Introduction
Chitosan, which is composed of a linear copolymer
compromising β-1,4-linked 2-amino-2-deoxy-β-D-glucose and units of
N-acetyl-D-glucosamine (1,2), is
derived from chitin after alkaline deacetylation. Chitosan
possesses a number of biological properties, including
antimicrobial, antifungal, biodegradable and biocompatible
properties. There is a clinical demand for these properties in
numerous fields, including pharmaceutical drug delivery (3), tissue engineering (4), implants (5), genetic engineering (6), vaccine adjuvants (7) and wound healing (8).
When chitosan is dissolved in acidic medium, the
amino groups of C-2 may be protonated to carry cations and then
interact with negative charges. This property is associated with
its antibacterial ability. When interacting with negative charges
of cell membranes, amino groups disrupt the membrane structure and
induce microbial death (9,10). However, chitosan cannot be dissolved
in neutral aqueous solutions or organic solvents, which limits its
application in certain fields. In addition, the antibacterial
effect has been indicated to be weaker in neutral environments
(10). Therefore, modification of
chitosan to enhance its solubility while maintaining its
antibacterial ability may allow for it to be applied in a broader
range of conditions.
N-[(2-hydroxy-3-trimethylammonium) propyl]
chitosan chloride (HTCC) derivatives, synthesized using an
alkylation reaction to introduce chains to obtain quaternary
ammonium groups, have a degree of substitution of 10-98% (11). As compared with chitosan, this
derivative has a better solubility that may be dissolved in neutral
or alkaline solutions. Furthermore, HTCC possesses the ability to
resist bacteria and fungi (11,12).
Most endodontic and periapical diseases are
attributed to infection with microbes and the principal aim of
treatment is to eliminate these pathogens. Root canal therapy is
currently the major treatment method for endodontic and periapical
diseases. However, based on aseptic conditions, the success rate of
this treatment ranges from 70 to 95% (13). One of the prime reasons for
endodontic failure is persistent infection in the root canal
(14), which troubles patients with
chronic bone defect. The composition of microbial species in filled
root canals, where Enterococcus faecalis is commonly
detected, is limited (15).
Enterococcus faecalis is gram-positive and
capable of growing in anaerobic or aerobic environments. Under the
microscope, it may be observed that E. faecalis exist on
their own, in pairs or in chains, and they are abundant in human
intestines. E. faecalis has been isolated from primary and
persistent endodontic infections. In asymptomatic primary
endodontic infections, the positive rate of E. faecalis
ranged from 4 to 40%, with a prevalence in the persistent lesions
of 24-77% (16,17). Furthermore, as compared with
untreated chronic apical periodontitis, E. faecalis was more
correlated with persistent root canal infection (18).
E. faecalis may survive non-culturable
conditions. It has been reported that E. faecalis that was
inoculated in filled root canals in vitro maintained
viability for a year without nutrients (19). E. faecalis may invade dentinal
tubules and form biofilms, which endow them with more viability and
virulence. Biofilms are environmental adaptations of E.
faecalis that protect and assist microorganisms against harsh
environments and antibiotics, and allows for higher internal
environment stability and viability (20).
Chitosan and quaternary chitosan have broad-spectrum
antibacterial properties (21,22), but
to date, only few studies have investigated the effect of chitosan
and quaternary chitosan, particularly HTCC, on E. faecalis
strains associated with endodontic infection. The aim of the
present study was to explore the efficiency of chitosan and HTCC in
resisting three strains of E. faecalis in the planktonic
state and bacterial biofilms. The antibacterial effect of chitosan
and HTCC in double-distilled water (DDW) and PBS on E.
faecalis was explored and analyzed, respectively. Both DDW and
PBS are common solvents used in root canal irrigations (23,24). A
Cell Counting Kit-8 (CCK-8) cytotoxicity assay was performed to
evaluate the biocompatibility of chitosan and HTCC at various
concentrations.
Materials and methods
Preparation of drugs at different
concentrations
HTCC (YJ201854; Cool Chemistry) was dissolved in DDW
and PBS (P1010; Beijing Solarbio Science & Technology Co. Ltd.)
and the concentration of the stock solution was 10,000
µg/ml. Chitosan (molecular weight, 150 kDa; substitution
degree, 85%; Laizhou Haili Biological Products Co. Ltd.) was
dissolved in 1% (v/v) acetic acid (Jiangsu Qiangsheng Chemical Co.
Ltd.) and the concentration of the initial stock solution was
10,000 µg/ml. DDW and PBS were used to dilute the chitosan
and HTCC solution. The double dilution method was used to prepare a
series of chitosan and HTCC solutions with different
concentrations, namely 2,500, 1,250, 625, 313, 156, 78, 39 and 20
µg/ml. All of these solutions were divided into two groups
according to the solvents; the DDW and PBS groups. The positive
control group was 2% (v/v) sodium hypochlorite (NaClO; Tianjin
Beichen Fangzheng Chemical).
Preparation of bacteria
Three strains of E. faecalis from different
sources were used in the present study: American Type Culture
Collection (ATCC) 29212, P25RC and P52Sa (25-27)
(provided by Professor Chengfei Zhang, Comprehensive Dental Care,
Faculty of Dentistry, University of Hong Kong, Hong Kong, China).
E. faecalis ATCC 29212 is a standard strain, while E.
faecalis P25RC and E. faecalis P52Sa were isolated from
the root canals and saliva of two patients (female; age, 18 years)
with refractory periapical periodontitis in June and September
2009, respectively, at The Hospital of Stomatology, Peking
University (Beijing, China). The participants of the study all
provided written informed consent. This study was approved by the
Medical Ethics Committee of the Affiliated Hospital of Qingdao
University (Qingdao, China).
Three strains of E. faecalis (ATCC 29212,
P25RC and P52Sa) were inoculated on solid brain heart infusion
(BHI) medium containing 1.5% (w/v) agar (cat. no. A8190; Beijing
Solarbio Science & Technology Co. Ltd.) and 3.85% (w/v) BHI
powder (cat. no. A0360; Beijing Solarbio Science & Technology
Co. Ltd.), and cultured anaerobically at 37˚C for 24 h. One colony
in BHI medium was then randomly collected, suspended in BHI broth
and incubated under anaerobic conditions overnight at 37˚C. The
bacterial suspension was adjusted to an optical density at 600 nm
(OD600) of 0.10 using a BioPhotometer plus (Eppendorf).
This was equal to a McFarland standard of 0.5, where the
concentration of E. faecalis was 7.5x107/ml.
Colony-forming unit (CFU) assay
The volume ratio of experimental solution to
bacterial suspension was 9:1 per well in 96-well plates (cat. no.
