Introduction
The menisci are crescent-shaped wedges of
fibrocartilage located on the medial and lateral aspects of the
knee. The primary function of the meniscus is to transmit load
across the tibiofemoral joint by increasing congruency, thereby
decreasing the resultant stress placed on the articular cartilage,
and to play a role in shock absorption, stability, lubrication,
nutrition, and proprioception to the knee joint (1-8).
As a consequence of its complex anatomical, biomechanical, and
functional characteristics, the menisci are prone to damage and
injury (9). Injuries to the menisci
are recognized as a cause of significant musculoskeletal morbidity
(10,11). Previous findings have shown that knee
injury is one of the strongest risk factors for the development of
knee osteoarthritis (KOA) (12,13).
Therefore, the challenge is to develop therapies and techniques
with the aim of preserving the menisci's distinct composition and
function, thereby delaying the development of knee
osteoarthritis.
Tougu Xiaotong capsule (TGXTC) is an effective
prescription in the treatment of KOA. TGXTC comprises Ligusticum
chuanxiong, Morinda officinalis, Sarcandra glabra and Paeonia
lactiflora (14,15). Numerous efficacious molecules are
present in TGXTC, which constitutes multi-drug, multi-path and
multi-target molecular mechanisms for the treatment of KOA, as well
as the non-linear regulation pattern existing in TGXTC
ligand-target interaction network (16). Previous findings have demonstrated
that TGXTC has therapeutic effects on KOA (17) via multiple targets, including the
inhibition of chondrocyte apoptosis (18), amelioration of the structure and
function of cartilage (19),
arresting cartilage degradation (15), promoting osteoblast proliferation and
calcium secretion (14), and
regulation of subchondral bone remodeling (20). However, the effect of TGXTC on
meniscus has not been reported.
In the present study, the effect of TGXTC on the
microstructure and ultrastructure of meniscus in KOA was observed,
so as to provide the experimental proofs for the application of
TGXTC in the clinical treatment of meniscus injury in KOA.
Materials and methods
Animals
A total of 27 male, 6-week-old SPF Sprague-Dawley
(SD) rats, qualified number SCXK (Hu) 2017-0005, were purchased
from the Shanghai Slack Laboratory Animal Co. (Shanghai, China).
The rats were raised in the Animal Experimental Center of Fujian
University of Traditional Chinese Medicine (permit no. SYXK
2014-0006; Fujian, China) at a room temperature of 20-26˚C, a
relative humidity of 40-70%, a 12 h light/dark cycle and free
access to food and water. The care and use of the laboratory
animals complied with the Guidance Suggestions for the Care and Use
of Laboratory Animals 2006 of the Ministry of Science and
Technology, China.
Drugs and reagents
TGXTC was prepared by the Second People's Hospital
of Fujian University of Traditional Chinese Medicine (Fujian,
China) (approval no. MIN ZIZHI Z20100006).
Experimental design
After one week of acclimation, 27 rats were randomly
divided into three groups: The normal group (non papain-induced
KOA; received equivalent amount of saline only), the model group
(papain-induced KOA by an injection of 0.2 ml 4% papain solution on
days 1, 4 and 7; received an equivalent amount of saline only) and
the TGXTC group [papain-induced KOA by an injection of 0.2 ml 4%
papain solution on days 1, 4 and 7; received a clinical oral dose
of TGXTC (0.31 g/kg/day)]. All the groups were treated once daily
for four consecutive weeks, after which the animals were
anesthetized by intraperitoneal injection 10% chloral hydrate, then
the sagittal plane of the intact knee (6 rats in each group) and
meniscus (3 rats in each group) were obtained and prepared into
paraffin section. Following hematoxylin and eosin (H&E)
staining, the structure changes in cartilage and meniscus were
observed and the area of calcification was analyzed. The content of
proteoglycan in meniscus was analyzed according to toluidine blue
staining. The ultrathin sections of the meniscuses were observed
through a transmission electron microscope (TEM).
Histology
The intact knee tissues were fixed in 4%
paraformaldehyde for 3 days and decalcified in 10% EDTA at room
temperature for approximately 8 weeks. The intact knee was
longitudinally cut and embedded in paraffin. Sagittal sections (4
µm) were prepared for H&E staining and toluidine blue staining
and observed under an optical microscope (DM4000B; Leica
Microsystems GmbH) and images were captured.
Mankin score
Following H&E staining, a modified Mankin
scoring principles was used to evaluate the degeneration of the
cartilage structure (21). According
to the Mankin scoring principles, the total score was 14 points;
1-5 point was identified as early osteoarthritis, 6-9 point was
identified as middle osteoarthritis, and 10-14 point was identified
as late osteoarthritis.
Microscopic image analysis
According to the H&E staining and toluidine blue
staining results, the area of calcification and the content of
proteoglycan in meniscus were assessed by image analysis system
(Motic Med 6.0) respectively.
Transmission electron microscopy
analysis
The meniscus specimens were cut into 2.5x1x1 mm
blocks, pre-fixed in 3% glutaraldehyde [Alfa Aesar (China)
Chemicals Co., Ltd.] and 1.5% paraformaldehyde solution (pH 7.3) at
4˚C for 4 days, post-fixed with 1% osmium tetroxide (Ted Pella,
Inc.) at 4˚C for 2 h following decalcification in 10% EDTA for one
week at 4˚C. The tissue specimens were dehydrated with graded
alcohol-acetone and embedded in Epoxy resin 618 (E-51, Ganxi
Chemical Co. Ltd.). The 1-µm resin semi-thin sections were
subsequently cut using a microtome and stained with azur-methylene
blue. The structure of the meniscus was observed under the optical
microscope (DM4000B; Leica Microsystems GmbH). The 70-nm ultrathin
sections were cut using an ultramicrotome (EM UC6; Leica
Microsystems GmbH), stained with 2% aqueous uranyl acetate and 0.3%
lead citrate. The ultrastructure of the meniscus was observed using
a transmission electron microscope (H7650; Hitachi, Ltd.) at 80
kV.
