer is one of the deadliest malignant tumor
types in the world. EGFR is a cell proliferation and signaling
receptor for epidermal growth factors. Furthermore, it is a
receptor tyrosine kinase that is frequently overexpressed and has a
central role in the development of NSCLC (27,28).
Studies on treatments targeting EGFR have opened up novel avenues
for the treatment of lung malignancies, but sensitivity to
treatment is significantly associated with EGFR mutation types.
Exon 19 deletion and exon 21 L858R mutation are the most common
types, which account for almost 90% of all EGFR mutations in lung
cancer (29,30). Due to the small proportion of
patients with uncommon mutations, evidence-based medical evidence
is only available from retrospective studies and case reports with
small samples. Certain studies have indicated that the proportion
of patients with uncommon mutations receiving first-line EGFR-TKIs
is up to 85.7%, and that proportion is higher for patients with
uncommon mutations combined with 19-DEL and L858R complex
mutations. Furthermore, the ORR, mPFS and OS of patients with
certain compound mutations are similar to those of patients with
common mutations (31).
In the present study, 2,984 patients of Uygur and
Han ethnicities with stage IIIB/IV NSCLC in Xinjiang were
retrospectively analyzed and the results indicated that Uygur and
Han people exhibited significant differences in EGFR mutations in
adenocarcinoma and squamous cell carcinoma. A meta-analysis by Wang
and Wang (26) suggested that the
overall EGFR mutation rate of Chinese patients was 37.5% and Wu
et al (32,33) reported that 37.9% of Chinese NSCLS
patients had EGFR mutations. However, the EGFR mutation rate of Han
people in the present study was 72.22%, which was higher than that
in other studies. The reason for this may be the use of ADx-ARMS, a
different and more sensitive method, in the present study. The
differences in the uncommon EGFR mutations were significant between
Uygur and Han people with lung adenocarcinoma, but not significant
between the two ethnic groups with lung squamous cell carcinoma. A
total of 2,984 patients with EGFR mutations were enrolled, among
whom 29 harbored uncommon mutations. It was indicated that the
proportion of patients harboring uncommon EGFR mutations was not
significantly different across different genders and smoking
statuses, which was similar to the results obtained by Sonobe et
al (34). The most common lymph
node metastasis sites in patients with uncommon mutations were
hilar lymph node, supraclavicular/subclavian lymph node, cervical
lymph node and mediastinal lymph node, and the most common distant
metastatic organs were the lung, bone, brain, liver and adrenal
gland. Comparison of the efficacy of EGFR-TKIs in patients with
uncommon EGFR mutations revealed that patients on treatment with
first-line EGFR-TKIs had an ORR of 43.75%, a DCR of 50% and mPFS of
5.5 months; the ORR and PFS of patients on treatment with
first-line EGFR-TKIs were inferior to those in patients with
classical mutations, and were also inferior to the previous
research of certain patients with uncommon mutations, but were
superior to those with wild-type EGFRs. The second-line EGFR-TKIs
had an ORR of 28.57%, a DCR of 42.85% and mPFS of 4.0 months. The
three-line EGFR-TKIs had an ORR of 33.33%, a DCR of 50.00% and mPFS
of 2.7 months. From this observation, it may be concluded that the
mPFS was shortened with the increasing lines of EGFR-TKIs, which
may be linked to the changes in patients' physical state, drug
tolerance and EGFR mutation kurtosis.
In the present study, the most common mutation site
was G719X. Among the 29 patients, 5 harbored G719X single mutations
and 6 harbored compound mutations. There was a point mutation of
G719 at exon 18: The glycine at position 719 was replaced by
serine, alanine or cysteine (G719S/A/C). Previous studies have
suggested that the affinity for ATP of the G719 mutant is between
that of wild-type EGFR and L858R (35). According to one study, patients with
G791X single mutations had an ORR of 36.8% (36), but it has also been reported that
patients with G719 mutations, whether single or double, had an ORR
of 53.3% and mPFS of 8.1 months (37). In the present study, 5 patients with
G719X mutations had an ORR of 80% and mPFS of 6 months, and 6
patients with compound mutations had an ORR of 50% and mPFS of 3.5
months. The best response achieved was PR; the ORR and PFS for
patients with single mutations were superior to those of patients
with compound mutations but inferior to those of patients with
classical mutations. It may be suggested that certain patients with
compound mutations harbored drug-resistant mutations whose affinity
for ATP was lower than that of G719 single mutations and the
possibility of bypass interference cannot be excluded.