3599; Corning Inc.). The final drug concentrations were 2,250,
1,125, 563, 282, 140, 70, 35 and 18 µg/ml. The group with
the concentration of 0 µg/ml contained DDW or PBS alone,
without any drugs. The different antibacterial effects of chitosan
or HTCC in DDW or PBS as the solvent was compared at the same
concentrations, with the PBS group used as the control. These
96-well plates were cultivated under anaerobic conditions at 37˚C
for 24 h. Following incubation, the solution in each well was
10-fold diluted at the appropriate concentration and plated on the
BHI medium supplemented with 1.5% (w/v) agar cultured overnight
anaerobically at 37˚C. Colonies on plates were counted to calculate
the bacterial concentration in each well. The residual bacterial
concentrations were compared pairwise to the different
antibacterial effects of chitosan or HTCC in DDW or PBS using the
independent-samples t-test. The experiment was performed in
triplicate and repeated three times independently.
Calculation of the inhibition rate
(IR)
To evaluate the capacity of bacteriostasis at
different concentrations, the IR values of each concentration
gradient were calculated to confirm the minimum bactericidal
concentration (MBC). In this experiment, the 0 µg/ml group
was the control group. The mean CFU (106/ml) was used in
the following formula: IR value=(CFU0 µg/ml - CFUN
µg/ml)/CFU0 µg/ml x100% (N=2,250, 1,125, 563, 282,
140, 70, 35 and 18).
Antibacterial effect on biofilm under
inverted fluorescent microscopy
The concentration of the bacterial suspension of
E. faecalis P25RC was adjusted to OD600=0.10. The
uncoated coverslips (18x18 mm; Sail Brand) were put into 6-well
plates (cat. no. 3516; Corning Inc.) and 2 ml bacterial suspension
was added to each well for culturing biofilms. The medium was
changed every other day. After constant culture for 7 days,
coverslips were randomly selected for staining and the formation of
bacterial biofilm was detected. The coverslips covered with
bacterial biofilms were divided into 7 groups: i) 78 µg/ml
chitosan solution diluted in PBS; ii) 156 µg/ml of HTCC
solution diluted in PBS; iii) PBS solution; iv) 78 µg/ml of
chitosan solution diluted in DDW; v) 156 µg/ml of HTCC
solution diluted in DDW; vi) DDW; vii) 2% NaClO. The volume of the
solution in each well was 2 ml and culture was performed for 24 h.
The coverslips were then rinsed with PBS and stained with the
Live-dead® Baclight™ bacterial viability kit
(cat. no. L-7012; Thermo Fisher Scientific, Inc.). The volume ratio
of propidium iodide/SYTO 9/PBS was 1.5:1.5:1,000 and incubation was
performed for 15 min in the dark. Once the residual dye was rinsed
out, the bacterial biofilms on coverslips were observed using an
inverted fluorescent microscope (magnification, x200; Olympus IX53,
Olympus Corp.). Images were processed with Image J software
(version 1.48; National Institutes of Health) and quantitative data
on the expression of green and red fluorescence were obtained. IR
was used to compare the different antibacterial effects of
experimental solutions on biofilm. The experiment was performed in
triplicate and repeated three times independently. The IR was
calculated using the following formula: IR value=mean red
fluorescence/(mean red fluorescence +mean green fluorescence)
x100%.
Biofilm on dentine observation by
scanning electron microscopy (SEM)
Teeth were extracted from 3 healthy volunteers (sex,
2 female and 1 male; age range, 18-30 years) at the Affiliated
Hospital of Qingdao University (Qingdao, China) in December 2019.
The third permanent molars were enrolled in the study and the
written informed consents were provided. Dentine blocks (5x5x1 mm)
were sliced from the third permanent molars. Following autoclave
sterilization (121˚C; 20 min), they were immersed in bacterial
solution of the E. faecalis P25RC strain
(OD600=0.10). These dentine blocks were cultured for 7
days and the old medium was changed by fresh BHI medium every other
day. After rinsing the surface with DDW, they were divided into 4
groups and placed in DDW, 78 µg/ml chitosan (dissolved in
DDW), 156 µg/ml HTCC (dissolved in DDW) and 2% NaClO
(diluted in DDW) for 24 h. The total volume of the solutions was 2
ml. After rinsing with PBS, the dentine blocks were fixed with 2.5%
glutaraldehyde (2 h; 4˚C) and observed under SEM (Tescan China,
Ltd.). The experiment was performed in triplicate and repeated
three times independently.
Cell proliferation-inhibition
test
MC3T3-E1 pre-osteoblasts (ATCC) were seeded into
96-well plates at 3x103 cells per well and cultured in
α-Minimum Essential Medium (αMEM; Beijing Solarbio Science &
Technology Co. Ltd.) supplemented with 10% (v/v) fetal bovine serum
(Hangzhou Sijiqing Biological Engineering Materials Co. Ltd.) and
1% (v/v) penicillin/streptomycin (Beijing Solarbio Science &
Technology Co. Ltd.) for 24 h. After removing the medium, 100
µl chitosan and HTCC solution at various concentrations were
added in 96-well plates, including 10,000, 5,000, 2,500, 1,250,
625, 31, 156, 78 and 39 µg/ml. These solutions were diluted
with cell culture medium. The control group was cell culture medium
without drugs and the blank group was cell culture medium without
any cells and drugs. After 24, 48 and 72 h, CCK-8 reagent (10%;
cat. no. HY-K0301; MedChemExpress) with αMEM was added following
the removal of residual solution and then incubated in the dark for
2 h. The absorbance (A) value (OD450) of each well was
detected using a microplate reader (Multiskan MK3; Thermo Fisher
Scientific, Inc.) and the mean A values of each group were used to
calculate the proliferation-IR (PIR) of MC3T3-E1 pre-osteoblasts.
The experiment was performed in duplicate and repeated three times
independently. The PIR was calculated as follows: PIR=(OD450
experimental group - OD450 blank
group)/(OD450 normal medium - OD450 blank
group) x100%, where the normal medium represents cell medium
without any other solutions.
Statistical analysis
Data analysis was performed using SPSS version 21
(IBM Corp.). The results were expressed by the mean ± standard
deviation. The independent-samples t-test was applied. P<0.05
was considered to indicate a statistically significant difference.
All experiments were performed in triplicate and repeated three
times independently.