Statistical analysis
Experimental data were processed and analyzed using
SPSS 22.0 software (IBM Corp.). The Shapiro-Wilk test was used to
determine the normality of all groups of data. If the data
exhibited a normal distribution, they were analyzed with one-way
analysis of variance followed by the least significant difference
or Games Howell post hoc tests. If data did not exhibit normal
distribution, the Kruskal-Wallis test was used and the Mann Whitney
U with Bonferroni's correction was applied as the post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Joint microstructure and Mankin
score
As shown in Figs. 1
and 2, in the normal group, the
joint space was uniform; the surface was smooth and the four-layer
structures (including surface layer, transitional layer, radiation
layer and calcification layer) of articular cartilage were arranged
regularly with the Mankin score: 0.33±0.52. Compared with the
normal group, the model group showed the joint space was uneven
with local stenosis or even adhesion; the surface of articular
cartilage was uneven and the four layers were disordered; the
number of chondrocytes in the surface layer was decreased or even
lost in some areas; the number of chondrocytes in the transitional
layer and the radiation layer appeared to be scarce, but the cells
in the calcified layer were relatively increased (Fig. 1).
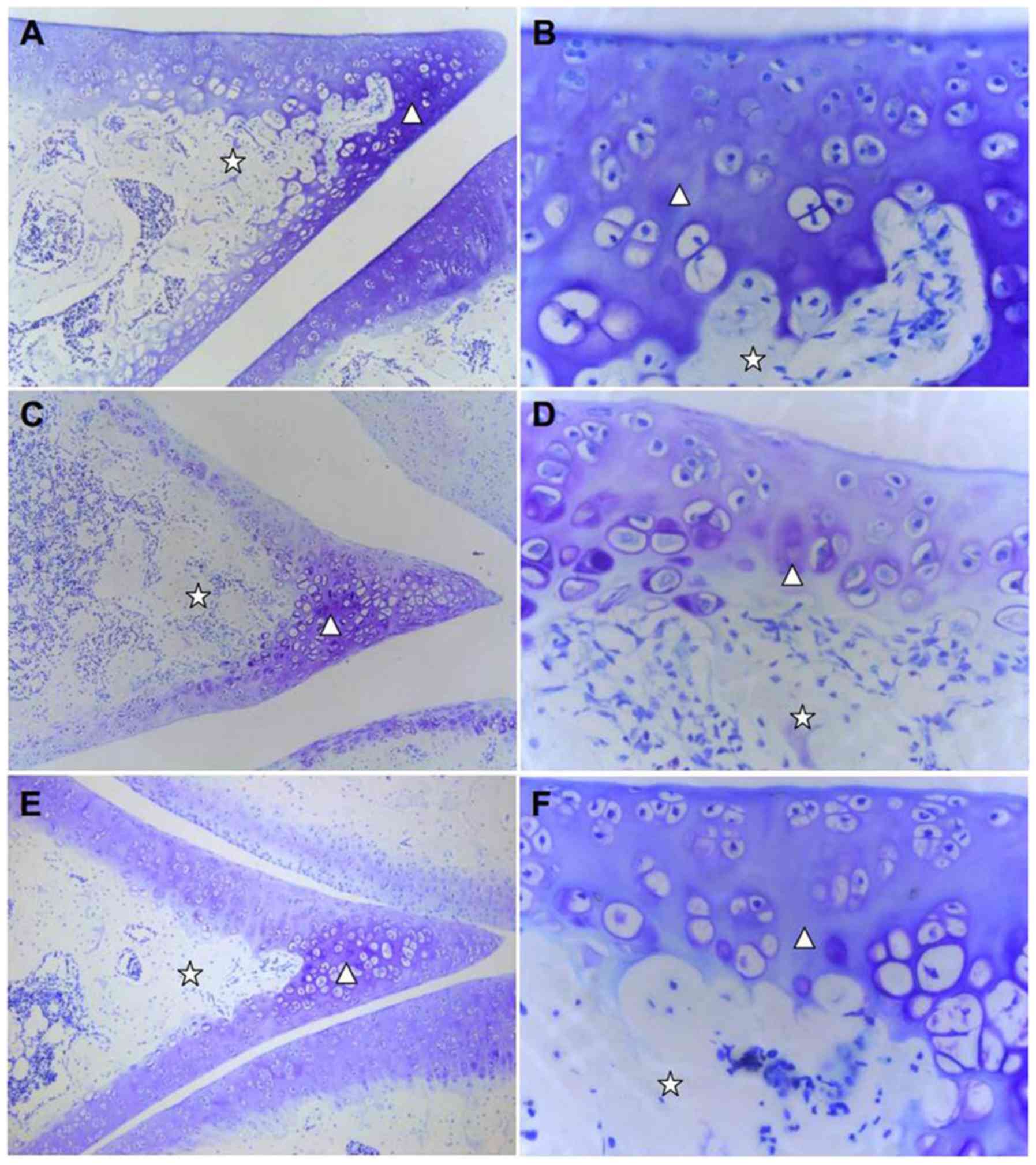
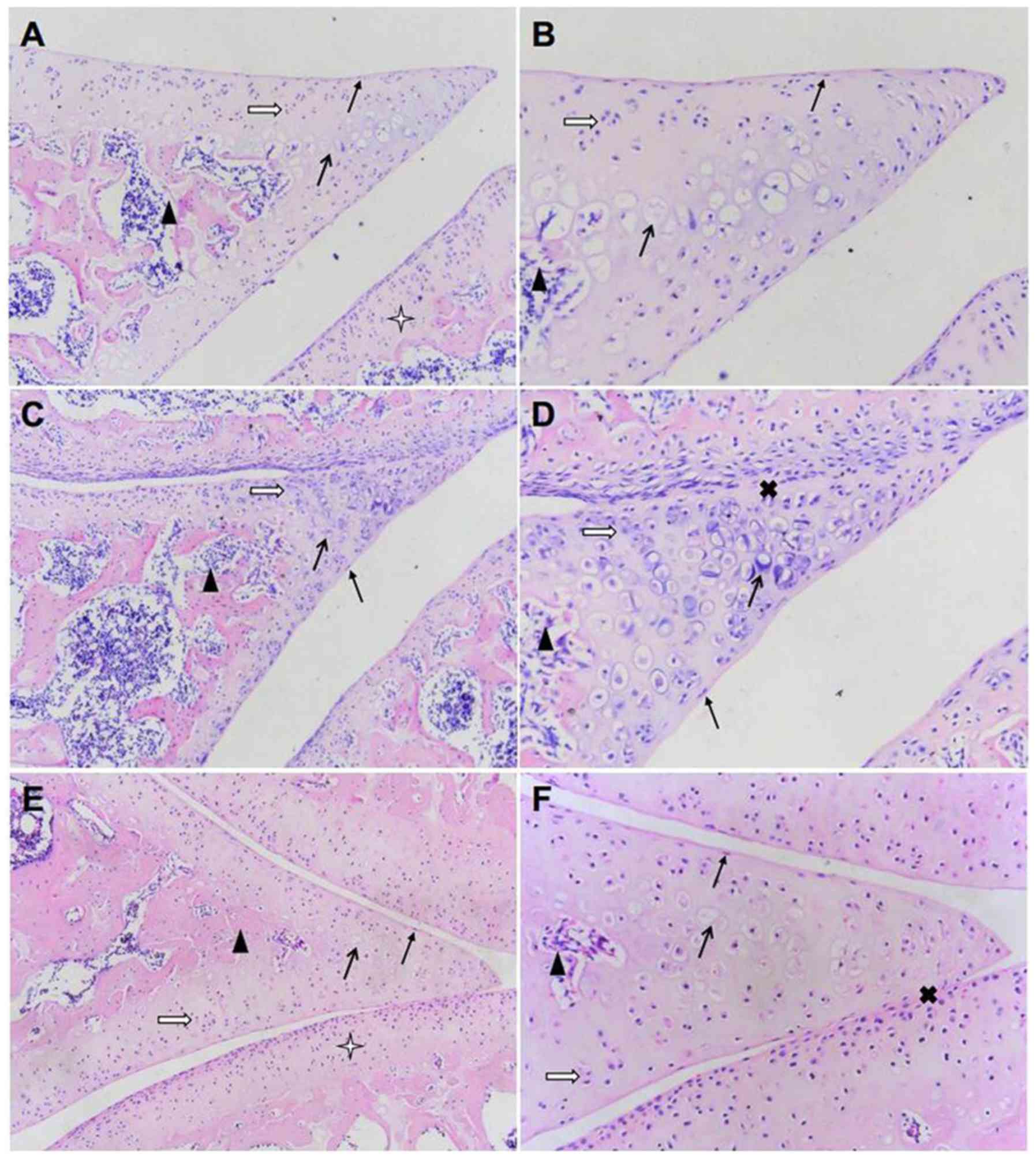 | Figure 1Effect of TGXTC treatment on the
microstructure of cartilage tissue. Following treatment with or
without TGXTC for four consecutive weeks, histopathological
alterations were evaluated by hematoxylin and eosin. The
morphological changes in the cartilage were observed under a
microscope and images were acquired at a magnification of (A, C and
E) x100 or (B, D and F) x200. (A) In the normal group, the joint
space was uniform, (B) the surface was smooth and the articular
cartilage structures were regular. (C) In the model group, the
joint space was uneven with local stenosis and adhesion, (D) the
surface of articular cartilage was uneven and the structure was