L861Q, as a mutation at position 861 on exon 20 of
the EGFR, was another common mutation site in the present study.
Among the 29 patients, 5 had L861Q single mutations and 3 had
compound mutations. A previous study reported that L861Q accounted
for 2% of EGFR mutations (38);
Yoshida et al (39) indicated
that patients with L861Q mutation were resistant to first-line
EGFR-TKIs, but certain other studies suggested that certain
EGFR-TKIs were effective for the treatment of L816Q mutation with
the efficacy being inferior to that for L858R and G719 mutations
(13,25). In the NEJ002 study, the efficacy of
gefitinib was retrospectively analyzed in 7 patients with uncommon
G719X mutations and 3 patients with uncommon L8861Q. It was
indicated that the median OS of the patients with uncommon
sensitive mutations in the gefitinib group was significantly
shorter than that in the classical sensitive mutation group, while
the median OS was not significantly different between patients with
uncommon sensitive mutations and those with common sensitive
mutations in the chemotherapy group (40). In the present study, the ORR was 40%
and the mPFS was 6.2 months in 5 patients with L861Q treated with
first-line EGFR-TKIs.
However, it should be noted that cohort size
(n=2,984) of the present study was relatively small, which may have
affected the uncommon EGRF mutation rate between Uygur and Han
people. The number of patients with uncommon EGFR mutations among
the Uygur people was too small, so large samples should be carried
out in the future to further verify the results of the present
study.
In conclusion, the incidence of EGFR gene mutations
is significantly higher in Han people who live in Xinjiang than in
Uygur people. The uncommon EGFR mutations may be divided into
different subtypes, which may result in different outcomes and
survival time of patients on treatment with EGFR-TKIs. The
mechanism of action may include the involvement of different
signaling pathways. In general, patients with uncommon EGFR
mutations have a lower ORR and shorter PFS than those with
classical mutations, but certain patients with uncommon EGFR
mutations have a higher ORR and longer PFS than patients with
wild-type mutations. Therefore, patients with different types of
uncommon mutations should be treated with different regimens. The
specific mechanism of action requires further investigation.
Supplementary Material
Table SI. Clinical and pathological
characteristics of Xinjiang Uygur and Han ethnicities.
Acknowledgements
Not applicable.
Funding
This study was supported by the Xinjiang Uygur
Autonomous Region Municipal Natural Science Foundation (grant no.
2016D01C376) and the Youth Medical Science and Technology Talents
Special Scientific Research Project of the Health and Family
Planning Commission in Xinjiang Uygur Autonomous Region (grant no.
WJWY-201907).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ contributed to the conception and design of the
study. QZ, YC and JZ were responsible for the collection and
analysis of the data and wrote the manuscript. JK and PA were
involved in the data analysis and interpretation. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Tumor Hospital Xinjiang Medical University
(G-201832). The patients provided written informed consent for the
participation of the present study and publication of any
associated data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–500.
2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Brooks DL, Wakefield L and Steeg P:
Abstract 4859: The role of LPAR1 and fibrosis in breast cancer
metastasis. Cancer Res. 77:4859. 2017.
|
|
3
|
Wang Y, Zhu B, Ye M and Chen Z: Study on
relationship between VEGF-C and lymphangiogenesis and lymph node
metastasis in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi.