Results
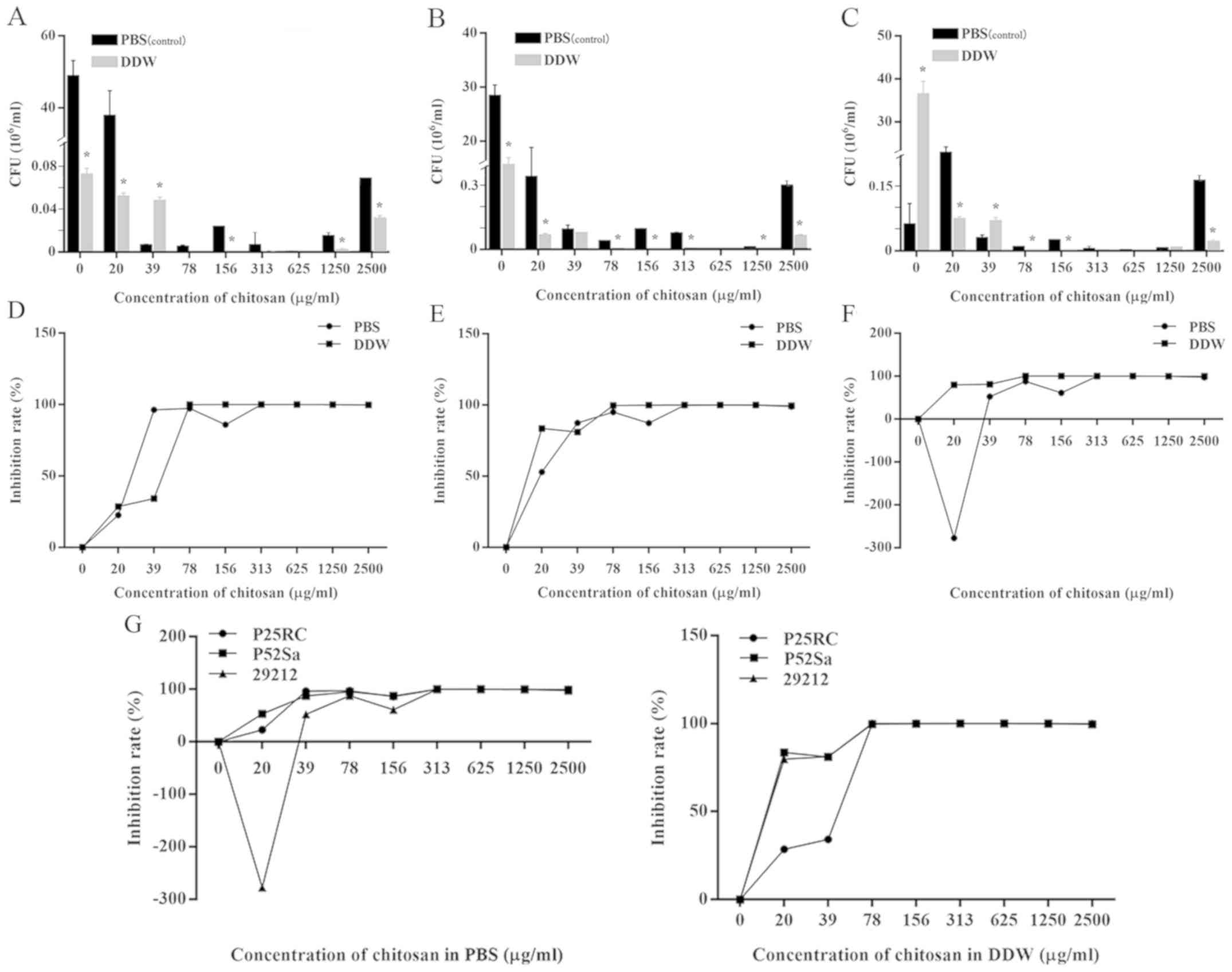
Antibacterial effects of chitosan
The antibacterial effect of chitosan in DDW or PBS
was enhanced as the concentration increased. The MBC of chitosan in
DDW on the three strains was 70 µg/ml, which was lower than
the MBC of 282 µg/ml for chitosan in PBS (Fig. 1D-F). The IR of the positive control
group (2% NaClO) was 100% (data not shown).
The results of the CFU (106/ml) assay
suggested that under most conditions, the antibacterial effect of
chitosan in DDW was greater than that in PBS at the same
concentration (P<0.05; Fig.
1A-C).
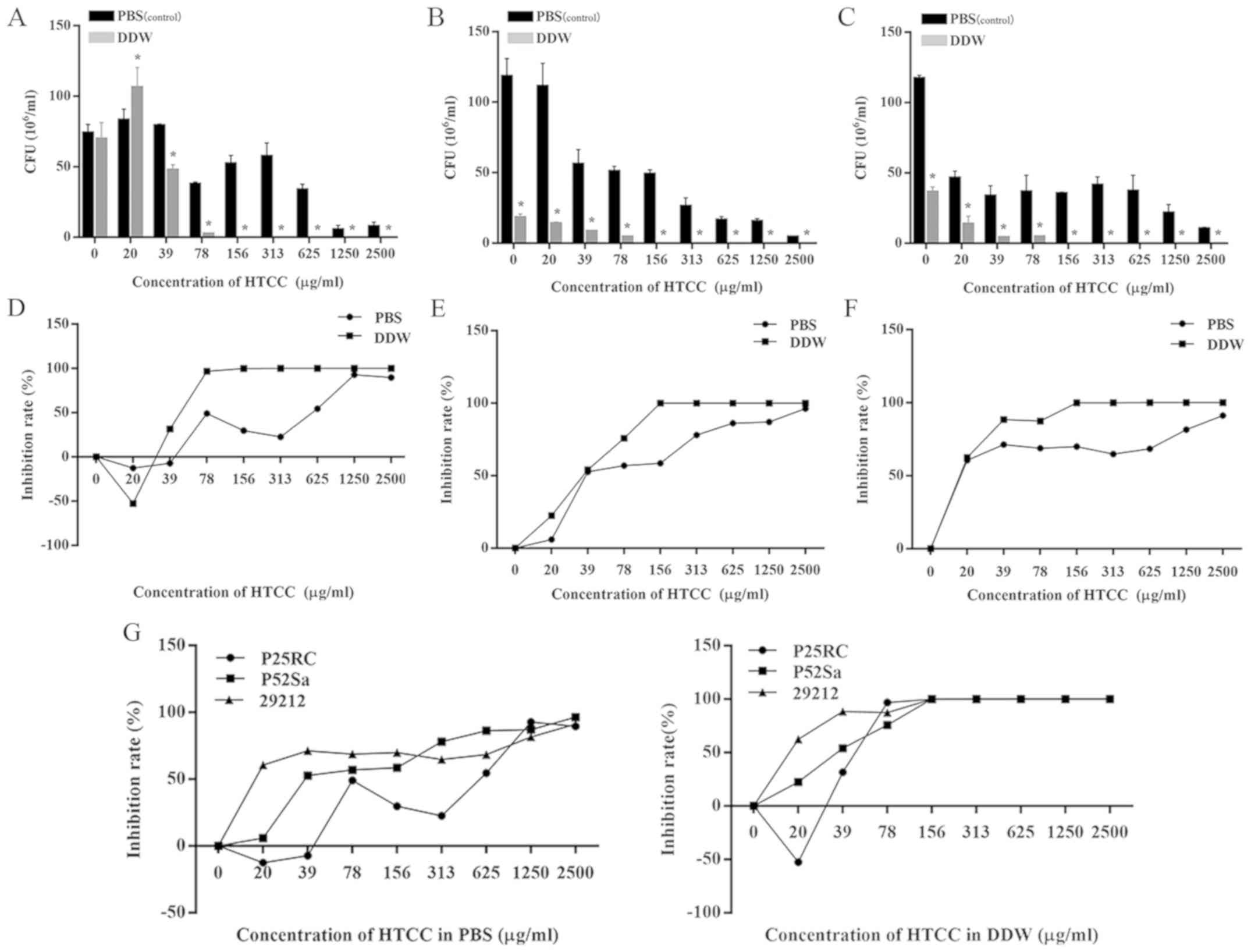
Antibacterial effects of HTCC
The results regarding the inhibitory effects of HTCC
are presented in Fig. 2. The MBC of
HTCC in DDW was 140 µg/ml on the three strains (Fig. 2D-F). The minimum inhibitory
concentration (MIC) for E. faecalis P25RC was 18
µg/ml, while that for the other two strains was outside the
gradient concentration in this experiment (Fig. 2D). Furthermore, it was clearly
demonstrated that, as compared with the negative control, the
bacterial concentrations were significantly increased when the
concentration of HTCC was below the MIC.