disordered. (E) In the TGXTC group, the narrow joint space and
adhesion areas were reduced, (F) the cartilage surface was smoother
and the cartilage structure was relatively in order. ➞, surface
cells;, isogenous group; ➔, calcified cells;p, subchondrial bone;
Ó, adhesion; ò, articular cartilage. |
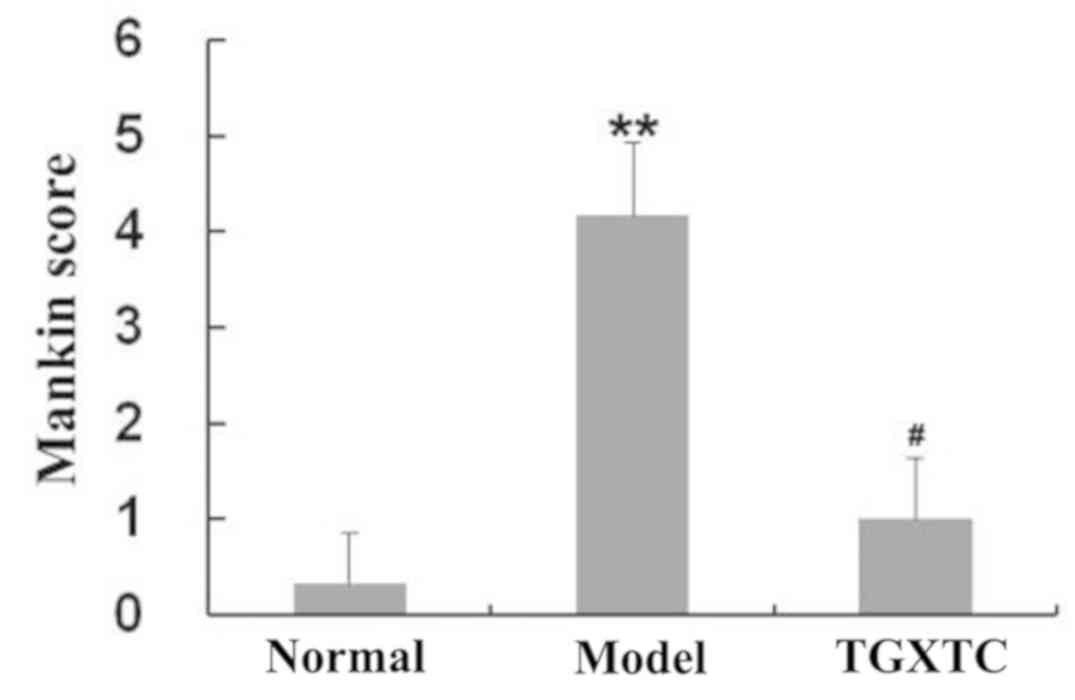
The Mankin score in the model group was 4.17±0.76,
which was significantly increased compared to the normal group
(0.33±0.52, P<0.05), suggesting that the early osteoarthritis
model was successfully established, which was consistent with our
previous results (22). After
treatment with TGXTC, the narrow joint space and adhesion areas
became relatively fewer, the cartilage surface smoother and the
thicker cartilage layer was clearly visible compared to those in
the model group. The chondrocytes status was larger and oval or
round in both the transitional layer and in the radiation layer or
in the calcification layer, and the nucleus in these layers were
more and clearly visible as evidenced by the significantly higher
Mankin score with 1.00±0.63 (P<0.05) (Fig. 2).
Microstructure observation of
meniscus
As shown in Fig. 1,
the surface of cartilage in meniscus was smooth, and the
chondrocytes were regularly distributed. The surface chondrocytes
were flat with diffused distribution. The middle layer cells were
larger, forming 2-4 isogenous group. The deep layer cells were
hypertrophic, which were near the subchondral bone. Cartilage
lacunae were deeply stained, and part of the cell nuclei were not
clear or disappeared, showing as calcified cells, i.e., temporary
calcified areas. However, in the model group, the surface of
meniscus was uneven and locally adhered to articular cartilage. The
boundary between articular cartilage and meniscus was blurred. The
cartilage layer became thinner. The surface cells were very scarce,
the middle layer chondrocyte proliferation was obvious, homologous
cells were aggregated, the number of hypertrophic chondrocyte in
the deep layer was increased, and the nuclei of some cells in the
cartilage lacunae were not clear or absent.