9:182–186. 2006.(In Chinese). PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Arrieta O, Campos-Parra AD, Zuloaga C,
Avilés A, Sánchez-Reyes R, Manríquez MEV, Covián-Molina E,
Martínez-Barrera L, Meneses A, Cardona A and Borbolla-Escoboza JR:
Clinical and pathological characteristics, outcome and mutational
profiles regarding non-small-cell lung cancer related to wood-smoke
exposure. J Thorac Oncol. 7:1228–1234. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Arrieta O, Cardona AF, Federico Bramuglia
G, Gallo A, Campos-Parra AD, Serrano S, Castro M, Avilés A, Amorin
E, Kirchuk R, et al: Genotyping non-small cell lung cancer (NSCLC)
in Latin America. J Thorac Oncol. 6:1955–1959. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee HJ, Kim YT, Kang CH, Zhao B, Tan Y,
Schwartz LH, Persigehl T, Jeon YK and Chung DH: Epidermal growth
factor receptor mutation in lung adenocarcinomas: Relationship with
CT characteristics and histologic subtypes. Radiology. 268:254–264.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yamane H, Ochi N, Yasugi M, Tabayashi T,
Yamagishi T, Monobe Y, Hisamoto A, Kiura K and Takigawa N:
Docetaxel for non-small-cell lung cancer harboring the activated
EGFR mutation with T790M at initial presentation. Onco Targets
Ther. 6:155–160. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rosell R, Moran T, Queralt C, Porta R,
Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M,
et al: Screening for epidermal growth factor receptor mutations in
lung cancer. N Engl J Med. 361:958–967. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shigematsu H, Lin L, Takahashi T, Nomura
M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et
al: Clinical and biological features associated with epidermal
growth factor receptor gene mutations in lung cancers. J Natl
Cancer Inst. 97:339–346. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pallis AG, Voutsina A, Kalikaki A,
Souglakos J, Briasoulis E, Murray S, Koutsopoulos A, Tripaki M,
Stathopoulos E, Mavroudis D and Georgoulias V: ‘Classical’ but not
‘other’ mutations of EGFR kinase domain are associated with
clinical outcome in gefitinib-treated patients with non-small cell
lung cancer. Br J Cancer. 97:1560–1566. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Frega S, Lorenzi M, Fassan M, Indraccolo
S, Calabrese F, Favaretto A, Bonanno L, Polo V, Zago G, Lunardi F,
et al: Clinical features and treatment outcome of non-small cell
lung cancer (NSCLC) patients with uncommon or complex epidermal
growth factor receptor (EGFR) mutations. Oncotarget. 8:32626–32638.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu J, Jin B, Chu T, Dong X, Yang H, Zhang
Y, Wu D, Lou Y, Zhang X, Wang H and Han B: EGFR tyrosine kinase
inhibitor (TKI) in patients with advanced non-small cell lung
cancer (NSCLC) harboring uncommon EGFR mutations: A real-world
study in China. Lung Cancer. 96:87–92. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen D, Song Z and Cheng G: Clinical
efficacy of first-generation EGFR-TKIs in patients with advanced
non-small-cell lung cancer harboring EGFR exon 20 mutations. Onco
Targets Ther. 9:4181–4186. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kuiper JL, Hashemi SMS, Thunnissen E,
Snijders PJF, Grünberg K, Bloemena E, Sie D, Postmus PE, Heideman
DA and Smit EF: Non-classic EGFR mutations in a cohort of Dutch
EGFR-mutated NSCLC patients and outcomes following EGFR-TKI
treatment. Br J Cancer. 115:1504–1512. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou J and Ben S: Comparison of
therapeutic effects of EGFR-tyrosine kinase inhibitors on 19Del and
L858R mutations in advanced lung adenocarcinoma and effect on
cellular immune function. Thoracic Cancer. 9:228–233.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li XY, Wu JZ, Cao HX, Ma R, Wu JQ, Zhong
YJ and Feng JF: Blockade of DNA methylation enhances the
therapeutic effect of gefitinib in non-small cell lung cancer
cells. Oncol Rep. 29:1975–1982. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rahman S, Kondo N, Yoneda K, Takuwa T,
Hashimoto M, Orui H, Okumura Y, Tanaka F, Kumamoto K, Mostafa MG,
et al: Frequency of epidermal growth factor receptor mutations in
Bangladeshi patients with adenocarcinoma of the lung. Int J Clin
Oncol. 19:45–49. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Smits AJ, Kummer JA, Hinrichs JW, Herder
GJ, Scheidel-Jacobse KC, Jiwa NM, Ruijter TE, Nooijen PT,
Looijen-Salamon MG, Ligtenberg MJ, et al: EGFR and KRAS mutations
in lung carcinomas in the Dutch population: Increased EGFR mutation
frequency in malignant pleural effusion of lung adenocarcinoma.
Cell Oncol (Dodr). 35:189–196. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou D: Detection of EGFR mutation by
DHPLC analysis in Chinese NSCLC and colorectal carcinoma patients
and its clinical implication. Oncol Prog, 2006.
|
|
23
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC Cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yoshida S, Miyata Y, Ohtsu A, Boku N,
Shirao K and Shimada Y: Significance of and problems in adopting
response evaluation criteria in solid tumor RECIST for assessing
anticancer effects of advanced gastric cancer. Gastric Cancer.