It was not possible to determine the definite
bactericidal concentration of HTCC in PBS in the experimental
ranges. The maximum IRs of E. faecalis strains P25RC, P52Sa
and ATCC 29212 were 92.79, 96.34 and 91.19%, respectively. The
final MIC of E. faecalis P25RC in PBS was 70 µg/ml
(Fig. 2D) and the CFU
(106/ml) of concentrations below the MIC were all higher
than the negative control groups. The antibacterial effect of HTCC
dissolved in PBS or DDW on three strains of E. faecalis was
concentration-dependent (Fig.
2).
To compare the antibacterial effect of HTCC in
different solvents, the CFU (106/ml) assay indicated
that, in most conditions, there were statistically significant
differences between HTCC in PBS and DDW at the same concentrations
(P<0.05; Fig. 2A-C). It was
indicated that the number of CFUs in the presence of HTCC in DDW
was generally lower than that in PBS. Furthermore, the IR curve of
HTCC in DDW on all E. faecalis strains was almost higher
compared with that in PBS, which all meant the antibacterial effect
of HTCC in DDW was greater compared with that dissolved in PBS.
(Fig. 2D-F).
In addition, the resistance of the three strains of
bacteria to chitosan and HTCC were presented in Figs. 1G and 2G, where lower IR curves indicate greater
bacterial resistance to chitosan or HTCC. Prior to reaching MBC,
the IR curves of E. faecalis P25RC were generally lower
compared with those of the other strains, apart from those for
chitosan in PBS. This finding suggest that the P25RC strain showed
the greatest resistance to chitosan and HTCC among the three
strains.
Effect on the viability of the
bacterial biofilm using fluorescence microscopy
Stained live and dead bacteria emitted green and red
fluorescence, respectively. According to the IR, 2% NaClO exerted
the greatest bactericidal effect among the 7 groups with the IR of
97.80%. The IR of group 4 was 94.00%, indicating that the
antibacterial effect of 78 µg/ml chitosan in DDW almost
reached that of 2% NaClO. Group 5 (156 µg/ml HTCC in DDW)
had an IR of 79.56% and the values of the other groups were far
below those of these groups (Fig.
3).
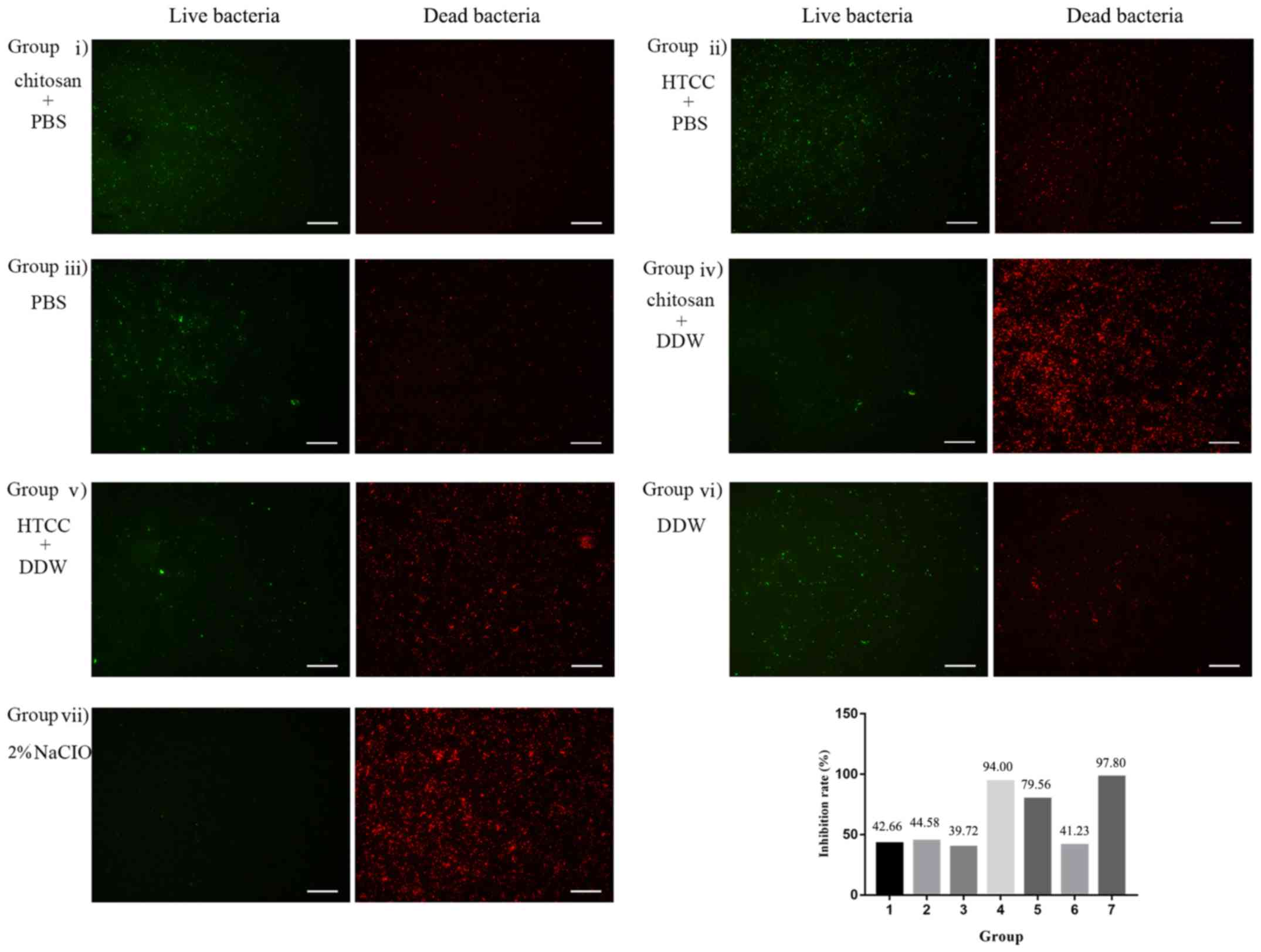 | Figure 3Bacterial biofilms of the E.
faecalis P25RC strain were treated with chitosan, HTCC or 2%
NaClO. Live bacteria emitted green fluorescence and dead bacteria
emitted red fluorescence under the inverted fluorescent microscope
(magnification x200; scale bar, 50 µm). Inhibition rates (%)
of bacteria, presented as a ratio of red/(red + green) fluorescence
x 100% in the biofilm in the 7 groups were presented in a bar
graph. Groups were as follows: i) Chitosan dissolved in PBS at a
concentration of 78 µg/ml, ii) HTCC dissolved in PBS at a
concentration of 156 µg/ml, iii) PBS, iv) Chitosan was
dissolved in DDW at a concentration of 78 µg/ml, v) HTCC was
dissolved in DDW at a concentration of 156 µg/ml, vi) DDW,
vii) 2% NaClO. E. faecalis, Enterococcus faecalis;
HTCC, N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan
chloride; DDW, double-distilled water. |
Effect on biofilm on dentine
surface
The effect of the treatments on biofilm on dentine
blocks was observed by SEM (Fig. 4).