Quantitative analysis of calcified
area in meniscus
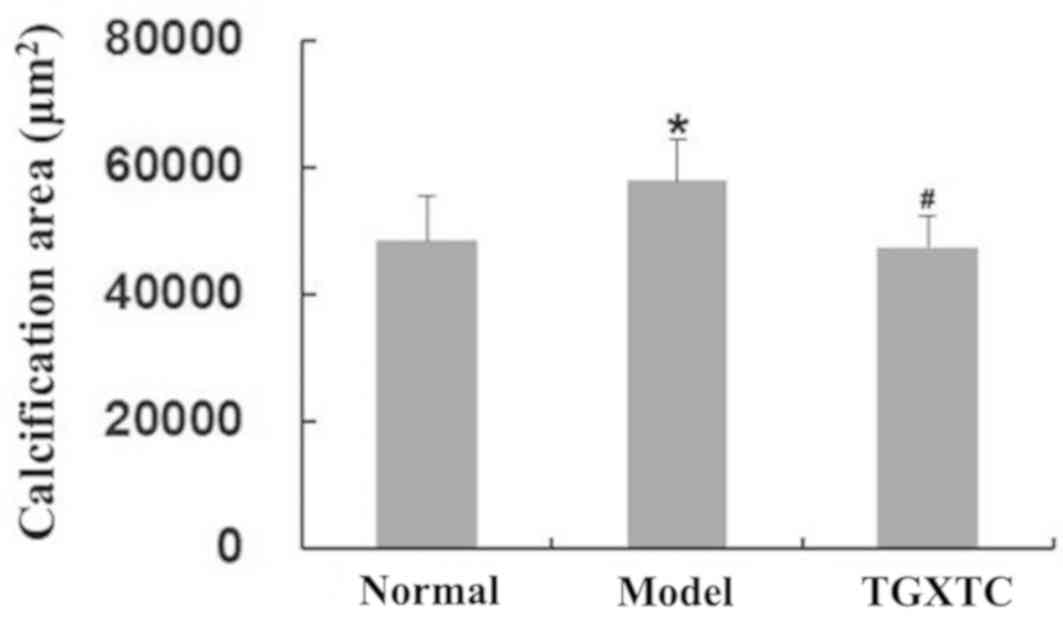
As shown in Fig. 3,
compared with the normal group (the calcified area was 48,406±6,943
µm2), the surface of meniscal cartilage in the model
group was uneven and the calcified area was significantly increased
to 57,795±6,521 µm2 (P<0.05). Compared with the model
group, the surface of the meniscal cartilage in the TGXTC group was
smooth and flat, and the damage of tissue structure was reduced,
leading to the calcified areas being significantly decreased at
47,423±5,051 µm2 (P<0.05). In the TGXTC group, the
surface cells were scattered and the thickness of the perichondrium
was the same. The middle layer cells formed homologous cell groups
with 2-3 cells. The deep layer cell proliferation was lower than
that of the model group.
Quantitative analysis of proteoglycan
in the meniscus cartilage matrix
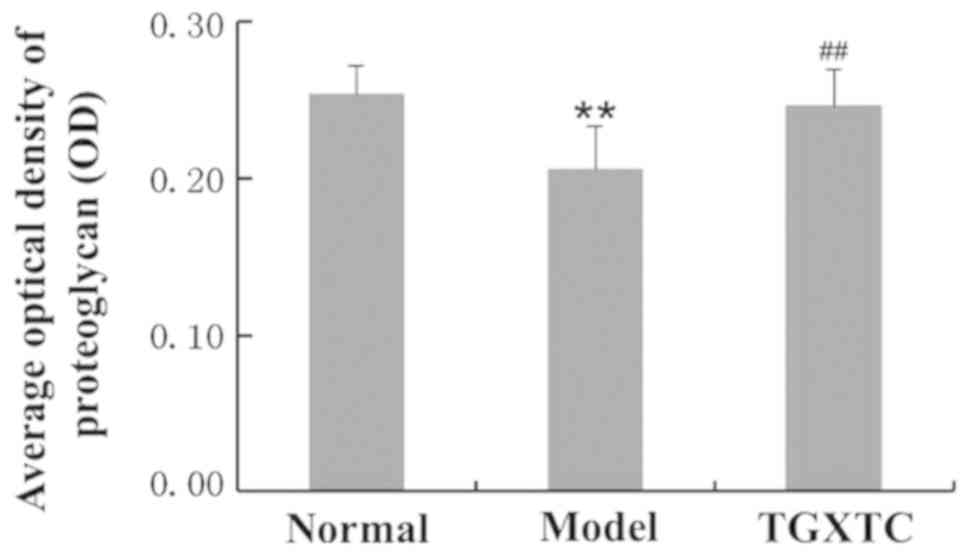
Toluidine blue staining results showed that the
meniscus cartilage matrix in the normal group was uniformly dyed
with dark purple-blue, while the color depth in the model group was
significantly decreased, showing light blue dye, whereas some areas
were unstained (Fig. 4). Image
quantitative analysis revealed that the optical density in the
model group (0.2060±0.0270) was significantly decreased compared to
that in the normal group at 0.2537±0.0185 (P<0.01). Compared
with the model group, the meniscus cartilage matrix in the TGXTC
group was deeply stained and uniform, and the optical density was
significantly increased to 0.2463±0.0230 (P<0.01) (Fig.5).
Ultrastructural observation of
meniscus
In the normal group, most of the cells in the
superficial layer of meniscus were fusiform with few and short
processes and little cytoplasm, and most of the cells in the inner
layer were triangular-like with more processes and abundant
cytoplasm, rich in the rough endoplasmic reticulum, mitochondria
and Golgi apparatus. The extracellular collagen fibrils were mainly
type II collagen fibrils of 35±5 nm, and were ordered and densely
packed. Compared with the normal group, in the model group, the
numbers of processes in the superficial and inner layers of
meniscal cartilage and organelles were reduced. The cells were
swollen, the number of cytoplasmic organelles was reduced and
swollen, glycogen was accumulated, and most of the nuclei were
deformed and heterochromatin agglutinated. Extracellular collagen
fibrils become slender (25±5 nm), disordered and sparse. Compared
with the model group, the TGXTC group had an increased number of
cell processes, rough endoplasmic reticulum, mitochondria and other
organelle swelling were alleviated, glycogen accumulation was
alleviated, nucleus morphology was approximate to normal, the
thickness and size of collagen fibrils around the cells were
increased, and arranged in a relatively orderly manner (Figs. 6 and 7).