3:128–133. 2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Machnicki MM, Glodkowska-Mrowka E,
Lewandowski T, Ploski R, Wlodarski P and Stoklosa T: ARMS-PCR for
detection of BRAF V600E hotspot mutation in comparison with
Real-Time PCR-based techniques. Acta Biochim Pol. 60:57–64.
2013.PubMed/NCBI
|
|
26
|
Chu H, Zhong C, Xue G, Liang X, Wang J,
Liu Y, Zhao S, Zhou Q and Bi J: Direct sequencing and amplification
refractory mutation system for epidermal growth factor receptor
mutations in patients with non-small cell lung cancer. Oncol Rep.
30:2311–2315. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ohsaki Y, Tanno S, Fujita Y, Toyoshima E,
Fujiuchi S, Nishigaki Y, Ishida S, Nagase A, Miyokawa N, Hirata S
and Kikuchi K: Epidermal growth factor receptor expression
correlates with poor prognosis in non-small cell lung cancer
patients with p53 overexpression. Oncol Rep. 7:603–610.
2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nicholson RI, Gee JM and Harper ME: EGFR
and cancer prognosis. Eur J Cancer. 37 (Suppl 4):S9–S15.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sharma SV, Bell DW, Settleman J and Haber
DA: Epidermal growth factor receptor mutations in lung cancer. Nat
Rev Cancer. 7:169–181. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Kobayashi S, Canepa HM, Bailey AS,
Nakayama S, Yamaguchi N, Goldstein MA, Huberman MS and Costa DB:
Compound EGFR mutations and response to EGFR tyrosine kinase
inhibitors. J Thorac Oncol. 8:45–51. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang S and Wang Z: EGFR mutations in
patients with non-small cell lung cancer from mainland China and
their relationships with clinicopathological features: A
meta-analysis. Int J Clin Exp Med. 7(1967)2014.PubMed/NCBI
|
|
33
|
Wu M, Zhao J, Song SW, Zhuo M, Wang X, Bai
H, Wang S, Yang L, An T, Zhang Y, et al: EGFR mutations are
associated with prognosis but not with the response to front-line
chemotherapy in the Chinese patients with advanced non-small cell
lung cancer. Lung Cancer. 67:343–347. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sonobe M, Manabe T, Wada H and Tanaka F:
Mutations in the epidermal growth factor receptor gene are linked
to smoking-independent, lung adenocarcinoma. Br J Cancer.
93:355–363. 2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yun CH, Boggon TJ, Li Y, Woo MS, Greulich
H, Meyerson M and Eck MJ: Structures of lung cancer-derived EGFR
mutants and inhibitor complexes: Mechanism of activation and
insights into differential inhibitor sensitivity. Cancer Cell.
11:217–227. 2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chiu CH, Yang CT, Shih JY, Huang MS, Su
WC, Lai RS, Wang CC, Hsiao SH, Lin YC, Ho CL, et al: Epidermal
growth factor receptor tyrosine kinase inhibitor treatment response
in advanced lung adenocarcinomas with G719X/L861Q/S768I mutations.
J Thorac Oncol. 10:793–799. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY
and Yang PC: Effectiveness of tyrosine kinase inhibitors on
‘uncommon’ epidermal growth factor receptor mutations of unknown
clinical significance in non-small cell lung cancer. Clin Cancer
Res. 17:3812–3821. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mitsudomi T and Yatabe Y: Epidermal growth
factor receptor in relation to tumor development: EGFR gene and
cancer. FEBS J. 277:301–308. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yoshida T, Ishii G, Goto K, Neri S,
Hashimoto H, Yoh K, Niho S, Umemura S, Matsumoto S, Ohmatsu H, et
al: Podoplanin-positive cancer-associated fibroblasts in the tumor
microenvironment induce primary resistance to EGFR-TKIs in lung
adenocarcinoma with EGFR mutation. Clin Cancer Res. 21:642–651.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Watanabe S, Minegishi Y, Yoshizawa H,
Maemondo M, Inoue A, Sugawara S, Isobe H, Harada M, Ishii Y, Gemma
A, et al: Effectiveness of gefitinib against non-small-cell lung
cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac
Oncol. 9:189–194. 2014.PubMed/NCBI View Article : Google Scholar
|