Except for the 2%NaClO group (Fig.
4A), 78 µg/ml chitosan in DDW had the best effect on
biofilms on dentine blocks (Fig.
4C). It was observed that most of the cells were killed and
rinsed away, and a minority remained together with debris. As
compared with the DDW group, HTCC solution was also able to kill
part of the bacteria (Fig. 4D). The
whole structure of the cells on the biofilm was disrupted with
membranolysis following treatment with chitosan or HTCC (Fig. 4E and F) and the dentine surface in the DDW group
was contaminated by more microorganisms (Fig. 4B).
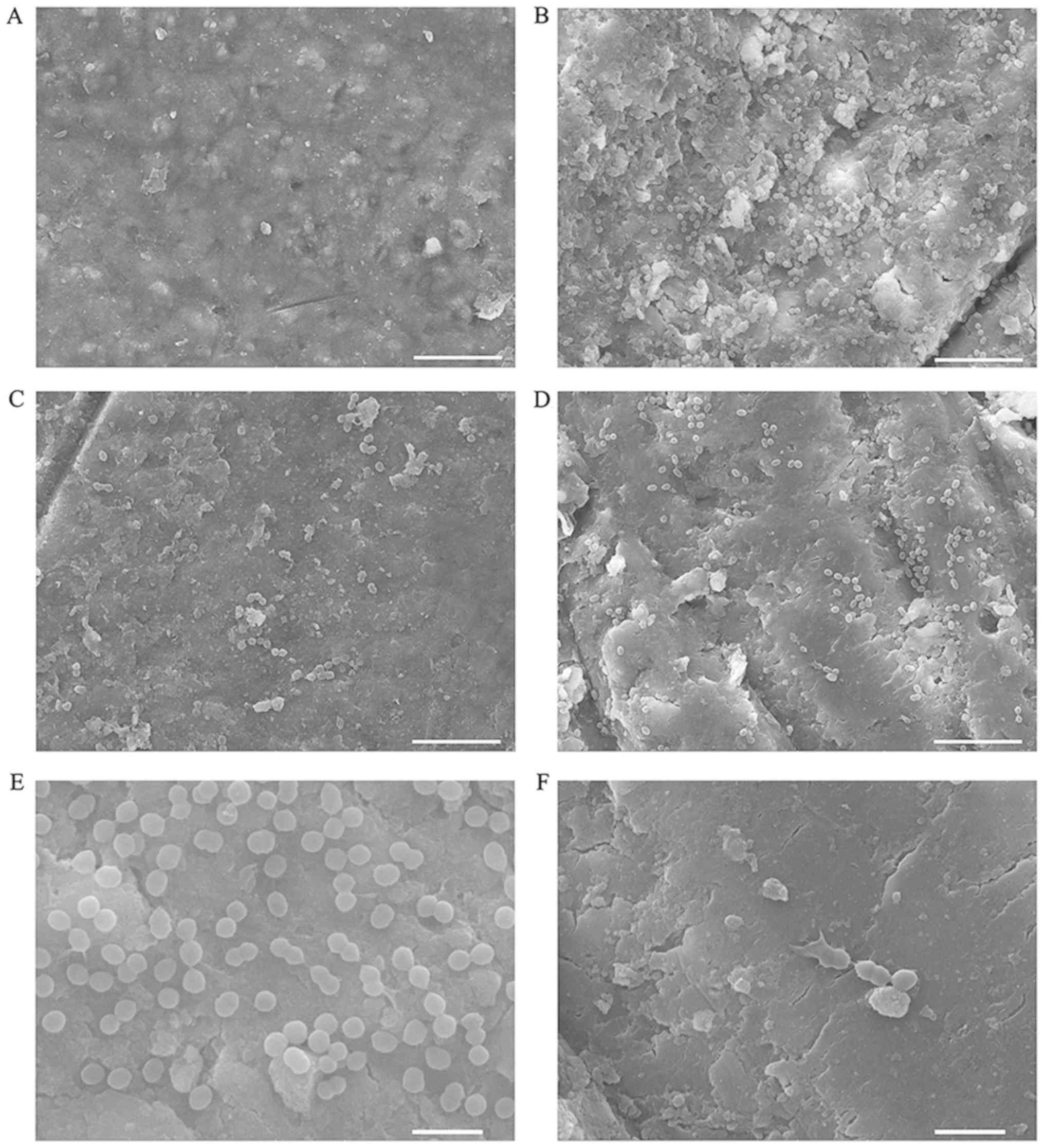 | Figure 4Bacterial biofilms of the E.
faecalis P25RC strain on dentine were observed by scanning
electron microscopy. (A) 2% NaClO, (B) DDW, (C) 78 µg/ml
chitosan in DDW, (D) 156 µg/ml HTCC in DDW (A-D,
magnification, x5,000; scale bar, 10 µm). (E) Normal
morphology of E. faecalis, (F) Rupture of membrane of E.
faecalis after treatment with 156 µg/ml HTCC solution.
(E and F, magnification, x20,000; scale bar, 2 µm). E.
faecalis, Enterococcus faecalis; DDW, double-distilled
water; HTCC, N-(2-hydroxyl) propyl-3-trimethyl ammonium
chitosan chloride. |
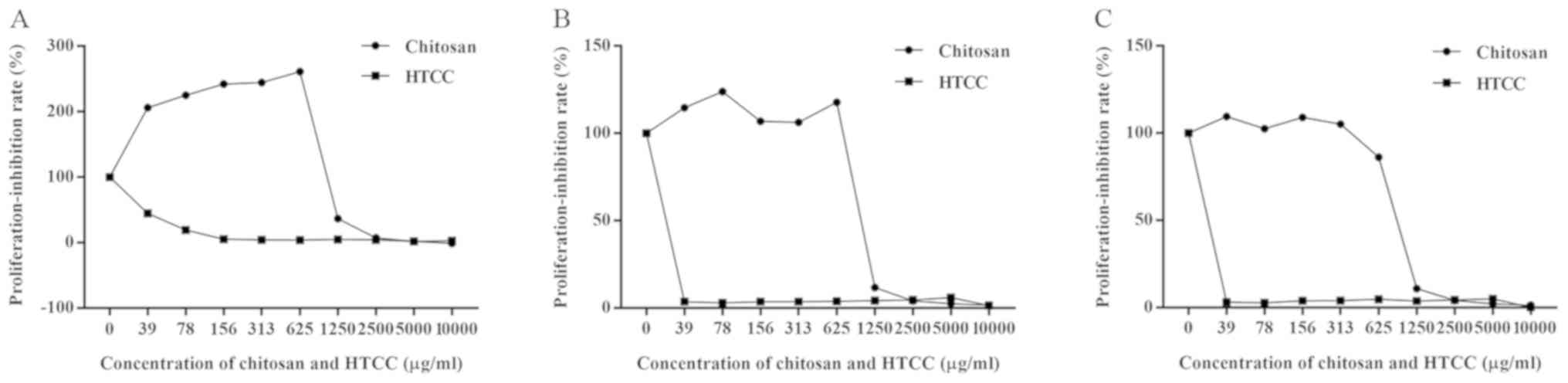
Cell proliferation-inhibition
test
According to the CCK-8 assay performed at 24 h of
incubation, the chitosan solution exhibited superior
biocompatibility and even promoted cell proliferation at <625
µg/ml (Fig. 5). In
particular, the cell proliferation was stimulated by up to 200%
(Fig. 5A). However, HTCC exhibited
higher toxicity than chitosan and only the 39 µg/ml
concentration had relatively lower cytotoxicity than the other
concentrations of HTCC.