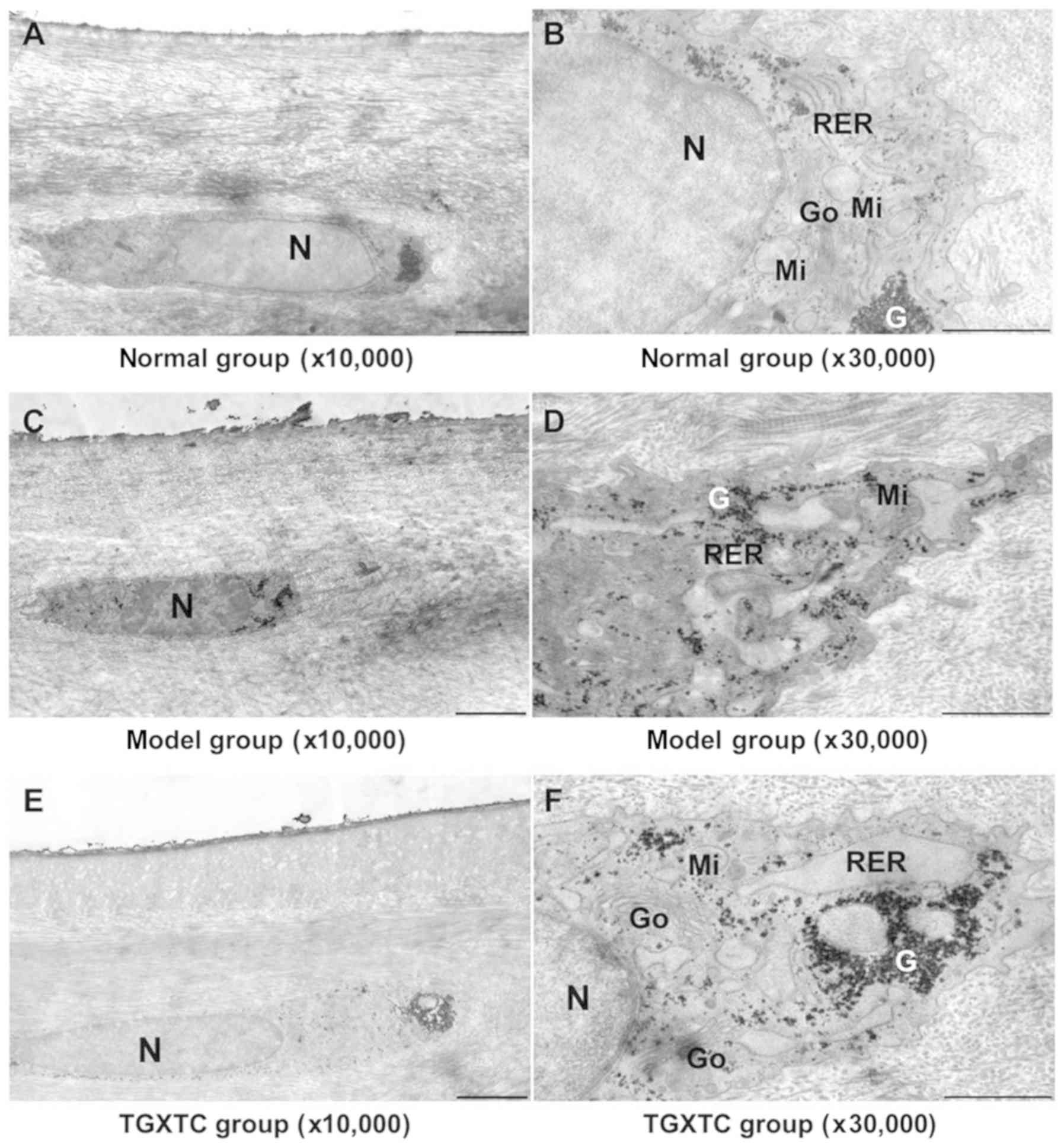 | Figure 6Effect of TGXTC treatment on the
ultrastructure of the cell in the superficial layer of meniscus.
Following treatment with or without TGXTC for 4 consecutive weeks,
the ultrastructure of the cells in the superficial layer of
meniscus was observed under a transmission electronic microscope
and images were acquired at a magnification of (A, C and E) x10,000
(scale bar, 2 µm) or (B, D and F) x30,000 (scale bar, 1 µm). (A) In
the normal group, the cells were fusiform with few and short
processes and little cytoplasm, and (B) the nuclei were elliptic
with uniform chromatin. (C) In the model group, the nuclei were
deformed and the heterochromatin was agglutinated, and (D) the
number of organelles was reduced and they were swollen. (E) In the
TGXTC group, the nuclear morphology was similar to in the normal
group and (F) organelle swelling was alleviated. TGXTC, Tougu
Xiaotong capsule; N, nuclei; RER, rough endoplasmic reticula; Go,
Golgi apparatus; Mi, mitochondria; G, glycogen. |
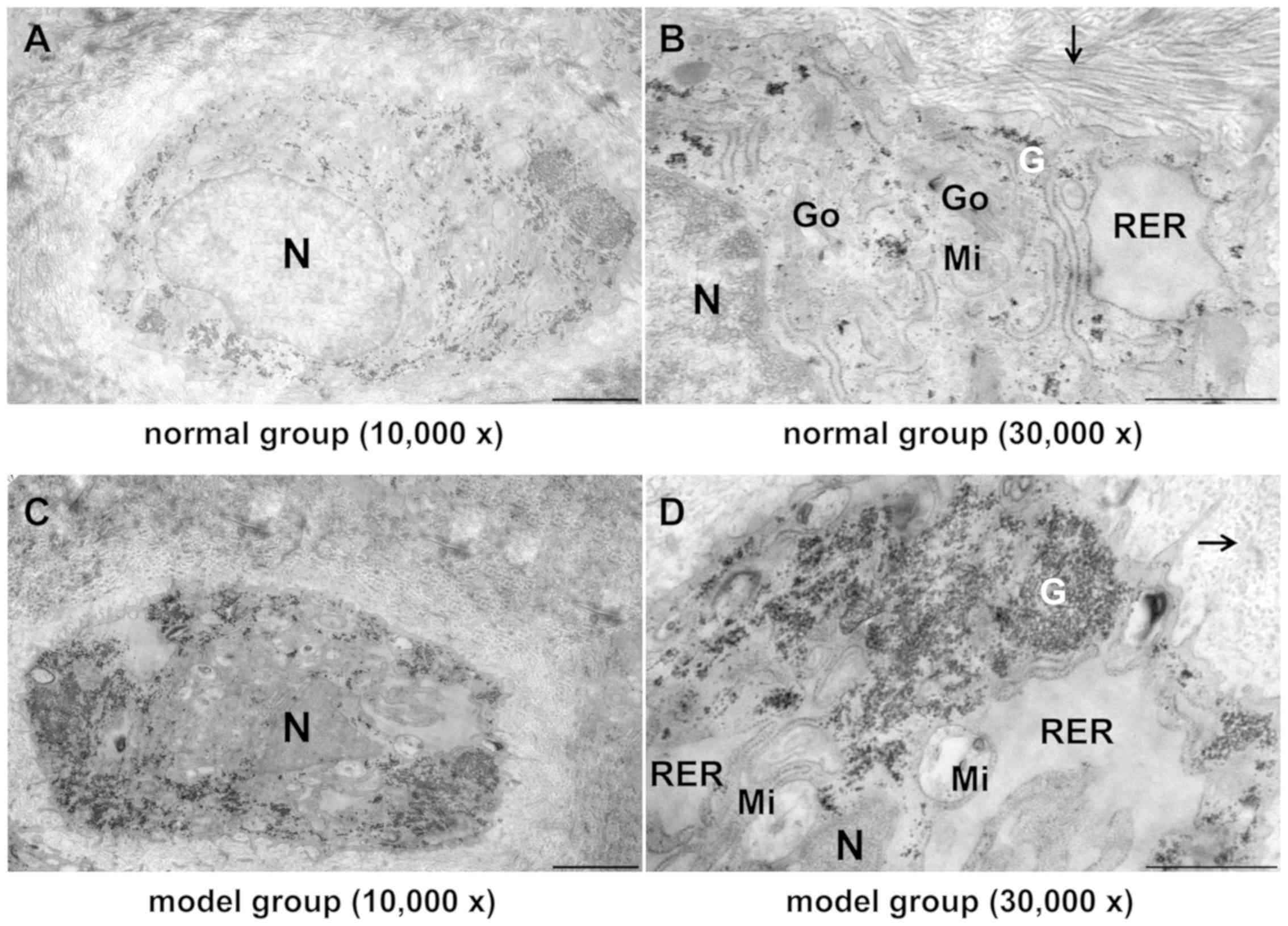 | Figure 7Effect of TGXTC treatment on the
ultrastructure of the cells in the inner layer of meniscus.
Following treatment with or without TGXTC for 4 consecutive weeks,
the ultrastructure of the cells in the inner layer of meniscus was
observed under a transmission electronic microscope and images were
acquired at a magnification of (A, C and E) x10,000 (scale bar, 2
µm) or (B, D and F) x30,000 (scale bar, 1 µm). (A) In the normal
group, the cells had abundant processes and cytoplasmic organelles,
and (B) the extracellular matrix contained ordered and densely
packed type II collagen fibrils. (C) In the model group, the number
of processes was reduced, the nuclei were deformed and the
heterochromatin was agglutinated, (D) the number of cytoplasmic
organelles was reduced and they were swollen with accumulated
glycogen, and the extracellular collagen fibrils become slender,
disordered and sparse. (E) In the TGXTC group, the cell processes
were increased and the nuclear morphology was similar to in the
normal, (F) organelle swelling and glycogen accumulation were
alleviated, and the thickness and size of collagen fibrils were
increased and orderly arranged. Black arrows indicate type II
collagen fibril; TGXTC, Tougu Xiaotong capsule; N, nuclei; RER,
rough endoplasmic reticula; Go, Golgi apparatus; Mi, mitochondria;
G, glycogen. |
Discussion
Prevention and treatment of meniscus
injury is significant to prevent the development of
osteoarthritis
In early research, there was a focus on the role of