Discussion
The present study indicated that chitosan and its
derivative HTCC displayed excellent antibacterial properties
against E. faecalis associated with endodontic infection. It
has been reported that the antibacterial properties of chitosan and
its derivatives are affected by certain aspects, including the
degree of deacetylation, degree of substitution and molecular
weight (28). However, the present
study indicated that the antibacterial effects of chitosan and HTCC
exhibited significant differences in different solvents. According
to the CFU assay, the residual bacterial concentration in the DDW
group, under most conditions, was lower than that in the PBS group
at the same drug concentration. This phenomenon appeared
simultaneously for chitosan and HTCC. These results indicated that
the antibacterial effect was attenuated when the compounds were
dissolved in PBS.
PBS is composed of Na+, K+,
Cl-, HPO42- and
H2PO4-. PBS is usually used as a
buffering solution and unlike DDW, it keeps the pH value stable
(7.2-7.4), which, in the present study, may have interfered with
the antibacterial action of chitosan and HTCC. The mechanism of the
antibacterial effect of chitosan is the interaction between the
positive charges on amino groups of chitosan and negative charges
on the bacterial surface, as well as changes in the permeability of
cell membranes, which induces the rupture of bacteria (29). To obtain HTCC, quaternary ammonium
groups were introduced in the chains of chitosan and the
antibacterial mechanism of this derivative is similar to that of
chitosan (30). It was speculated
that the ions of PBS have a pivotal role in disrupting the
attraction between charges of drugs and E. faecalis,
suggesting that the cations of chitosan and HTCC may bind to anions
in PBS and further result in less contact between the positive
charges of drugs and the negative charges on the bacterial surface.
Furthermore, Chung et al (31) reported that more negative charges on
the cell surface of waterborne pathogens led to an enhanced
interaction with chitosan. Therefore, in the present study, cations
of PBS may also lead to a decline of negative charges on cell
surfaces by competing with positive charges of chitosan or HTCC and
reduce the probability of contact between chitosan or HTCC and
pathogens, finally weakening the antibacterial properties of
chitosan and HTCC. In addition, PBS contains a buffer pair, which
attenuates changes in the pH value. This change in pH value due to
different solvents may affect the protonation of amino groups
(-NH3+) on C-2 of chitosan and HTCC and
further intensify the difference in the antibacterial effect in PBS
and DDW. These speculations indicated that the mechanism of the
antibacterial action of chitosan and HTCC may be associated with
the electrostatic attraction between opposite charges.
Chitosan has been used in root canal therapy for its
antibacterial effect. Ong et al (32) reported that a chitosan-propolis
nanoparticle formulation partly reduced the number of bacteria in
biofilm and pre-formed biofilm at a concentration of 200
µg/ml. In addition, photo-dynamic therapy combined with 3
mg/ml chitosan performed best among various groups (33). 2% Chlorhexidine with chitosan in gel
had the strongest effect against E. faecalis (34). This combination also performed well
in the form of root canal sealer (35). However, the antibacterial effect of
chitosan in a gradient concentration had not been tested in
previous reports. Based on the CFU assay in the present study, it
was observed that the residual bacterial concentration decreased
with the increase of drug concentration in general, until the MBC
was reached. In addition, a suitable concentration may be
determined based on the IR. Kong et al (36) suggested that the antibacterial
activity of chitosan microspheres on E. coli was
proportional to the drug concentration, indicating that enhancement
of the disinfecting effect of chitosan and HTCC may be achieved by
adjusting the concentration.
In addition, the IR curve indicated that the value
of certain low concentrations of chitosan and HTCC was negative,
suggesting that at these concentrations, the residual bacterial
concentration was higher than that in the control groups and that
chitosan and HTCC promoted proliferation instead of inhibiting it.
Chitosan has a hydrolytic susceptibility to certain types of
enzymes from bacteria and may be hydrolyzed to serve as a source of
energy for bacteria (37), which may
lead to bacterial proliferation. According to the MBC, the
antibacterial effect of chitosan was better than that of HTCC. Ji
et al (38) compared the
antibacterial properties of chitosan and HTCC on periodontal
pathogens, indicating that chitosan had a better antibacterial
effect than HTCC, which was consistent with the results of the
present study. However, HTCC is more soluble than chitosan and may
be used as an alternative in root canal therapy.
The drug resistance of clinical strains isolated
from the oral cavities of patients with refractory periapical
periodontitis was stronger than that of the standard strains. Based
on the IR curve, when the concentration of HTCC was below the MBC,
the antibacterial rate of E. faecalis P25RC was the lowest
among the three strains, which was the same as that of chitosan in
DDW, indicating that E. faecalis P25RC may have the highest
drug resistance due to having been isolated from an infectious root
canal. It has been reported that the IRs in clinical strains of
Candida albicans were lower than the standard strains
following photodynamic therapy in biofilms (39). Therefore, E. faecalis P25RC
was selected and then used in the bacterial biofilm experiments to
form more resistant biofilms.
In persistent infectious root canals, E.
faecalis exists in the form of biofilms. According to the IR
curve, 78 µg/ml chitosan in DDW had an IR of 94.00%, which
was close to the IR of 2% NaClO (97.80%). Prior to reaching the
higher cytotoxicity concentration (>625 µg/ml), the IR of
chitosan can be improved by increasing the concentration. However,
chitosan and HTCC were dissolved in PBS and the antibacterial
effects on the biofilms were much worse than those in DDW. It may
be speculated that PBS weakened the antibacterial effect by
interfering with the electrostatic attraction between the amino
groups of chitosan or HTCC and cell membranes. In addition, the
resistance of biofilms to drugs was stronger than that in the
planktonic state. It has been reported that the resistance of
biofilms to antimicrobials, antibodies and phagocytosis is 1,000
times stronger than that of planktonic bacteria (37). It was indicated that in DDW, the
anti-biofilm effect of chitosan was stronger than that of HTCC,
which was consistent with the planktonic state of E.
faecalis. Furthermore, biofilms on dentine blocks were
subjected to SEM and broken cells along with debris were observed.
The morphology and complement of cells were damaged. According to
the quantity of residual bacteria, 78 µg/ml chitosan in DDW
also had a better effect.
Due to the potential of application of chitosan and
its derivatives in disinfection and obturation of root canals, its
biocompatibility was also detected in the present study. The
traditional materials in clinical obturation are substances
including zinc oxide, resin, calcium hydroxide and glass ionomers.
However, nearly each of those materials has certain deficits. For
instance, cytotoxicity has been reported for the components of zinc
oxide and resin sealers and there is insufficient evidence for the
biocompatibility of glass ionomers (40-42).
E. faecalis exhibited resistance to calcium hydroxide via a
proton pump mechanism (43). Hence,
the composition of materials for root canal disinfection and
obturation requires further exploration. According to the CCK-8
assay results, chitosan exhibited clear cytotoxicity when the
concentration was >625 µg/ml. However, below that
concentration, chitosan did not inhibit the proliferation of
MC3T3-E1 pre-osteoblasts at 24, 48 and 72 h, and it even promoted
the proliferation at certain concentrations. The range of available
concentrations for the application of chitosan was expanded while
ensuring bactericidal efficacy and biocompatibility. However, HTCC
exhibited higher cytotoxicity within the concentration range used
in this study and it may be used in the irrigation of root canal
disinfection due to its excellent solubility. HTCC has been
reported as a component in an injectable thermosensitive hydrogel
that may be applied in periodontal therapy and had no acute
toxicity even if exhibiting higher cytotoxicity (44,45).
Therefore, chitosan and HTCC, both of which have strong
antibacterial effects, may be used as alternatives for root canal
disinfection and obturation.
In conclusion, chitosan and HTCC have antibacterial
effects on E. faecalis in the state of plankton and biofilm.
Charge interference reduces the antibiotic efficacy of chitosan and
quaternary chitosan on E. faecalis by affecting the
electrostatic interaction between amino groups and cell membrane.
Chitosan has better biocompatibility, which means it may be used in
long-term root canal disinfection and filling. In addition, HTCC
may also serve as a new material for the disinfection of root
canals.
Acknowledgements
The authors are grateful to Professor Chengfei Zhang
(Comprehensive Dental Care, Faculty of Dentistry, University of
Hong Kong, Hong Kong, China), Professor Xiguang Chen (College of
Marine Life Science, Ocean University of China, Qingdao, China) and
Dr Min Lin (College of Materials Science and Engineering, Qingdao
University, Qingdao, China) for their support by supplying
materials and providing technical guidance.
Funding
The present study was supported by a grant for Young
Scientists from The Affiliated Hospital of Qingdao University in
Shandong, China (grant no. 2646).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW and JD conceived and supervised the present
study. NW, YJ, YZ and XW performed the experiments. NW, LM and HZ
analyzed the data. SW and LM provided materials, as well as
technical and administrative support. NW and SW wrote and reviewed
the manuscript. SW and JD gave the final approval of the version.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of Qingdao University Health Science Center (Qingdao,
China). All the volunteers in this investigation have been provided
written informed consents.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fan Z, Qin Y, Liu S, Xing R, Yu H, Chen X,
Li K and Li P: Synthesis, characterization, and antifungal
evaluation of diethoxyphosphoryl polyaminoethyl chitosan
derivatives. Carbohydr Polym. 190:1–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Moreno JAS, Mendes AC, Stephansen K,
Engwer C, Goycoolea FM, Boisen A, Nielsen LH and Chronakis IS:
Development of electrosprayed mucoadhesive chitosan microparticles.
Carbohydr Polym. 190:240–247. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chi J, Jiang Z, Qiao J, Peng Y, Liu W and
Han B: Synthesis and anti-metastasis activities of
norcantharidin-conjugated carboxymethyl chitosan as a novel drug
delivery system. Carbohydr Polym. 214:80–89. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Saravanan S, Vimalraj S, Thanikaivelan P,
Banudevi S and Manivasagam G: A review on injectable chitosan/beta
glycerophosphate hydrogels for bone tissue regeneration. Int J Biol
Macromol. 121:38–54. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Palla-Rubio B, Araujo-Gomes N,
Fernandez-Gutierrez M, Rojo L, Suay J, Gurruchaga M and Goni I:
Synthesis and characterization of silica-chitosan hybrid materials
as antibacterial coatings for titanium implants. Carbohydr Polym.
203:331–341. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kwak SY, Lew TTS, Sweeney CJ, Koman VB,
Wong MH, Bohmert-Tatarev K, Snell KD, Seo JS, Chua NH and Strano
MS: Chloroplast-selective gene delivery and expression in planta
using chitosan-complexed single-walled carbon nanotube carriers.
Nat Nanotechnol. 14:447–455. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carroll EC, Jin L, Mori A, Munoz-Wolf N,
Oleszycka E, Moran HBT, Mansouri S, McEntee CP, Lambe E, Agger EM,
et al: The vaccine adjuvant chitosan promotes cellular immunity via
DNA sensor cGAS-STING-dependent induction of type I interferons.
Immunity. 44:597–608. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen C, Liu Y, Wang H, Chen G, Wu X, Ren
J, Zhang H and Zhao Y: Multifunctional chitosan inverse opal
particles for wound healing. ACS Nano. 12:10493–10500.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shariatinia Z: Pharmaceutical applications
of chitosan. Adv Colloid Interface Sci. 263:131–194.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kong M, Chen XG, Xing K and Park HJ:
Antimicrobial properties of chitosan and mode of action: A state of
the art review. Int J Food Microbiol. 144:51–63. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shagdarova B, Lunkov A, Il'ina A and
Varlamov V: Investigation of the properties of
N-[(2-hydroxy-3-trimethylammonium) propyl] chloride chitosan
derivatives. Int J Biol Macromol. 124:994–1001. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hoque J, Adhikary U, Yadav V, Samaddar S,
Konai MM, Prakash RG, Paramanandham K, Shome BR, Sanyal K and
Haldar J: Chitosan derivatives active against multidrug-resistant
bacteria and pathogenic fungi: In vivo evaluation as topical
antimicrobials. Mol Pharm. 13:3578–3589. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Weiger R, Axmann-Krcmar D and Lost C:
Prognosis of conventional root canal treatment reconsidered. Endod
Dent Traumatol. 14:1–9. 1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sjogren U, Figdor D, Persson S and
Sundqvist G: Influence of infection at the time of root filling on
the outcome of endodontic treatment of teeth with apical
periodontitis. Int Endod J. 30:297–306. 1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sundqvist G, Figdor D, Persson S and
Sjogren U: Microbiologic analysis of teeth with failed endodontic
treatment and the outcome of conservative re-treatment. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 85:86–93. 1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Stuart CH, Schwartz SA, Beeson TJ and
Owatz CB: Enterococcus faecalis: Its role in root canal
treatment failure and current concepts in retreatment. J Endod.
32:93–98. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rocas IN, Siqueira JF Jr and Santos KR:
Association of Enterococcus faecalis with different forms of
periradicular diseases. J Endod. 30:315–320. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang C, Du J and Peng Z: Correlation
between Enterococcus faecalis and persistent intraradicular
infection compared with primary intraradicular infection: A
systematic review. J Endod. 41:1207–1213. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sedgley CM, Lennan SL and Appelbe OK:
Survival of Enterococcus faecalis in root canals ex vivo.