articular cartilage in the pathogenesis of osteoarthritis. With
advances in research, increasing attention has been given to the
role of subchondral bone and synovium (23,24).
However, research regarding the role of meniscus in osteoarthritis
is relatively rare. Mechanical loading is critical to joint health.
However, abnormal mechanical loading can lead to the onset and
progression of osteoarthritis (OA) (25). Specifically, OA results from an
imbalance of anabolism and catabolism in the joint, which may be
influenced by the biological and mechanical environment (26). Thus, biomechanical studies are
critical to understanding OA development, prevention, and
treatment. The menisci are integral to the normal function of the
knee joint and play an important role in load distribution, shock
absorption, stability, lubrication, and proprioception (1). If meniscus is injured or the location
was changed, it can easily lead to knee instability and
biomechanical changes, and damaged cartilage and other structures,
ultimately lead to osteoarthritis (9). Therefore, the prevention and treatment
of meniscus injury is very important in delaying the occurrence and
development of osteoarthritis.
Current situation of meniscus injury
treatment and the unique role of traditional Chinese medicine
Previously, treatment of meniscus injury was mainly
based on excision. However, with the improvement in understanding
the anatomical structure and physiological function of the
meniscus, its importance was recognized and meniscus retention
became imperative (27). In
addition, owing to the particular supply of meniscus blood, the
marginal part of the meniscus with rich blood supply healed well
after suture, while repair of the central free edge, which was
distant from the blood supply, was unsatisfactory (28,29).
Therefore, conservative treatment was studied to promote meniscus
healing. Nowadays, the conservative treatment of meniscus injury
occurs mainly through intra-articular injection of growth factor or
active ingredients in the extracellular matrix (30). In recent years, tissue-engineering
research has led to the promotion of meniscus repair through
construction of scaffolds and culturing stem cells, but the
majority of such studies were in the pre-clinical experimental
stage (31,32). Previous studies had proven that
Traditional Chinese Medicine could play a unique role in
conservative treatment. For example, Cheng et al (33) found that the addition or subtraction
of Taohong Siwu Decoction and Wuling Powder could promote the
rehabilitation of joint function after meniscus injury surgery.
Tang (34) found that compound
Longxuejiesan powder had an ideal therapeutic effect on an acute
meniscus injury. Song and Li (35)
found that Huoxue Xiaozhong Decoction treatment for meniscus injury
patients receiving arthroscopic meniscus plasty and suture could
relieve pain and promote the recovery of joint function.
Repair effect of TGXTC on meniscus
tissue structure
Although significant progress has been made in
understanding KOA pathophysiological pathways, much remains to be
done to develop a specific therapy that could effectively retard or
prevent the progression of the disease. To this end, the literature
suggests that in addition to cartilage and subchondral bone, other
articular tissues, including the meniscus, should be targeted.
Previous findings had shown that TGXTC was clinically effective in
the treatment of KOA through multiple targets (14-20).
However, whether TGXTC has a protective effect on meniscus remains
unclear. In the present study, TGXTC was demonstrated to be able to
efficiently reduce the destruction of the cartilage structure of
the meniscus and improve the composition and function of the
meniscus cartilage matrix.
In this experiment, the KOA model was induced by
injecting 0.2 ml of 4% papain solution on days 1, 4 and 7. In
addition, the Mankin score results showed that the model of early
KOA was successfully established. Quantitative analysis of the
calcified area in meniscus showed that in the model group, the
meniscus lost its normal shape, the surface layer of meniscal
cartilage was damaged and the surface cells were absent, while the
middle chondrocyte proliferation was obvious, which could be
related to the cell stress proliferation response caused by the
injury. In addition, the calcified area in meniscus was
significantly increased, which could be related to the increase of
calcified cells, leading to meniscal cartilage degeneration. In the
TGXTC group, the structure of meniscus gradually returned to normal
shape, and the abnormal proliferation of the middle chondrocyte and
the area of calcified area was significantly reduced, which
indicated that TGXTC could improve the structure of meniscus, then
reduce the brittleness of meniscus and restore its elasticity.
Effect of TGXTC on the proteoglycan
synthesis of meniscus
The central region of the meniscus is similar to
articular cartilage, which is also hyaline cartilage containing
collagen protein, proteoglycan and water molecules, but the
composition ratio of the two components is not the same (36). Previous finings have shown that
meniscus injury is associated with the process of KOA. When KOA
occurs, meniscus degeneration will occur progressively, resulting
in corresponding changes in the composition and morphology of
meniscal hyaline cartilage (37).
In the TGXTC group, the content of proteoglycan in
meniscal cartilage increased significantly, indicating that TGXTC
could promote the synthesis and secretion of proteoglycan in
meniscal chondrocyte, increase the content of cartilage matrix
components and enhance the ability of binding water, then increase
the elasticity of meniscus, and finally improve the function of the
meniscus.
Effect of TGXTC on the ultrastructure
of meniscus
In the present study, we found that in the early
stage of KOA, there were fewer chondrocyte processes, fewer
organelles, and organelles synthesizing proteins and
energy-supplying became swelling and degeneration in the meniscus,
which indicated that cells and organelles degenerated. The
synthesis and secretion function of cells was weakened, which
reflected that collagen fibrils around cells became thin, sparse
and disorderly arranged.
Following treatment with TGXTC, cell processes
increased, organelles in cytoplasm decreased, swelling and
degeneration were restored, glycogen accumulated was consumed, and
extracellular collagen fibril morphology was restored, which
indicated that TGXTC could alleviate the degeneration of
chondrocytes and organelles in early osteoarthritis meniscus of
rats, restore the function of oxidative productive organelles, and
improve the function of cell synthesizing and secreting
proteins.
In conclusion, TGXTC may decrease meniscus fragility
by reducing the area of calcified area and increase the water
binding capacity and elasticity by promoting the synthesis and
secretion of proteoglycan in the meniscus cartilage matrix.
Therefore, TGXTC could improve meniscus function by reducing the
degree of meniscal cartilage lesion and improving meniscal
cartilage structure. Subsequently, TGXTC could be used in the
treatment of meniscus injury in early osteoarthritis, so as to
control the occurrence and development of osteoarthritis.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Fujian Province (grant no. 2015J01690).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WC and CT conceived and designed the study, and
reviewed and edited the manuscript. XLu and FF performed the drug
intervention and model replication experiment. JC, X Lu and MH
performed the histology experiment. GW and YH evaluated the
degeneration of cartilage structure by modified Mankin scoring
principles. X Lu, X Liu, RL and ZL performed the transmission
electron microscopy experiment. YH, XLiu and WC performed the
microscopic image analysis. GW and YH designed the experiment and
wrote the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All experimental rats and procedures were approved
by Animal Care and Usage Committee of Fujian University of
Traditional Chinese Medicine (Fuzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kettelkamp DB and Jacobs AW: Tibiofemoral
contact area - determination and implications. J Bone Joint Surg
Am. 54:349–356. 1972.PubMed/NCBI
|
|
2
|
Seedhom BB: Loadbearing function of the
menisci. Physiotherapy. 62(223)1976.PubMed/NCBI
|
|
3
|
Seedhom BB and Hargreaves DJ: Transmission
of the load in the knee joint with special reference to the role in
the menisci: Part II. Experimental results, discussion and
conclusion. Eng Med. 8:220–228. 1979.
|
|
4
|
Ahmed AM and Burke DL: In-vitro
measurement of static pressure distribution in synovial joints -
Part I: Tibial surface of the knee. J Biomech Eng. 105:216–225.
1983.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mow VC, Gu WY and Chen HC: Structure and
function of articular cartilage and meniscus. In: Basic Orthopaedic
Biomechanics and Mechano-Biology. 3rd edition. Mow VC and Huiskes R
(eds). Lippincott Williams & Wilkins, Philadelphia, 2005.
|
|
6
|
McDermott ID, Masouros SD and Amis AA:
Biomechanics of the menisci of the knee. Curr Orthop. 22:193–201.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chevrier A, Nelea M, Hurtig MB, Hoemann CD
and Buschmann MD: Meniscus structure in human, sheep, and rabbit
for animal models of meniscus repair. J Orthop Res. 27:1197–1203.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Englund M, Guermazi A and Lohmander LS:
The meniscus in knee osteoarthritis. Rheum Dis Clin North Am.
35:579–590. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fox AJ, Wanivenhaus F, Burge AJ, Warren RF
and Rodeo SA: The human meniscus: A review of anatomy, function,
injury, and advances in treatment. Clin Anat. 28:269–287.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Fox AJ, Bedi A and Rodeo SA: The basic
science of human knee menisci: Structure, composition, and
function. Sports Health. 4:340–351. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rath E and Richmond JC: The menisci: Basic
science and advances in treatment. Br J Sports Med. 34:252–257.