Int Endod J. 38:735–742. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen W, Liang J, He Z and Jiang W:
Differences in the chemical composition of Enterococcus
faecalis biofilm under conditions of starvation and alkalinity.
Bioengineered. 8:1–7. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Verlee A, Mincke S and Stevens CV: Recent
developments in antibacterial and antifungal chitosan and its
derivatives. Carbohydr Polym. 164:268–283. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheah WY, Show PL, Ng IS, Lin GY, Chiu CY
and Chang YK: Antibacterial activity of quaternized chitosan
modified nanofiber membrane. Int J Biol Macromol. 126:569–577.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Conte MC, da Silveira Teixeira C,
Bortoluzzi EA, Felippe WT, Dos Santos LGP, Pandolfo MT, da Agostim
Cancelier P and da Fonseca Roberti Garcia L: Effect of medicaments
used in endodontic regeneration on the morphological
characteristics of bovine radicular dentin: Experimental immature
tooth model. Microsc Res Tech. 83:354–361. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tonini R, Giovarruscio M, Gorni F, Ionescu
A, Brambilla E, Mikhailovna IM, Luzi A, Maciel Pires P and Sauro S:
In vitro evaluation of antibacterial properties and smear layer
removal/sealer penetration of a novel silver-citrate root canal
irrigant. Materials (Basel). 13(E194)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang S, Heng BC, Qiu S, Deng J, Shun Pan
Cheung G, Jin L, Zhao B and Zhang C: Lipoteichoic acid of
Enterococcus faecalis inhibits osteoclastogenesis via
transcription factor RBP-J. Innate Immun. 25:13–21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang S, Deng Z, Ye X, Geng X and Zhang C:
Enterococcus faecalis attenuates osteogenesis through
activation of p38 and ERK1/2 pathways in MC3T3-E1 cells. Int Endod
J. 49:1152–1164. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu X, Wang Q, Zhang C, Cheung GS and Shen
Y: Prevalence, phenotype, and genotype of Enterococcus
faecalis isolated from saliva and root canals in patients with
persistent apical periodontitis. J Endod. 36:1950–1955.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sahariah P, Gaware VS, Lieder R,
Jonsdottir S, Hjalmarsdottir MA, Sigurjonsson OE and Masson M: The
effect of substituent, degree of acetylation and positioning of the
cationic charge on the antibacterial activity of quaternary
chitosan derivatives. Mar Drugs. 12:4635–4658. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kravanja G, Primozic M, Knez Z and Leitgeb
M: Chitosan-based (Nano)materials for novel biomedical
applications. Molecules. 24(E1960)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tan H, Ma R, Lin C, Liu Z and Tang T:
Quaternized chitosan as an antimicrobial agent: Antimicrobial
activity, mechanism of action and biomedical applications in
orthopedics. Int J Mol Sci. 14:1854–1869. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chung YC, Su YP, Chen CC, Jia G, Wang HL,
Wu JC and Lin JG: Relationship between antibacterial activity of
chitosan and surface characteristics of cell wall. Acta Pharmacol
Sin. 25:932–936. 2004.PubMed/NCBI
|
|
32
|
Ong TH, Chitra E, Ramamurthy S,
Siddalingam RP, Yuen KH, Ambu SP and Davamani F: Chitosan-propolis
nanoparticle formulation demonstrates anti-bacterial activity
against Enterococcus faecalis biofilms. Plos One.
12(e0174888)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Camacho-Alonso F, Julian-Belmonte E,
Chiva-Garcia F and Martinez-Beneyto Y: Bactericidal efficacy of
photodynamic therapy and chitosan in root canals experimentally
infected with Enterococcus faecalis: An in vitro study.
Photomed Laser Surg. 35:184–189. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Savitha A, SriRekha A, Vijay R, Ashwija
Champa C and Jaykumar T: An in vivo comparative evaluation of
antimicrobial efficacy of chitosan, chlorhexidine gluconate gel and
their combination as an intracanal medicament against
Enterococcus faecalis in failed endodontic cases using real
time polymerase chain reaction (qPCR). Saudi Dent J. 31:360–366.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Loyola-Rodriguez JP, Torres-Mendez F,
Espinosa-Cristobal LF, García-Cortes JO, Loyola-Leyva A, González
FJ, Soto-Barreras U, Nieto-Aguilar R and Contreras-Palma G:
Antimicrobial activity of endodontic sealers and medications
containing chitosan and silver nanoparticles against
Enterococcus faecalis. J Appl Biomater Funct Mater.
17(2280800019851771)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kong M, Chen XG, Liu CS, Liu CG, Meng XH
and Yu le J: Antibacterial mechanism of chitosan microspheres in a
solid dispersing system against E. coli. Colloids Surf B
Biointerfaces. 65:197–202. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li Y, Chen XG, Liu N, Liu CS, Liu CG, Meng
XH, Yu LJ and Kenendy JF: Physicochemical characterization and
antibacterial property of chitosan acetates. Carbohydrate Polymers.
67:227–232. 2007.
|
|
38
|
Ji QX, Zhong de Y, Lu R, Zhang WQ, Deng J
and Chen XG: In vitro evaluation of the biomedical properties of
chitosan and quaternized chitosan for dental applications.
Carbohydr Res. 344:1297–1302. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ma J, Shi H, Sun H, Li J and Bai Y:
Antifungal effect of photodynamic therapy mediated by curcumin on
Candida albicans biofilms in vitro. Photodiagnosis Photodyn Ther.
27:280–287. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schwarze T, Leyhausen G and Geurtsen W:
Long-term cytocompatibility of various endodontic sealers using a
new root canal model. J Endod. 28:749–753. 2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Troiano G, Perrone D, Dioguardi M,
Buonavoglia A, Ardito F and Lo Muzio L: In vitro evaluation of the
cytotoxic activity of three epoxy resin-based endodontic sealers.
Dent Mater J. 37:374–378. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Patel E, Pradeep P, Kumar P, Choonara YE
and Pillay V: Oroactive dental biomaterials and their use in
endodontic therapy. J Biomed Mater Res B Appl Biomater.
108:201–212. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Evans M, Davies JK, Sundqvist G and Figdor
D: Mechanisms involved in the resistance of Enterococcus
faecalis to calcium hydroxide. Int Endod J. 35:221–228.
2002.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ji QX, Chen XG, Zhao QS, Liu CS, Cheng XJ
and Wang LC: Injectable thermosensitive hydrogel based on chitosan
and quaternized chitosan and the biomedical properties. J Mater Sci
Mater Med. 20:1603–1610. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ji QX, Zhao QS, Deng J and Lu R: A novel
injectable chlorhexidine thermosensitive hydrogel for periodontal
application: Preparation, antibacterial activity and toxicity
evaluation. J Mater Sci Mater Med. 21:2435–2442. 2010.PubMed/NCBI View Article : Google Scholar
|