2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Silverwood V, Blagojevic-Bucknall M, Jinks
C, Jordan JL, Protheroe J and Jordan KP: Current evidence on risk
factors for knee osteoarthritis in older adults: A systematic
review and meta-analysis. Osteoarthritis Cartilage. 23:507–515.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Muthuri SG, McWilliams DF, Doherty M and
Zhang W: History of knee injuries and knee osteoarthritis: A
meta-analysis of observational studies. Osteoarthritis Cartilage.
19:1286–1293. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liao N, Huang Y, Ye J, Chen W, Li ZF, Lin
R, Li X, Zheng L and Liu X: Protective effects of Tougu Xiaotong
capsule on tumor necrosis factor-α-injured UMR-106 cells. Exp Ther
Med. 10:1908–1914. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen S, Huang Y, Chen W, Wu G, Liao N, Li
X, Huang M, Lin R, Yu C, Li X, et al: Protective effects of the
Tougu Xiaotong capsule on morphology and osteoprotegerin/nuclear
factor-κB ligand expression in rabbits with knee osteoarthritis.
Mol Med Rep. 13:419–425. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zheng CS, Ye HZ, Xu XJ and Liu XX:
Computational pharmacology study of tougu xiaotong granule in
preventing and treating knee osteoarthritis. Chin J Integr Med.
15:371–376. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lin MN and Liu XX: Observation of Tougu
Xiaotong Recipe for the treatment of 30 patients with knee
osteoarthritis. Fujian J Tradit Chin Med Chin. 36:15–16, 20 (In
Chinese).
|
|
18
|
Li XH, Wu MX, Ye HZ, Chen WL, Lin JM,
Zheng LP and Liu XX: Experimental study on the suppression of
sodium nitroprussiate-induced chondrocyte apoptosis by Tougu
Xiaotong Capsule-containing serum. Chin J Integr Med. 17:436–443.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li X, Lang W, Ye H, Yu F, Li H, Chen J,
Cai L, Chen W, Lin R, Huang Y, et al: Tougu Xiaotong capsule
inhibits the tidemark replication and cartilage degradation of
papain-induced osteoarthritis by the regulation of chondrocyte
autophagy. Int J Mol Med. 31:1349–1356. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu G, Zhang J, Chen W, Chen S, Huang Y,
Lin R, Huang M, Li Z, Zheng L and Li X: Tougu Xiaotong capsule
exerts a therapeutic effect on knee osteoarthritis by regulating
subchondral bone remodeling. Mol Med Rep. 19:1858–1866.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mankin HJ, Dorfman H, Lippiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteo-arthritic human hips. II. Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg
Am. 53:523–537. 1971.PubMed/NCBI
|
|
22
|
Tan C, Zhang J, Chen W, Feng F, Yu C, Lu
X, Lin R, Li Z, Huang Y, Zheng L, et al: Inflammatory cytokines via
upregulation of aquaporins deteriorated the pathogenesis of early
osteoarthritis. PLoS ONE. 14(e0220846): https://doi.org/10.1371/journal.pone.0220846.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Aaron RK, Racine J and Dyke JP:
Contribution of circulatory disturbances in subchondral bone to the
pathophysiology of osteoarthritis. Curr Rheumatol Rep.
19(49)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hügle T and Geurts J: What drives
osteoarthritis?-synovial versus subchondral bone pathology.
Rheumatology (Oxford). 56:1461–1471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Griffin TM and Guilak F: The role of
mechanical loading in the onset and progression of osteoarthritis.
Exerc Sport Sci Rev. 33:195–200. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guilak F, Fermor B, Keefe FJ, Kraus VB,
Olson SA, Pisetsky DS, Setton LA and Weinberg JB: The role of
biomechanics and inflammation in cartilage injury and repair. Clin
Orthop Relat Res. 423:17–26. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Beaufils P and Pujol N: Management of
traumatic meniscal tear and degenerative meniscal lesions. Save the
meniscus. Orthop Traumatol Surg Res. 103 (8S):S237–S244.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Vaquero J and Forriol F: Meniscus tear
surgery and meniscus replacement. Muscles Ligaments Tendons J.
6:71–89. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Makris EA, Hadidi P and Athanasiou KA: The
knee meniscus: Structure-function, pathophysiology, current repair
techniques, and prospects for regeneration. Biomaterials.
32:7411–7431. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ghazi ZL, Chevrier A, Farr J, Rodeo SA and
Buschmann MD: Augmentation techniques for meniscus repair. J Knee
Surg. 31:99–116. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guo W, Xu W, Wang Z, Chen M, Hao C, Zheng
X, Huang J, Sui X, Yuan Z, Zhang Y, et al: Cell-free strategies for
repair and regeneration of meniscus injuries through the
recruitment of endogenous stem/progenitor cells. Stem Cells Int.
2018(5310471)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pillai MM, Gopinathan J, Selvakumar R and
Bhattacharyya A: human knee meniscus regeneration strategies: A
review on recent advances. Curr Osteoporos Rep. 16:224–235.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cheng T, Wang Q, Zhou J and Wen J:
Clinical study of internal traditional chinese medicine on
functional recovery after sugery under arthroscope for meniscus
injury treatment. Chin J Trad Med Traum Orthop. 23:27–30. 2015.(In
Chinese).
|
|
34
|
Tang HY: Effect of compound longxuejie
powder in the acute phase of meniscus injury. China Health Stand
Manage. 7:150–151. 2016.(In Chinese).
|
|
35
|
Song SF and Li BH: Effects of Jiawei
Huoxue Xiaozhong Decoction on VAS Score and Knee Joint Function in
Meniscus Injury after Arthroscopic Meniscus suture. Med J Liaoning.
31:52–54. 2017.(In Chinese).
|
|
36
|
Gallo MC, Wyatt C, Pedoia V, Kumar D, Lee
S, Nardo L, Link TM, Souza RB and Majumdar S: T1ρ and T2 relaxation
times are associated with progression of hip osteoarthritis.
Osteoarthritis Cartilage. 24:1399–1407. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Englund M, Guermazi A, Roemer F W,
Aliabadi P, Yang M, Lewis CE, Torner J, Nevitt MC, Sack B and
Felson DT: Meniscal tear in knees without surgery and the
development of radiographic osteoarthritis among middle-aged and
elderly persons: The Multicenter Osteoarthritis Study. Arthritis
Rheum. 60:831–839. 2014.
|